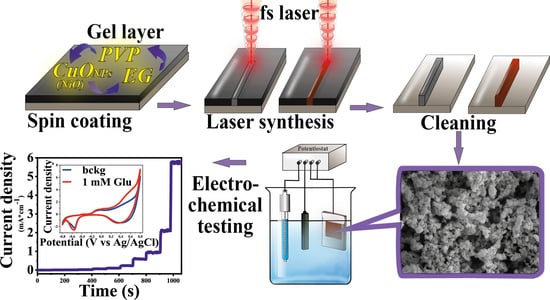
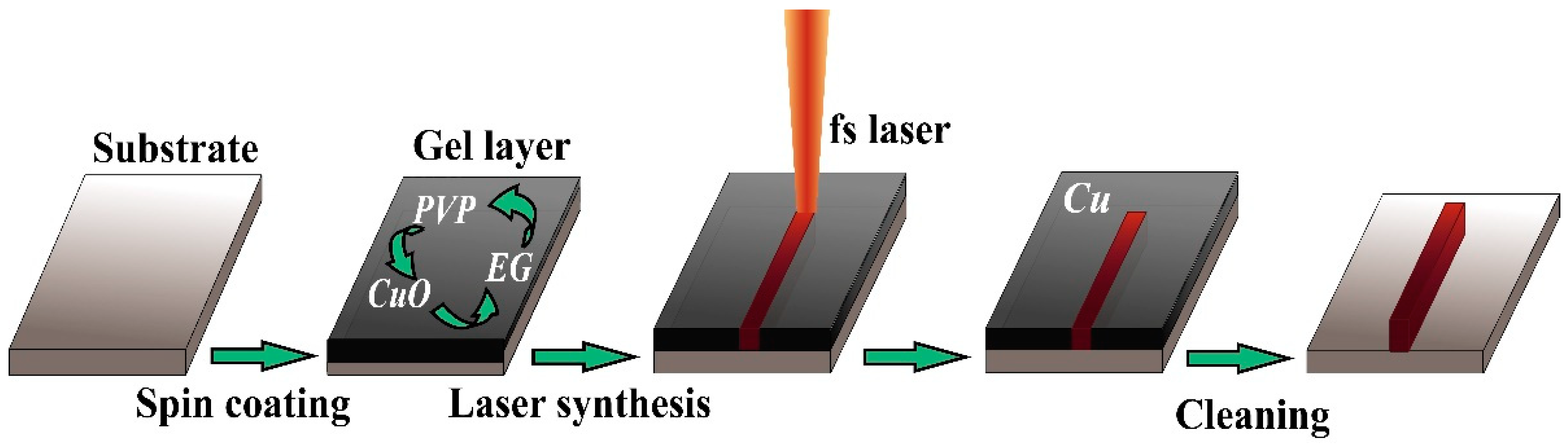
Copper and Nickel Microsensors Produced by Selective Laser Reductive Sintering for Non-Enzymatic Glucose Detection
Abstract
1. Introduction
2. Materials and Methods
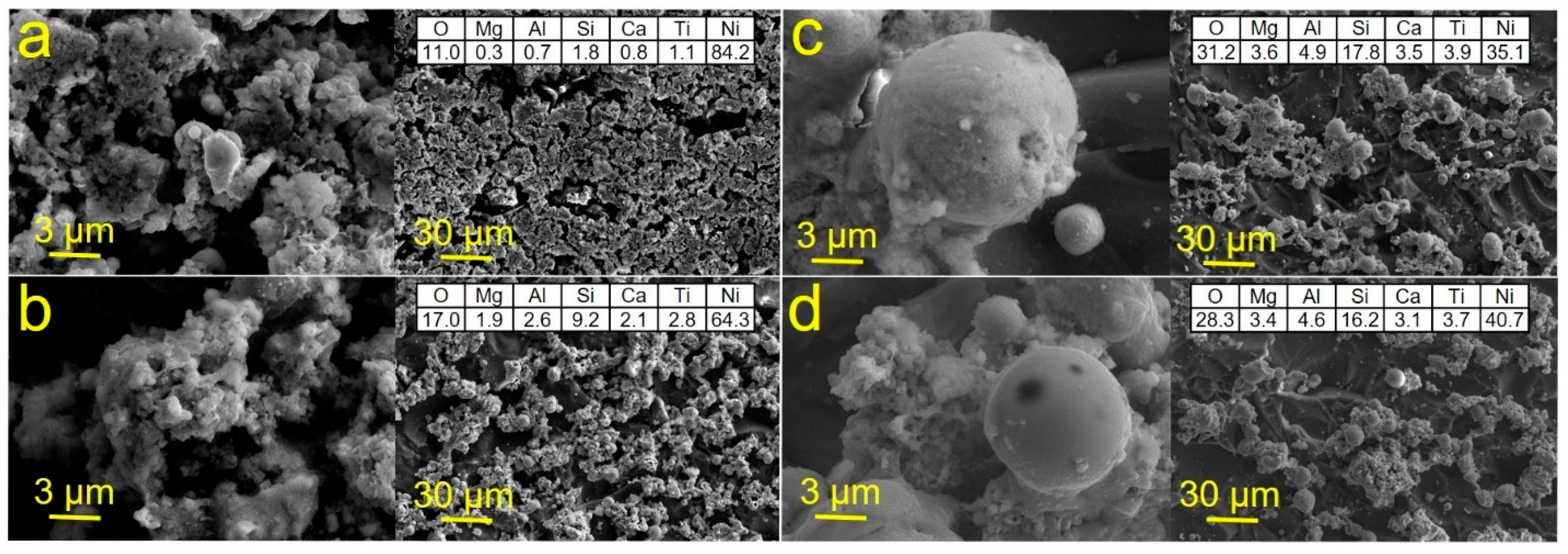
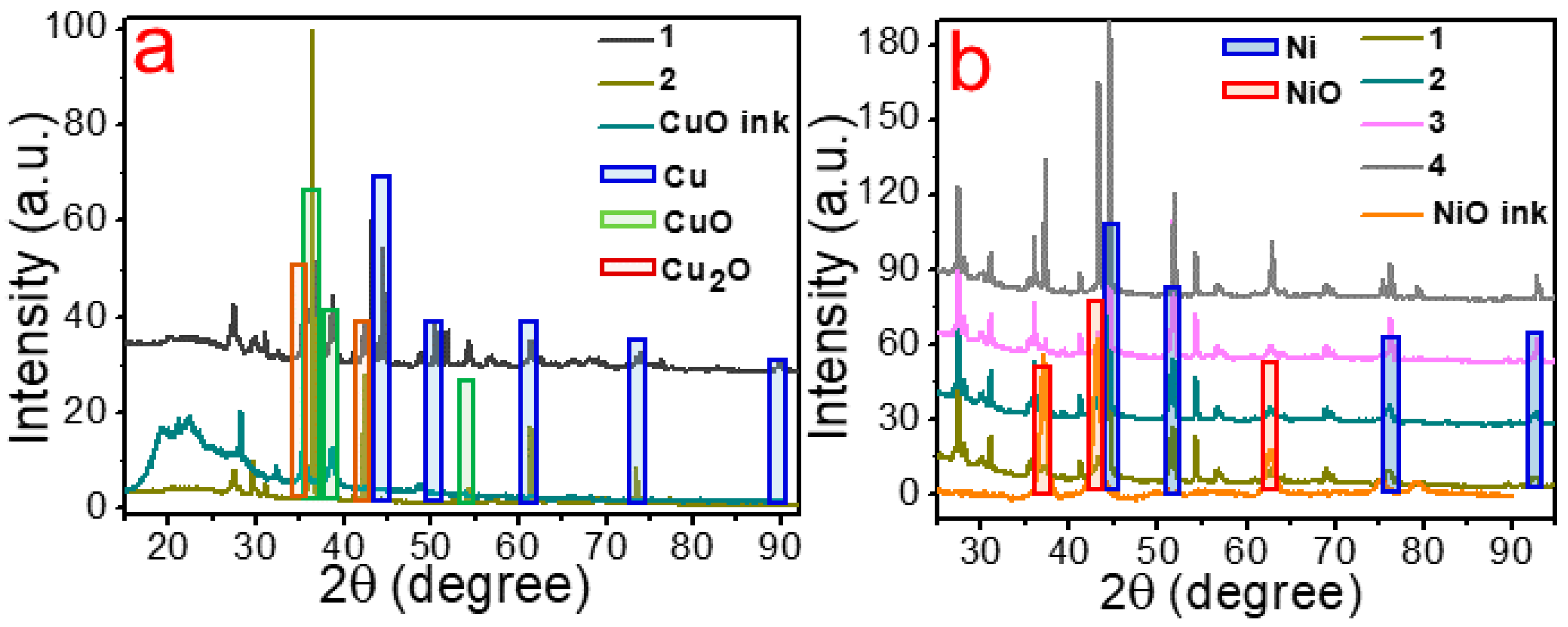
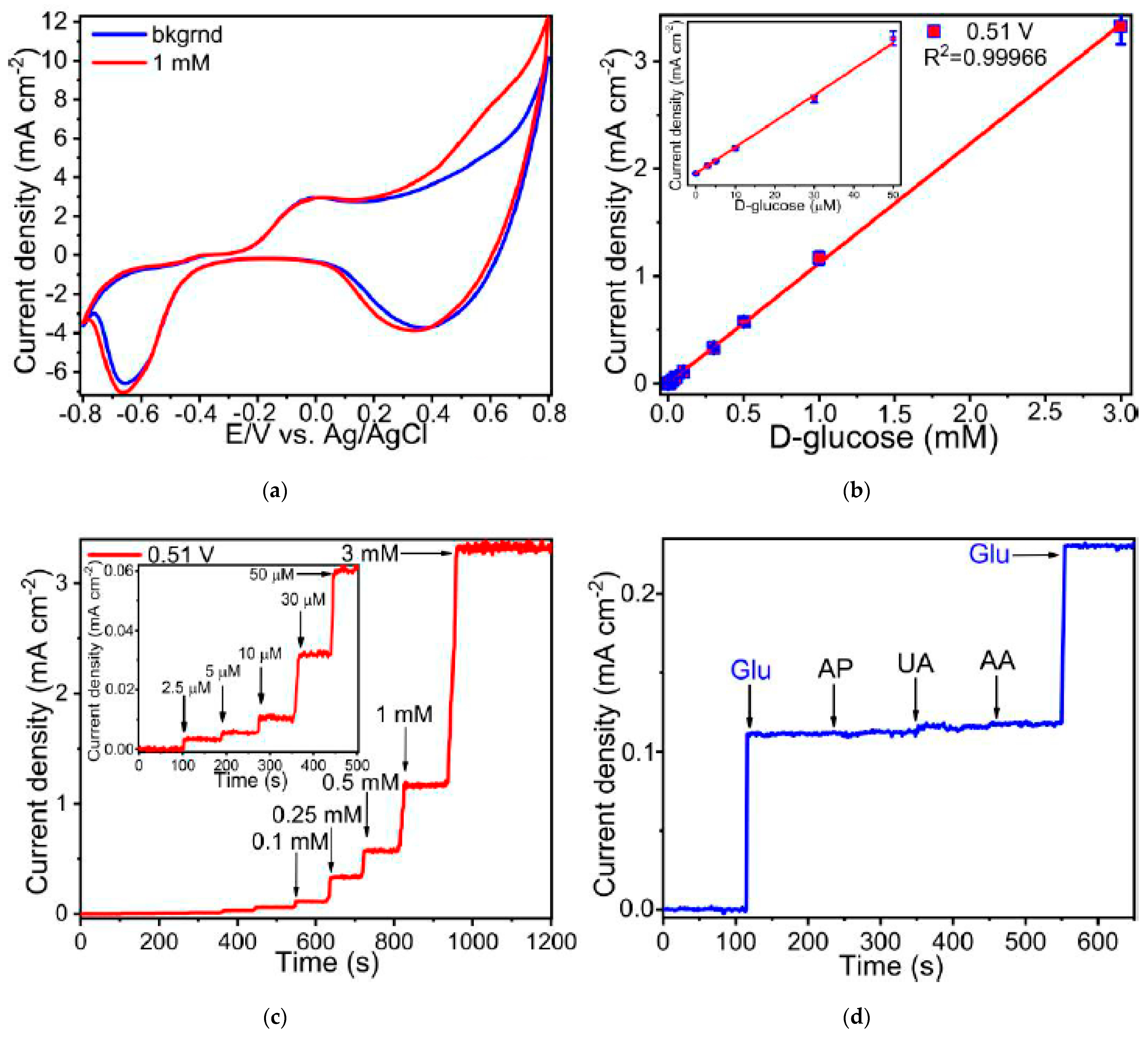
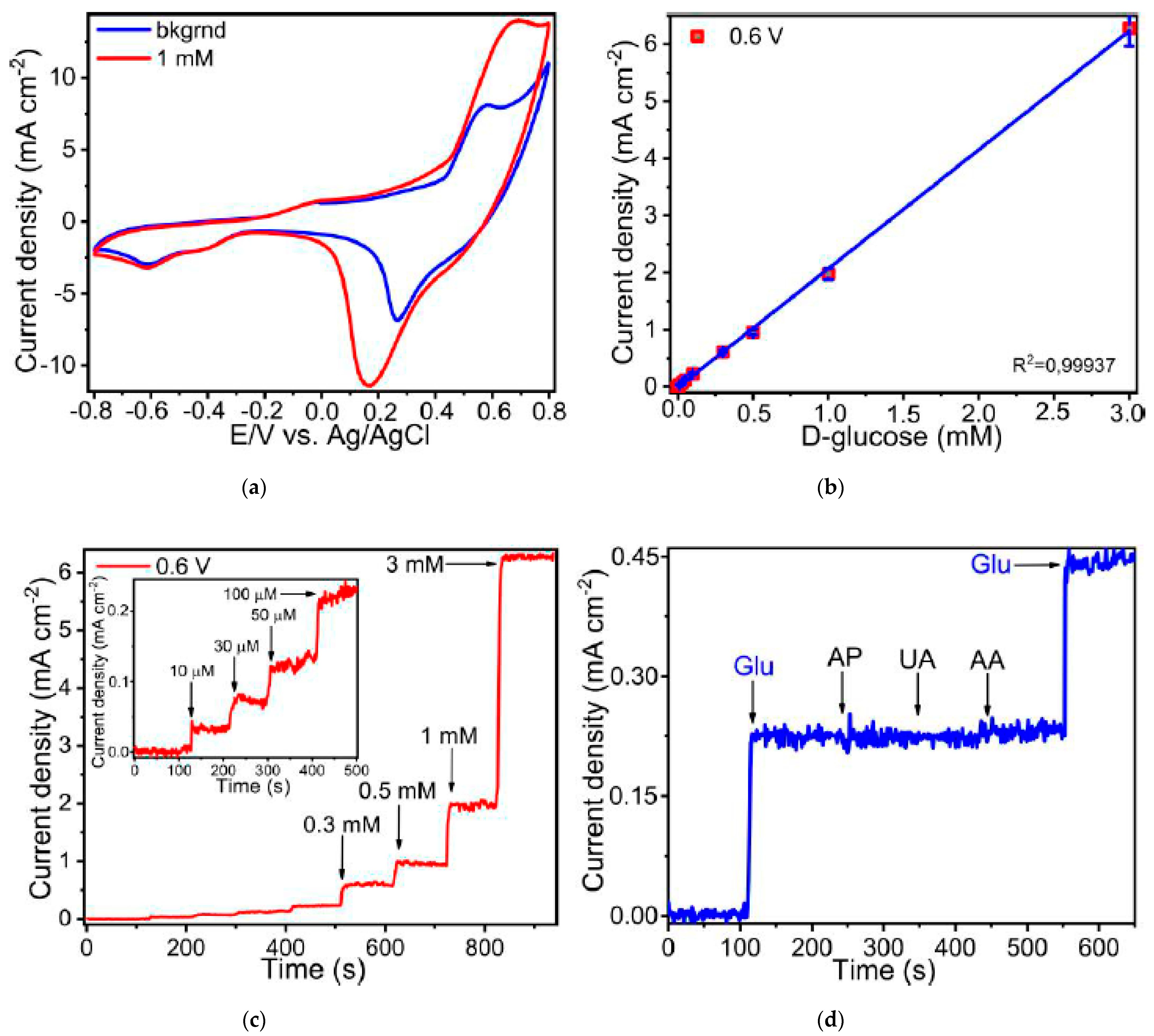
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tørring, M.L.; Frydenberg, M.; Hamilton, W.; Hansen, R.P.; Lautrup, M.D.; Vedsted, P. Diagnostic interval and mortality in colorectal cancer: U-shaped association demonstrated for three different datasets. J. Clin. Epidemiol. 2012, 65, 669–678. [Google Scholar] [CrossRef]
- Sun, J.Y.; Shi, Y.; Cai, X.Y.; Liu, J. Potential diagnostic and therapeutic value of circular RNAs in cardiovascular diseases. Cell. Signal. 2020, 71, 109604. [Google Scholar] [CrossRef] [PubMed]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef]
- Park, S.S.; Tatum, C.E.; Lee, Y. Dual electrochemical microsensor for simultaneous measurements of nitric oxide and oxygen: Fabrication and characterization. Electrochem. Commun. 2009, 11, 2040–2043. [Google Scholar] [CrossRef]
- Nasiri, S.; Khosravani, M.R. Progress and challenges in fabrication of wearable sensors for health monitoring. Sensors Actuators A Phys. 2020, 312, 112105. [Google Scholar] [CrossRef]
- Batra, B.; Narwal, V.; Kalra, V.; Sharma, M.; Rana, J.S. Folic acid biosensors: A review. Process Biochem. 2020, 92, 343–354. [Google Scholar] [CrossRef]
