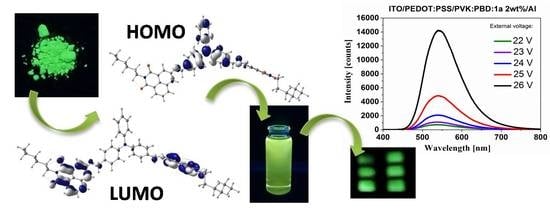
New Acceptor–Donor–Acceptor Systems Based on Bis-(Imino-1,8-Naphthalimide)
Abstract
1. Introduction
2. Materials, Methods and Synthesis
2.1. Materials
2.2. Structural Characterization
3. Result and Discussion
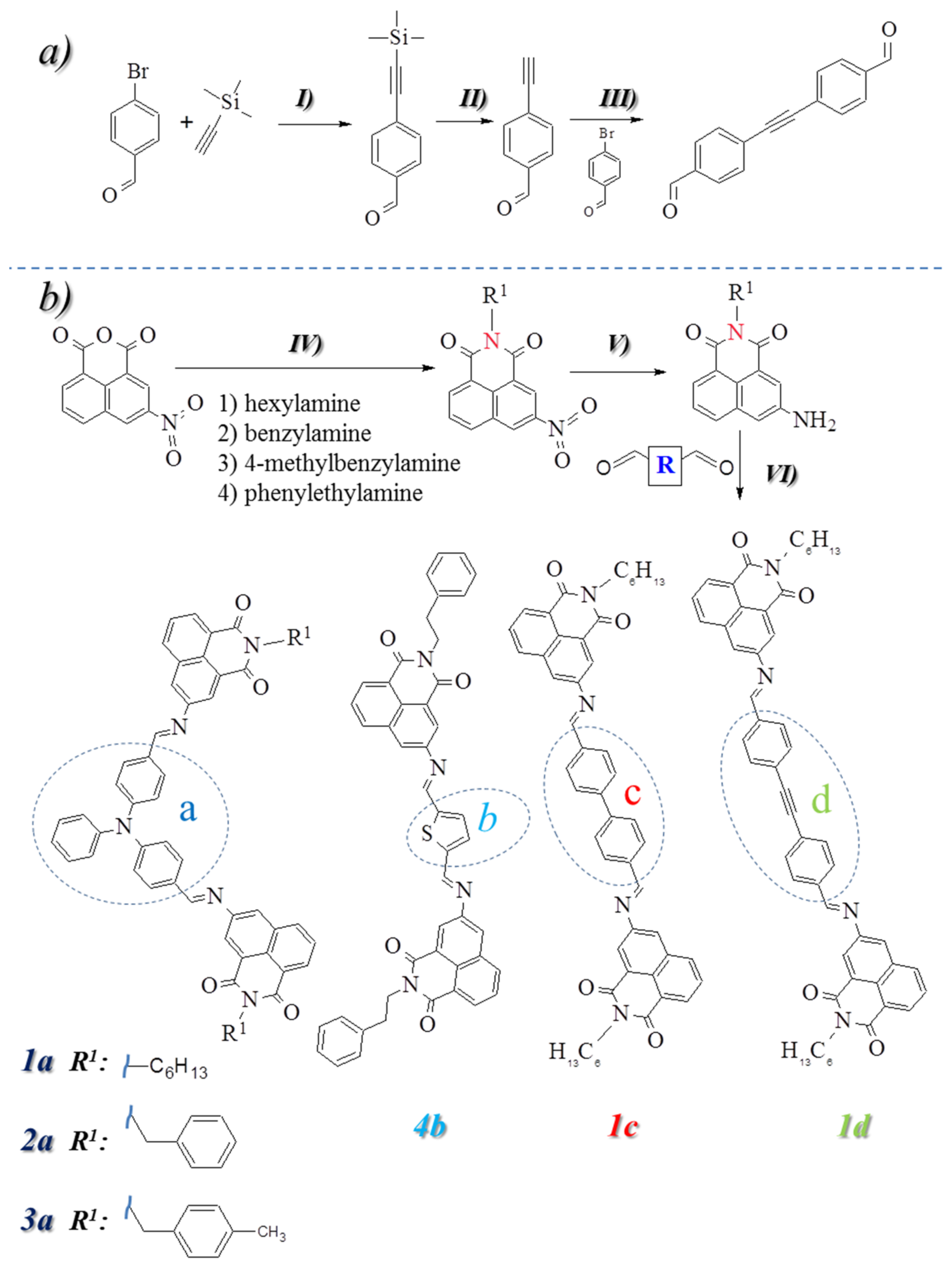
3.1. Synthesis and Structural Characterization
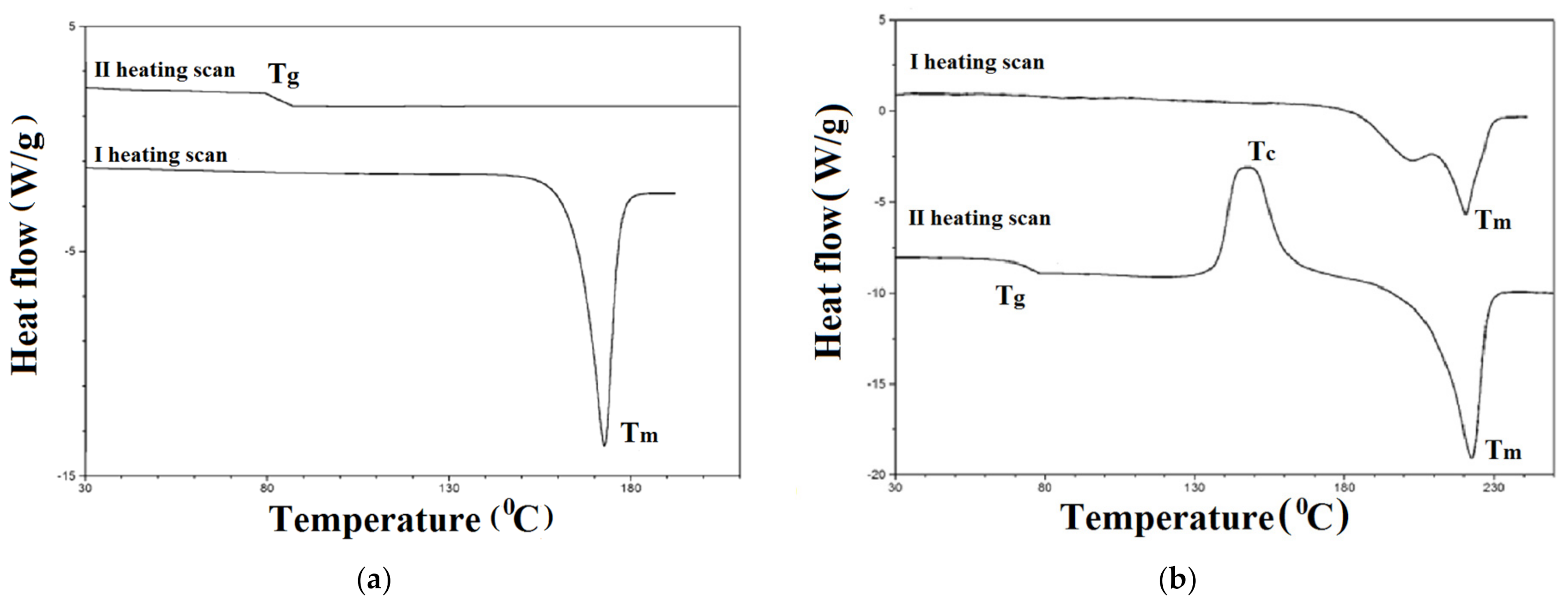
3.2. Thermal Characterization
3.3. Electrochemical Investigations
3.4. Theoretical Calculations
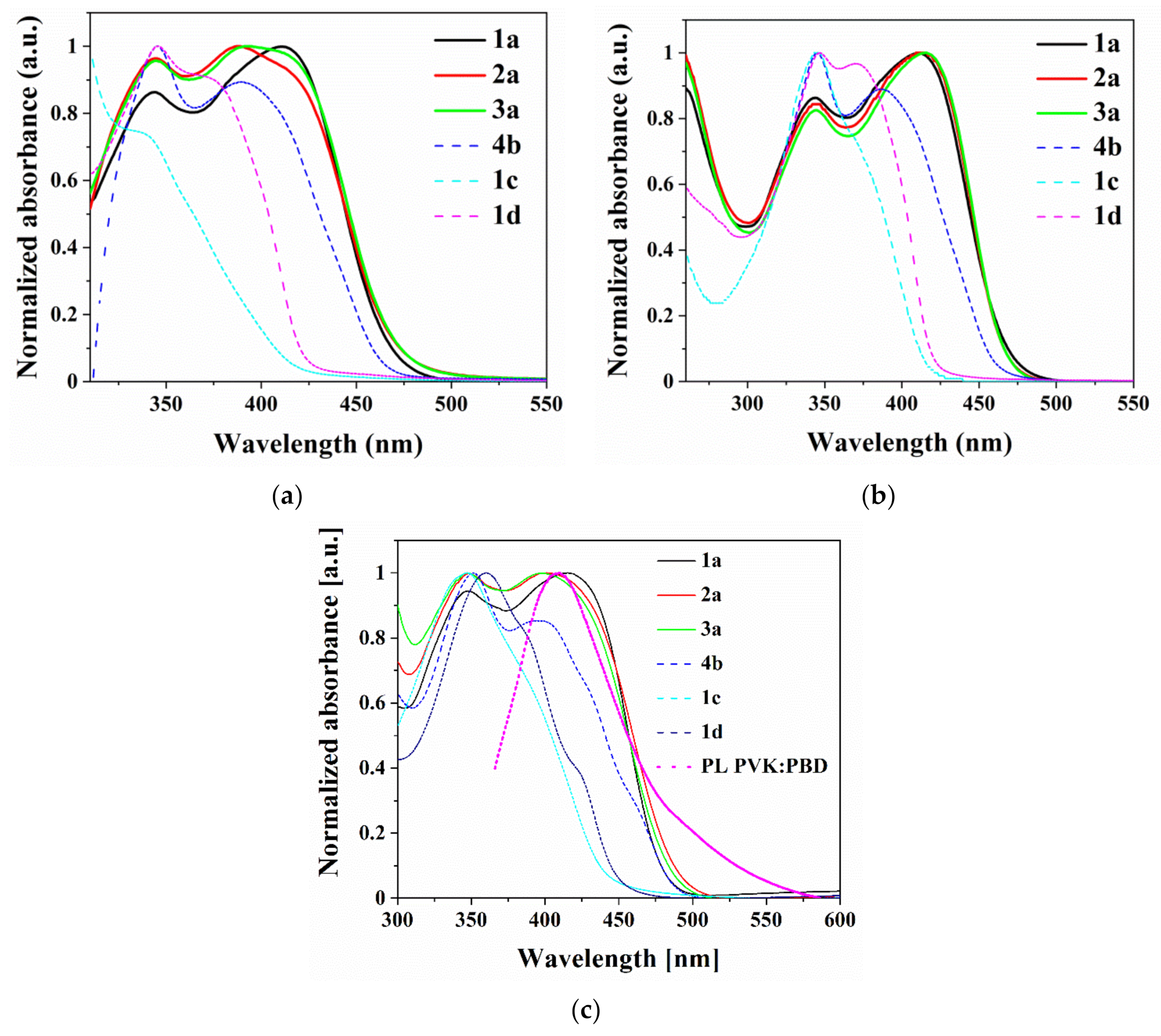
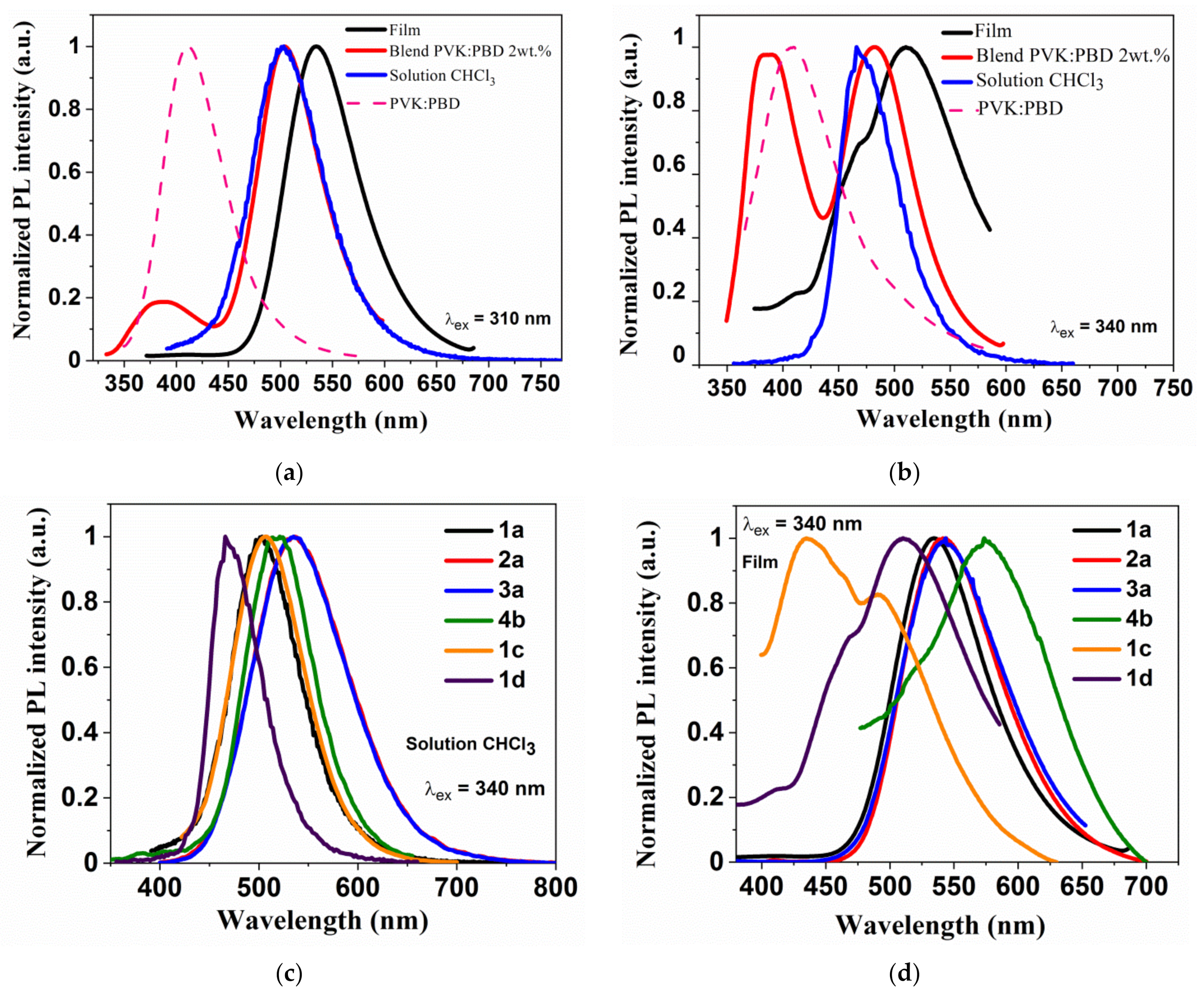
3.5. Photophysical Properties
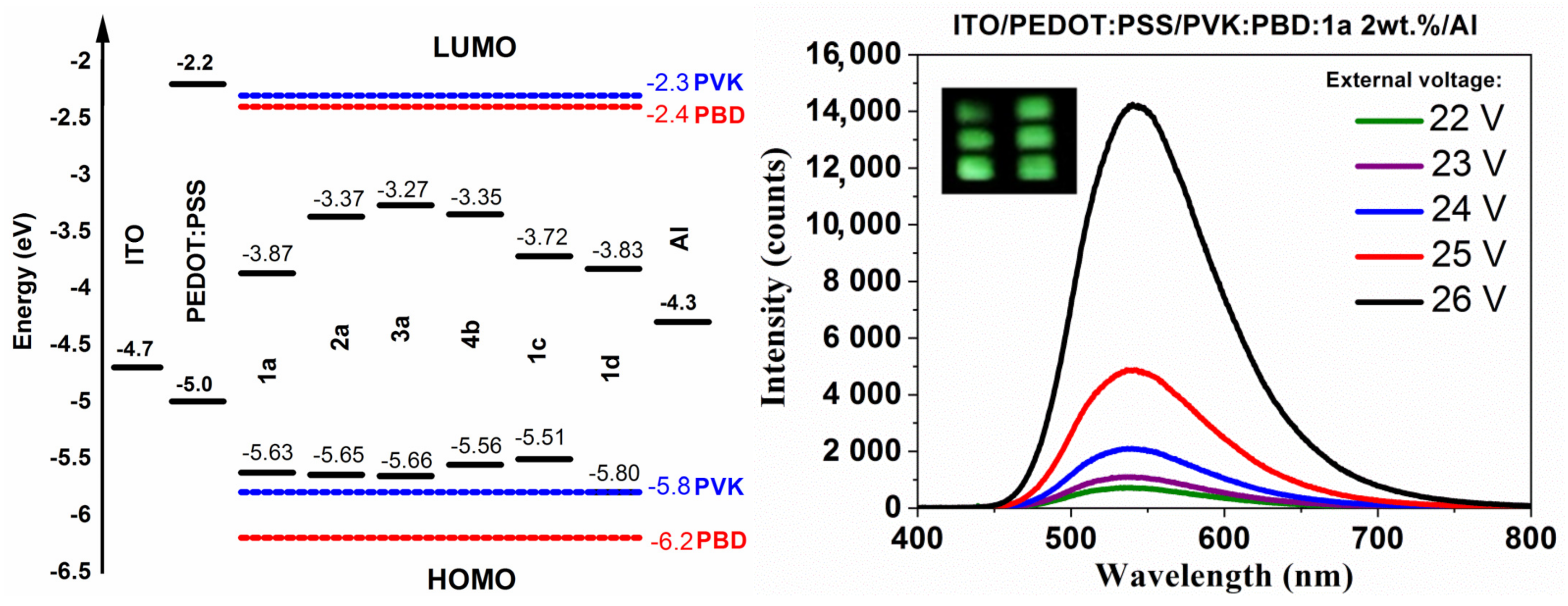
3.6. Electroluminescence Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, R.F.; Chang, Y.F. A theoretical study on photophysical properties of triphenylamine-cored molecules with naphthalimide arms and different π-conjugated bridges as organic solar cell materials. Phys. Chem. Chem. Phys. 2015, 17, 2094–2103. [Google Scholar] [CrossRef]
- Grabchev, I.; Staneva, D. Photophysical properties of new polymerizable 1,8-Naphthalimides and their copolymers with methylmethacrylate. Z. Nat. 2003, 58, 558–562. [Google Scholar] [CrossRef]
- Refat, M.S.; Al Didamony, H.; Abou El-Nour, K.M.; Grabchev, I.; El-Zayat, L. Synthesis and characterizations of charge-transfer complexes of 1,8-naphthalimides with different acceptors. Bulg. Chem. Commun. 2010, 42, 279–299. [Google Scholar]
- Bag, S.S.; Pradhan, M.K.; Kundu, R.; Jana, S. Highly solvatochromic fluorescent naphthalimides: Design, synthesis, photophysical properties and fluorescence switch-on sensing of ct-DNA. Bioorg. Med. Chem. Lett. 2013, 23, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Bijak, K.; Janeczek, H.; Grucela-Zajac, M.; Schab-Balcerzak, E. New room-temperature thermotropic perylene-based bisimides: Synthesis, liquid crystalline, light-emitting and electrochemical properties. Opt. Mater. 2013, 35, 1042–1050. [Google Scholar] [CrossRef]
- Schab-Balcerzak, E.; Iwan, A.; Krompiec, M.; Siwy, M.; Tapa, D.; Sikora, A.; Palewicz, M. New thermotropic azomethine-naphthalene diimides for optoelectronic applications. Synth. Met. 2010, 160, 2208–2218. [Google Scholar] [CrossRef]
- Bijak, K.; Grucela-Zajac, M.; Janeczek, H.; Wiacek, M.; Schab-Balcerzak, E. New azomethine-phthalic diimides:Synthesis and thermal, optical and electrochemical characterization. Synth. Met. 2013, 175, 146–154. [Google Scholar] [CrossRef]
- Grucela-Zajac, M.; Bijak, K.; Kula, S.; Filapek, M.; Wiacek, M.; Janeczek, H.; Skorka, L.; Gasiorowski, J.; Hingerl, K.; Sariciftci, N.S.; et al. (Photo)physical properties of new molecular glasses end-capped with thiophene rings composed of diimide and imine units. J. Phys. Chem. C 2014, 118, 13070–13086. [Google Scholar] [CrossRef]
- Grucela-Zajac, M.; Bijak, K.; Zaleckas, E.; Grigalevicius, S.; Wiacek, M.; Janeczek, H.; Schab-Balcerzak, E. Electronic and thermal properties of compounds bearing diimide, azomethine and triphenylamine units. Opt. Mater. 2014, 37, 543–551. [Google Scholar] [CrossRef]
