Fabrication of CZTSe/CIGS Nanowire Arrays by One-Step Electrodeposition for Solar-Cell Application
Abstract
1. Introduction
2. Materials and Methods
- The membrane is easy to remove by chemical dissolution in dichloromethane with negligible damages to the nanostructures;
- The polycarbonate and the dichloromethane can be recovered, after the membrane dissolution, using a simple batch distillation [25].
2.1. CBD-ZnS(O,OH) Buffer Layer Deposition
2.2. ZnO/AZO Deposition
3. Results and Discussion
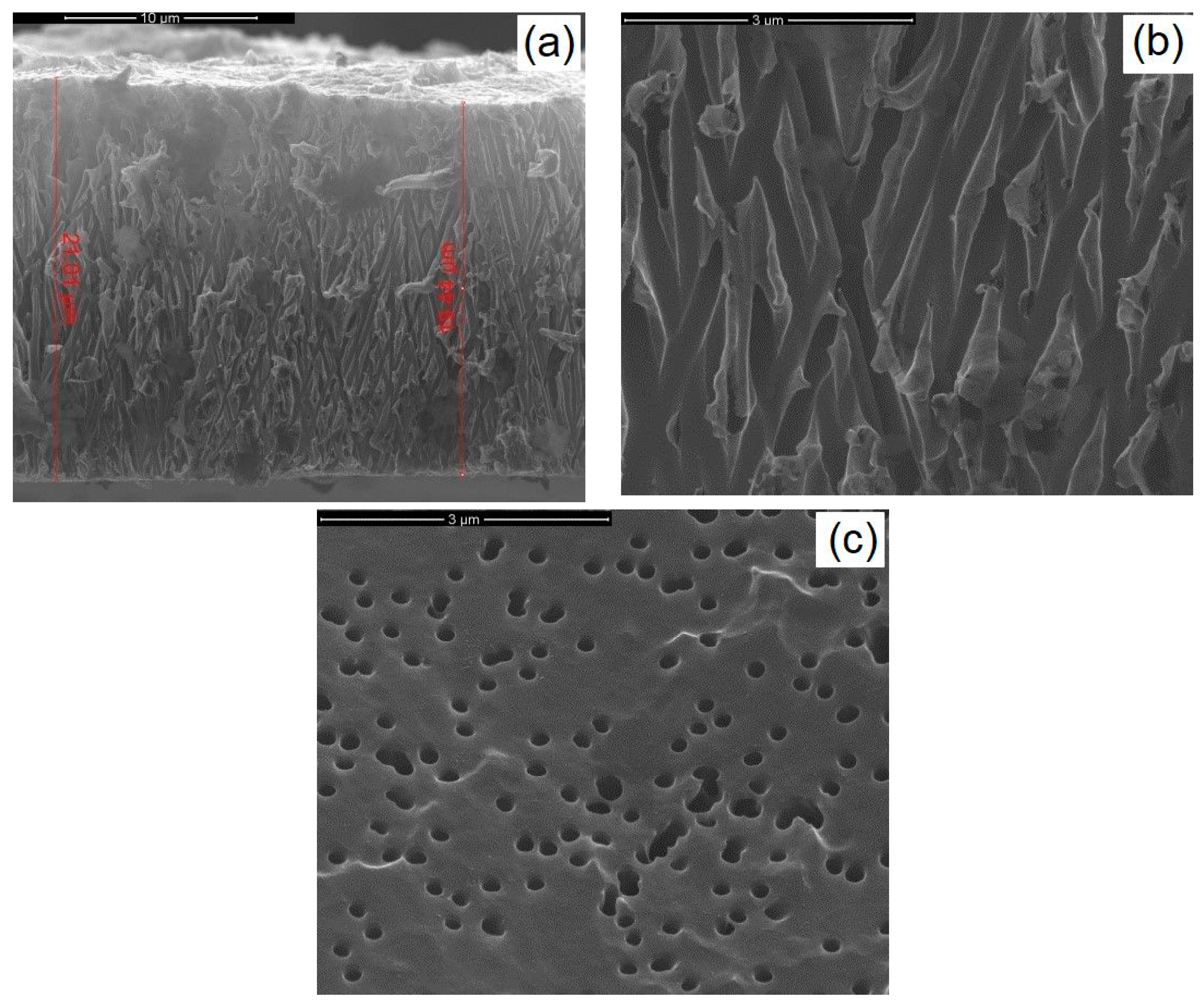
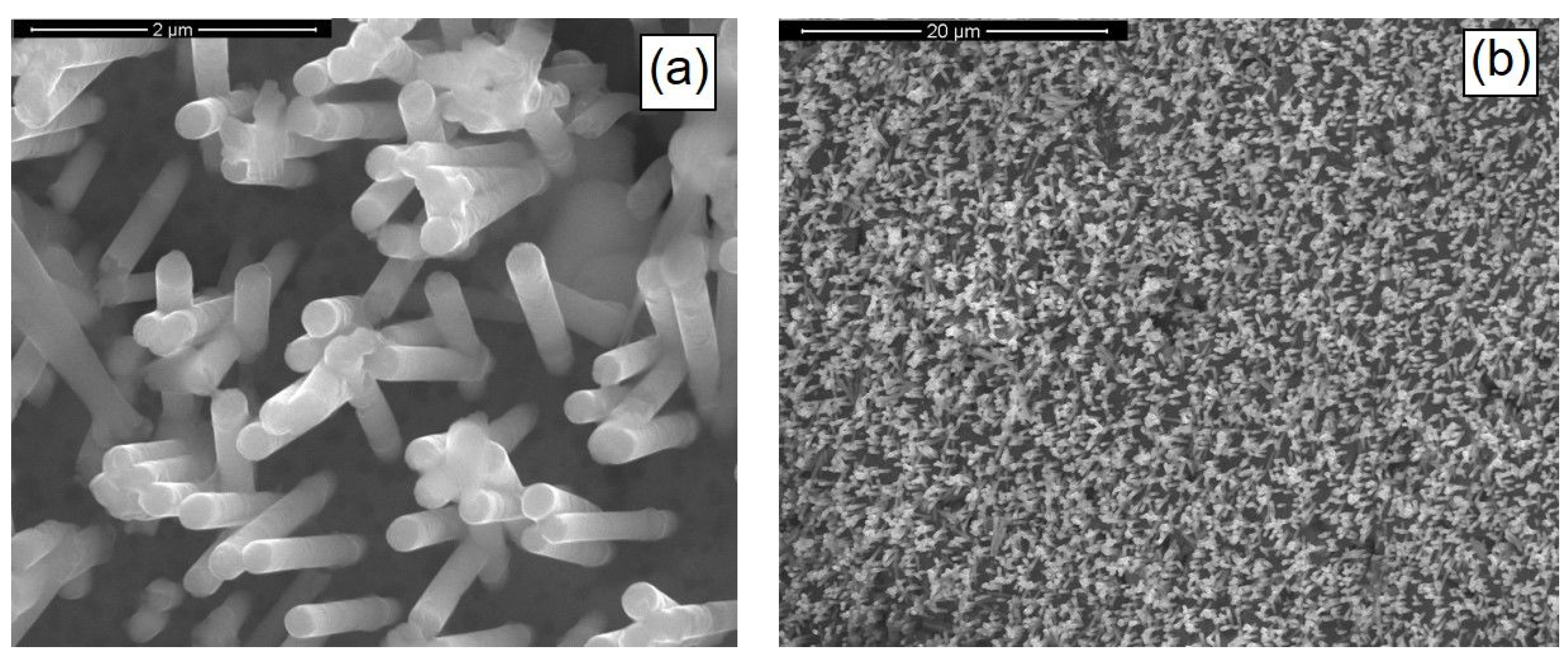
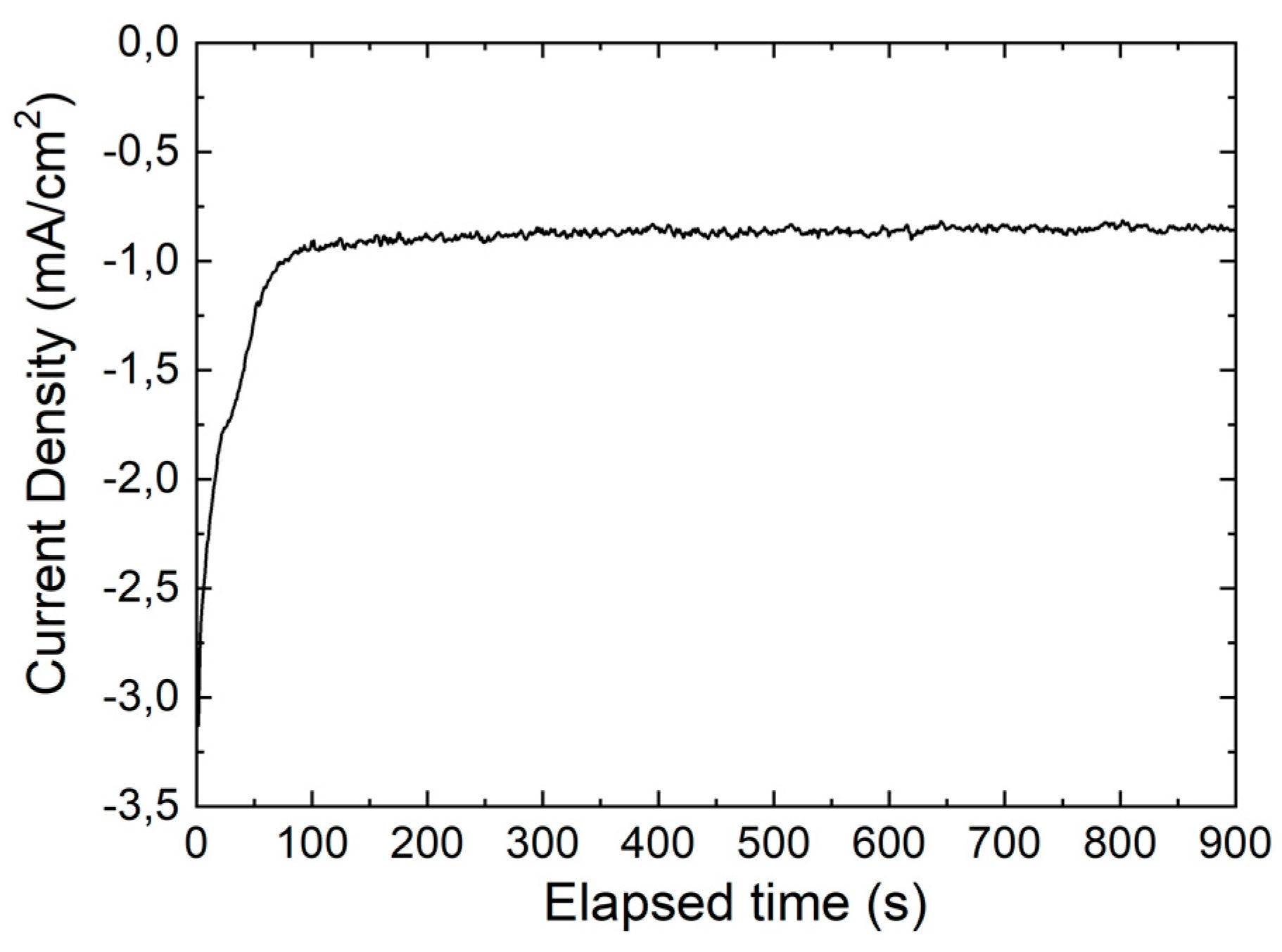
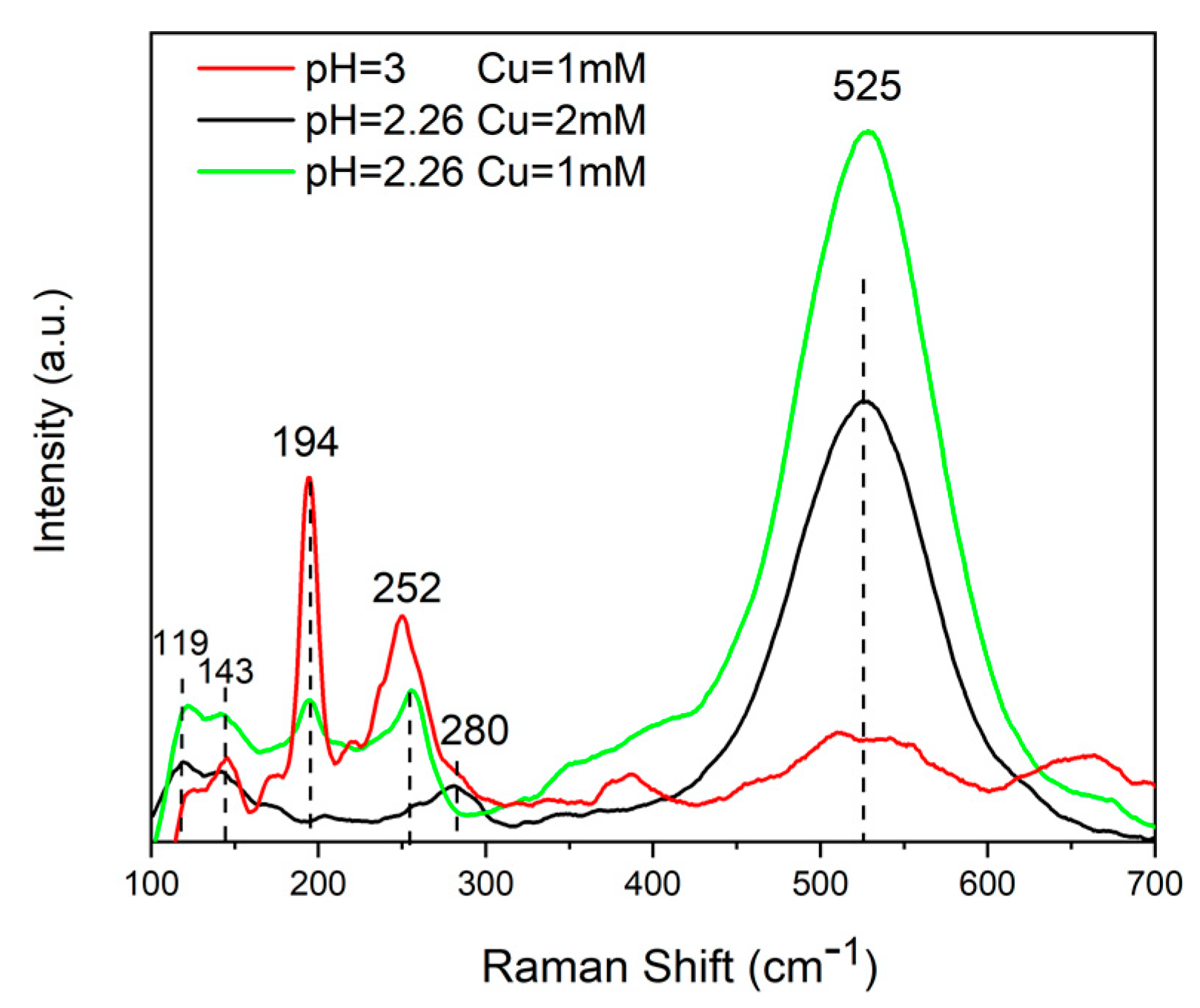
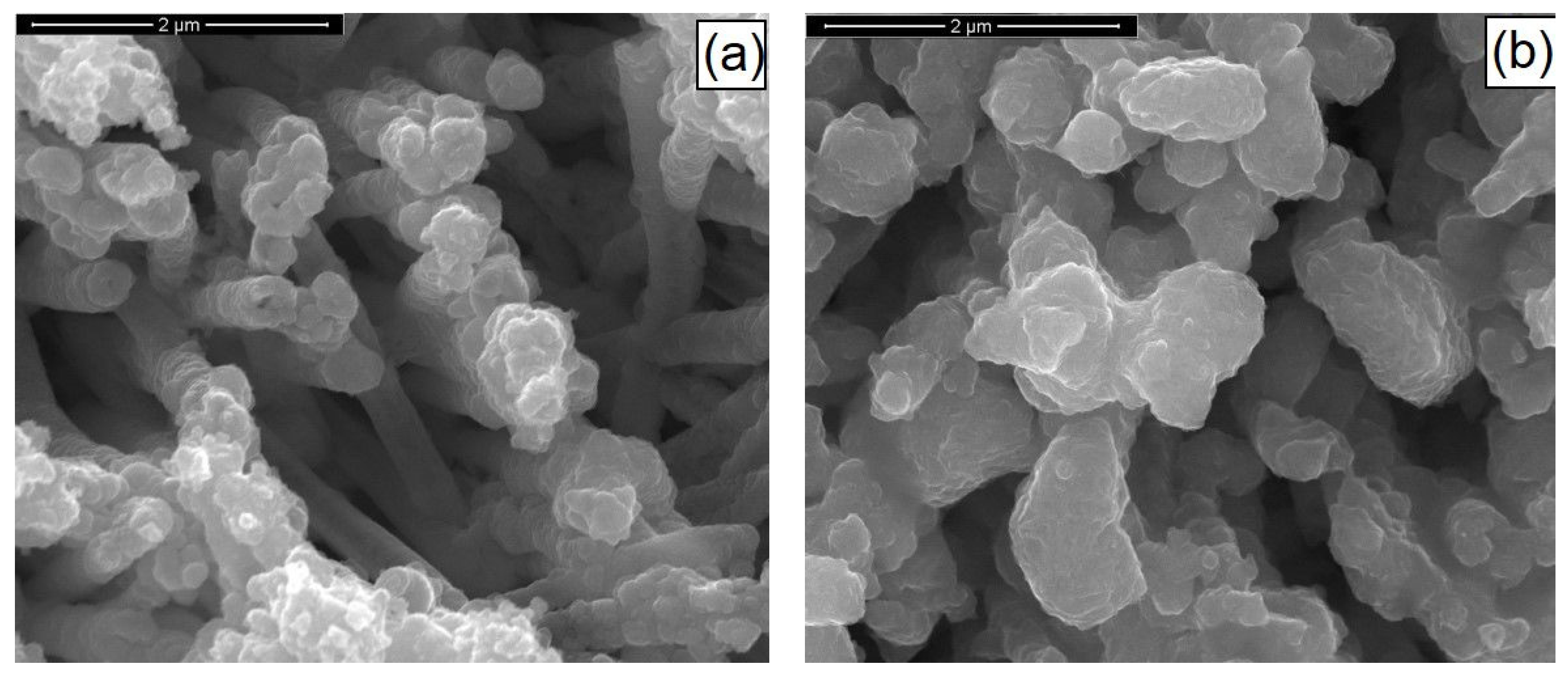
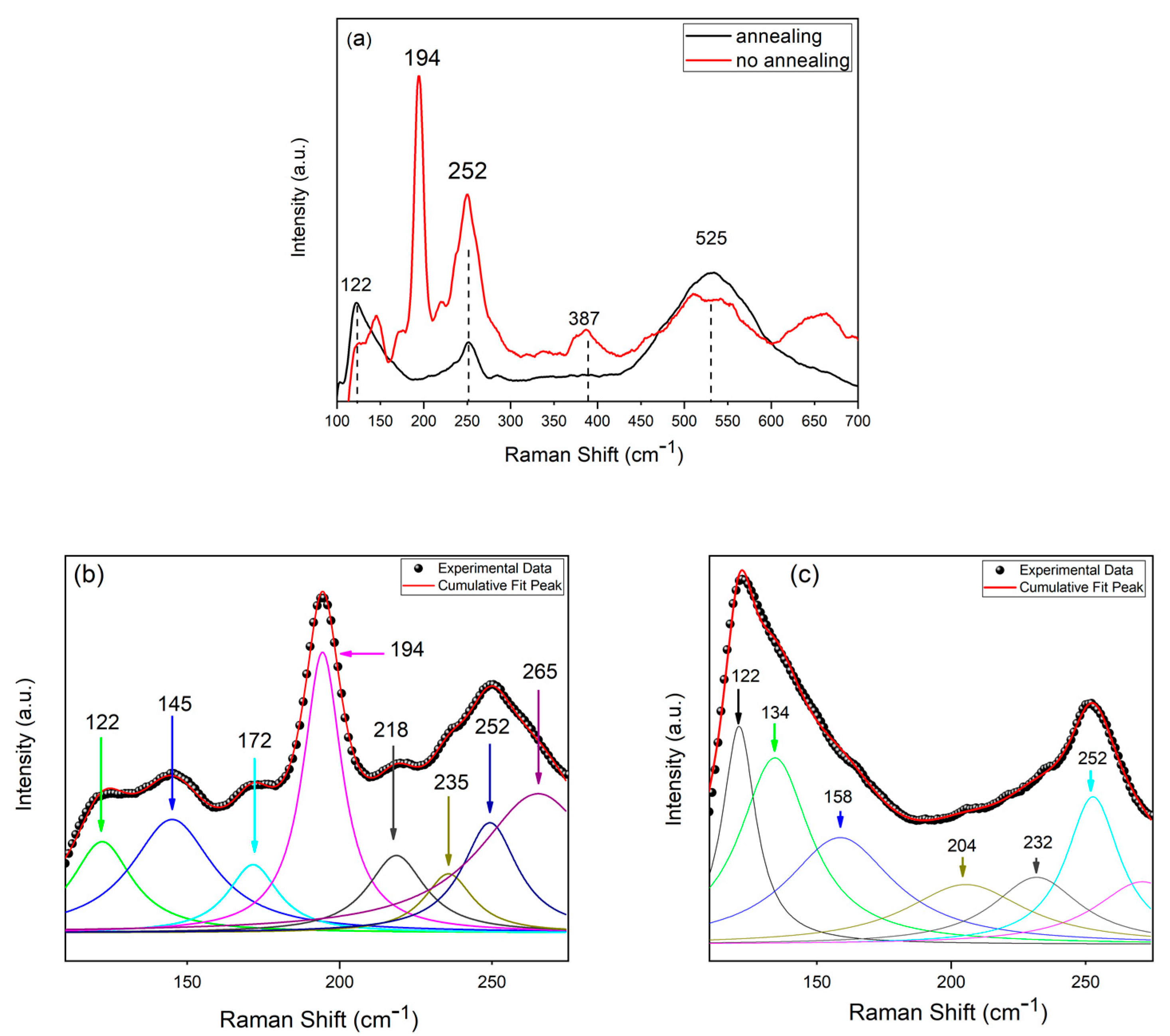
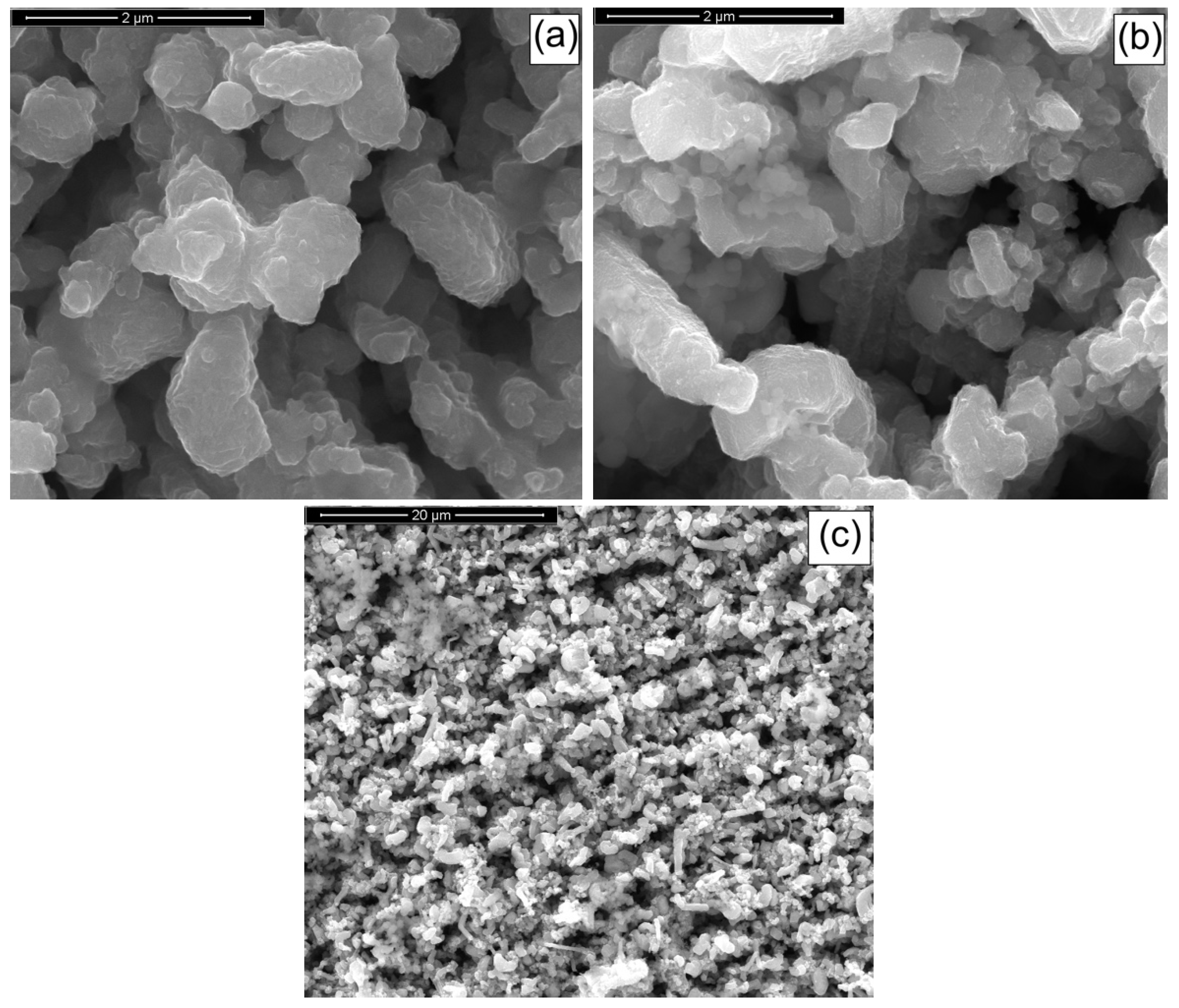
3.1. CZTS Nanowires
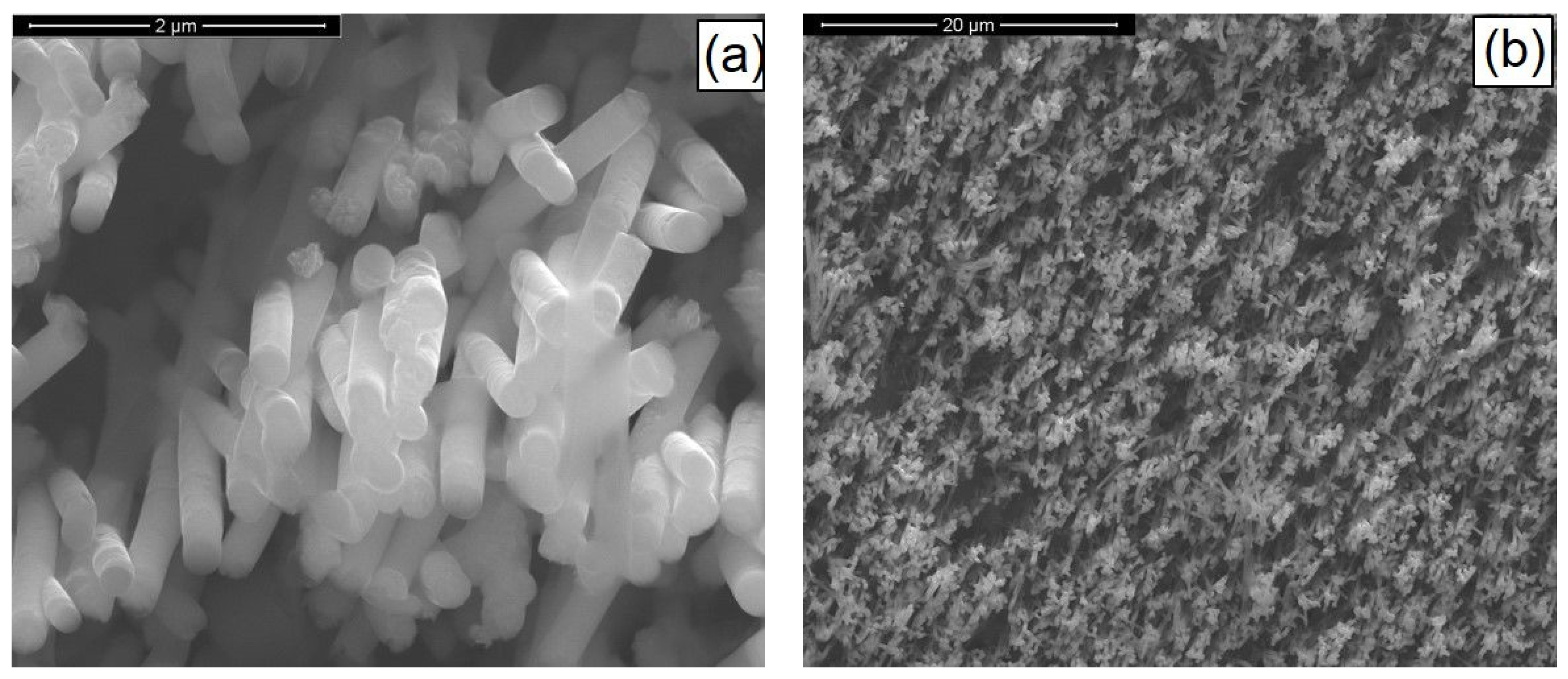
3.2. CIGS Nanowires
3.3. CZTSe/ZnS/ZnO-AZO Fabrication
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thony, P. Semiconductor nanowires for solar cells. In Semiconductor Nanowires Materials, Synthesis, Characterization and Applications, 2nd ed.; Arbiol, J., Xiong, Q., Eds.; Woodhead Publishing Series in Electronic and Optical Materials: Number 77; Elsevier: Amsterdam, The Netherlands, 2015; pp. 411–439. [Google Scholar]
- Green, M.; Dunlop, E.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Hao, X. Solar cell efficiency tables (version 57). Prog. Photovolt. Res. Appl. 2021, 29, 3. [Google Scholar] [CrossRef]
- Schock, H.W. High efficiency thin-film solar cells. Green 2013, 2, 149–157. [Google Scholar]
- Steinberger, H. Health, safety and environmental risks from the operation of CdTe and CIS thin-film modules. Prog. Photovolt. Res. Appl. 1998, 6, 99. [Google Scholar] [CrossRef]
- Raugei, M.; Fthenakis, V. Cadmium flows and emissions from CdTe PV: Future expectations. Energy Policy 2010, 38, 5223. [Google Scholar] [CrossRef]
