SiC-Coated Carbon Nanotubes with Enhanced Oxidation Resistance and Stable Dielectric Properties
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Samples Preparation
2.3. Measurements
3. Results and Discussion
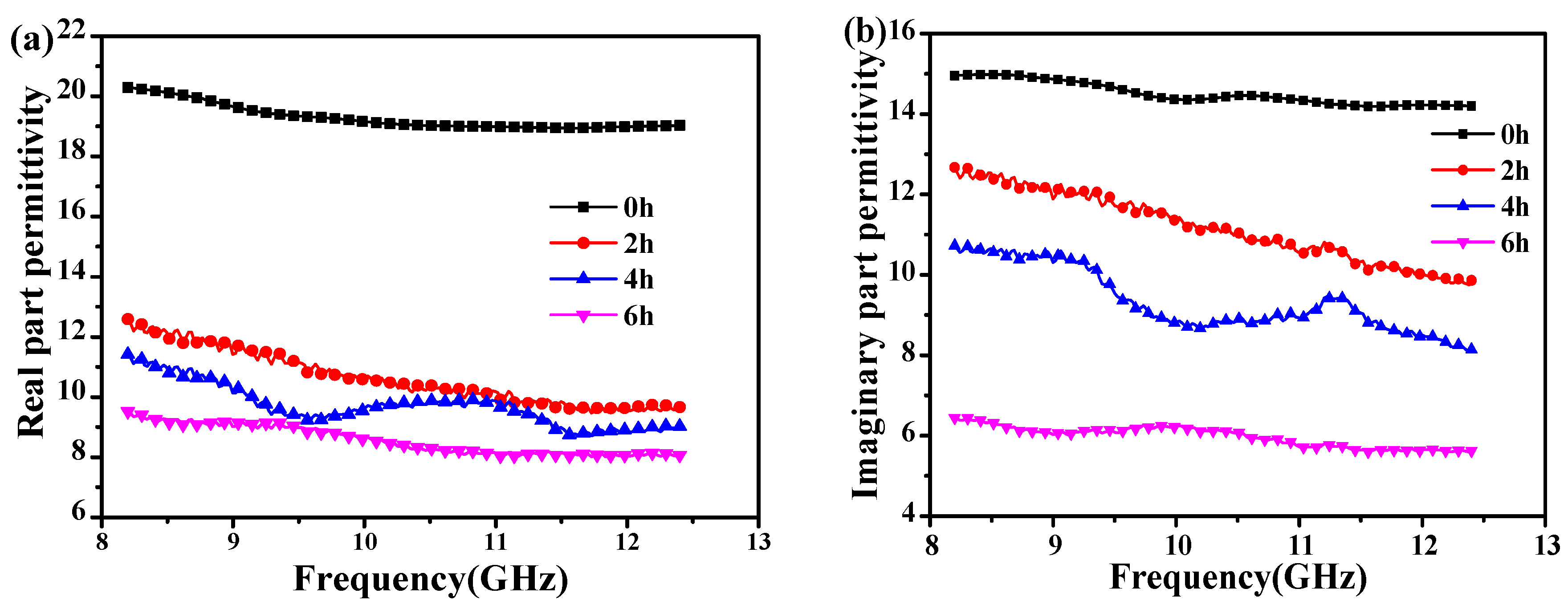
3.1. Dielectric Properties of CNTs Annealed at 400 °C with Different Time
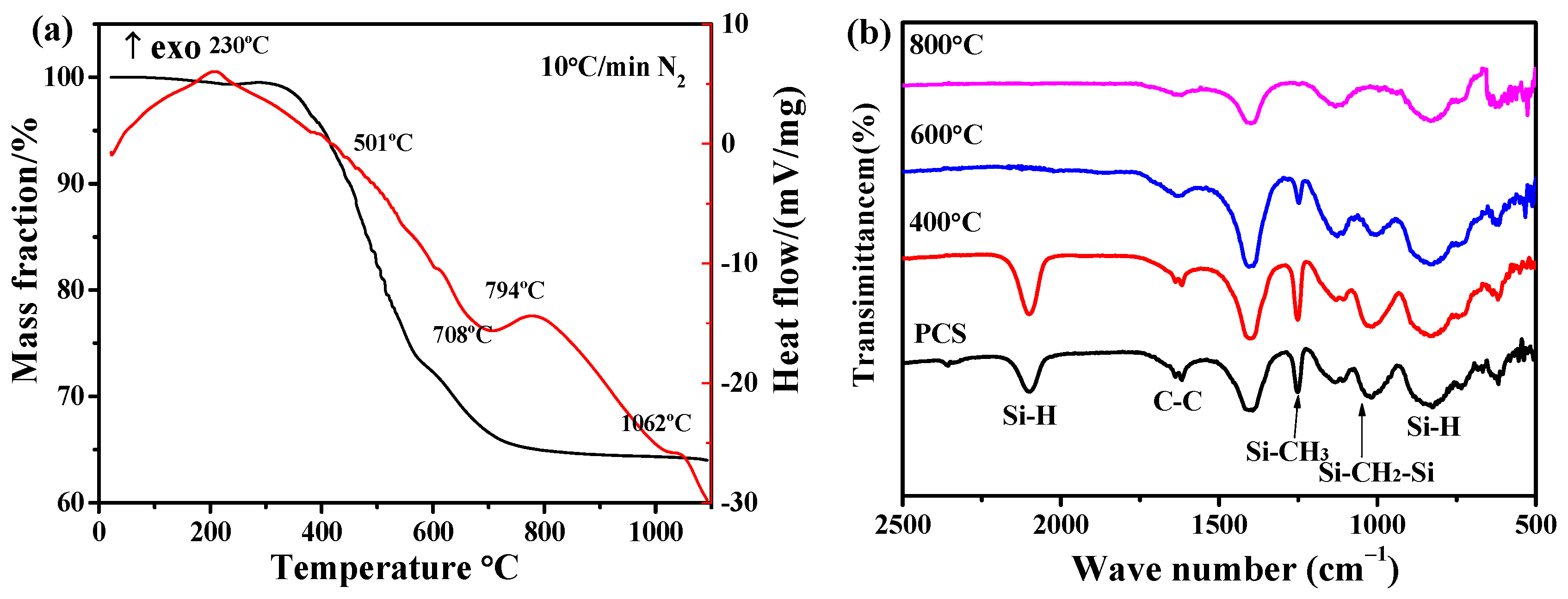
3.2. The Pyrolysis Mechanism and Products of PCS at Different Temperatures
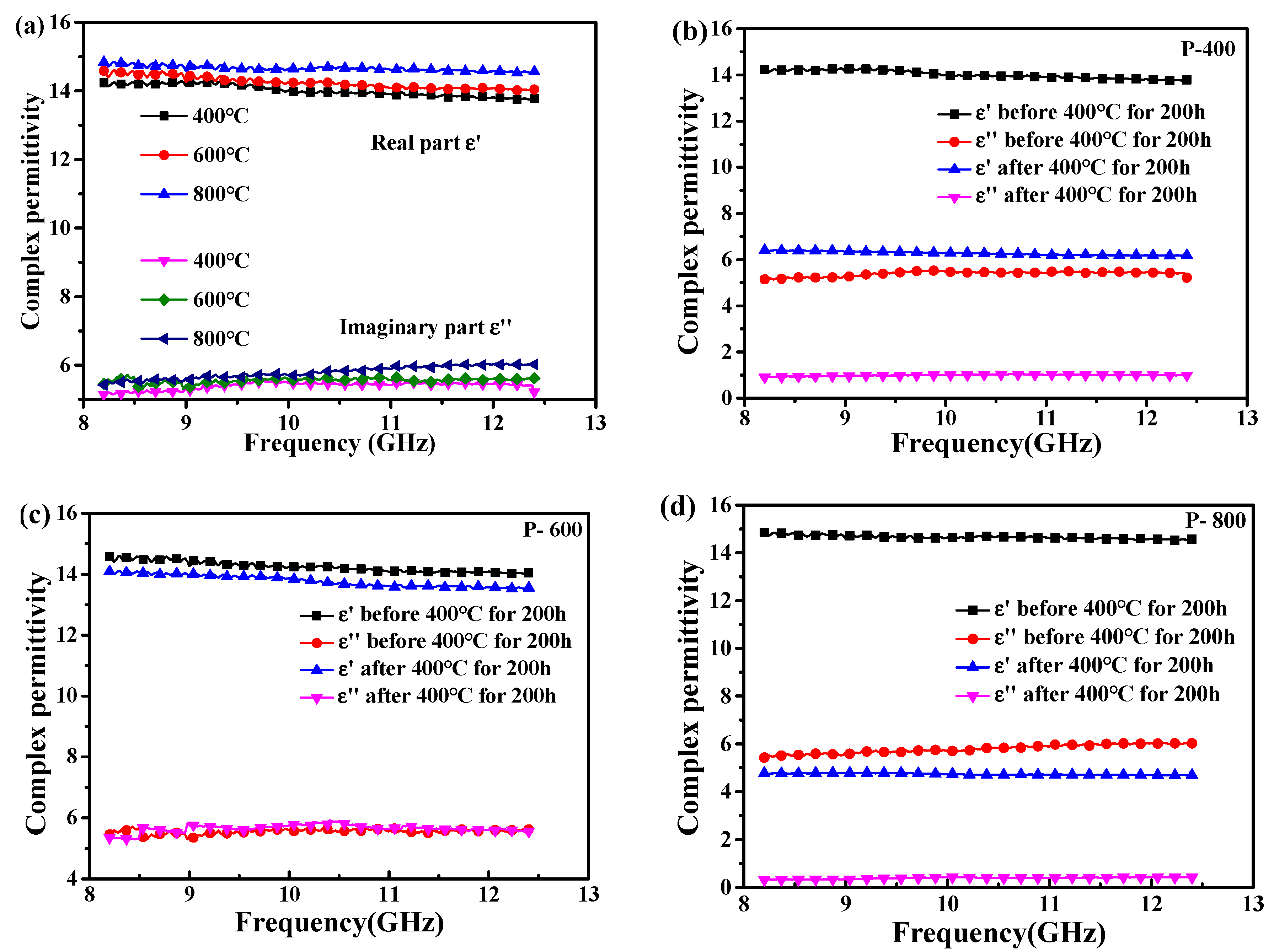
3.3. Dielectric Properties of SiC-Coated CNTs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, G.; Duan, Y.; Pang, H. Microwave Absorption of Crystalline Fe/MnO@C Nanocapsules Embedded in Amorphous Carbon. Nano-Micro Lett. 2020, 12, 1–16. [Google Scholar] [CrossRef]
- Li, Y.; Qing, Y.; Zhou, Y.; Zhao, B.; Zhi, Q.; Fan, B.; Zhang, R. Unique nanoporous structure derived frSom Co3O4–C and Co/CoO–C composites towards the ultra-strong electromagnetic absorption. Compos. B Eng. 2021, 9, 1205–1214. [Google Scholar]
- Huang, L.; Duan, Y.; Dai, X.; Zeng, Y.; Ma, G.; Liu, Y.; Gao, S.; Zhang, W. Bioinspired Metamaterials: Multibands Electromagnetic Wave Adaptability and Hydrophobic Characteristics. Small 2019, 15, e1902730. [Google Scholar] [CrossRef]
- Wang, L.; Shi, X.; Zhang, J.; Zhang, Y.; Gu, J. Lightweight and robust rGO/sugarcane derived hybrid carbon foams with outstanding EMI shielding performance. J. Mater. Sci. Technol. 2020, 52, 119–126. [Google Scholar] [CrossRef]
- Zhang, C.; Yin, S.; Long, C.; Dong, B.; He, D.; Cheng, Q. Hybrid metamaterial absrober for ultra-low and dual-broadband absorption. Opt. Express 2021, 29, 14078–14086. [Google Scholar] [CrossRef]
- Lv, H.; Yang, Z.; Liu, B.; Wu, G.; Lou, Z.; Fei, B.; Wu, R. A flexible electromagnetic wave-electrivity harvester. Nat. Commun. 2021, 12, 834–841. [Google Scholar] [CrossRef]
- Lv, H.; Yang, Z.; Xu, H.; Wang, L.; Wu, R. An electrical switch-driven flexible electromagentic absorber. Adv. Funct. Mater. 2020, 30, 1907251–1907258. [Google Scholar] [CrossRef]
