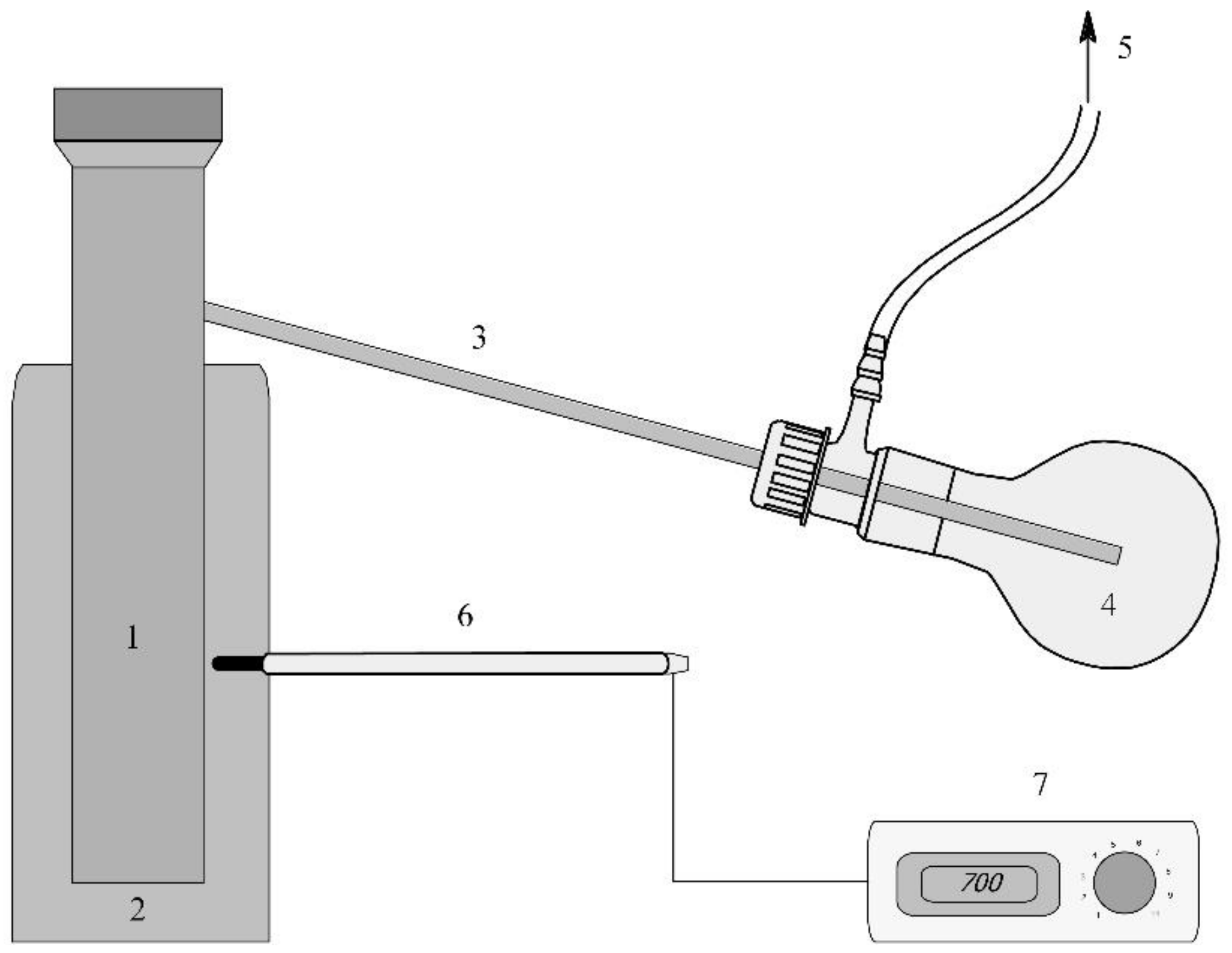
3.1. Adsorbent Characterisation
The results of CHN elementary analysis (
Table 2) indicate that the carbon content in the obtained sorbent did not exceed 65% along with a relatively large amount of nitrogen (above 5%). The obtained biochar contains a higher carbon concentration than reported by Ozcimen and Karaosmanoglu [
10], most probably due to the differences in the waste preparation method and pyrolysis conditions. The degree of carbonisation expressed by the H/C molar ratio, according to the data in
Table 2, indicates clearly that a pyrolysis temperature increase resulted in the H/C molar ratio systematically decreasing. The observed H/C ratio, 0.29 (973.15 K), is lower than the value obtained for the biochar studied by Ozcimen and Karaosmanoglu, 0.47 [
10], and similar to that of the activated carbon, 0.26, obtained by Chen et al. [
11]. These low H/C ratios suggest that the discussed biochar is strongly carbonised and does not contain organic residues for rape plant, such as cellulose. On the other hand, the relatively high content of nitrogen (5.6%) in biochar may also have a positive effect on the adsorption process. Nitrogen can form highly polar functional groups on the surface of the biochar, resulting in greater hydrophilicity of the surface.
The nitrogen adsorption–desorption isotherms are shown in
Figure 2. The observed curves indicate that the surface area of the studied biochar increases systematically with increasing pyrolysis temperature. The literature data (Wang et al. [
12]) indicate that when the preparation temperature is equal to or above 873 K, the specific surface area of the obtained biochar is larger than that of most other materials. This is most probably due to the gradual evaporation and breakdown of volatile organic components as the pyrolysis temperature was increased from 673 to 973 K, resulting in the formation of a microporous structure.
The biochar obtained at 973.15 K characterises the mean pore diameter (
Sp), calculated according to the Barrett–Joyner–Halenda (BJH) algorithm, as being equal to 1.9 nm (
Figure 2c). The carbon material was also characterised by surface area of
ABET = 166.99 m
2·g
−1 and pore volume
Vp = 0.08 cm
3·g
−1. Nitrogen adsorption/desorption studies revealed that the obtained isotherm is of type IV, according to International Union of Pure and Applied Chemistry (IUPAC) classification, indicating the existence of mesopores.
Wang et al. demonstrated that the specific surface area of biochar, produced from other precursors (peanut shell, sludge, bamboo reed, etc.) at 573–973 K, was in the range of 3.75–54.05 m
2·g
−1 [
13]. In this experiment, the specific surface area of biochar prepared from waste rapeseed cake was much larger. The latter confirms the correct choice of pyrolysis conditions and biochar activation method.
Based on the obtained results, the biochar at the highest temperature of pyrolysis (973.15 K) revealed the most promising physicochemical properties of metal ion adsorption, and hence the material was chosen for further studies.