- Ito, K.; Nakazawa, T. Electrical and optical properties of stannite-type quaternary semiconductor thin films. Jpn. J. Appl. Phys. 1988, 27, 2094. [Google Scholar] [CrossRef]
- Gupta, N.; Alapatt, G.F.; Podila, R.; Singh, R.; Poole, K.F. Prospects of nanostructure-based solar cells for manufacturing future generations of photovoltaic modules. Int. J. Photoenergy 2009, 2009, 154059. [Google Scholar] [CrossRef]
- Battaglia, M.; Sunseri, C.; Piazza, S.; Inguanta, R. Cu2ZnSnSe4 nanotubes and nanowires by template electrosynthesis. Adv. Sci. Technol. 2014, 93, 241–246. [Google Scholar] [CrossRef]
- Katagiri, H.; Ishigaki, N.; Ishida, T.; Saito, K. Characterization of Cu2ZnSnS4 Thin Films Prepared by Vapor Phase Sulfurization. Jpn. J. Appl. Phys. 2001, 40, 500. [Google Scholar] [CrossRef]
- Araki, H.; Mikaduki, A.; Kubo, Y.; Sato, T.; Jimbo, K.; Maw, W.S.; Katagiri, H.; Yamazaki, M.; Oishi, K.; Takeuchi, A. Preparation of Cu2ZnSnS4 thin films by sulfurization of stacked metallic layers. Thin Solid Films 2008, 517, 1457. [Google Scholar] [CrossRef]
- Shi, C.; Shi, G.; Chen, Z.; Yang, P.; Yao, M. Deposition of Cu2ZnSnS4 thin films by vacuum thermal evaporation from single quaternary compound source. Mater. Lett. 2012, 73, 89. [Google Scholar] [CrossRef]
- Jimbo, K.; Kimura, R.; Kamimura, T.; Yamada, S.; Mav, W.S.; Araki, H.; Oishi, K.; Katagiri, H. Cu2ZnSnS4-type thin film solar cells using abundant materials. Thin Solid Films 2007, 515, 5997. [Google Scholar] [CrossRef]
- Ericson, T.; Kubbart, T.; Scragg, J.J.; Platzer-Björkman, C. Reactive sputtering of precursors for Cu2ZnSnS4 thin film solar cells. Thin Solid Films 2012, 520, 7093. [Google Scholar] [CrossRef]
- Liu, F.; Li, Y.; Zhang, K.; Wang, B.; Yan, C.; Lai, Y.; Zhang, Z.; Liu, J. In situ growth of Cu2ZnSnS4 thin films by reactive magnetron co-sputtering. Sol. Energy Mater. Sol. Cells 2010, 94, 2431. [Google Scholar] [CrossRef]