- Qing, Y.; Wen, Q.; Luo, F.; Zhou, W.; Zhu, D. Graphene nanosheets/BaTiO3 ceramics as highly efficient electromagnetic interference shielding materials in the X-band. J. Mater. Chem. C 2015, 4, 371–375. [Google Scholar]
- Zong, M.; Huang, Y.; Zhang, N. Reduced graphene oxide-CoFe2O4 composite: Synthesis and electromagnetic absorption properties. Appl. Surf. Sci. 2015, 345, 272–278. [Google Scholar] [CrossRef]
- Zong, M.; Huang, Y.; Wu, H.; Zhao, Y.; Wang, Q.; Sun, X. One-pot hydrothermal synthesis of RGO/CoFe2O4 composite and its excellent microwave absorption properties. Mater. Lett. 2014, 114, 52–55. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.; Yin, P.; Guo, A.; Huang, A.; Guo, L.; Wang, G. Tunable High-Performance Microwave Absorption of Co1-xS Hollow Spheres Constructed by Nanosheets within Ultralow Filler Loading. Adv. Funct. Mater. 2018, 28, 1800761. [Google Scholar] [CrossRef]
- Qing, Y.; Zhou, W.; Luo, F.; Zhu, D. Titanium carbide (MXene) nanosheets as promising microwave absorbers. Ceram. Int. 2016, 42, 16412–16416. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nat. Cell Biol. 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Che, R.C.; Peng, L.-M.; Duan, X.F.; Chen, Q.; Liang, X.L. Microwave Absorption Enhancement and Complex Permittivity and Permeability of Fe Encapsulated within Carbon Nanotubes. Adv. Mater. 2004, 16, 401–405. [Google Scholar] [CrossRef]
- Yu, Z.; Lv, X.; Mao, K.; Yang, Y.; Liu, A. Role of in-situ formed free carbon on electromagnetic absorption properties of polymer-derived SiC ceramics. J. Adv. Ceram. 2020, 9, 617–628. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.; Wang, L.; Li, J.; Feng, T.; Fan, J.; Chen, L.; Gu, J. Superior wave-absorbing performances of silicone rubber composites via introducing covalently bonded SnO2@MWCNT absorbent with encapsulation structure. Compos. Commun. 2020, 22, 100486. [Google Scholar] [CrossRef]
- Che, R.; Zhi, C.; Liang, C.; Zhou, X. Fabrication and microwave absorption of carbon nanotubes∕CoFe2O4 spinel nanocomposite. Appl. Phys. Lett. 2006, 88, 033105. [Google Scholar] [CrossRef]