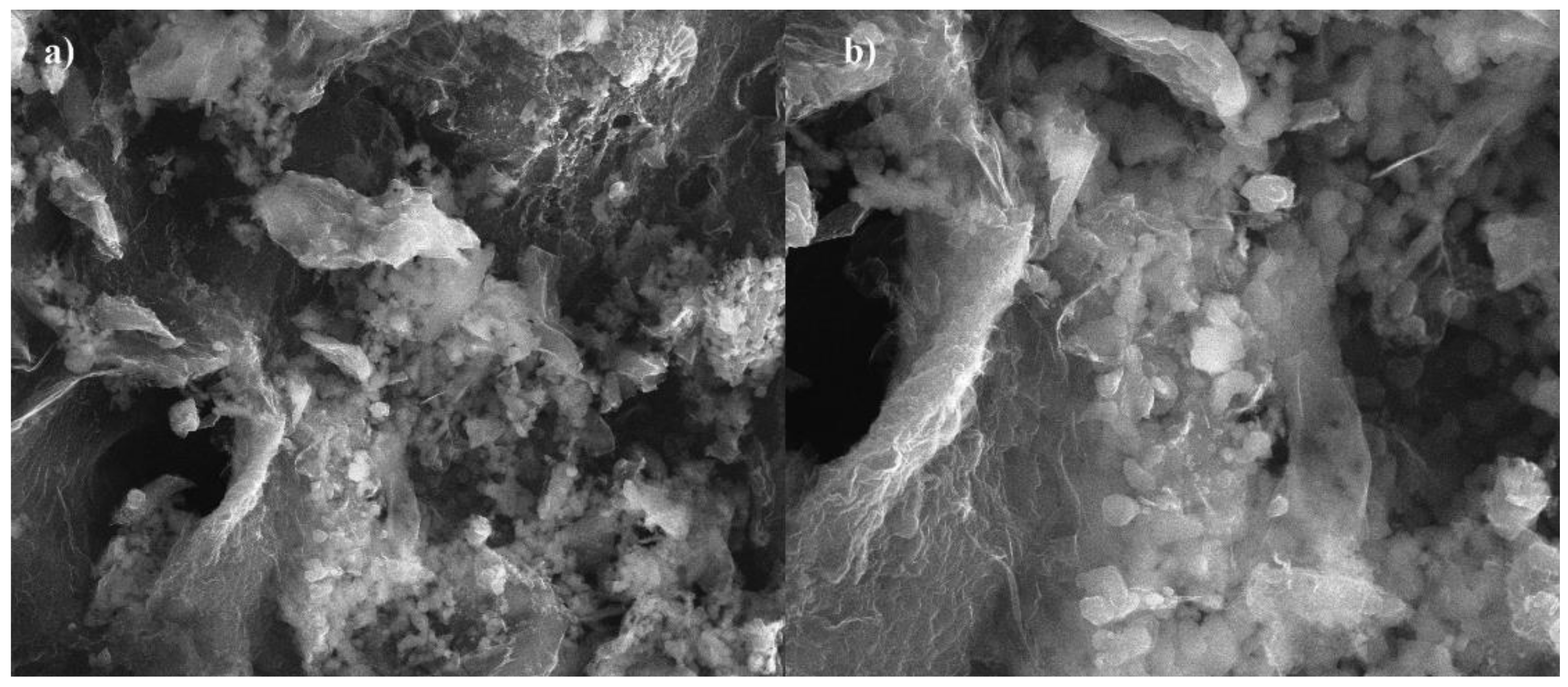
SEM images of the sorption material are presented at
Figure 3. The prepared carbon sorbent is composed of irregularly shaped particles of varying grain sizes that tend to agglomerate. The external surface of the obtained biochar also has deep slits, which could be formed upon the removal of volatile organic matter during pyrolysis.
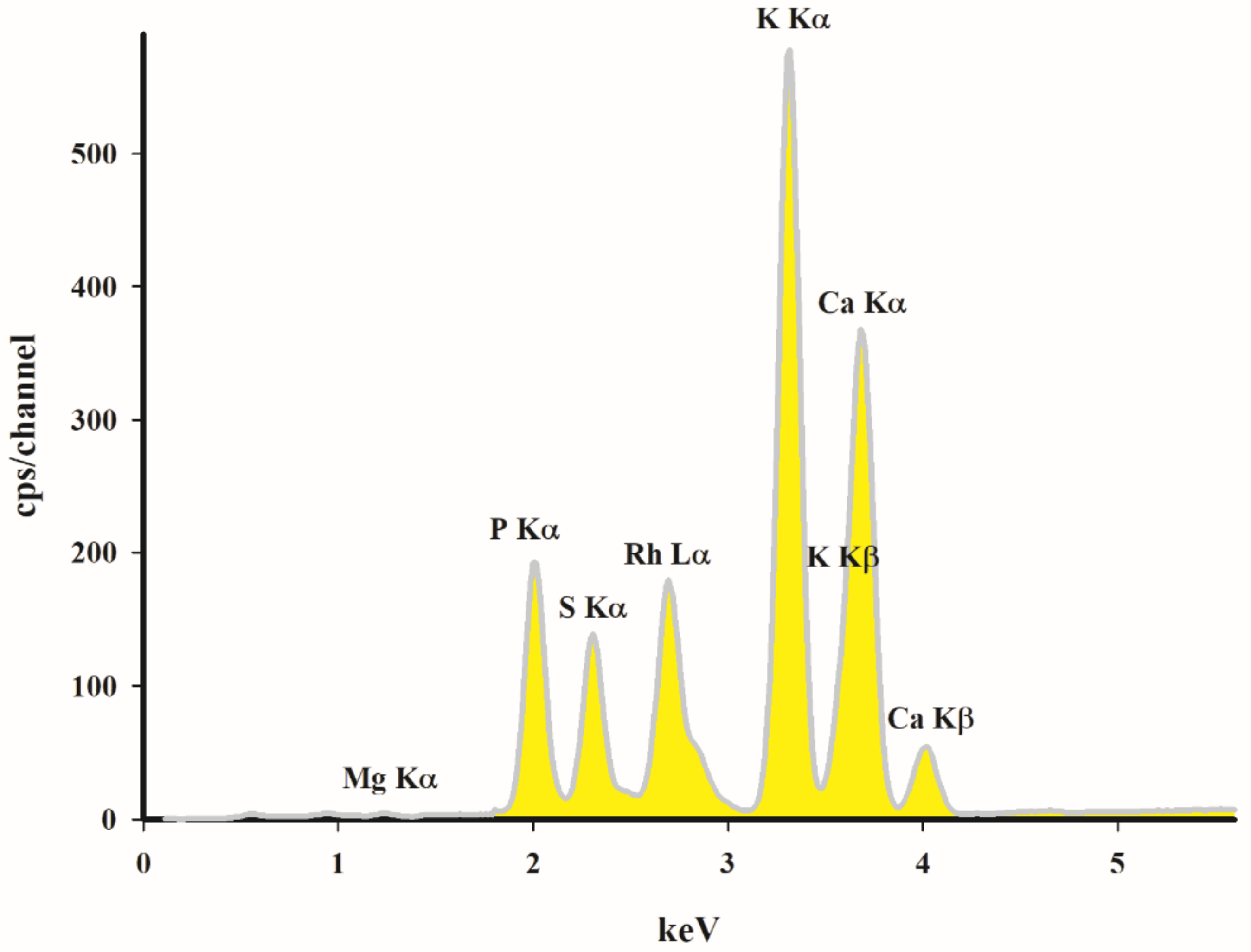
The EDXRF analysis (
Figure 4) revealed that the studied porous material possesses significant amounts of potassium, calcium, phosphorus and magnesium. The presence of these metals in biochar is beneficial for the metal adsorption process. They can exchange or precipitate with the studied metals and reduce their availability. The reported adsorption kinetic results indicate that the adsorption of heavy metals by the cation exchange mechanism in the biochar occurs at the early stage of the reaction. Park et al. and Lu et al. reported that the effects of cation exchange on the adsorption of heavy metals for rice straw and sludge biochar were equal to 62.3% (Cu
2+) and 51.8% (Pb
2+), respectively [
14,
15].