- Blair, E.O.; Corrigan, D.K. A review of microfabricated electrochemical biosensors for DNA detection. Biosens. Bioelectron. 2019, 134, 57–67. [Google Scholar] [CrossRef]
- Pundir, C.S.; Lata, S.; Narwal, V. Biosensors for determination of D and L- amino acids: A review. Biosens. Bioelectron. 2018, 117, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Pundir, C.S.; Jakhar, S.; Narwal, V. Determination of urea with special emphasis on biosensors: A review. Biosens. Bioelectron. 2019, 123, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, P.; Joseph, Y. Enzyme-based biosensors for choline analysis: A review. TrAC—Trends Anal. Chem. 2019, 110, 367–374. [Google Scholar] [CrossRef]
- Khanmohammadi, A.; Aghaie, A.; Vahedi, E.; Qazvini, A.; Ghanei, M.; Afkhami, A.; Hajian, A.; Bagheri, H. Electrochemical biosensors for the detection of lung cancer biomarkers: A review. Talanta 2020, 206, 120251. [Google Scholar] [CrossRef] [PubMed]
- Sehit, E.; Altintas, Z. Significance of nanomaterials in electrochemical glucose sensors: An updated review (2016-2020). Biosens. Bioelectron. 2020, 159, 112165. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, H.; Yang, B.; Li, Z.; Lei, L.; Zhang, X. A non-enzymatic hydrogen peroxide sensor based on vertical NiO nanosheets supported on the graphite sheet. J. Electroanal. Chem. 2015, 749, 62–67. [Google Scholar] [CrossRef]
- Revathi, C.; Rajendra Kumar, R.T. Enzymatic and Nonenzymatic Electrochemical Biosensors; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; ISBN 9780081025772. [Google Scholar]