- Song, W.-L.; Cao, M.-S.; Hou, Z.-L.; Yuan, J.; Fang, X.-Y. High-temperature microwave absorption and evolutionary behavior of multiwalled carbon nanotube nanocomposite. Scr. Mater. 2009, 61, 201–204. [Google Scholar] [CrossRef]
- Li, Y.; Yang, M.; Xu, B.; Sun, Q.; Zhang, W.; Zhang, Y.; Meng, F. Synthesis, structure and antioxidant performance of boron nitride (hexagonal) layers coating on carbon nanotubes (multi-walled). Appl. Surf. Sci. 2018, 450, 284–291. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, L.; Xu, Y.; Cheng, L.; Lou, J.; Zhang, J.; Yu, L. Microstructure and properties of 3D needle-punched carbon/silicon carbide brake materials. Compos. Sci. Technol. 2007, 67, 2390–2398. [Google Scholar] [CrossRef]
- Krenkel, W.; Berndt, F. C/C–SiC composites for space applications and advanced friction systems. Mater. Sci. Eng. A 2005, 412, 177–181. [Google Scholar] [CrossRef]
- Xiao, P.; Li, Z.; Zhu, Z.; Xiong, X. Preparation, Properties and Application of C/C-SiC Composites Fabricated by Warm Compacted-in situ Reaction. J. Mater. Sci. Technol. 2010, 26, 283–288. [Google Scholar] [CrossRef]
- Allebrandt (Probst), D.; Hoche, H.; Scheerer, H.; Broszeit, E.; Berger, C. Oxidation resistance of SiAlCN: H-coatings. Surf. Coat. Technol. 2007, 201, 5172–5175. [Google Scholar] [CrossRef]
- Probst, D.; Hoche, H.; Zhou, Y.; Hauser, R.; Stelzner, T.; Scheerer, H.; Broszeit, E.; Berger, C.; Riedel, R.; Stafast, H.; et al. Development of PE-CVD Si/C/N:H films for tribological and corrosive complex-load conditions. Surf. Coat. Technol. 2005, 200, 355–359. [Google Scholar] [CrossRef]
- Hu, C.; Niu, Y.; Li, H.; Ren, M.; Zheng, X.; Sun, J. SiC Coatings for Carbon/Carbon Composites Fabricated by Vacuum Plasma Spraying Technology. J. Therm. Spray Technol. 2011, 21, 16–22. [Google Scholar] [CrossRef]
- Zou, J.; Liu, L.; Chen, H.; Khondaker, S.I.; McCullough, R.D.; Huo, Q.; Zhai, L. Dispersion of Pristine Carbon Nanotubes Using Conjugated Block Copolymers. Adv. Mater. 2008, 20, 2055–2060. [Google Scholar] [CrossRef]