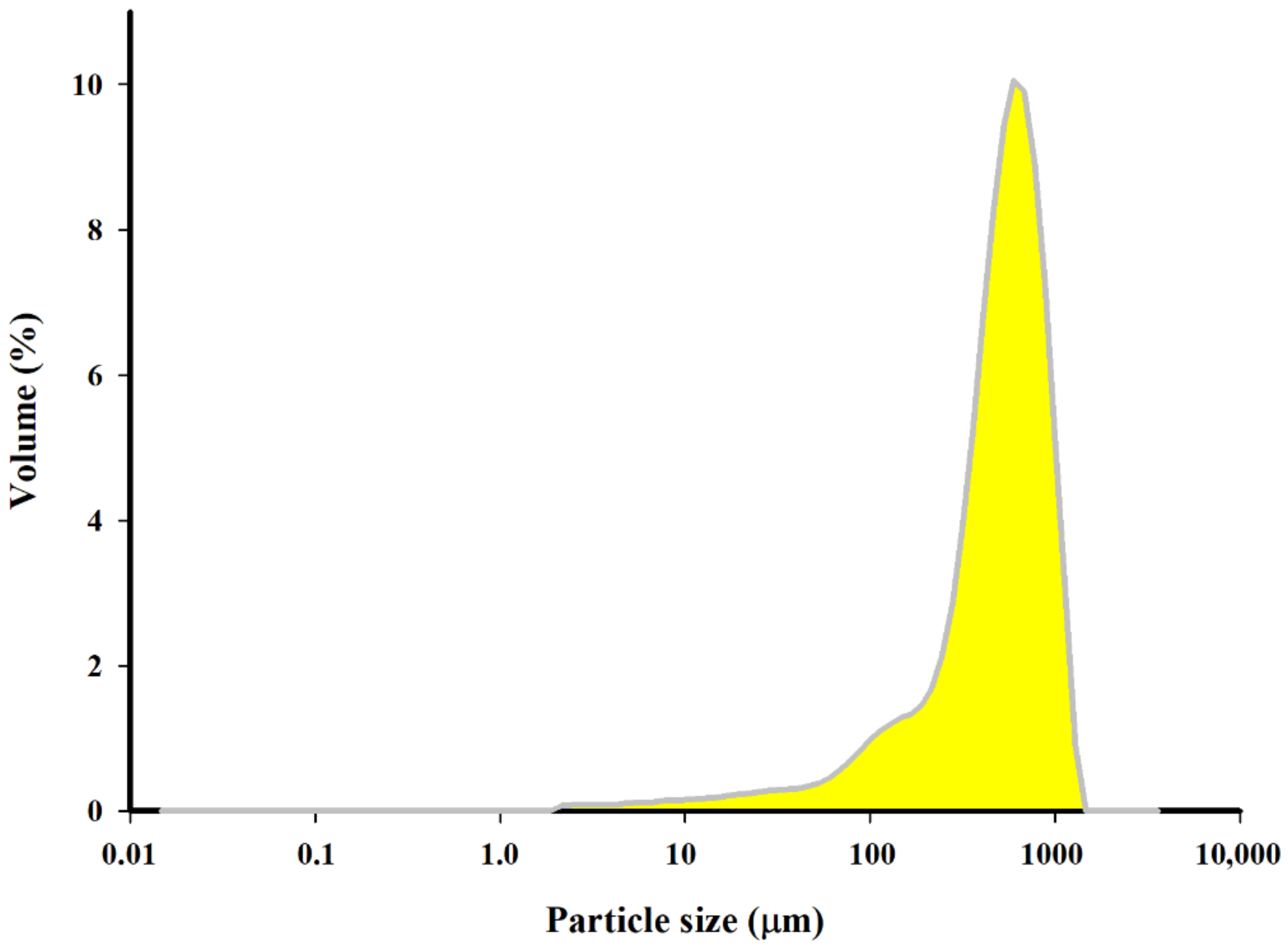
Granulometric analysis of the samples suspended in methanol was performed with a MasterSizer 3000 laser apparatus. The obtained biochar revealed the dominant fraction with a size of 119–844 μm and an average specific surface of 45.2 m
2·kg
−1 (
Figure 5).
TGA-DTA analysis was carried out in air and in a nitrogen atmosphere (100 cm
3·min
−1) and with a heating rate of 5 K·min
−1. The results are listed in
Table 3, while TGA-DTA curves are plotted in
Figure 6. The TG curve (
Figure 6A) of the sample exhibits four stages.
In the first endotherm, 304.6–321.3 K moisture is released. The second endotherm (321.3–468.5 K) can be related to the degradation of an unstable oxygen species (i.e., carboxyl, hydroxyl or lactone groups,) along with further loss of water. In the third exoenergetic stage (468.9–792.0 K), 59.06% of mass loss is noted due to the nitrogen-containing groups decomposition and destruction of the sample carbon material’s microstructure. The fourth stage (792.0–1273.2 K) can be assigned to the decomposition of the most thermally stable sulphur groups. The TG curve recorded under a nitrogen atmosphere (
Figure 6B) is a three-stage process. In the first stage, the sample loses a small amount of adsorption water (299.2–311.5 K). The second stage (311.5–754.0 K) (
Figure 6B) can be caused by the sample decomposition after treatment with acetone and n-heptane, which unblocks the pores and causes the detachment of oxygen and nitrogen functional groups. In the third stage (onset 754.0 K), sulphur groups most probably decompose on the surface of biochar.
The obtained results of the TGA-DTA tests clearly indicate that oxygen, nitrogen and sulphur groups are present on the surface of the obtained biochar. This is closely related to the method of raw material modification prior to the pyrolysis process with the use of ammonium peroxodisulphate. The presence of various functional groups on the surface of the sorbent significantly improves the sorption capacity and significantly extends the potential application time of the material.
3.2. Adsorption Process
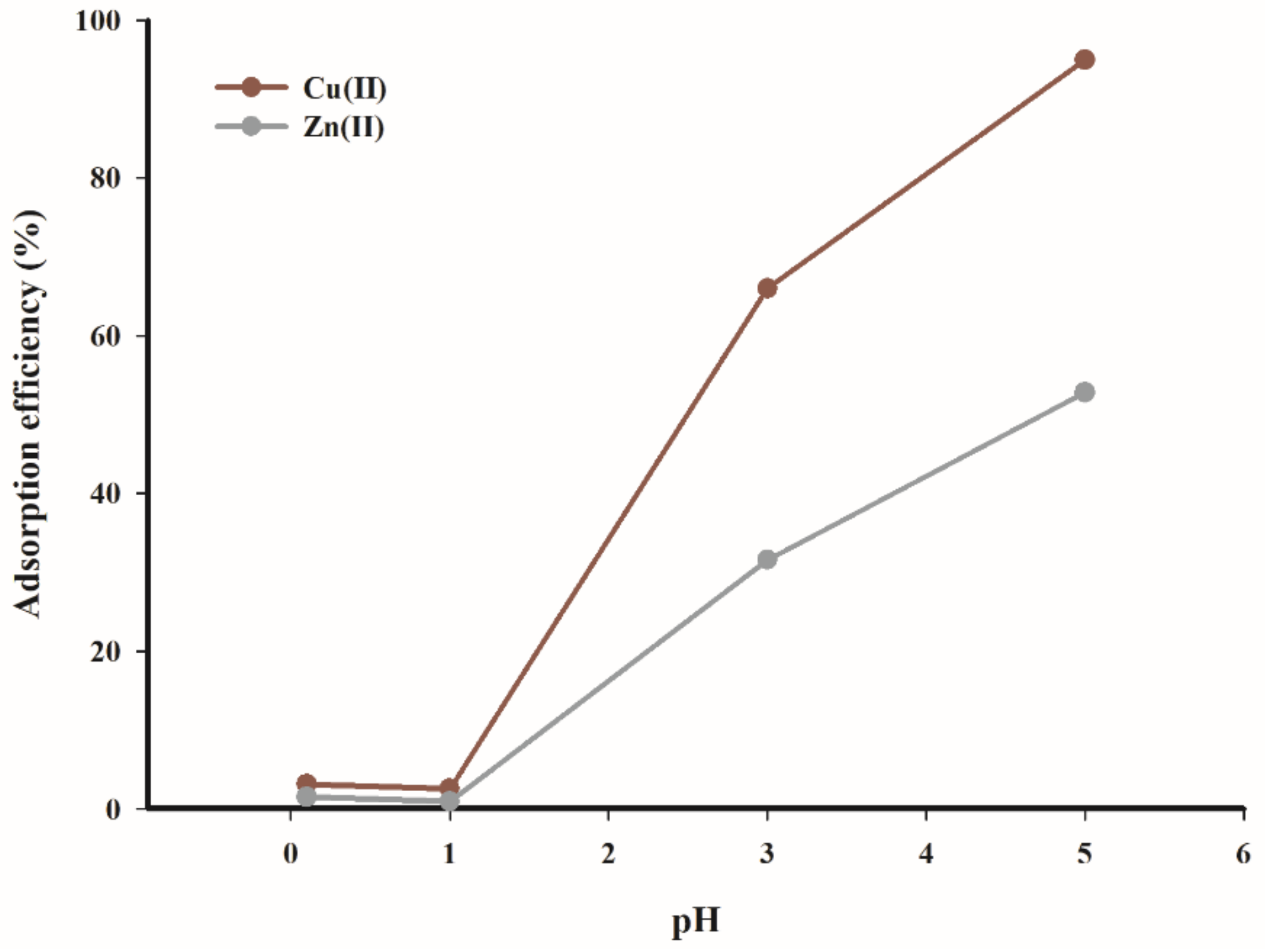
The effect of pH on the adsorption rate of copper(II) and zinc(II) ions was tested and obtained results are presented in
Figure 7. The data in
Figure 7 clearly demonstrate that the adsorption of copper(II) and zinc(II) ions was not favourable at low pH (0–3). The calculated difference in adsorption efficiency in the pH range of 0.1–5 was equal to 90%, while the highest adsorption rate (95%) was obtained for a solution of pH 5.