- Fernandes, P.A.; Salome, P.M.P.; de Cunha, A.F. Growth and Raman scattering of Cu2ZnSnS4 thin films. Thin Solid Films 2009, 517, 2519. [Google Scholar] [CrossRef]
- Scragg, J.J.; Dale, P.J.; Peter, M.L. Towards sustainable materials for solar energy conversion: Preparation and photoelectrochemical characterization of Cu2ZnSnS4. Electrochem. Commun. 2008, 10, 639. [Google Scholar] [CrossRef]
- Ennaoui, A.; Lux-Steiner, M.; Weber, A.; Abou-Ras, D.; Kötschau, I.; Schock, H.-W.; Schurr, R.; Hölzing, A.; Jost, S.; Hock, R.; et al. Cu2ZnSnS4 thin film solar cells from electroplated precursors: Novel low-cost perspective. Thin Solid Films 2009, 517, 2511. [Google Scholar] [CrossRef]
- Oliveri, R.L.; Ferrara, G.; Livreri, P.; Piazza, S.; Sunseri, C.; Inguanta, R. Investigation of Annealing Conditions on Electrochemically Deposited CZTS Film on Flexible Molybdenum Foil. J. Electrochem. Soc. 2016, 163, D532. [Google Scholar] [CrossRef]
- Tanaka, K.; Kato, M.; Goto, K.; Nakano, Y.; Uchik, H. Face-to-Face Annealing Process of Cu2ZnSnS4 Thin Films Deposited by Spray Pyrolysis Method. Jpn. J. Appl. Phys. 2012, 51, 10NC26. [Google Scholar] [CrossRef]
- Yoo, H.; Kim, J. Comparative study of Cu2ZnSnS4 film growth. Sol. Energy Mater. Sol. Cells 2011, 95, 239. [Google Scholar] [CrossRef]
- Moholkar, A.V.; Shinde, S.S.; Babar, A.R.; Sim, K.; Kwon, Y.; Rajpure, K.Y.; Patil, P.S.; Bhosale, C.H.; Kimb, J.H. Development of CZTS thin films solar cells by pulsed laser deposition: Influence of pulse repetition rate. Sol. Energy 2011, 85, 1354. [Google Scholar] [CrossRef]
- Su, Z.; Yan, C.; Tang, D.; Sun, K.; Han, Z.; Liu, F.; Lai, Y.; Li, J.; Liu, Y. Fabrication of Cu2ZnSnS4 nanowires and nanotubes based on AAO templates. Cryst. Eng. Comm. 2012, 14, 782. [Google Scholar] [CrossRef]