- Gong, X.; Gu, Y.; Zhang, F.; Liu, Z.; Li, Y.; Chen, G.; Wang, B. High-performance non-enzymatic glucose sensors based on CoNiCu alloy nanotubes arrays prepared by electrodeposition. Front. Mater. 2019, 6, 1–9. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, W.; Lu, W.; Qin, X.; Xu, X. Surface plasmon aided high sensitive non-enzymatic glucose sensor using Au/NiAu multilayered nanowire arrays. Biosens. Bioelectron. 2018, 111, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, W.; Chen, L.; Jia, J. Three-Dimensional Copper Foam Supported CuO Nanowire Arrays: An Efficient Non-enzymatic Glucose Sensor. Electrochim. Acta 2017, 235, 519–526. [Google Scholar] [CrossRef]
- S. Panov, M.; M. Khairullina, E.; S. Vshivtcev, F.; N. Ryazantsev, M.; I. Tumkin, I. Laser-Induced Synthesis of Composite Materials Based on Iridium, Gold and Platinum for Non-Enzymatic Glucose Sensing. Materials 2020, 13, 3359. [Google Scholar] [CrossRef] [PubMed]
- Panov, M.S.; Vereshchagina, O.A.; Ermakov, S.S.; Tumkin, I.I.; Khairullina, E.M.; Skripkin, M.Y.; Mereshchenko, A.S.; Ryazantsev, M.N.; Kochemirovsky, V.A. Non-enzymatic sensors based on in situ laser-induced synthesis of copper-gold and gold nano-sized microstructures. Talanta 2017, 167, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Panov, M.; Aliabev, I.; Khairullina, E.; Mironov, V.; Tumkin, I. Fabrication of nickel-gold microsensor using in situ laser-induced metal deposition technique. J. Laser Micro Nanoeng. 2019, 14, 266–269. [Google Scholar] [CrossRef]