- Rybakiewicz, R.; Djurado, D.; Cybulski, H.; Dobrzynska, E.; Kulszewicz-Bajer, I.; Boudinet, D.; Verilhac, J.M.; Zagorska, M.; Pron, A. Arylene bisimides with trirylamine N-substituents as new solution processable organic semiconductors: Synthesis, spectroscopic, electrochemical and electronic properties. Synth. Met. 2011, 161, 1600–1610. [Google Scholar] [CrossRef]
- Sakai, N.; Mareda, J.; Vauthey, E.; Matile, S. Core-substituted naphthalenediimides. Chem. Commun. 2010, 46, 4225–4237. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, M.; Shi, G.; Wang, L.; Yin, H.; Mei, C. A novel fluorescent molecule based on 1,8-naphthalimide: Synthesis, spectral properties, and application in cell imaging. Res. Chem. Intermed. 2010, 36, 1021–1026. [Google Scholar] [CrossRef]
- Saito, G.; Velluto, D.; Resmini, M. Synthesis of 1,8-naphthalimide-based probes with fluorescent switch triggered by flufenamic acid. R. Soc. Opern Sci. 2018, 5, 172137. [Google Scholar] [CrossRef] [PubMed]
- Duke, R.M.; Veale, E.B.; Pfeffer, F.M.; Kruger, P.E.; Gunnlaugsson, T. Colorimetric and fluorescent anion sensors: An overview of recent developments in the use of 1,8-naphthalimide-based chemosensors. Chem. Soc. Rev. 2010, 39, 3936–3953. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.J.; Choi, M.G.; Jeong, Y.A.; Lee, S.H.; Chang, S.K. Colorimetric determination of Cu2+ in simulated wastewater using naphthalimide-based Schiff base. Tetrahedron Lett. 2017, 58, 474–477. [Google Scholar] [CrossRef]
- Ulla, H.; Kiran, M.R.; Garudachari, B.; Satyanarayan, M.; Umesh, G.; Isloor, A. Blue emitting halogen-phenoxy substituted 1,8-naphthalimides for potential organic light emitting diode applications. Opt. Mater. 2014, 37, 311–321. [Google Scholar] [CrossRef]
- Yordanova, S.; Grabchev, I.; Stoyanov, S.; Milusheva, V.; Petkov, I. Synthesis and functional characteristics of two new yellow-green fluorescent PAMAM dendrimers periphery modified with 1,8-naphthalimides. Inorg. Chim. Acta 2014, 409, 89–95. [Google Scholar] [CrossRef]
- Bojinov, V.B.; Simeonov, D.B. Synthesis of highly photostable blue-emitting 1,8-naphthalimides and their acrylonitrile copolymers. Polym. Degrad. Stab. 2010, 95, 43–52. [Google Scholar] [CrossRef]
- Prezhdo, O.V.; Uspenskii, B.V.; Prezhdo, V.V.; Boszczyk, W.; Distanov, V.B. Synthesis and spectral-luminescent characteristics of N-sustituted 1,8-naphthalimides. Dye. Pigment. 2007, 72, 42–46. [Google Scholar] [CrossRef]
- Cao, X.; Meng, L. Large red-shifted fluorescent emission via intermolecular π-π stacking in 4-ethynyl-1,8-naphthalimide-based supramolecular assemblies. Langmuir 2014, 30, 11753–11760. [Google Scholar] [CrossRef]
- Sonalin, S.; Sakthivel, K.; Nagarajan, S. Functionalization of 1,8-naphthalimides-an approach towards air-stable n-type organic semiconductors. Mater. Today Proc. 2018, 5, 16592–16597. [Google Scholar] [CrossRef]
- Zhu, M.; Miao, J.; Hu, Z.; Chen, Y.; Liu, M.; Murtaza, I.; Meng, H. A novel A-D-A small molecule with 1,8-naphthalimide as a potential non-fullerene acceptor for solution processable solar cells. Dye. Pigment. 2017, 142, 39–50. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, P.; Liu, Y.; Yu, G.; Sun, X.; Niu, H.; Zhu, D. Luminescent properties of a novel naphthalimide-fluorene molecule. Synth. Met. 2005, 150, 33–38. [Google Scholar] [CrossRef]