- Inguanta, R.; Spanò, T.; Oliveri, R.L.; Piazza, S.; Sunseri, C. Electrodeposition and Photo-electrochemical Behavior of CIGS Thin Films and Nanowire Arrays for Solar Cell. Chem. Eng. Trans. 2013, 32, 343–348. [Google Scholar]
- Wang, C.E.; Tanaka, S.; Shimizu, T.; Shingubara, S. Fabrication of vertical Cu2ZnSnS4 Nanowires Arrays by Two-Step Electroplating Method into Anodic Aluminum Oxide Template. J. Mater. Sci. Nanotechnol. 2014, 1, 1–4. [Google Scholar]
- Insinga, M.G.; Oliveri, R.L.; Sunseri, C.; Inguanta, R. Template electrodeposition and characterization of nanostructured Pb as a negative electrode for lead-acid battery. J. Power Sources 2019, 413, 107–116. [Google Scholar] [CrossRef]
- Insinga, M.G.; Moncada, A.; Oliveri, R.L.; Ganci, F.; Piazza, S.; Sunseri, C.; Inguanta, R. Nanostructured Pb electrode for innovative leadacid battery. Chem. Eng. Trans. 2017, 60, 49–54. [Google Scholar]
- Jackson, P.; Hariskos, D.; Lotter, E.; Paetel, S.; Wuerz, R.; Menner, R.; Wischmann, W.; Powalla, M. New world record efficiency for Cu(In,Ga)Se2 thin-film solar cells beyond 20%. Prog. Photovolt. 2011, 19, 894–897. [Google Scholar] [CrossRef]
- Repins, I.; Contreras, M.A.; Egaas, B.; DeHart, C.; Scharf, J.; Perkins, C.L.; To, B.; Noufi, R. 19.9%-efficient ZnO/CdS/CuInGaSe2 solar cell with 81.2%fill factor. Prog. Photovolt. Res. Appl. 2008, 16, 235–239. [Google Scholar] [CrossRef]
- Chung, Y.-D.; Cho, D.-H.; Park, N.-M.; Lee, K.-S.; Kim, J. Effect of annealing on CdS/Cu(In,Ga)Se2 thin-film solar cells. Curr. Appl. Phys. 2011, 11, S65–S67. [Google Scholar] [CrossRef]
- Hones, C.; Fuchs, A.; Zweigart, S.; Siebentritt, S. Improved chemically deposited Zn(O,S)buffers for Cu(In,Ga)(S,Se)2 solar cells by controlled incorporation of indium. Photovolt. IEEE J. 2016, 6, 319–325. [Google Scholar] [CrossRef]