- Smikhovskaia, A.V.; Andrianov, V.S.; Khairullina, E.M.; Lebedev, D.V.; Ryazantsev, M.N.; Panov, M.S.; Tumkin, I.I. In situ laser-induced synthesis of copper-silver microcomposite for enzyme-free D-glucose and L-alanine sensing. Appl. Surf. Sci. 2019, 488, 531–536. [Google Scholar] [CrossRef]
- Smikhovskaia, A.V.; Panov, M.S.; Tumkin, I.I.; Khairullina, E.M.; Ermakov, S.S.; Balova, I.A.; Ryazantsev, M.N.; Kochemirovsky, V.A. In situ laser-induced codeposition of copper and different metals for fabrication of microcomposite sensor-active materials. Anal. Chim. Acta 2018, 1044, 138–146. [Google Scholar] [CrossRef]
- Baranauskaite, V.E.; Novomlinskii, M.O.; Tumkin, I.I.; Khairullina, E.M.; Mereshchenko, A.S.; Balova, I.A.; Panov, M.S.; Kochemirovsky, V.A. In situ laser-induced synthesis of gas sensing microcomposites based on molybdenum and its oxides. Compos. Part B Eng. 2019, 157, 322–330. [Google Scholar] [CrossRef]
- Manzanares Palenzuela, C.L.; Pumera, M. (Bio)Analytical chemistry enabled by 3D printing: Sensors and biosensors. TrACTrends Anal. Chem. 2018, 103, 110–118. [Google Scholar] [CrossRef]
- Tamura, K.; Mizoshiri, M.; Sakurai, J.; Hata, S. Ni-based composite microstructures fabricated by femtosecond laser reductive sintering of NiO/Cr mixed nanoparticles. Jpn. J. Appl. Phys. 2017, 56. [Google Scholar] [CrossRef]
- Cheng, T.S.; Nasir, M.Z.M.; Ambrosi, A.; Pumera, M. 3D-printed metal electrodes for electrochemical detection of phenols. Appl. Mater. Today 2017, 9, 212–219. [Google Scholar] [CrossRef]