- Greil, P. Polymer Derived Engineering Ceramics. Adv. Eng. Mater. 2000, 2, 339–348. [Google Scholar] [CrossRef]
- Birot, M.; Pillot, J.-P.; Dunogues, J. Comprehensive Chemistry of Polycarbosilanes, Polysilazanes, and Polycarbosilazanes as Precursors of Ceramics. Chem. Rev. 1995, 95, 1443–1477. [Google Scholar] [CrossRef]
- Gupta, R.K.; Mishra, R.; Mukhopadhyay, K.; Tiwari, R.K.; Ranjan, A.; Saxena, A.K. A New Technique for Coating Silicon Carbide onto Carbon Nanotubes Using a Polycarbosilane Precursor. Silicon 2009, 1, 125–129. [Google Scholar] [CrossRef]
- Luo, M.; Li, Y.; Jin, S.; Sang, S.; Zhao, L. Oxidation resistance of multi-walled carbon nanotubes coated with polycarbosilane-derived SiCxOy ceramic. Ceram. Int. 2011, 37, 3055–3062. [Google Scholar] [CrossRef]
- Ding, D.-H.; Zhou, W.-C.; Zhou, X.; Luo, F.; Zhu, D.-M. Influence of pyrolysis temperature on structure and dielectric properties of polycarbosilane derived silicon carbide ceramic. Trans. Nonferrous Met. Soc. China 2012, 22, 2726–2729. [Google Scholar] [CrossRef]
- Fang, X.; Jiang, L.; Pan, L. High-thermally conductive AlN-based microwave attenuating composite ceramics with spherical graphite as attenuating agent. J. Adv. Ceram. 2021, 10, 301–319. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Cheng, L.; Wang, Y.; Yu, Z.; Huang, M.; Tu, H.; Xia, H. Polymer–ceramic conversion of a highly branched liquid polycarbosilane for SiC-based ceramics. J. Mater. Sci. 2008, 43, 2806–2811. [Google Scholar] [CrossRef]
- Jang, Y.-S.; Jank, M.; Maier, V.; Durst, K.; Travitzky, N.; Zollfrank, C. SiC ceramic micropatterns from polycarbosilanes. J. Eur. Ceram. Soc. 2010, 30, 2773–2779. [Google Scholar] [CrossRef]