- Friedlmeier, T.M.; Jackson, P.; Bauer, A.; Hariskos, D.; Kiowski, O.; Wuerz, R.; Powalla, M. Improved photocurrent in Cu(In,Ga)Se2solar cells: From 20.8% to 21.7% efficiencywith CdS buffer and 21.0% Cd-free. IEEE J. Photovolt. 2015, 5, 1487–1491. [Google Scholar] [CrossRef]
- Nakada, T.; Furumi, K.; Kunioka, A. High-efficiency cadmium-free Cu(In,Ga)Se2thin-film solar cells with chemically deposited ZnS buffer layers. IEEE Trans. Electron Devices 1999, 46, 2093–2097. [Google Scholar] [CrossRef]
- Oliveri, R.L.; Inguanta, R.; Ferrara, G.; Piazza, S.; Sunseri, C.; Parisi, A.; Curcio, L.; Adamo, G.; Cino, A.C.; Busacca, A. CuInSe2/Zn(S,O,OH) junction on Mo foil by electrochemical and chemical route for photovoltaic applications. In Proceedings of the 2014 Fotonica AEIT Italian Conference on Photonics Technologies, Fotonica AEIT, Naples, Italy, 12–14 May 2014; pp. 1–4. [Google Scholar] [CrossRef]
- Downs, R.T.; Hall-Wallace, M. The American Mineralogist crystal structure database. Am. Mineral. 2003, 88, 247. [Google Scholar]
- Battaglia, M.; Piazza, S.; Sunseri, C.; Inguanta, R. Amorphous silicon nanotubes via galvanic displacement deposition. Electrochem. Commun. 2013, 34, 134–137. [Google Scholar] [CrossRef]
- Inguanta, R.; Ferrara, G.; Piazza, S.; Sunseri, C. Nanostructures fabrication by template deposition into anodic alumina membranes. Chem. Eng. Trans. 2009, 17, 957–962. [Google Scholar]
- Inguanta, R.; Garlisi, C.; Spanò, T.; Piazza, S.; Sunseri, C. Growth and photoelectrochemical behaviour of electrodeposited ZnO thin films for solar cells. J. Appl. Electrochem. 2013, 43, 199–208. [Google Scholar] [CrossRef]
- Kroger, F.A. Cathodic deposition and characterization of metallic or semiconducting binary alloys or compounds. J. Electrochem. Soc. 1978, 125, 2028. [Google Scholar] [CrossRef]