- Do, T.T.; Takeda, Y.; Manzhos, S.; Bell, J.; Tokito, S.; Sonar, P. Naphthalimide and capped anthraquinone based solution-processable n-channel organic semiconductors: Effect of alkyl chain engineering on charge transport. J. Mater. Chem. C 2018, 6, 3774–3786. [Google Scholar] [CrossRef]
- Bojinov, V.; Grabchev, I. Synthesis of new polymerizable 1,8-naphthalimide dyes containing a 2-hydroxyphenylbenzotriazole fragment. Dye. Pigment. 2003, 59, 277–283. [Google Scholar] [CrossRef]
- Pluczyk, S.; Laba, K.; Schab-Balcerzak, E.; Bijak, K.; Kotowicz, S.; Lapkowski, M. Electrochemical and spectroelectrochemical properties of new polymers with diimide subunits. J. Electroanal. Chem. 2017, 795, 90–96. [Google Scholar] [CrossRef]
- Kolosov, D.; Adamovich, V.; Djurovich, P.; Thompson, M.E.; Adachi, C. 1,8-Naphthalimides in phosphorescent organic LEDs: The interplay between dopant, exciplex, and host emission. J. Am. Chem. Soc. 2002, 124, 9945–9954. [Google Scholar] [CrossRef] [PubMed]
- Kucheryavy, P.; Li, G.; Vyas, S.; Hadad, C.; Glusac, K.D. Electronic properties of 4-substituted naphthalimides. J. Phys. Chem. A 2009, 113, 6453–6461. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Jin, R. Optical and charge transport properties of N-butyl-1,8-naphthalimide derivatives as organic light-emitting materials: A theoretical study. J. Lumin. 2014, 149, 125–132. [Google Scholar] [CrossRef]
- Jin, R.; Tang, S. Theoretical study on optical and electronic properties of bipolar molecules with 1,8-naphthalimide and triphenylamine moieties as organic light-emitting materials. J. Mol. Graph. Model. 2013, 42, 120–128. [Google Scholar] [CrossRef]
- Chai, W.; Jin, R. Theoretical investigations into optical and charge transfer properties of donor-acceptor 1,8-naphthalimide derivatives as possible organic light-emitting materials. J. Mol. Struct. 2016, 1103, 177–182. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Wang, Y.; Sun, H.; Lu, Z.; Wu, H.; Peng, J.; Huang, Y. Synthesis and luminescent properties of blue sextuple-hydrogen-bond self-assembly molecular duplexes bearing 4-phenoxy-1,8-naphthalimide moieties. Opt. Mater. 2012, 34, 1535–1542. [Google Scholar] [CrossRef]
- Liu, J.; Tu, G.; Zhou, Q.; Cheng, Y.; Geng, Y.; Wang, L.; Ma, D.; Jing, X.; Wang, F. Highly efficient green light emitting polyfluorene incorporated with 4-diphenylamino-1,8-naphthalimides as green dopant. J. Mater. Chem. 2006, 16, 1431–1438. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Xiao, H.; Li, G.; Liu, Y.; Li, C.; Huang, H.; Chen, X.; Bo, Z. 1,8-naphthalimide-based planar small molecular acceptor for organic solar cells. ACS Appl. Mater. Interfaces 2016, 8, 5475–5483. [Google Scholar] [CrossRef]
