Corrosion of Steel Rebars in Anoxic Environments. Part I: Electrochemical Measurements
Abstract
1. Introduction
2. Methodology
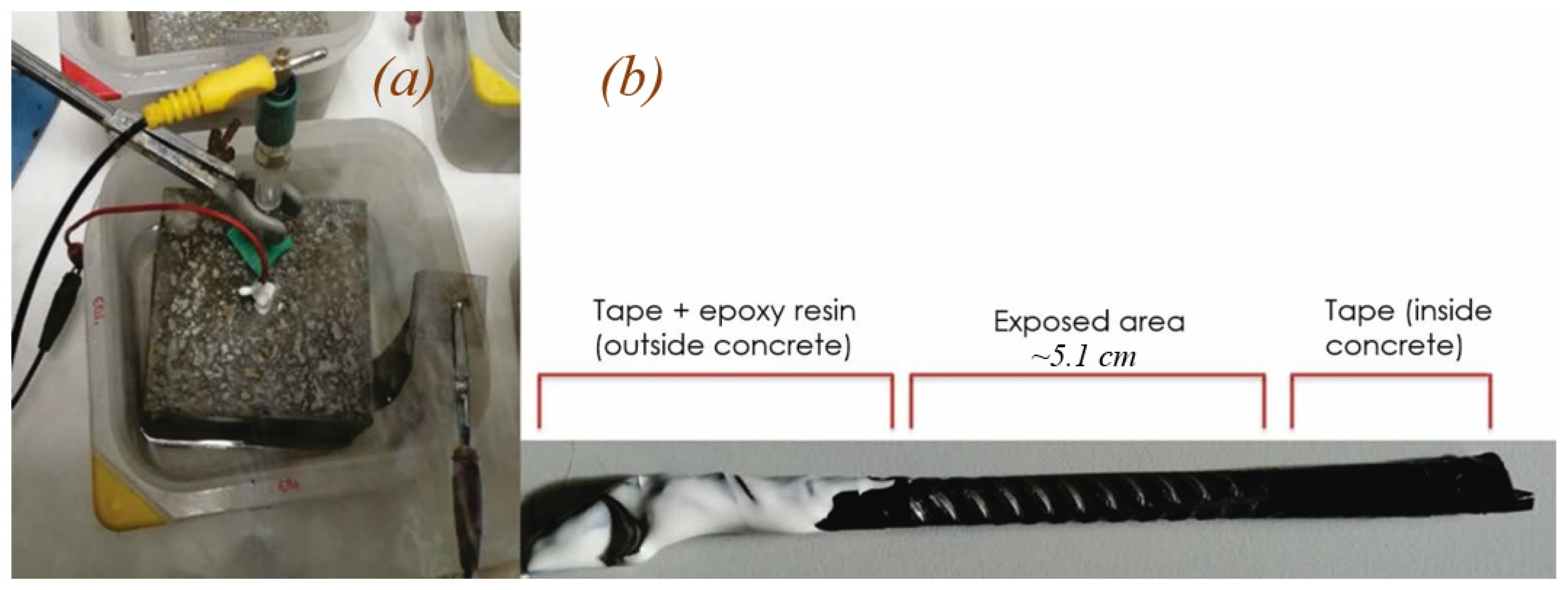
2.1. Samples and Test Conditions
2.2. Electrotechnical Techniques
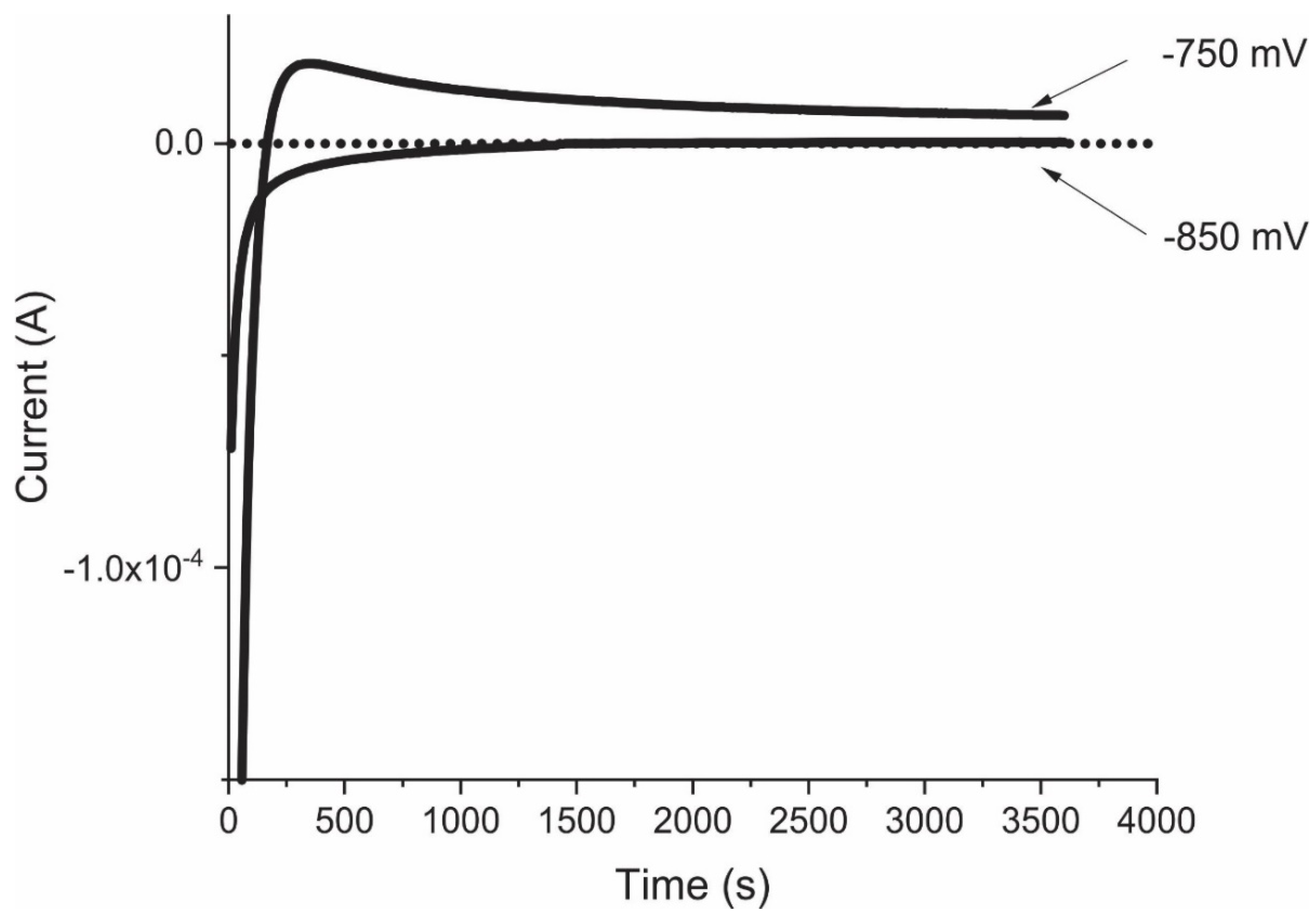
2.2.1. Chronoamperometry
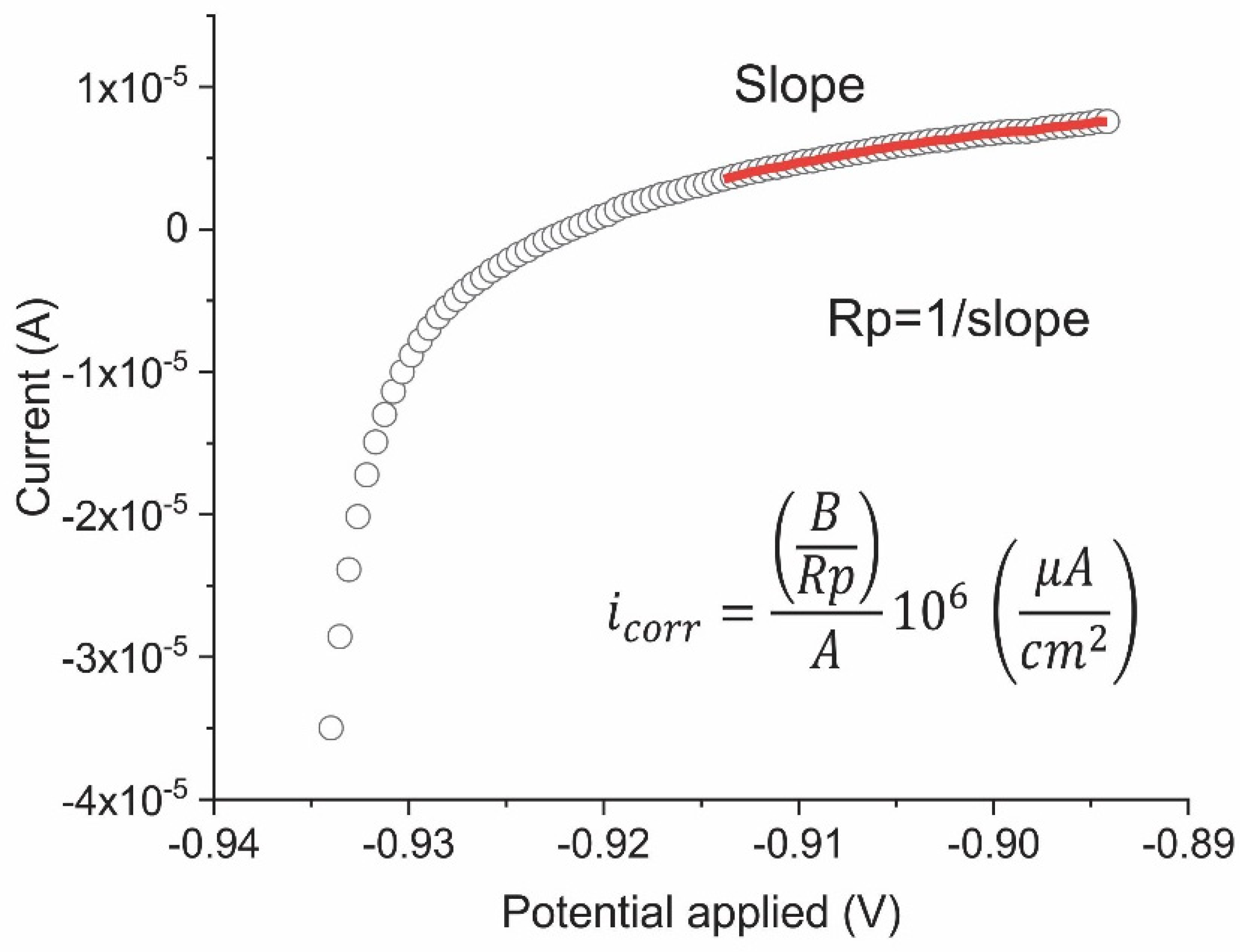
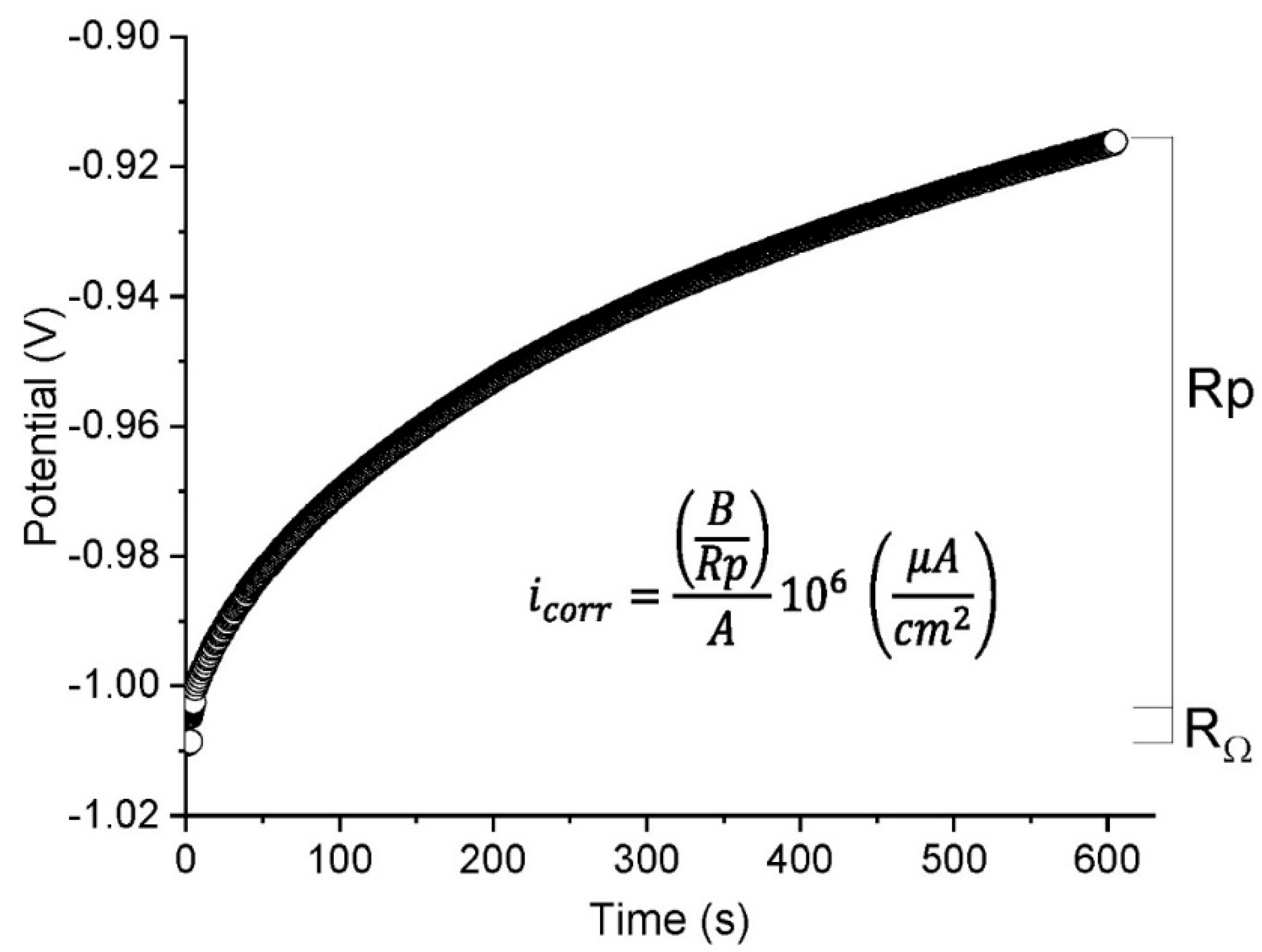
2.2.2. Linear Polarisation Resistance (LPR)
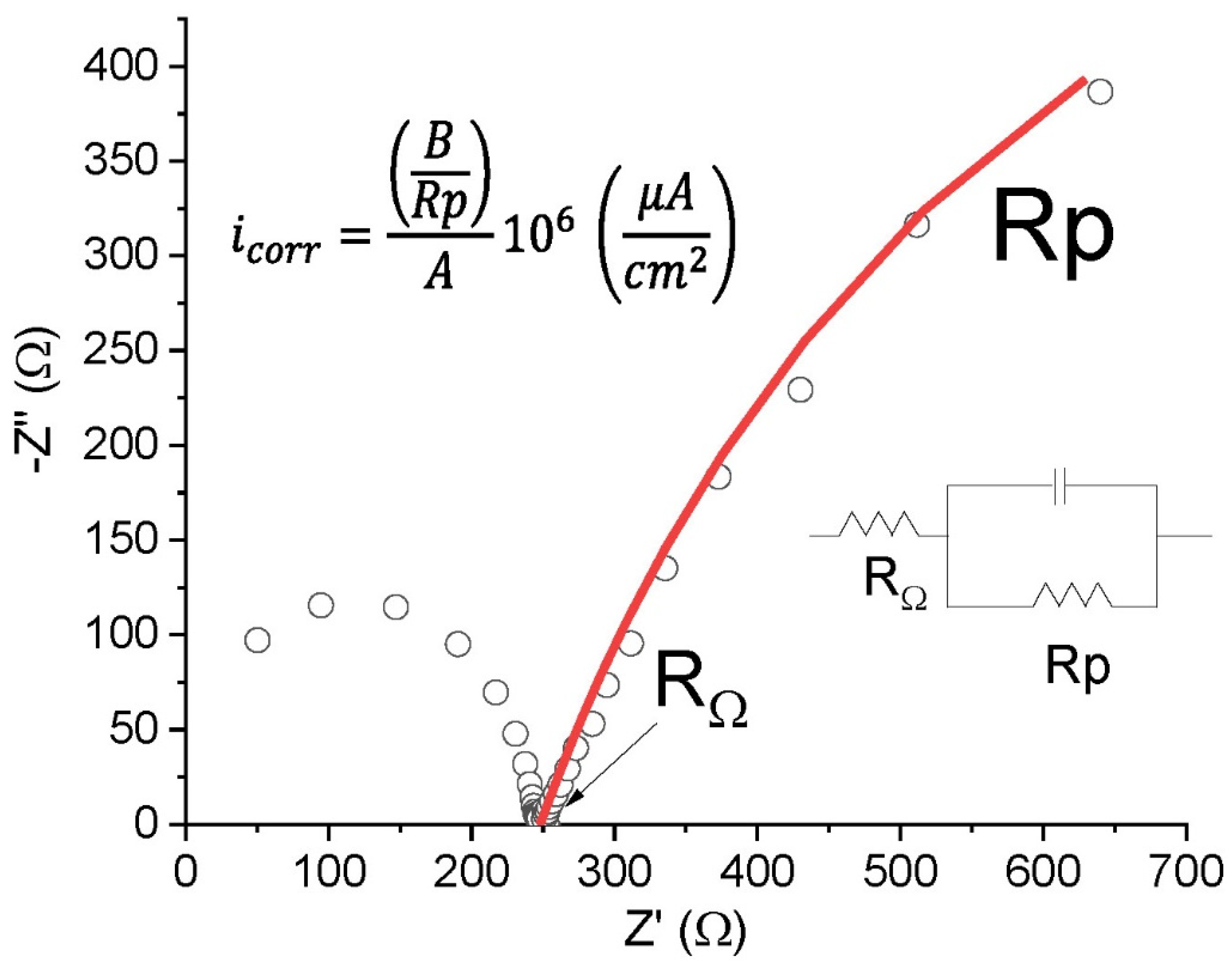
2.2.3. Electrochemical Impedance Spectroscopy (EIS)