- Vauche, L.; Dubois, J.; Laparre, A.; Pasquinelli, M.; Bodnar, S.; Grand, P.P.; Jaime, S. Rapid thermal processing annealing challenges for large scale Cu2ZnSnS4 thin films. Phys. Status Solidi A 2015, 212, 103–108. [Google Scholar] [CrossRef]
- Lee, K.D.; Seo, S.-W.; Lee, D.-K.; Kim, H.; Jeong, J.-H.; Ko, M.J.; Kim, B.S.; Kim, D.H.; Kim, J.Y. Preparation of Cu2ZnSnS4 thin films via electrochemical deposition and rapid thermal annealing. Thin Solid Film 2013, 546, 294–298. [Google Scholar] [CrossRef]
- Fernandesa, P.A.; Salomè, P.M.P.; da Cunha, A.F. Study of polycrystalline Cu2ZnSnS4 films by Raman scattering. J. Alloy. Compd. 2011, 509, 7600–7606. [Google Scholar] [CrossRef]
- Ramanathan, K.; Keane, J.; Noufi, R. Properties of High-Efficiency CIGS Thin-Film Solar Cells. In Proceedings of the NREL 31st IEEE Photovoltaics Specialists, Lake Buena Vista, FL, USA, 3–7 January 2005; pp. 3–7. [Google Scholar]
- Farinella, M.; Inguanta, R.; Spanò, T.; Livreri, P.; Piazza, S.; Sunseri, C. Electrochemical deposition of CZTS thin films on flexible substrate. Energy Procedia 2014, 44, 105–110. [Google Scholar] [CrossRef]
- Inguanta, R.; Livreri, P.; Piazza, S.; Sunseri, C. Fabrication and photoelectrochemical behavior of ordered CIGS nanowire arrays for application in solar cells. Electrochem. Solid-State Lett. 2010, 13, K22–K25. [Google Scholar] [CrossRef][Green Version]
- Inguanta, R.; Piazza, S.; Sunseri, C.; Cino, A.; di Dio, V.; la Cascia, D.; Miceli, R.; Rando, C.; Zizzo, G. An electrochemical route towards the fabrication of nanostructured semiconductor solar cells. In Proceedings of the SPEEDAM 2010—International Symposium on Power Electronics, Electrical Drives, Automation and Motion, Pisa, Italy, 14–16 June 2010; Volume 5542264, pp. 1166–1171. [Google Scholar]
- Tanino, H.; Maeda, T.; Fujikake, H.; Nakanishi, H.; Endo, S.; Irie, T. Raman spectra of CuInSe2. Phys. Rev. B 1992, 1, 1323–1325. [Google Scholar]