- Gautam, P.; Sharma, R.; Misra, R.; Keshtov, M.L.; Kuklin, S.A.; Sharma, G.D. Donor-acceptor-acceptor (D-A-A) type 1,8-naphthalimides as non-fullerene small molecule acceptors for bulk heterojunction solar cells. Chem. Sci. 2017, 8, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Thomas, K.R.J. Bis-naphthalimides bridged by electron acceptors: Optical and self-assembly characteristics. RSC Adv. 2016, 6, 71638–71651. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, H.; Zhang, X.; Wu, Y.; Li, G.; Li, C.; Chen, X.; Ma, W.; Bo, Z. 1,8-Naphthalimide-based nonfullerene acceptors for wide optical band gap polymer solar cells with an ultrathin active layer thickness of 35nm. J. Mater. Chem. C 2016, 4, 5656–5663. [Google Scholar] [CrossRef]
- Zhengneng, J.; Najun, L.; Chuanfeng, W.; Huajiang, J.; Jianmei, L.; Qizhong, Z. Synthesis and fluorescence property of some novel 1,8-naphthalimide derivatives containing a thiophene ring at the C-4 position. Dye. Pigment. 2013, 96, 204–210. [Google Scholar] [CrossRef]
- Gan, J.A.; Song, Q.L.; Hou, X.Y.; Chen, K.; Tian, H. 1,8-naphthalimides for non-doping OLEDs: The tunable emission color from blue, green to red. J. Photochem. Photobiol. A Chem. 2004, 162, 399–406. [Google Scholar] [CrossRef][Green Version]
- Arunchai, R.; Sudyoadsuk, T.; Prachumrak, N.; Namuangruk, S.; Promarak, V.; Sukwattanasinitt, M.; Rashatasakhon, P. Synthesis and characterization of new triphenylamine-1,8-naphthalimides for organic light-emitting diode applications. New J. Chem. 2015, 39, 2807–2814. [Google Scholar] [CrossRef]
- Schab-Balcerzak, E.; Grucela, M.; Malecki, G.; Kotowicz, S.; Siwy, M.; Janeczek, H.; Golba, S.; Praski, A. Azomethine diimides and-capped with anthracene moieties: Experimental and theoretical investigations. J. Mol. Struct. 2017, 1128, 462–470. [Google Scholar] [CrossRef]
- Bijak, K.; Filapek, M.; Wiacek, M.; Janeczek, H.; Grucela, M.; Smolarek, K.; Mackowski, S.; Schab-Balcerzak, E. Preparation and characterization of new aliphatic-tailed five- and six-membered azomethine-diimides. Mater. Chem. Phys. 2016, 171, 97–108. [Google Scholar] [CrossRef]
- Nowak, E.M.; Sanetra, J.; Grucela, M.; Schab-Balcerzak, E. Azomethine naphthalene diimides as component of active layers in bulk heterojunction solar cells. Mater. Lett. 2015, 157, 93–98. [Google Scholar] [CrossRef]
- Iwan, A.; Boharewicz, B.; Tazbir, I.; Sikora, A.; Schab-Balcerzak, E.; Grucela-Zajac, M.; Skórka, Ł. Structural and electrical properties of mixture based on P3HT:PCBM and low band gap naphthalene diimide-imines. Synth. Met. 2014, 189, 183–192. [Google Scholar] [CrossRef]
- Schab-Balcerzak, E.; Siwy, M.; Filapek, M.; Kula, S.; Malecki, G.; Laba, K.; Lapkowski, M.; Janeczek, H.; Domanski, M. New core-substituted with electron-donating group 1,8-naphthalimides towards optoelectronic applications. J. Lumin. 2015, 166, 22–39. [Google Scholar] [CrossRef]
- Kotowicz, S.; Korzec, M.; Siwy, M.; Golba, S.; Malecki, J.G.; Janeczek, H.; Mackowski, S.; Bednarczyk, K.; Libera, M.; Schab-Balcerzak, E. Novel 1,8-naphthalimides substituted at 3-C position: Synthesis and evaluation of thermal, electrochemical and luminescent properties. Dye. Pigment. 2018, 158, 65–78. [Google Scholar] [CrossRef]