- Gu, W.; Cui, X.; Zheng, J.; Yu, J.; Zhao, Y.; Ji, G. Heterostructure design of Fe3N alloy/porous carbon nanosheet composites for efficient microwave attenuation. J. Mater. Sci. Technol. 2021, 67, 265–272. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, B.; Xiang, H.; Dai, F.-Z.; Wu, S.; Zhou, Y. One-step synthesis and electromagnetic absorption properties of high entropy rare earth hexaborides (HE REB6) and high entropy rare earth hexaborides/borates (HE REB6/HE REBO3) composite powders. J. Adv. Ceram. 2021, 10, 62–77. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Li, X. The Kinetics of Oxidation Curing of Polycarbosilane Fibers. Korean J. Chem. Eng. 2004, 21, 901–904. [Google Scholar] [CrossRef]
- Ly, H.; Taylor, R.; Day, R. Conversion of polycarbosilane (PCS) to SiC-based ceramic Part Ⅰ: Characterisation of PCS and curing products. J. Mater. Sci. 2001, 36, 4037–4043. [Google Scholar] [CrossRef]
- Yajima, S.; Hayashi, J.; Omori, M. Development of a silicon carbide fiber with high tensile strength. Nature 1976, 261, 683–685. [Google Scholar] [CrossRef]
- Ly, H.; Taylor, R.; Day, R. Conversion of polycarbosilane (PCS) to SiC-based ceramic Part Ⅱ: Pyrolysis and characterisation. J. Mater. Sci. 2001, 36, 4045–4057. [Google Scholar] [CrossRef]
- Huang, Y.; Wan, C. Controllable fabrication and multifunctional applications of graphene/ceramic composites. J. Adv. Ceram. 2020, 9, 271–291. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Qing, Y.; Zhao, J.; Huang, S. SiC-Coated Carbon Nanotubes with Enhanced Oxidation Resistance and Stable Dielectric Properties. Materials 2021, 14, 2770. https://doi.org/10.3390/ma14112770
Li R, Qing Y, Zhao J, Huang S. SiC-Coated Carbon Nanotubes with Enhanced Oxidation Resistance and Stable Dielectric Properties. Materials. 2021; 14(11):2770. https://doi.org/10.3390/ma14112770
Chicago/Turabian StyleLi, Rong, Yuchang Qing, Juanjuan Zhao, and Shiwen Huang. 2021. "SiC-Coated Carbon Nanotubes with Enhanced Oxidation Resistance and Stable Dielectric Properties" Materials 14, no. 11: 2770. https://doi.org/10.3390/ma14112770
APA StyleLi, R., Qing, Y., Zhao, J., & Huang, S. (2021). SiC-Coated Carbon Nanotubes with Enhanced Oxidation Resistance and Stable Dielectric Properties. Materials, 14(11), 2770. https://doi.org/10.3390/ma14112770