- Rincon, C.; Ramirez, F.J. Lattice vibrations in CuInSe2 and CuGaSe2 by Raman spectroscopy. J. Appl. Phys. 1992, 72, 4321–4324. [Google Scholar] [CrossRef]
- Nagels, P.; Sleeckx, E.; Callaerts, R.; Tichy, L. Structural and optical properties of amorphous selenium prepared by plasma-enhanced CVD. Solid State Commun. 1995, 94, 49. [Google Scholar] [CrossRef]
- Park, J.H.; Yang, I.S.; Cho, H.Y. Micro-Raman spectroscopy on polycrystalline CuInSe2 formation. J. Appl. Phys. A 1994, 58, 125–128. [Google Scholar] [CrossRef]
- Genduso, G.; Inguanta, R.; Sunseri, C.; Piazza, S.; Kelch, C.; Sáez-Araoz, R.; Zykov, A.; Fischer, C.-H. Deposition of very thin uniform indium sulfide layers over metallic nano-rods by the Spray-Ion Layer Gas Reaction method. Thin Solid Films 2013, 548, 91–97. [Google Scholar] [CrossRef]
- Piazza, S.; Genduso, G.; Inguanta, R.; Sunseri, C.; Lux-Steiner, M.C.; Fischer, C.-H. Nickel-indium sulphide core-shell nanostructures obtained by spray-ILGAR deposition. Chem. Eng. Trans. 2013, 32, 2239–2244. [Google Scholar]
- Sáez-Araoz, R.; Krammer, J.; Harndt, S.; Koehler, T.; Krueger, M.; Pistor, P.; Hergert, F.; Jasenek, A.; Lux-Steiner, M.C.; Fischer, C.-H. ILGAR In2S3 buffer layers for Cd-free Cu(In,Ga)(S,Se)2 solar cells with certified efficiencies above 16%. Prog. Photovolt. Res. Appl. 2012, 20, 855–861. [Google Scholar] [CrossRef]



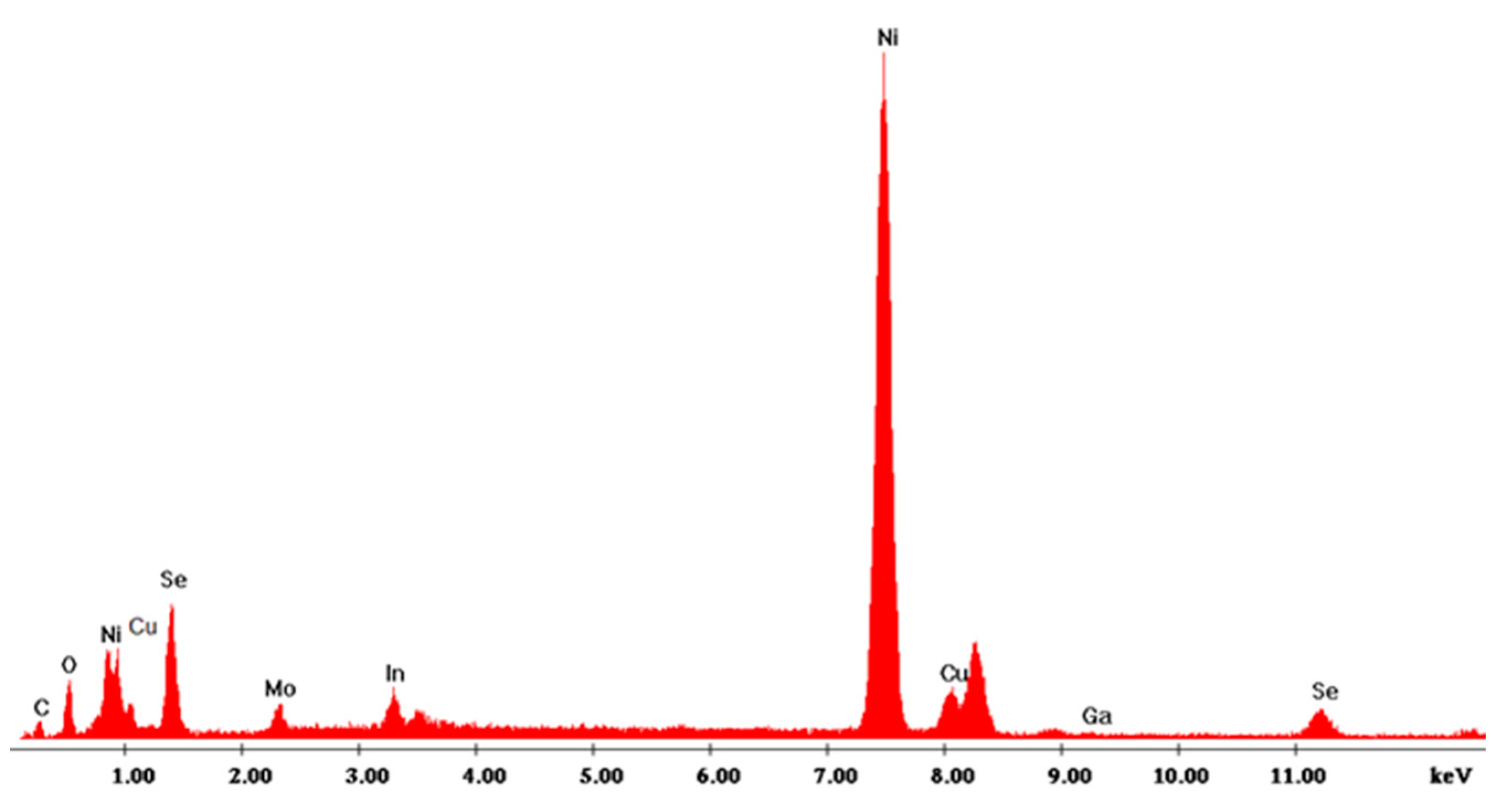
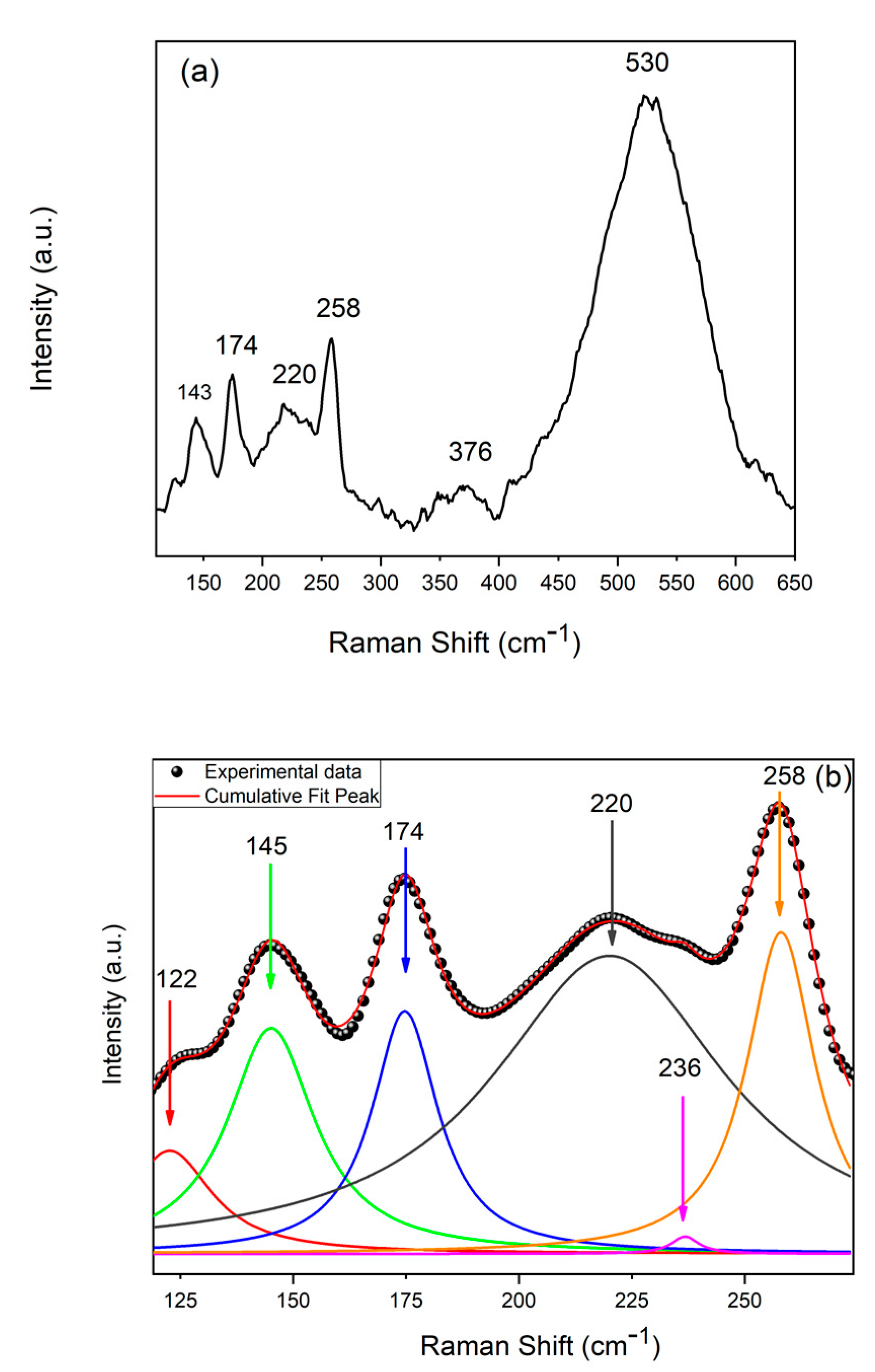

| pH | Cu (mM) | Cu (%) | Zn (%) | Sn (%) | Se (%) | Cu/(Zn+Sn) | Zn/Sn |
|---|---|---|---|---|---|---|---|
| 2.26 | 2 | 53.2 ± 1.6 | 1.5 ± 0.5 | 29.1 ± 1.3 | 16.2 ± 1.2 | 1.74 ± 0.6 | 0.05 ± 0.03 |
| 2.26 | 1 | 36.2 ± 1.2 | 9.1 ± 0.9 | 11.5 ± 1.2 | 43.2 ± 1.6 | 1.75 ± 0.5 | 0.8 ± 0.1 |
| 3 | 1 | 26.8 ± 1.5 | 15.9 ± 1.1 | 12.4 ± 1.6 | 44.9 ± 1.6 | 0.94 ± 0.3 | 1.28 ± 0.5 |
| Compound | Raman Shift (cm−1) |
|---|---|
| CZTSe | 172, 194–197, 231–235, 239–254 |