- Mikroyannidis, J.A.; Ye, S.; Liu, Y. Electroluminesent divinylene- and trivinylene-molecules with terminal naphthalimide or phthalimide segments. Synth. Met. 2009, 159, 492–500. [Google Scholar] [CrossRef]
- Gudeika, D.; Lygaitis, R.; Mimaitė, V.; Grazulevicius, J.; Jankauskas, V.; Lapkowski, M.; Data, P. Hydrazones containing electron-accepting and electron-donating moieties. Dye. Pigment. 2011, 91, 13–19. [Google Scholar] [CrossRef]
- Steidl, L.; Jhaveri, S.J.; Ayothi, R.; Sha, J.; McMullen, J.D.; Ng, S.Y.C.; Zipfel, W.R.; Zentel, R.; Ober, C.K. Non-ionic photo-acid generators for applications in two-photon lithography. J. Mater. Chem. 2009, 19, 505–513. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Y.; Chen, S.; Wang, N.; Qi, Y.; Zhang, X.; Yang, M.; Huang, Y.; Li, M.; Yu, J.; et al. Facile Access to Twisted Intramolecular Charge-Transfer Fluorogens Bearing Highly Pretwisted Donor–Acceptor Systems Together with Readily Fine-Tuned Charge-Transfer Characters. Small 2017, 13, 1604113. [Google Scholar] [CrossRef]
- Gudeika, D. A review of investigation on 4-substituted 1,8-naphthalimide derivatives. Synth. Met. 2020, 262, 116328. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Lua, H.Y.; Chen, C.; Li, M.; Chen, C.-F. 1,8-Naphthalimide-based circularly polarized TADF enantiomers as the emitters for efficient orange-red OLEDs. Org. Electron. 2019, 70, 71–77. [Google Scholar] [CrossRef]
- Kumar, S.; Muhammad, S.; Koh, J.; Khalid, M.; Ayub, K. A combined experimental and computational study of 2,2’-(diazene-1,2-diylbis(4,1-phenylene))bis(6-(butylamino)-1H-benzo[de]isoquinoline-1,3(2H)-dione): Synthesis, optical and nonlinear optical properties. Optik 2019, 182, 162952. [Google Scholar] [CrossRef]
- Staneva, D.; Grabchev, I.; Soumillion, J.P.; Bojinov, V. A new fluorosensor based on bis-1,8-naphthalimide for metal cations and protons. J. Photochem. Photobiol. A Chem. 2007, 189, 192–197. [Google Scholar] [CrossRef]
- Esteban-Gómez, D.; Fabbrizzi, L.; Licchelli, M.; Sacchi, D. A two-channel chemosensor for the optical detection of carboxylic acids, including cholic acid. J. Mater. Chem. 2005, 15, 2670–2675. [Google Scholar] [CrossRef]
- Korzec, M.; Kotowicz, S.; Łaba, K.; Łapkowski, M.; Małecki, J.G.; Smolarek, K.; Mackowski, S.; Schab-Balcerzak, E. Naphthalene Diimides Prepared by a Straightforward Method and Their Characterization for Organic Electronics. Eur. J. Org. Chem. 2018, 15, 1756–1760. [Google Scholar] [CrossRef]
- Korzec, M.; Malarz, K.; Mrozek-Wilczkiewicz, A.; Rzycka-Korzec, R.; Schab-Balcerzak, E.; Polański, J. Live cell imaging by 3-imino-(2-phenol)-1,8-naphthalimides: The effect of ex vivo hydrolysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 238, 118442. [Google Scholar] [CrossRef]
- McEwan, J.A.; Clulow, A.J.; Nelson, A.; Yepuri, N.R.; Burn, P.L.; Gentle, I.R. Dependence of Organic Interlayer Diffusion on Glass-Transition Temperature in OLEDs. ACS Appl. Mater. Interfaces 2017, 9, 14153–14161. [Google Scholar] [CrossRef]