- Tan, C.; Nasir, M.Z.M.; Ambrosi, A.; Pumera, M. 3D Printed Electrodes for Detection of Nitroaromatic Explosives and Nerve Agents. Anal. Chem. 2017, 89, 8995–9001. [Google Scholar] [CrossRef] [PubMed]
- Bariya, M.; Shahpar, Z.; Park, H.; Sun, J.; Jung, Y.; Gao, W.; Nyein, H.Y.Y.; Liaw, T.S.; Tai, L.C.; Ngo, Q.P.; et al. Roll-to-Roll Gravure Printed Electrochemical Sensors for Wearable and Medical Devices. ACS Nano 2018, 12, 6978–6987. [Google Scholar] [CrossRef]
- Su, C.H.; Kung, C.W.; Chang, T.H.; Lu, H.C.; Ho, K.C.; Liao, Y.C. Inkjet-printed porphyrinic metal-organic framework thin films for electrocatalysis. J. Mater. Chem. A 2016, 4, 11094–11102. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.T.; Li, D.W.; Long, Y.T. Recent developments and applications of screen-printed electrodes in environmental assays-A review. Anal. Chim. Acta 2012, 734, 31–44. [Google Scholar] [CrossRef]
- Barreca, D.; Massignan, C.; Daolio, S.; Fabrizio, M.; Piccirillo, C.; Armelao, L.; Tondello, E. Composition and microstructure of cobalt oxide thin films obtained from a novel cobalt(II) precursor by chemical vapor deposition. Chem. Mater. 2001, 13, 588–593. [Google Scholar] [CrossRef]
- Kochemirovsky, V.A.; Skripkin, M.Y.; Tveryanovich, Y.S.; Mereshchenko, A.S.; Gorbunov, A.O.; Panov, M.S.; Tumkin, I.I.; Safonov, S. V Laser-induced copper deposition from aqueous and aqueous–organic solutions: State of the art and prospects of research. Russ. Chem. Rev. 2015, 84, 1059–1075. [Google Scholar] [CrossRef]
- Arakane, S.; Mizoshiri, M.; Sakurai, J.; Hata, S. Three-dimensional Cu microfabrication using femtosecond laser-induced reduction of CuO nanoparticles. Appl. Phys. Express 2017, 10. [Google Scholar] [CrossRef]