| CuSe | 240–260 |
| CuSe2 | 142, 233, 260–270, 450 |
| Cu2-xSe | 200, 260, 460 |
| Cu3Se2 | 190, 510, 560 |
| Cu2SnSe3 | 180, 200–230, 250, 360 |
| CuxSe | 260 |
| ZnSe | 252–256 |
| SnSe | 130, 150 |
| SnSe2 | 119, 185 |
| Se | 235, 253, 440 |
| Cu/(Zn + Sn) Before | Cu/(Zn + Sn) After | Zn/Sn Before | Zn/Sn After | S/(S + Se) |
|---|---|---|---|---|
| 0.94 ± 0.06 | 0.22 ± 0.03 | 1.28 ± 0.07 | 0.9 ± 0.06 | 0.47 ± 0.06 |
| Ga (mM) | Cu (%) | In (%) | Ga (%) | Se (%) | Cu/(In + Ga) | Ga/(In + Ga) |
|---|---|---|---|---|---|---|
| 5.7 | 37.2 ± 1.4 | 12.2 ± 0.9 | 0.7 ± 0.2 | 49.6 ± 1.4 | 2.9 ± 0.8 | 0.054 ± 0.03 |
| 57 | 34.7 ± 1.2 | 12.6 ± 0.9 | 3.3 ± 0.3 | 49.4 ± 1.8 | 2.2 ± 0.6 | 0.2 ± 0.08 |
| 114 | 38.4 ± 1.5 | 9.8 ± 0.8 | 1 ± 0.3 | 50.8 ± 1.6 | 3.5 ± 0.8 | 0.09 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveri, R.L.; Patella, B.; Di Pisa, F.; Mangione, A.; Aiello, G.; Inguanta, R. Fabrication of CZTSe/CIGS Nanowire Arrays by One-Step Electrodeposition for Solar-Cell Application. Materials 2021, 14, 2778. https://doi.org/10.3390/ma14112778
Oliveri RL, Patella B, Di Pisa F, Mangione A, Aiello G, Inguanta R. Fabrication of CZTSe/CIGS Nanowire Arrays by One-Step Electrodeposition for Solar-Cell Application. Materials. 2021; 14(11):2778. https://doi.org/10.3390/ma14112778
Chicago/Turabian StyleOliveri, Roberto Luigi, Bernardo Patella, Floriana Di Pisa, Alfonso Mangione, Giuseppe Aiello, and Rosalinda Inguanta. 2021. "Fabrication of CZTSe/CIGS Nanowire Arrays by One-Step Electrodeposition for Solar-Cell Application" Materials 14, no. 11: 2778. https://doi.org/10.3390/ma14112778
APA StyleOliveri, R. L., Patella, B., Di Pisa, F., Mangione, A., Aiello, G., & Inguanta, R. (2021). Fabrication of CZTSe/CIGS Nanowire Arrays by One-Step Electrodeposition for Solar-Cell Application. Materials, 14(11), 2778. https://doi.org/10.3390/ma14112778