In the case of zinc(II) ions, the significant rise in the adsorption efficiency was observed at pH = 5. At same time, the highest process performance, below 53%, was obtained.
The observed dependencies are consistent with the results reported by Hawari and Mulligan, as well as Rahman and Islam for the adsorption of copper cations on various natural sorbents, which determine the optimal pH range as 4–5.5 [
16,
17]. Similar results were obtained by Gao et al. during an investigation of the kinetics and mechanism of copper(II) ion adsorption on an activated carbon obtained from pinewood sawdust [
18]. The optimum pH was in the range 4–6 for copper(II) ions; in a strongly acidic medium, the reaction proceeds with less efficiency due to the protonation of functional groups on the sorbent surface. At a low pH, both metal cations were adsorbed to a lesser extent on the charged surface of biochar due to electrostatic repulsions. An increase in pH caused a charge change on the sorbent surface, generating more negatively charged groups. Furthermore, the number of competing hydronium ions decreased in an environment with an increase in pH value.
The analysis of data presented in
Figure 7 indicates that the tested biochar adsorbent exhibits higher affinity for copper(II) ions than for zinc(II) ions.
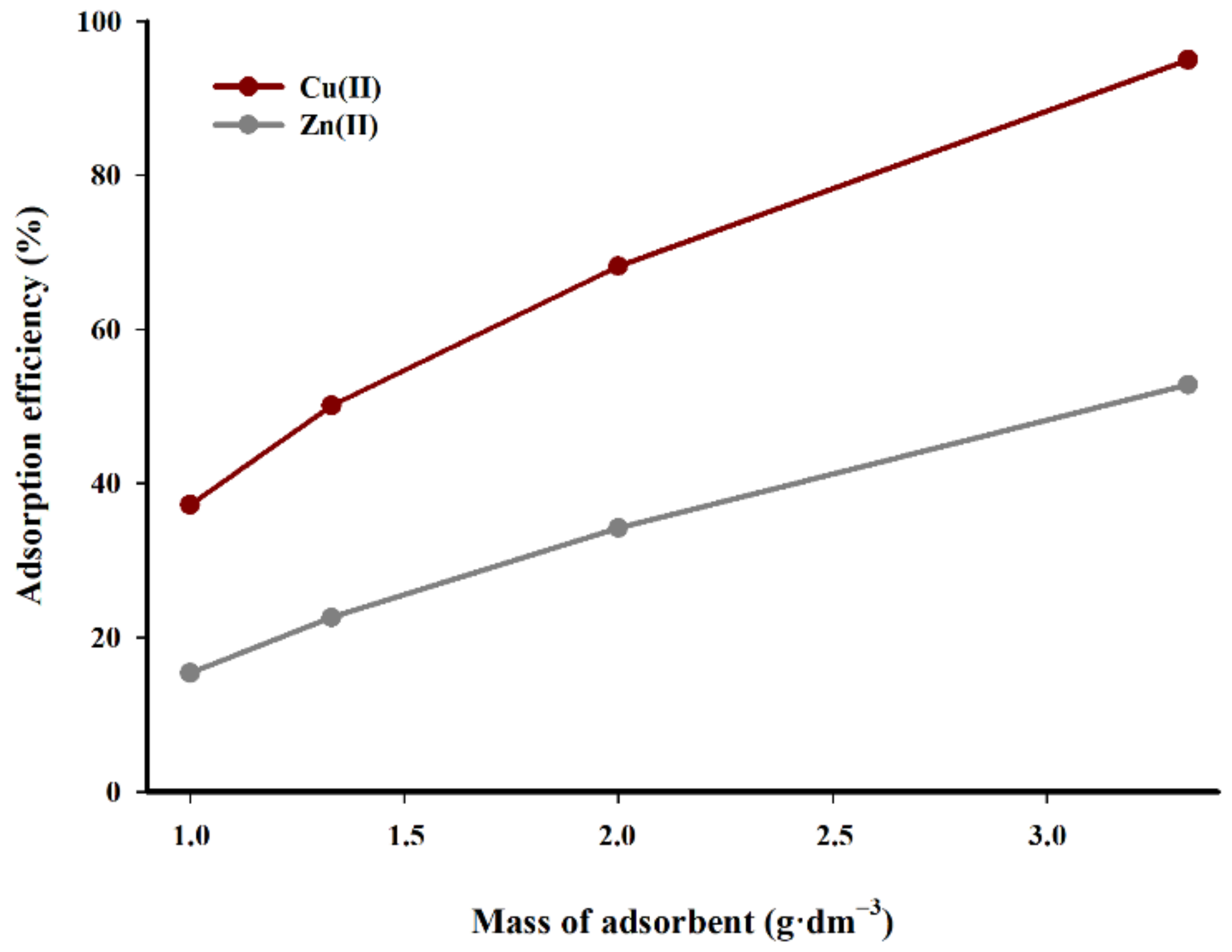
The studies of the effect of sorbent concentration (g·dm
−3) on the efficiency of the adsorption of copper(II) and zinc(II) are presented in
Figure 8. The research performed by different authors has demonstrated that the ratio of adsorbent to the solution volume has a considerable impact on the efficiency of the adsorption process [
19,
20,
21,
22]. The efficiency value increases with the increase in adsorbent mass.
Analogous relations can be observed in
Figure 8. It is evident that a higher concentration of a sorbent in the suspension improves the adsorption efficiency of copper(II) cations. The increase in sorbent concentration in the suspension from 1.0 to 3.33 g·dm
−3 results in a performance increase of almost 60%, while in the case of zinc, the adsorption efficiency is less pronounced. The efficiency of the process increases linearly with the rise in the mass of the adsorbent used in relation to the volume of solution. The difference in adsorption efficiency between the limit tested sorbent concentrations in suspension is over 37%. It can be assumed that when optimising the sorption efficiency, the concentration of sorbent in the suspension should oscillate within 3 g·dm
−3.
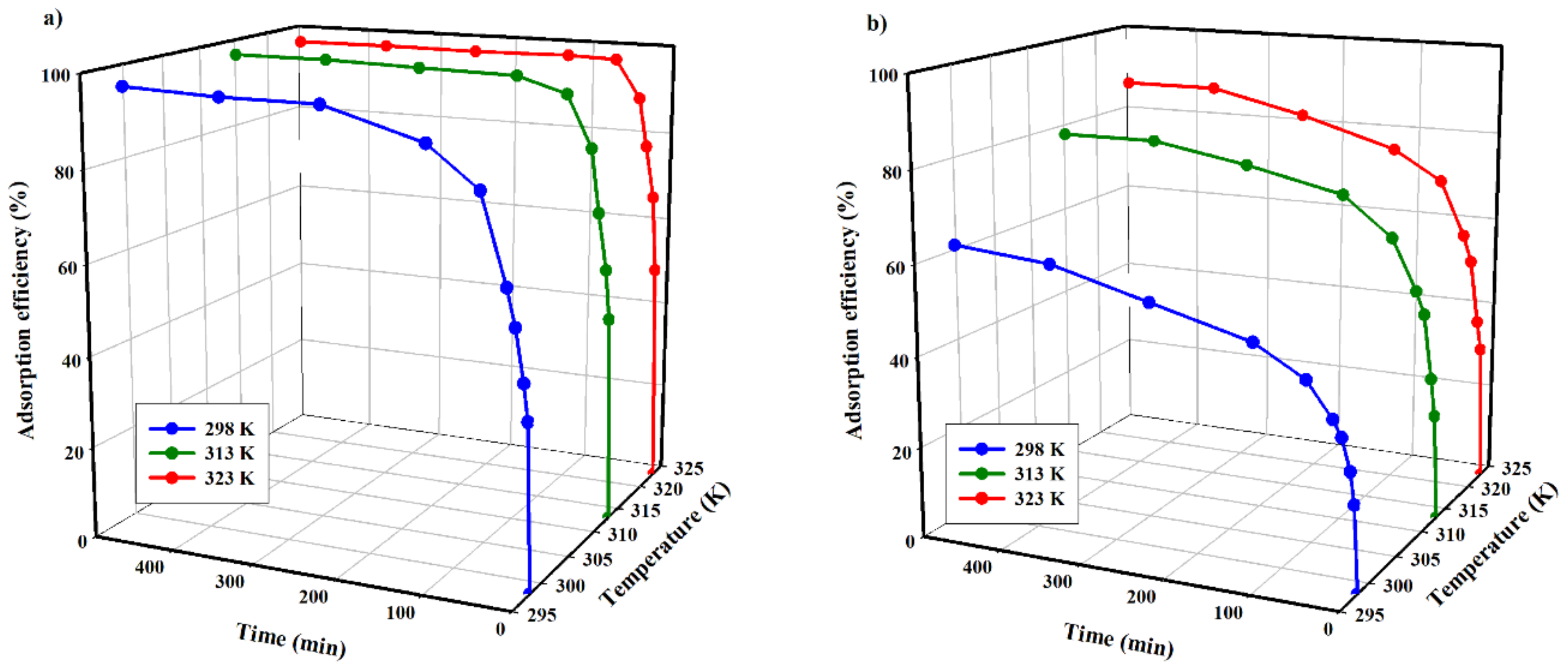
The efficiency of copper(II) and zinc(II) adsorption on the tested carbon adsorbent is presented in
Figure 9.