- Mizoshiri, M.; Nishitani, K.; Hata, S. Effect of heat accumulation on femtosecond laser reductive sintering of mixed CuO/NiO nanoparticles. Micromachines 2018, 9, 264. [Google Scholar] [CrossRef]
- Mizoshiri, M.; Arakane, S.; Sakurai, J.; Hata, S. Direct writing of Cu-based micro-temperature detectors using femtosecond laser reduction of CuO nanoparticles. Appl. Phys. Express 2016, 9. [Google Scholar] [CrossRef]
- Guo, M.M.; Xia, Y.; Huang, W.; Li, Z. Electrochemical fabrication of stalactite-like copper micropillar arrays via surface rebuilding for ultrasensitive nonenzymatic sensing of glucose. Electrochim. Acta 2015, 151, 340–346. [Google Scholar] [CrossRef]
- Hou, L.; Zhao, H.; Bi, S.; Xu, Y.; Lu, Y. Ultrasensitive and highly selective sandpaper-supported copper framework for non-enzymatic glucose sensor. Electrochim. Acta 2017, 248, 281–291. [Google Scholar] [CrossRef]
- Mei, L.; Zhang, P.; Chen, J.; Chen, D.; Quan, Y.; Gu, N.; Zhang, G.; Cui, R. Non-enzymatic sensing of glucose and hydrogen peroxide using a glassy carbon electrode modified with a nanocomposite consisting of nanoporous copper, carbon black and nafion. Microchim. Acta 2016, 183, 1359–1365. [Google Scholar] [CrossRef]
- Shi, L.; Zhu, X.; Liu, T.; Zhao, H.; Lan, M. Encapsulating Cu nanoparticles into metal-organic frameworks for nonenzymatic glucose sensing. Sens. Actuators B Chem. 2016, 227, 583–590. [Google Scholar] [CrossRef]