2.2.4. Chronopotentiometry (CP)
2.2.5. Cyclic Polarisation Curves
3. Results and Discussion
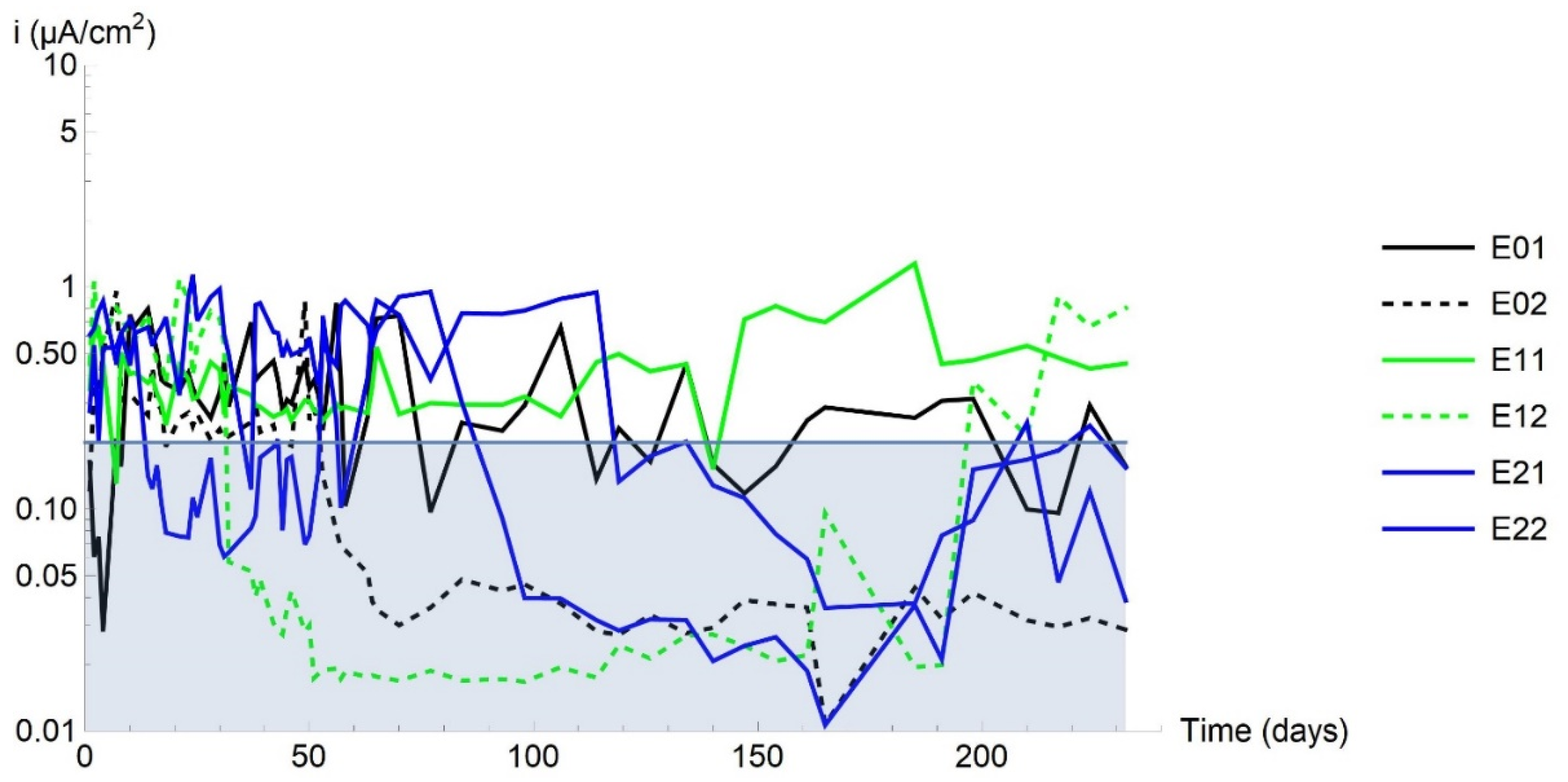
3.1. Corrosion Rate
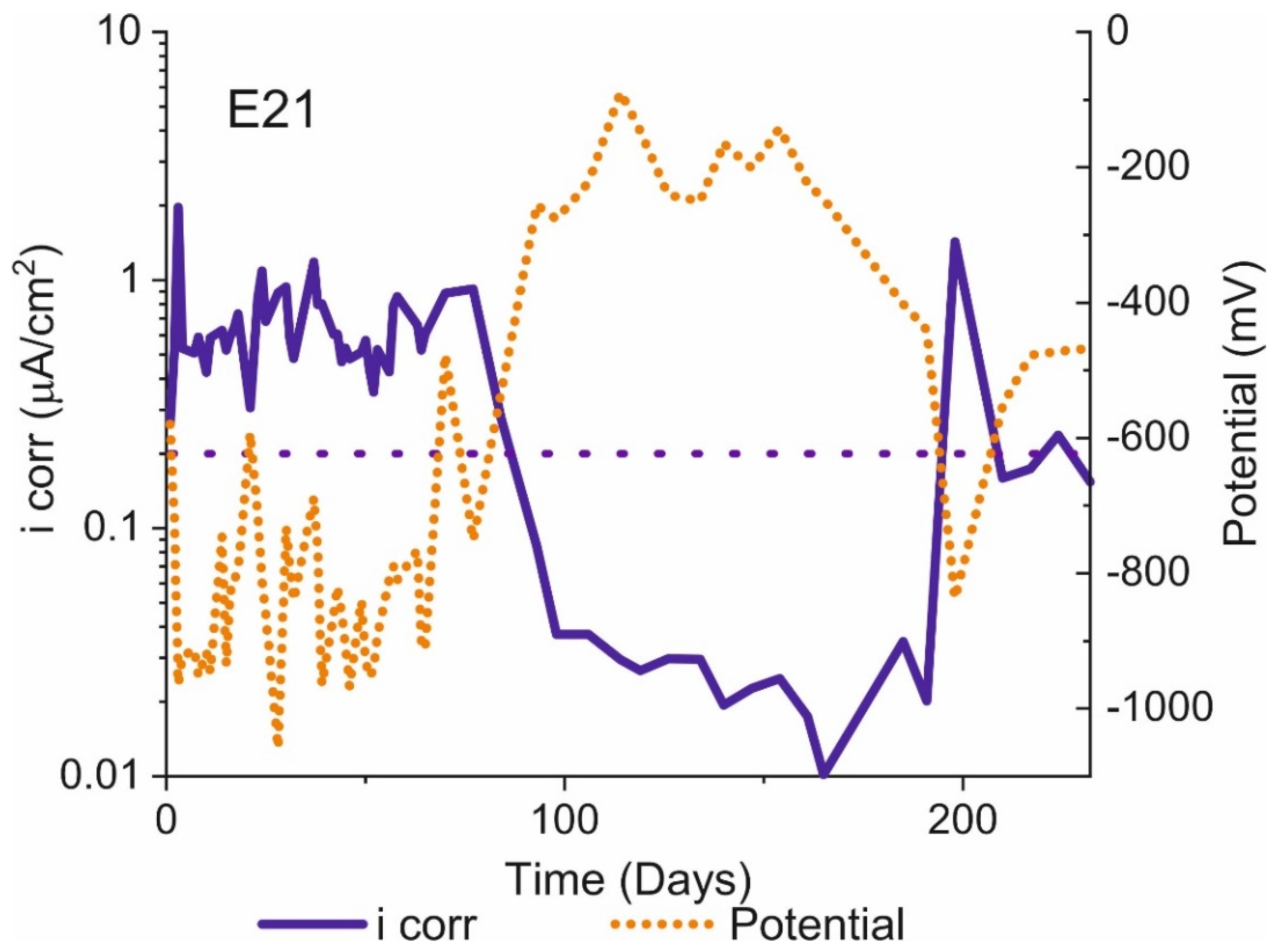
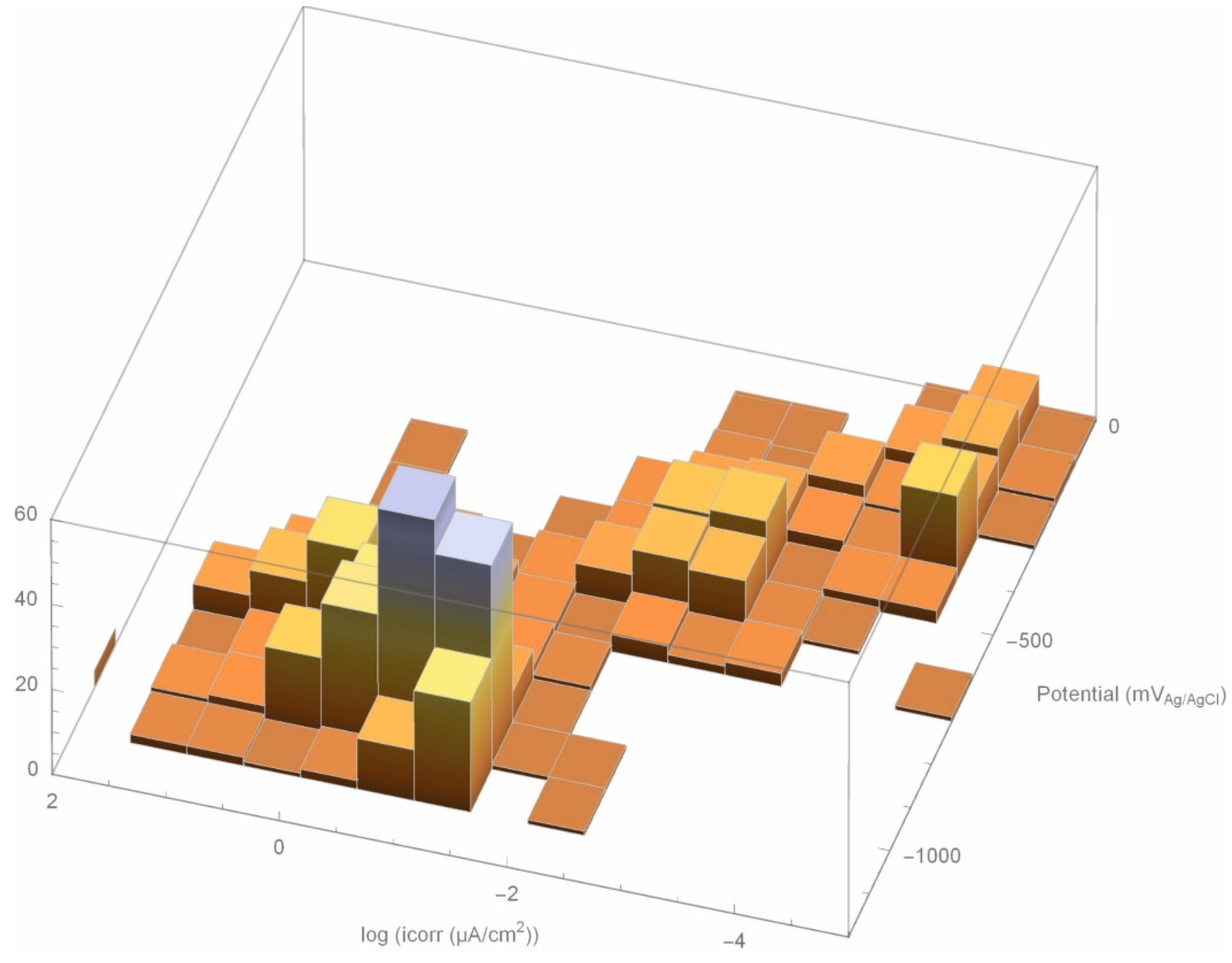
3.2. Activation/Passivation Intervals
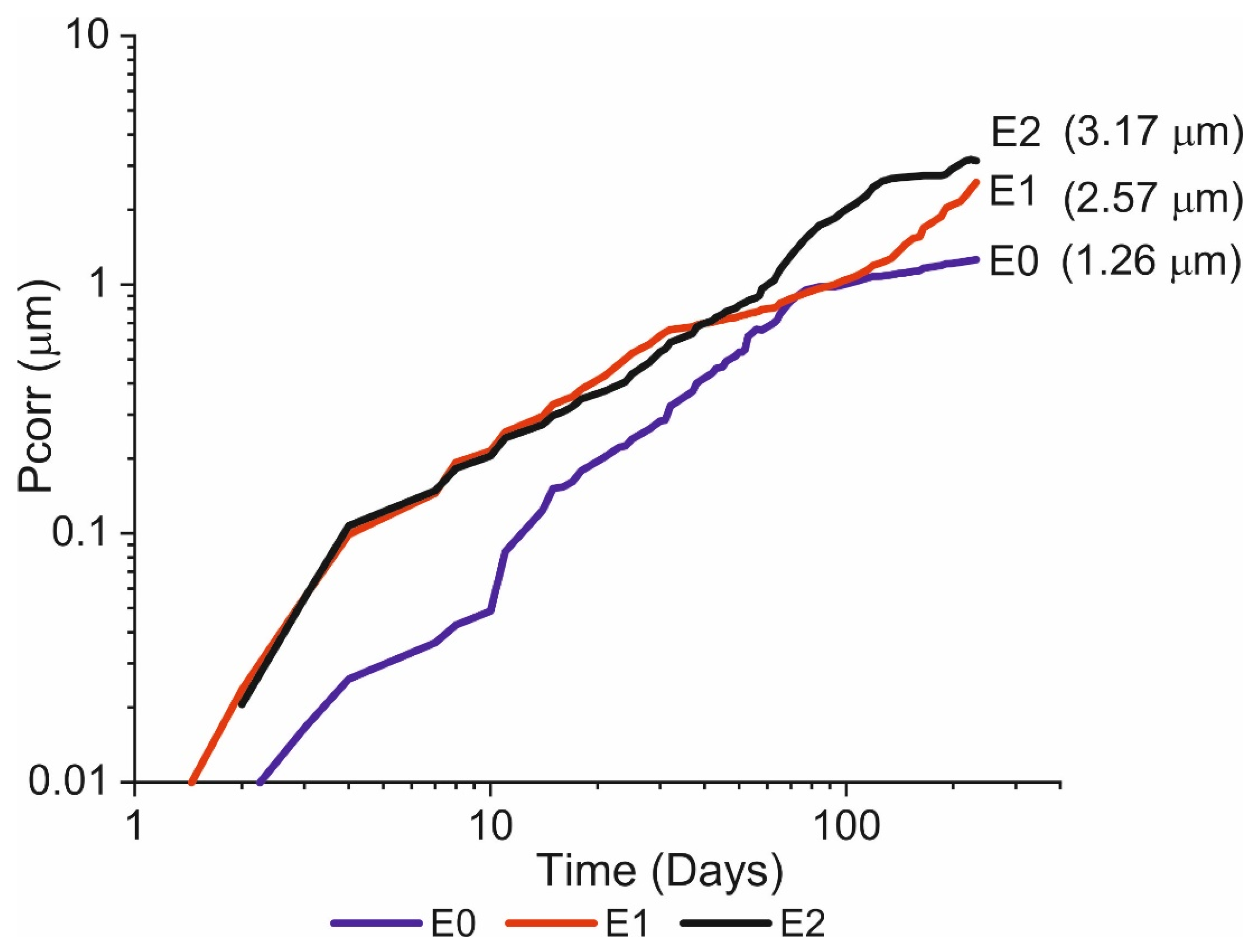
3.3. Corrosion-Induced Section Loss
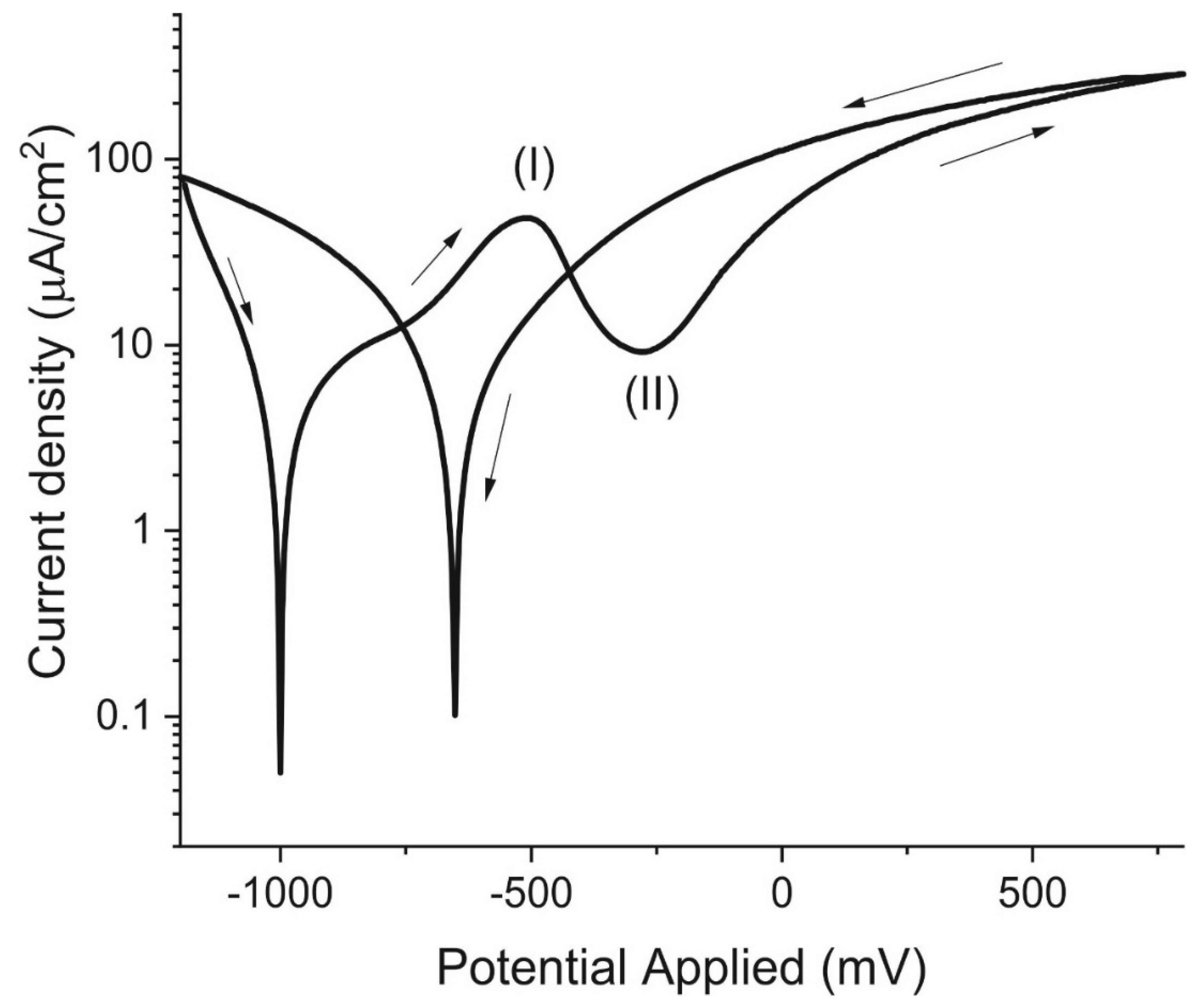
3.4. Cyclical Polarisation Curves
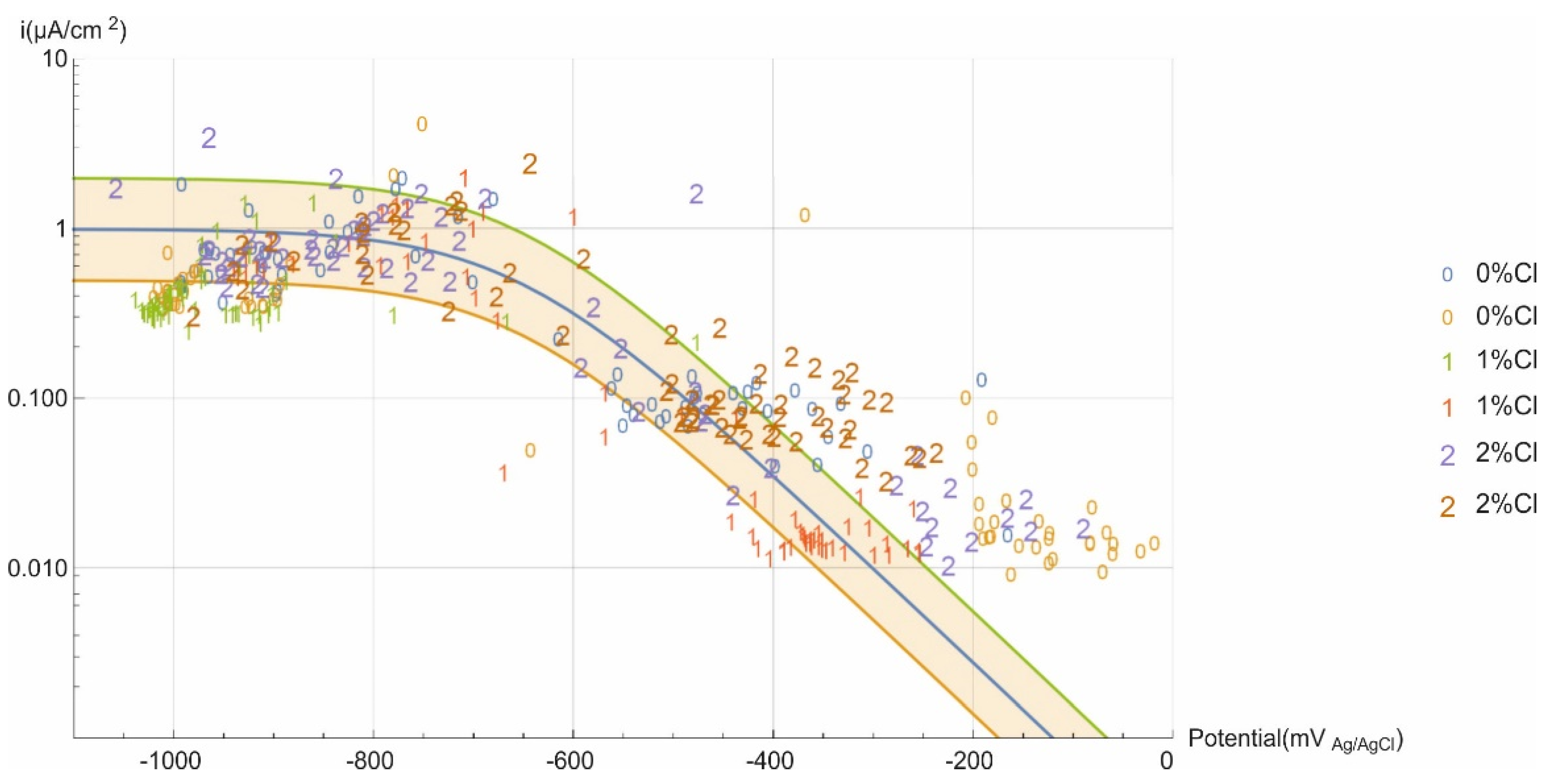