The analysis of data shown in
Figure 9 indicates that efficiency values are affected by the temperature increase for both tested cations. The lowest efficiency was obtained for the experiments performed at the lowest temperature (298.15 K), while the maximum efficiency for copper(II) and zinc(II) ions was 96 and 63%, respectively. At 323.15 K, the values of the process efficiency were the highest for both cations, at 98 and 87%, respectively.
At the same time, it is clear that the temperature of the process impacts the adsorption of zinc(II) ions to a larger extent than copper(II) ions. In the case of Zn2+, increasing the process temperature by 25 K results in an increase in efficiency of about 21%. An analogous increase in process temperature, in the case of Cu2+, results in a 2% larger process efficiency.
Furthermore, it is evident that the adsorption process temperature results in a shortened time necessary to reach thermodynamic equilibrium in the system. In the case of Zn(II), adsorption at 298.15 K, during an 8 h experiment, appeared to be too low to reach an equilibrium state. Increasing the process temperature by 15 K results in achieving equilibrium after approximately 6 h, while a further temperature increase by 10 K does not reduce the equilibrium time.
Such thermodynamic dependence of the copper(II) and zinc(II) adsorption process on temperature can be related to its endothermic nature. This may be attributed to an increased penetration of the studied cations inside micropores at higher temperatures or the creation of new active sites. The endothermic adsorptions may be due to a stronger interaction between pre-adsorbed water and the adsorbent than the interaction between copper(II) and zinc(II) ions and the adsorbent.
The temperature increase may impact the kinetic energy of both cations, which in turn may lead to an increase in their mobility and promote spontaneous adsorption. On the other hand, the temperature increase causes the solution viscosity to decrease, which results in a rise in ionic diffusion rate and faster adsorption to the surface of the tested biochar. The endothermic adsorption of the studied cations on biochar appears to be uncommon behaviour. However, several authors have reported endothermic adsorption of heavy metal cations on different types of adsorbents of natural origin [
23,
24].
Considering the technological nature of the research, the recommended temperature of adsorption seems to be ca. 313.15 K. It should be noted that the temperature of acidic scrubber liquid waste fluctuates within this temperature.
The next stage of research involved the determination of the optimal time for the adsorption process. Studies were conducted for standard solutions with initial concentrations of copper(II) and zinc(II) ions of 100, 200 and 300 mg·dm
−3, as well as 50, 100 and 200 mg·dm
−3, respectively (
Figure 10).
The adsorption progress indicates that a high degree of removal of the tested ions was achieved after two hours of the adsorbent contact with the adsorbate (
Figure 10). The efficiency of the adsorption process for copper(II) ions after this contact time was 97, 65 and 45%, respectively, for increasing initial concentrations of Cu
2+ in solutions. The analysis of curves in
Figure 10 indicates that the increase in the initial concentration of Cu
2+ ions from 100 to 300 mg·dm
−3 reduces the adsorption efficiency by approximately 52%.
Analogous studies of zinc(II) adsorption indicate that the same time of contact with the adsorbate resulted in the adsorption efficiency of 100, 71 and 38%, respectively, for increasing initial concentrations of Zn2+. Here, there is also a substantial impact of the concentration of the researched cation in the solution on the efficiency of the adsorption in the studied carbon adsorbent. The increase in Zn2+ concentration from 50 to 200 mg·dm−3 results in an adsorption efficiency decrease of ca. 62%, in relation to starting zinc(II) concentration (50 mg·dm−3).
The equilibrium point for both cations, depending on the initial concentration, was reached relatively slowly. In the case of a copper(II) cation solution of 100 mg·dm−3, the thermodynamic equilibrium was found after ca. two hours of the adsorption process, while in the case of solutions of concentration of 200 and 300 mg·dm−3, the equilibrium time was reached after 6 h. For the initial zinc(II) ion concentration (50 mg·dm−3), the equilibrium point was reached after 60 min, whereas at 200 mg·dm−3 the time was extended to ca. 6 h.
The data show that the adsorption equilibrium of metal ions reported by others is much slower for carbon sorbents when compared to inorganic, hybrid or ion exchange resin adsorbents [
20,
25,
26,
27].
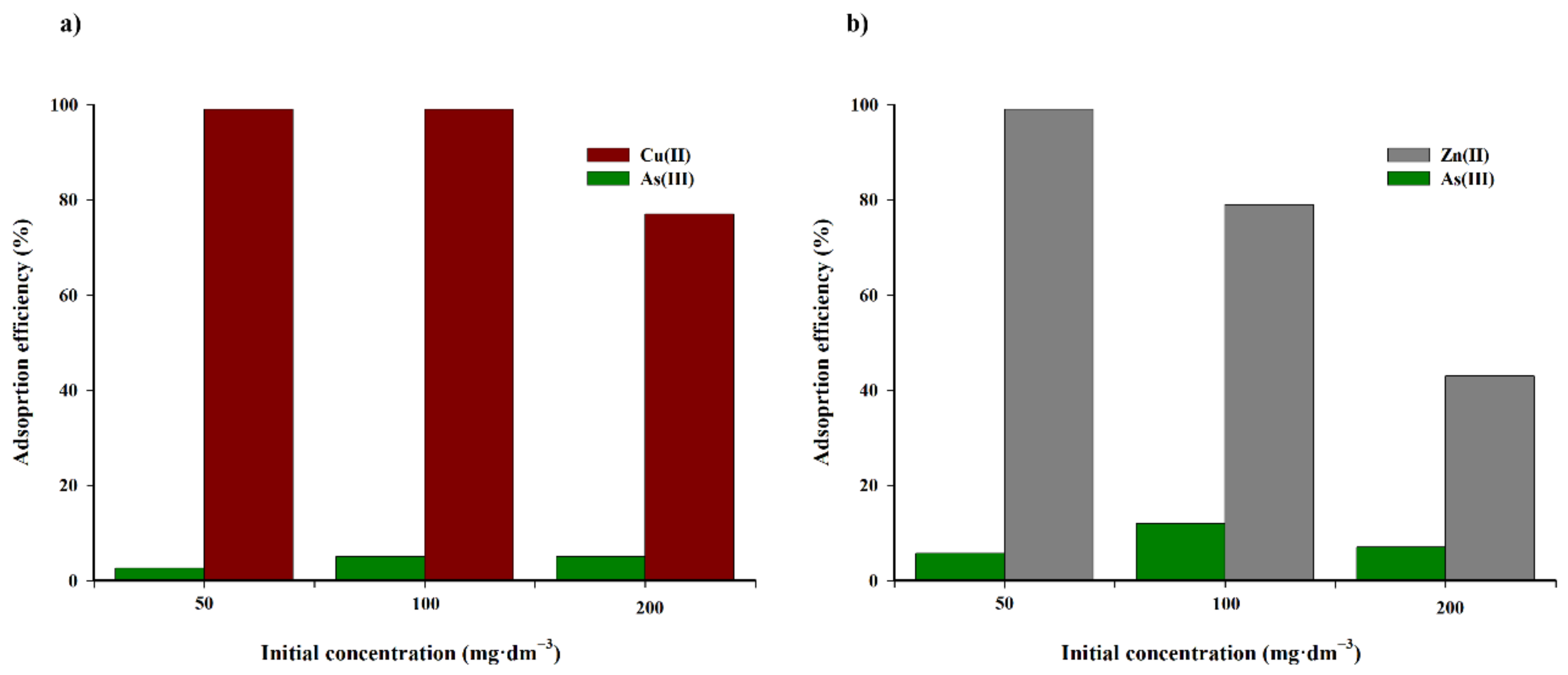
To conclude this part of the research, the selectivity of the biochar sorbent is effective for a mixture of copper(II) and zinc(II) in the presence of arsenic(III) ions in the range of 50–200 mg·dm
−3, which is presented in
Figure 11.