- Nie, H.; Yao, Z.; Zhou, X.; Yang, Z.; Huang, S. Nonenzymatic electrochemical detection of glucose using well-distributed nickel nanoparticles on straight multi-walled carbon nanotubes. Biosens. Bioelectron. 2011, 30, 28–34. [Google Scholar] [CrossRef]
- Huo, K.; Fu, J.; Zhang, X.; Xu, P.; Gao, B.; Mooni, S.; Li, Y.; Fu, J. Phase separation induced rhizobia-like Ni nanoparticles and TiO2nanowires composite arrays for enzyme-free glucose sensor. Sens. Actuators B Chem. 2017, 244, 38–46. [Google Scholar] [CrossRef]
- Yang, J.; Yu, J.H.; Rudi Strickler, J.; Chang, W.J.; Gunasekaran, S. Nickel nanoparticle-chitosan-reduced graphene oxide-modified screen-printed electrodes for enzyme-free glucose sensing in portable microfluidic devices. Biosens. Bioelectron. 2013, 47, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Yu, J.; He, J.; Yang, H.; Ye, Y.; Song, Y. A green and simple strategy to prepare graphene foam-like three-dimensional porous carbon/Ni nanoparticles for glucose sensing. Sens. Actuators B Chem. 2017, 239, 172–179. [Google Scholar] [CrossRef]


| Material of Electrode | CuO or NiO, g | PVP, g | EG, g |
|---|---|---|---|
| Cu | 3 | 0.65 | 1.35 |
| Ni | 1.5 | 0.65 | 1.35 |
| Material of Electrode | Laser Fluence, J/cm2 | Scanning Speed, mm/s | Pitch Size, µm |
|---|---|---|---|
| Cu (Glass) [35] | 0.0154 | 5 | 5 |
| Cu (Sitall) | 0.0192 | 5 | 5 |
| Ni (Sitall) | 0.0192 | 5 | 5 |
| Electrode Material | Sensitivity (μA mM−1 cm−2) | Linear Range (mM) | Limit of Detection (μM) | Ref. |
|---|---|---|---|---|
| Cu on glass-ceramics | 1110 ± 6,45 | 0.003−3 | 0.91 | This work |
| Ni on glass-ceramics | 2080 ± 18,53 | 0.01−3 | 2.1 | This work |