- Skórka, Ł.; Kurzep, P.; Wiosna-Sałyga, G.; Łuszczyńska, B.; Wielgus, I.; Wróbel, Z.; Ulański, J.; Kulszewicz-Bajer, I. New diarylaminophenyl derivatives of carbazole: Effect of substituent position on their redox, spectroscopic and electroluminescent properties. Synth. Met. 2017, 228, 1–8. [Google Scholar] [CrossRef]
- Sęk, D.; Kotowicz, S.; Kula, S.; Siwy, M.; Szłapa-Kula, A.; Małecki, J.G.; Maćkowski, S.; Schab-Balcerzak, E. Thermal, spectroscopic, electrochemical, and electroluminescent characterization of malononitrile derivatives with triphenylamine structure. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 210, 136–147. [Google Scholar] [CrossRef]
- Korzec, M.; Kotowicz, S.; Rzycka-Korzec, R.; Schab-Balcerzak, E.; Małecki, J.G.; Czichy, M.; Łapkowski, M. Novel β-ketoenamines versus azomethines for organic electronics: Characterization of optical and electrochemical properties supported by theoretical studies. J. Mater. Sci. 2020, 55, 3812–3832. [Google Scholar] [CrossRef]
- Kim, K.-W.; Kim, G.-H.; Park, C.-E.; Choi, J.-H. 1,8-Naphthalimide Derivatives Containing Ethynyl Linkage and Blue Light Emitting Properties. Bull. Korean Chem. Soc. 2017, 38, 956–959. [Google Scholar] [CrossRef]
- Kula, S.; Szlapa-Kula, A.; Kotowicz, S.; Filapek, M.; Bujak, K.; Siwy, M.; Janeczek, H.; Maćkowski, S.; Schab-Balcerzak, E. Phenanthro[9,10-d]imidazole with thiophene rings toward OLEDs application. Dye. Pigment. 2018, 159, 646–654. [Google Scholar] [CrossRef]
- Adaci, M.; Murata, Y.; Nakamura, S. Spectral Similarity and Difference of Naphthalenetetracarboxylic Dianhydride, Perylenetetracarboxylic Dianhydride, and Their Derivatives. J. Phys. Chem. 1995, 99, 14240–14246. [Google Scholar] [CrossRef]
- Kotowicz, S.; Siwy, M.; Filapek, M.; Malecki, J.G.; Smolarek, K.; Grzelak, J.; Mackowski, S.; Slodek, A.; Schab-Balcerzak, E. New donor-acceptor-donor molecules based on quinoline acceptor unit with Schiff base bridge: Synthesis and characterization. J. Lumin. 2017, 183, 458–469. [Google Scholar] [CrossRef]
- Glowacki, I.; Szamel, Z. The nature of trapping sites and recombination centres in PVK and PVK–PBD electroluminescent matrices seen by spectrally resolved thermoluminescence. J. Phys. D Appl. Phys. 2010, 43, 295101. [Google Scholar] [CrossRef]
- Bujak, P.; Kulszewicz-Bajer, I.; Zagorska, M.; Maurel, V.; Wielgus, I.; Pron, A. Polymers for electronics and spintronics. Chem. Soc. Rev. 2013, 42, 8895–8999. [Google Scholar] [CrossRef]
- Hussain, S.A. An Introduction to Fluorescence Resonance Energy Transfer (FRET). arXiv 2009, arXiv:0908.1815. [Google Scholar]
- Grykien, R.; Luszczynska, B.; Glowacki, I.; Kurach, E.; Rybakiewicz, R.; Kotwica, K.; Zagorska, M.; Pron, A.; Tassini, P.; Maglione, M.G.; et al. Photo- and electroluminescent properties of bithiophene disubstituted 1,3,4-thiadiazoles and their application as active components in organic light emitting diodes. Opt. Mater. 2014, 37, 193–199. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, M.H.; Lee, C.J.; Park, S.K. Driving Characteristics of Poly(N-vinylcarbazole) and 2-(4-biphenylyl)-5-(4-tert-butylphenyl)-1,3-4-oxadiazole-Based Polymer Light Emitting Diodes. Electron. Mater. Lett. 2013, 9, 663–668. [Google Scholar] [CrossRef]