Arsenic(III) ions are adsorbed to a small extent from the solution on the tested biochar. Furthermore, the presence of arsenic(III) in the solution only has a slight impact on the increase in the adsorption rate of copper(II) and zinc(II) cations.
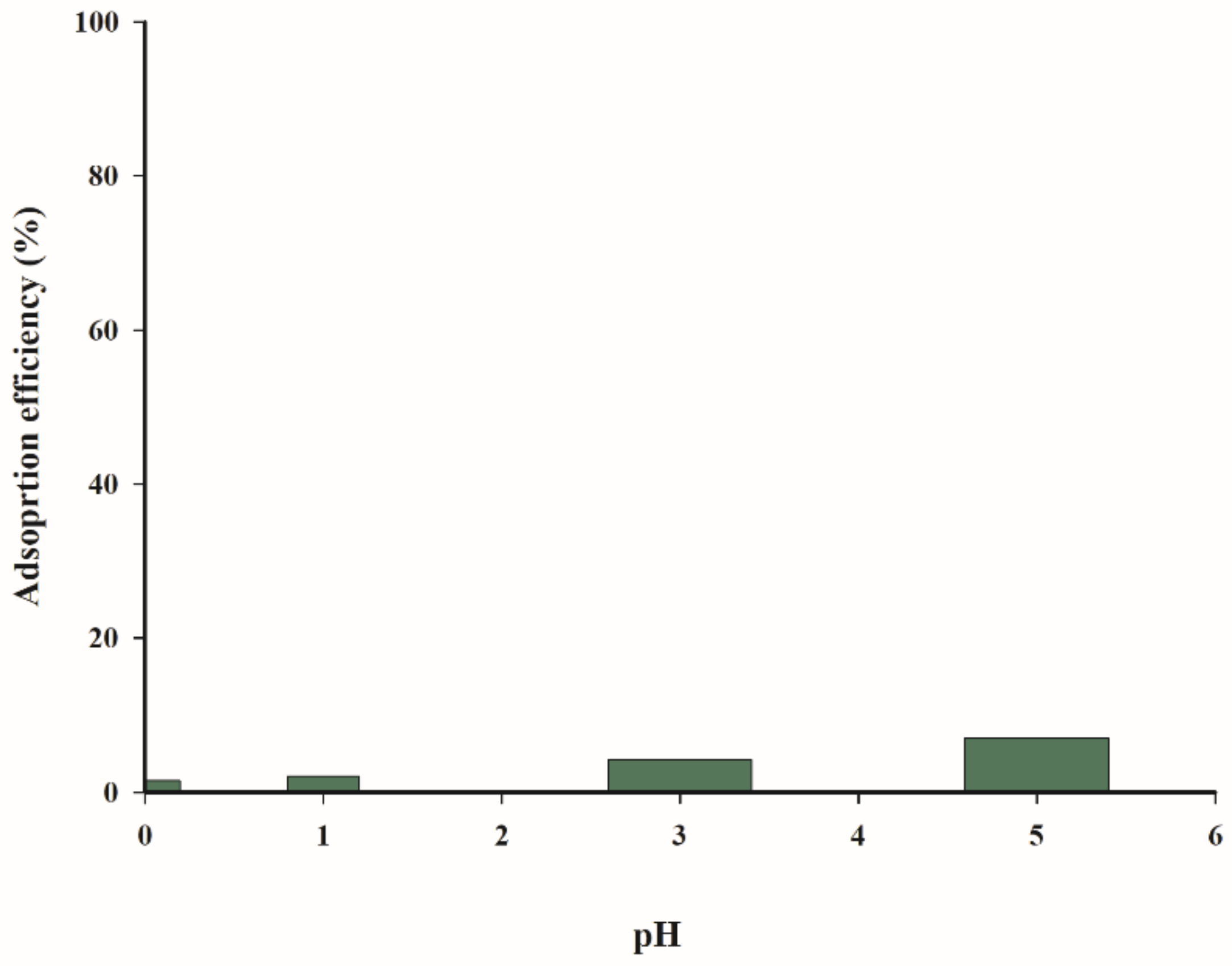
The solution acidity does not increase the adsorption of arsenic(III) ions in the studied pH range (1–5), because of a constant low level of only a few to several percent (
Figure 12).
3.3. Adsorption Kinetics Study
On the basis of the experimental data, kinetic models were calculated in order to precisely design the adsorption process. For that purpose, the equation of the pseudo first-order by the Lagergren kinetic model and Ho’s equation of pseudo-second order kinetic model were applied.
The linear model of pseudo-first order kinetics is defined according to Equation (3):
where
qe and
qt define the amounts of metal ions adsorbed in a point of equilibrium and at time
t (min), and
k1 (1·min
−1) is the speed constant for the pseudo-first order model.
The calculated kinetics parameters for the adsorption of studied metal ions onto the biochar, at initial concentration of 100 and 200 mg·dm
−3, are listed in
Table 4.
The linear correlation coefficients indicate that the first-order kinetic model of pseudo-first order adsorption provides a satisfactory description of the adsorption of copper(II) and zinc(II) cations on the tested sorbent. However, the adsorption capacity results based on this kinetic model qe,cal are substantially different from the values obtained experimentally in all cases.
The linear model of the pseudo-second order equation by Ho is:
where
qe and
qt determine the amounts of metal cations adsorbed at a point of equilibrium and at time
t (min), and
k2 (g·mg
−1·min
−1) is the speed constant for the pseudo-second order model, respectively.
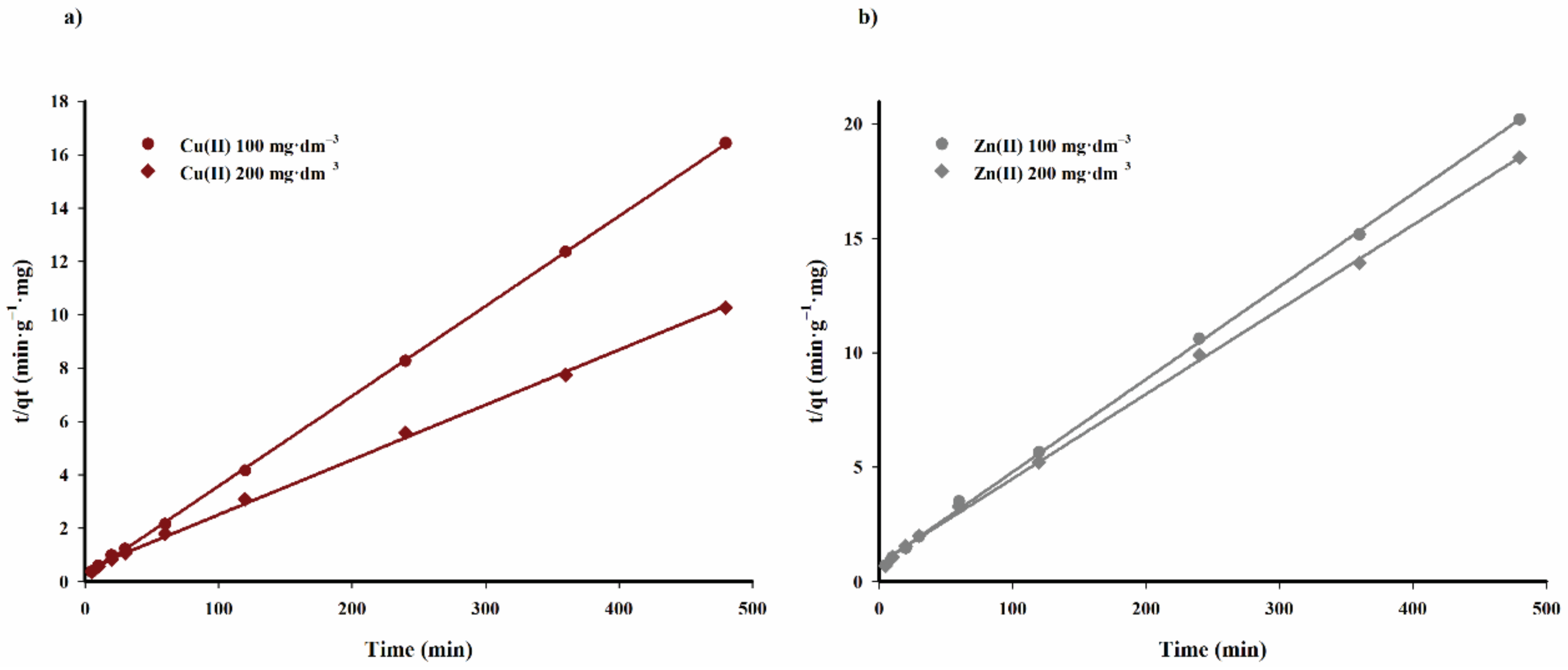
The values of the constant rate of adsorption process
k2 and calculated adsorption capacity q
e,calc. for specific concentrations of lithium and cobalt(II) ions in solution are listed in
Table 5, calculated on the basis of straight lines shown in
Figure 13.