| Cu MPs | 2432 | 0−4.711 | 0.19 | [36] |
| Cu coating | 2149.1 | 0.001−4.6 | 0.03 | [37] |
| carbon electrode/nanoporous Cu | 33.75 | 0.0006−3.369 | 2.6 | [38] |
| Cu NPs | 412 | 0−0.7 | 2.76 | [39] |
| Ni NPs on carbon nanotubes | 1438 | 0.001−1 | 0.5 | [40] |
| rhizobia-like Ni NPs | 50.97 | 0.001−7 | 0.18 | [41] |
| Ni NP/chitosan | 318.4 | 0−9 | 4.1 | [42] |
| 3D porous carbon/Ni NPs | 207.3 | 0.015−6.45 | 4.8 | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tumkin, I.I.; Khairullina, E.M.; Panov, M.S.; Yoshidomi, K.; Mizoshiri, M. Copper and Nickel Microsensors Produced by Selective Laser Reductive Sintering for Non-Enzymatic Glucose Detection. Materials 2021, 14, 2493. https://doi.org/10.3390/ma14102493
Tumkin II, Khairullina EM, Panov MS, Yoshidomi K, Mizoshiri M. Copper and Nickel Microsensors Produced by Selective Laser Reductive Sintering for Non-Enzymatic Glucose Detection. Materials. 2021; 14(10):2493. https://doi.org/10.3390/ma14102493
Chicago/Turabian StyleTumkin, Ilya I., Evgeniia M. Khairullina, Maxim S. Panov, Kyohei Yoshidomi, and Mizue Mizoshiri. 2021. "Copper and Nickel Microsensors Produced by Selective Laser Reductive Sintering for Non-Enzymatic Glucose Detection" Materials 14, no. 10: 2493. https://doi.org/10.3390/ma14102493
APA StyleTumkin, I. I., Khairullina, E. M., Panov, M. S., Yoshidomi, K., & Mizoshiri, M. (2021). Copper and Nickel Microsensors Produced by Selective Laser Reductive Sintering for Non-Enzymatic Glucose Detection. Materials, 14(10), 2493. https://doi.org/10.3390/ma14102493