3.5. Tafel Parameters Estimation of Current Limit Density
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, J.; Li, T.; Fang, J.-X.; Jin, W.-L. Numerical simulation of steel corrosion in chloride contaminated concrete. Constr. Build. Mater. 2019, 228, 116745. [Google Scholar] [CrossRef]
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China. NPJ Mater. Degrad. 2017, 1, 4. [Google Scholar] [CrossRef]
- Schueremans, L.; Van Gemert, D.; Giessler, S. Chloride penetration in RC-structures in marine environment–Long term assessment of a preventive hydrophobic treatment. Constr. Build. Mater. 2007, 21, 1238–1249. [Google Scholar] [CrossRef]
- Val, D.V.; Stewart, M.G. Life-cycle cost analysis of reinforced concrete structures in marine environments. Struct. Saf. 2003, 25, 343–362. [Google Scholar] [CrossRef]
- Zhu, W.; François, R.; Fang, Q.; Zhang, D. Influence of long-term chloride diffusion in concrete and the resulting corrosion of reinforcement on the serviceability of RC beams. Cem. Concr. Compos. 2016, 71, 144–152. [Google Scholar] [CrossRef]
- Wei, J.; Wang, C.-G.; Wei, X.; Mu, X.; He, X.-Y.; Dong, J.-H.; Ke, W. Corrosion Evolution of Steel Reinforced Concrete Under Simulated Tidal and Immersion Zones of Marine Environment. Acta Met. Sin. 2019, 32, 900–912. [Google Scholar] [CrossRef]
- Lu, P.; Kursten, B.; Macdonald, D.D. Deconvolution of the Partial Anodic and Cathodic Processes during the Corrosion of Carbon Steel in Concrete Pore Solution under Simulated Anoxic Conditions. Electrochim. Acta 2014, 143, 312–323. [Google Scholar] [CrossRef]