The data listed in
Table 5 indicate that the pseudo-second order kinetic model accurately describes the kinetics of the adsorption process of copper(II) and zinc(II) cations on the tested biochar sorbent. The adsorption capacity values calculated on the basis of the discussed model are highly correlated with the experimental data.
The adsorption rate constant k2 decreases with an increasing concentration of copper(II) ions in the solution. However, the effect of zinc(II) cation concentration in the solution on this parameter is minimal.
The parameters listed in
Table 4 and
Table 5 clearly show that the pseudo-second order kinetic model reflects the kinetics of the investigated process better and can be sufficient to predict the adsorption of copper(II) and zinc(II) cations on the tested biochar sorbent. The presented results are consistent with the works of Demiral and Gungor as well as Adebisi et al. [
28,
29]. The process of adsorption of copper(II) and zinc(II) cations on carbon sorbents presented in these works is also best described by pseudo-second order kinetics.
To assess the adsorption capacity and the sorption mechanism of the biochar, the Langmuir and Freundlich isotherm models were applied.
The Langmuir isotherm equation is given by the equation:
where
qe (mg·g
−1) is the real adsorption at sorption equilibrium,
ce (mg·dm
−3) is the concentration of cation in the solution at equilibrium point,
qm (mg·g
−1) is the maximum adsorption on the sorbent surface, and
K (dm
3·mg
−1) is a constant associated with the adsorption energy.
Freundlich isotherm adsorption on heterogeneous (energetically heterogeneous) surfaces and on microporous adsorbents is given by equation:
where
qe (mg·g
−1) is the real adsorption at sorption equilibrium,
ce (mg·dm
−3) is the concentration of cation at equilibrium,
KF (mg·g
−1) is a constant, expressing maximum adsorption on the sorbent surface, and 1/
n is a characteristic constant related to the intensity of the adsorption process. The linear form of this equation is most often used for calculations:
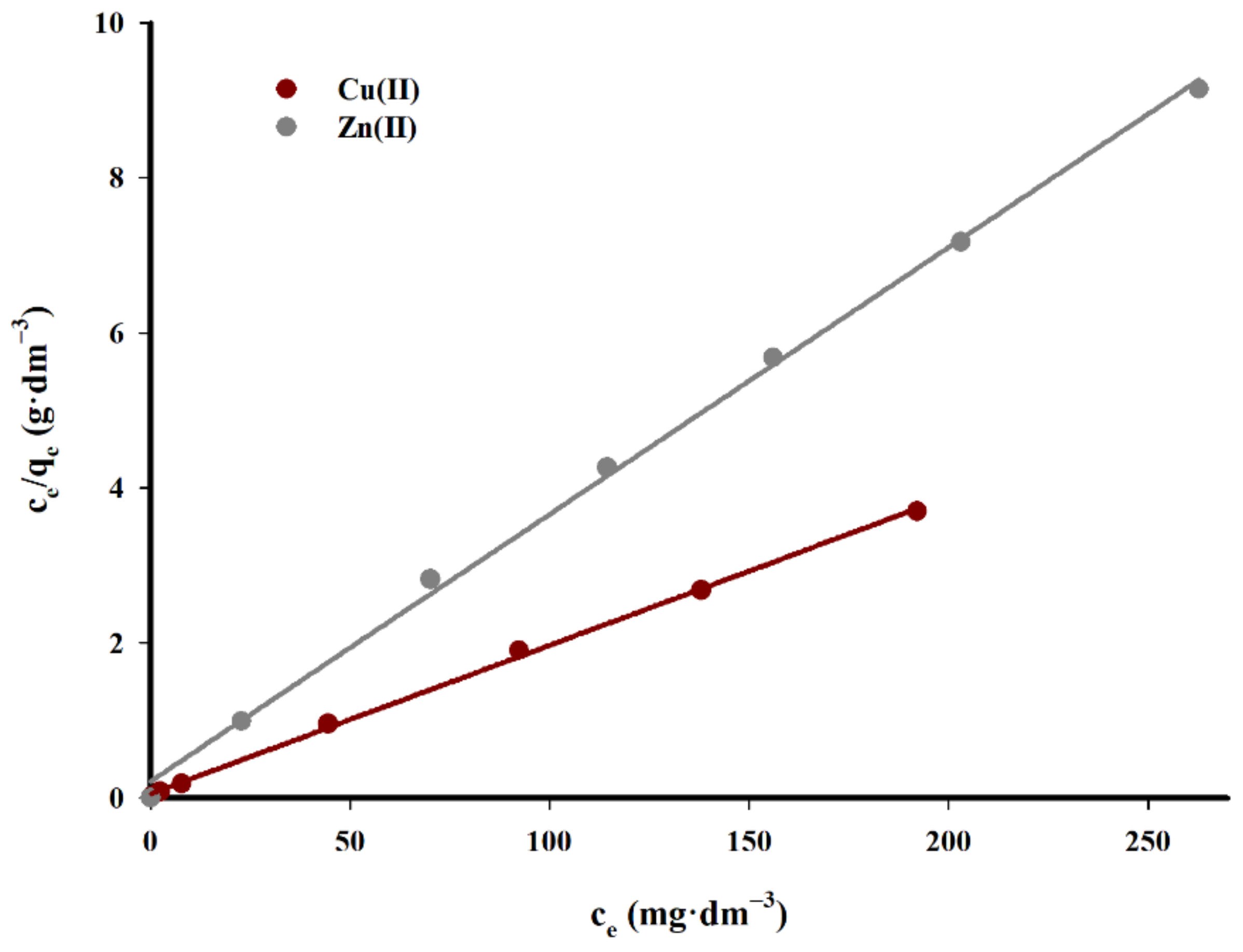
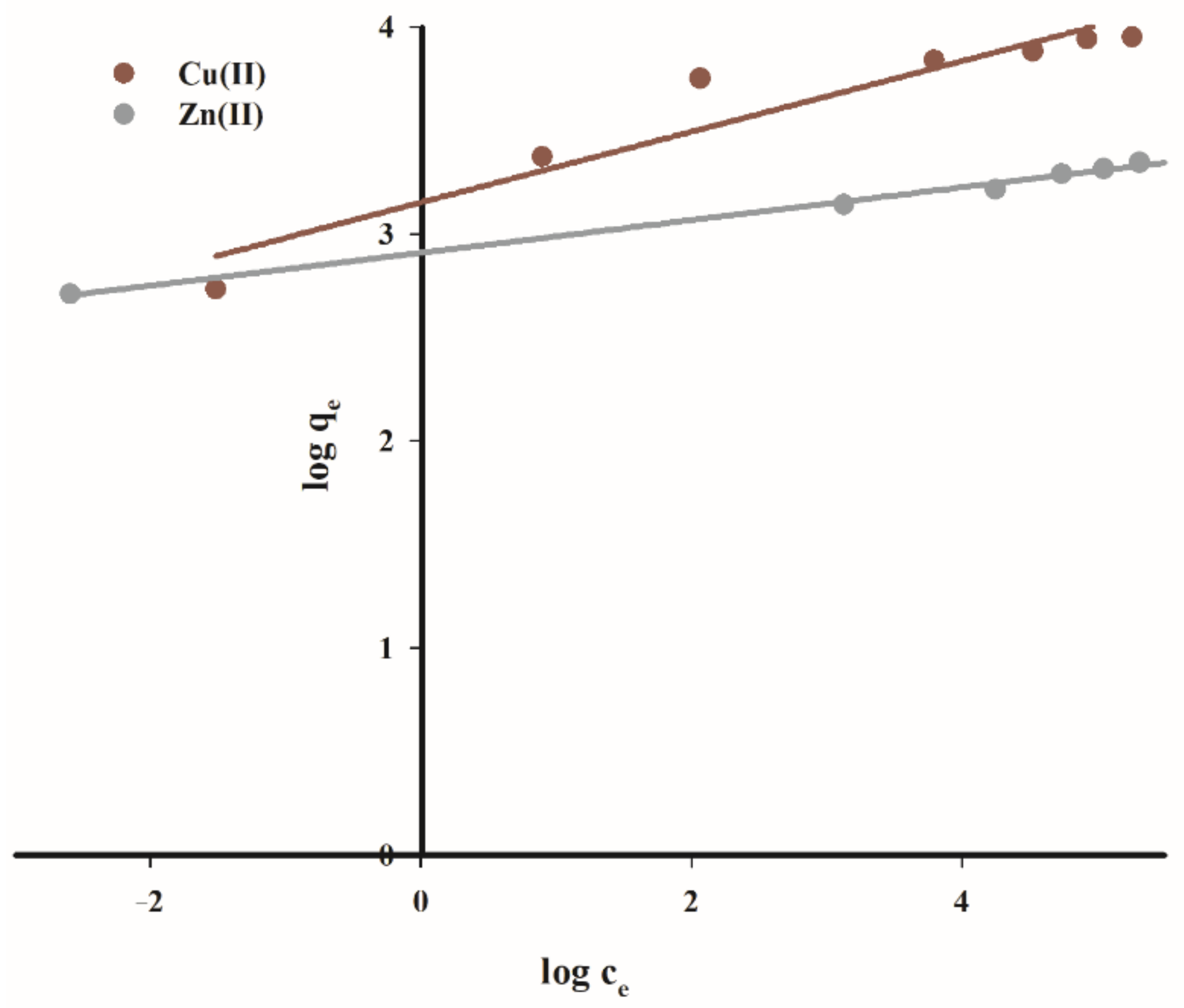
Figure 14 and
Figure 15 show a graph of studied isotherms for the sorption of copper(II) and zinc(II) cations on the tested carbon sorbent, while in