- Yang, X.; Neher, D.; Hertel, D.; Daubler, T.K. Highly Efficient Single-Layer Polymer Electrophosphorescent Devices. Adv. Mater. 2004, 16, 161–166. [Google Scholar] [CrossRef]
- Kotwica, K.; Bujak, P.; Wamil, D.; Pieczonka, A.; Wiosna-Salyga, G.; Gunka, P.A.; Jaroch, T.; Nowakowski, R.; Luszczynska, B.; Witkowska, E.; et al. Structural, Spectroscopic, Electrochemical, and Electroluminescent Properties of Tetraalkoxydinaphthophenazines: New Solution-Processable Nonlinear Azaacenes. J. Phys. Chem. C 2015, 119, 10700–10708. [Google Scholar] [CrossRef]
- Yersin, H. Highly Efficient OLEDs with Phosphorescent Materials; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; ISBN 978-527-40594-7. [Google Scholar]
- Wen, L.; Li, F.; Xie, J.; Wu, C.; Zheng, Y.; Chen, D.; Xu, S.; Guo, T.; Qu, B.; Chen, Z.; et al. Electroplex emission at PVK/Bphen interface for application in white organic light-emitting diodes. J. Lumin. 2011, 131, 2252–2254. [Google Scholar] [CrossRef]


| Code | Imide 13C (ppm) | Imine | |
|---|---|---|---|
| 1H (ppm) | 13C (ppm) | ||
| 1a | 164.1, 164.0 | 8.6 (s, 2H) | 161.2 |
| 2a | - | 8.6 (s, 2H) | - |
| 3a | 164.3, 165.1 | 8.6 (s, 2H) | 161.2 |
| 4b | 163.9, 164.8 | 8.9 (s, 2H) | 154.1 |
| 1c | 164.0, 161.5 | 8.7 (s, 2H) | 150.3 |
| 1d | 164.4, 160.9 | 8.7 (s, 2H) | 149.9 |
| Compound | TGA | DSC | ||||
|---|---|---|---|---|---|---|
| I Heating Scan | II Heating Scan | |||||
| T5 a | Tmax b | Tm c | Tg e | Tc d | Tm c | |
| (°C) | (°C) | (°C) | (°C) | (°C) | (°C) | |
| 1a | 426 | 464 | 173 | 86 | nd | nd |
| 2a | 431 | 479 | 259 | 140 | nd | nd |
| 3a | 446 | 488 | 209 | 138 | nd | nd |
| 4b | 428 | 498 | 255 | 118 | 170 | 226 |
| 1c | 387 | 432 | 220 | 74 | 148 | 225 |
| 1d | 285 | 275, 479 | 251 | 127 | nd | nd |
| Compound | Method | Ered 1 | Ered(onset) | Eox 1 | Eox(onset) | EA | IP | Eg |
|---|---|---|---|---|---|---|---|---|
| [V] | [V] | [V] | [V] | [eV] | [eV] | [eV] | ||
| 1a | CV | −1.72 a | −1.23 | 0.73 a | 0.53 | −3.87 | −5.63 | 1.76 |
| DPV | −1.46 | −1.22 | 0.50 | 0.36 | −3.88 | −5.46 | 1.58 | |
| 2a | CV | −1.82 b | −1.73 | 0.69 a | 0.55 | −3.37 | −5.65 | 2.28 |
| DPV | −1.89 | −1.77 | 0.61 | 0.53 | −3.33 | −5.63 | 2.30 | |
| 3a | CV | −1.96 b | −1.83 | 0.67 a | 0.56 | −3.27 | −5.66 | 2.39 |
| DPV | −1.99 | −1.88 | 0.58 | 0.48 | −3.22 | −5.58 | 2.36 | |
| 4b | CV | −1.75 b | −1.53 | 0.76 a | 0.46 | −3.35 | −5.56 | 2.21 |
| DPV | −1.67 | −1.57 | 0.78 | 0.56 | −3.43 | −5.66 | 2.23 | |
| 1c | CV | −1.92 a | −1.38 | 0.76 a | 0.41 | −3.72 | −5.51 | 1.79 |
| DPV | −1.96 | −1.28 | 0.70 | 0.38 | −3.82 | −5.48 | 1.66 | |
| 1d | CV | −1.66 a | −1.27 | 0.90 a | 0.70 | −3.83 | −5.80 | 1.97 |
| DPV | −1.62 | −1.19 | 0.80 | 0.70 | −3.91 | −5.80 | 1.89 |
| Compound | Medium | UV-vis | PL | ||||
|---|---|---|---|---|---|---|---|
| λmax (nm) (ε·104) a | λem(nm) | Stokes Shift | Φ (%) | τ c (ns) | X2 | ||
| (cm−1) b | |||||||
| 1a | CHCl3 d | 345(2.5), 411(9.8) | 501 | 6356 | 25 | 16.9 | 1.045 |