Table 6 the calculated parameters of constants and values R
2 for both considered isotherms are listed.
As demonstrated by the presented data, the adsorption of copper(II) on the tested carbon sorbent is best reflected by the Langmuir isotherm. It has been proved that the sorbent used has a varied surface structure, and during the adsorption process reactions occur mainly in the monolayer with no additional interactions that could give rise to the effects of multilayer adsorption.
The maximum sorption capacity calculated for the studied carbon biochar sorbent for copper(II) ions is 52.2 mg·g−1.
In the case of zinc(II) cations, a very good fit of straight lines was obtained for both tested models with a slight predominance of Langmuir isotherms. The maximum sorption capacity in for zinc(II) ions is was 29.0 mg·g−1.
The comparison of the selected maximum adsorption capacities for various types of carbon sorbents are listed in
Table 7 and
Table 8. A comparison of the data in
Table 7 and
Table 8 indicates that the sorbent obtained from waste rapeseed cake by pyrolysis reveals a relatively good value of sorption capacity in relation to copper(II) and zinc(II) ions. The advantage of the studied biochar is also high selectivity in relation to the ion mixture of Cu
2+/As
3+ and Zn
2+/As
3+. The high level of separation permits the recovery of these metals, which is undoubtedly beneficial from an ecological and economic point of view.
The arsenic remaining in waste acidic scrubber liquids after sorption should also be removed. The best method appears to be the precipitation of arsenic in the form of sparingly soluble compounds. Battaglia-Brunet et al. suggested the precipitation of arsenic in the form of arsenic sulphide with high efficiency [
38]. According to Grzesiak, arsenic can also be precipitated in the form of calcium arsenate, chloro-arsenate of lead and iron arsenate [
9].
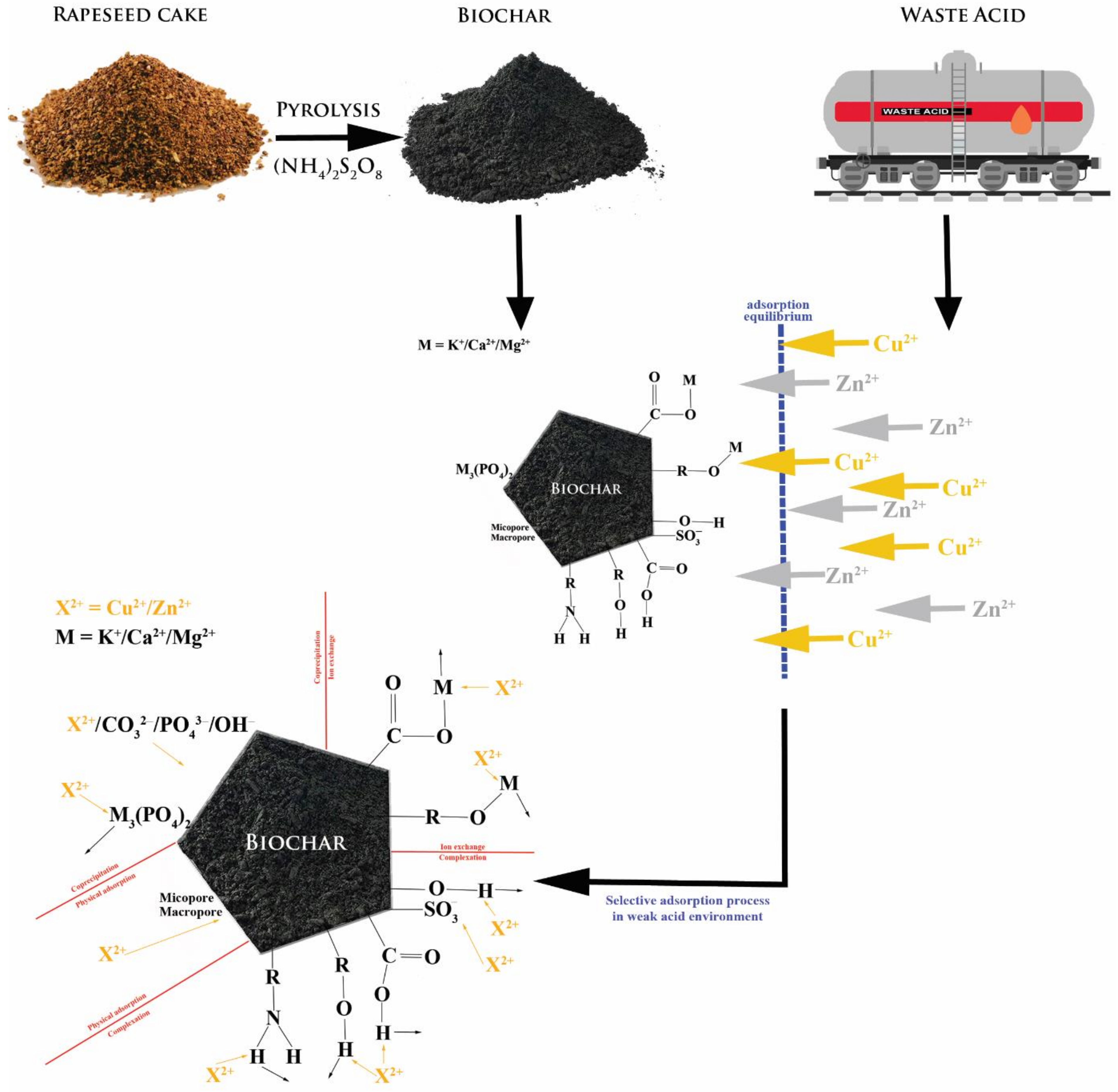
Based on the experimental data, the mechanism of adsorbent/adsorbate interactions was proposed (
Figure 16). It should be emphasised that these interactions are mostly affected by the pH of the reaction. On the other hand, the interactions seem to be caused by the electrostatic and chemical nature.