| NMP d | 342(2.5), 413(2.8) | 544 | 5831 | - | - | - | |
| FILM | 345, 413 | 537 | 5591 | 3.8 | 12.5 | 0.930 | |
| PVK:PBD e | 310 sh, 344 sh, 424 | 388, 504 | - | 11.3 | - | - | |
| 2a | CHCl3 d | 246(14.1), 260 sh, 345(8.1), 415(10.0) | 533 | 5335 | 26 | 19.1 | 1.017 |
| NMP d | 345(3.5), 387(6.9),414sh | 516 | 6460 | - | - | - | |
| FILM | 348, 402 | 544 | 6493 | 4.0 | 16.2 | 1.061 | |
| PVK:PBD e | 310 sh, 344 sh, 429 sh | 384, 499 | - | 7.5 | - | - | |
| 3a | CHCl3 d | 246(14.0), 260 sh, 345(10.1), 415(12.1) | 533 | 5335 | 14 | 21.0 | 1.063 |
| NMP d | 345(3.8), 393(8.9), 414 sh | 514 | 6460 | - | - | - | |
| FILM | 345, 397 | 544 | 6807 | 3.7 | 15.3 | 1.098 | |
| PVK:PBD e | 310 sh, 344 sh, 429 sh | 384, 499 | - | 7.3 | - | - | |
| 4b | CHCl3 d | 246 sh, 274(23.2), 345(5.8), 390(4.7) | 515 | 6224 | 1 | 22.0 | 0.935 |
| NMP d | 345(10.1), 387(5.6) | 450 | 3618 | - | - | - | |
| FILM | 348, 397 | 577 | 4221 | 1.7 | 2.16 | 1.019 | |
| PVK:PBD e | 310 sh, 344 sh | 384 | - | 2.3 | - | - | |
| 1c | CHCl3 d | 243(3.6), 340(9.1) | 503 | 9445 | 7 | 16.7 | 1.123 |
| NMP d | 267(21.2), 340 (4.6) | 517 | 10069 | - | - | - | |
| FILM | 345 | 435, 490 sh | 5997 | 4.8 | 11.0 | 1.056 | |
| PVK:PBD e | 310 sh, 344 sh | 399, 477 | - | 3.6 | - | - | |
| 1d | CHCl3 d | 345(9.8), 376(8.8) | 470 | 5319 | 3 | 11.2 | 1.012 |
| NMP d | 345(17.8), 376(8.8) | 525 | 7548 | - | - | - | |
| FILM | 360, 389 sh, 424 sh | 471 sh, 509 | - | 4.6 | 9.0 | 1.047 | |
| PVK:PBD e | 310 sh, 344 sh | 386, 498 | - | 8.4 | - | - | |
| Compound | λEL | UELMax | EL Intensity |
|---|---|---|---|
| (nm) | (V) | (Counts) | |
| 1a | 670 a | 37 | 2207 |
| 530 b | 24 | 16,214 | |
| 540 c | 26 | 14,228 | |
| 555 d | 28 | 195,060 | |
| 2a | 675 a | 12 | 608 |
| 535 c | 9 | 267 | |
| 3a | 675 a | 16 | 489 |
| 532 c | 11 | 226 | |
| 1c | 675 a | 19 | 125 |
| 525 c | 23 | 1837 | |
| 1d | 529 c | 23 | 7091 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotowicz, S.; Korzec, M.; Pająk, A.K.; Golba, S.; Małecki, J.G.; Siwy, M.; Grzelak, J.; Maćkowski, S.; Schab-Balcerzak, E. New Acceptor–Donor–Acceptor Systems Based on Bis-(Imino-1,8-Naphthalimide). Materials 2021, 14, 2714. https://doi.org/10.3390/ma14112714
Kotowicz S, Korzec M, Pająk AK, Golba S, Małecki JG, Siwy M, Grzelak J, Maćkowski S, Schab-Balcerzak E. New Acceptor–Donor–Acceptor Systems Based on Bis-(Imino-1,8-Naphthalimide). Materials. 2021; 14(11):2714. https://doi.org/10.3390/ma14112714
Chicago/Turabian StyleKotowicz, Sonia, Mateusz Korzec, Agnieszka Katarzyna Pająk, Sylwia Golba, Jan Grzegorz Małecki, Mariola Siwy, Justyna Grzelak, Sebastian Maćkowski, and Ewa Schab-Balcerzak. 2021. "New Acceptor–Donor–Acceptor Systems Based on Bis-(Imino-1,8-Naphthalimide)" Materials 14, no. 11: 2714. https://doi.org/10.3390/ma14112714
APA StyleKotowicz, S., Korzec, M., Pająk, A. K., Golba, S., Małecki, J. G., Siwy, M., Grzelak, J., Maćkowski, S., & Schab-Balcerzak, E. (2021). New Acceptor–Donor–Acceptor Systems Based on Bis-(Imino-1,8-Naphthalimide). Materials, 14(11), 2714. https://doi.org/10.3390/ma14112714