- Lu, P.; Sharifi-Asl, S.; Kursten, B.; Macdonald, D.D. The irreversibility of the passive state of carbon steel in the alkaline con-crete pore solution under simulated anoxic conditions. J. Electrochem. Soc. 2015, 162, C572. [Google Scholar] [CrossRef]
- Page, C.L.; Lambert, P.; Vassie, P.R.W. Investigations of reinforcement corrosion. 1. The pore electrolyte phase in chlo-ride-contaminated concrete. Mater. Struct. 1991, 24, 243–252. [Google Scholar] [CrossRef]
- Lambert, P.; Page, C.L.; Vassie, P.R.W. Investigations of reinforcement corrosion. 2. Electrochemical monitoring of steel in chloride-contaminated concrete. Mater. Struct. 1991, 24, 351–358. [Google Scholar] [CrossRef]
- Walsh, M.T.; Sagüés, A.A. Steel corrosion in submerged concrete structures—Part 1: Field observations and corrosion distri-bution modeling. Corrosion 2016, 72, 518–533. [Google Scholar] [CrossRef]
- Tinnea, R. Corrosion in Public Aquariums. Mater. Perform. 2015, 54, 38–41. [Google Scholar]
- Mercier-Bion, F.; Li, J.; Lotz, H.; Tortech, L.; Neff, D.; Dillmann, P. Electrical properties of iron corrosion layers formed in anoxic environments at the nanometer scale. Corros. Sci. 2018, 137, 98–110. [Google Scholar] [CrossRef]
- Angst, U.M.; Geiker, M.R.; Alonso, M.C.; Polder, R.; Isgor, O.B.; Elsener, B.; Wong, H.; Michel, A.; Hornbostel, K.; Gehlen, C.; et al. The effect of the steel–concrete interface on chloride-induced corrosion initiation in concrete: A critical review by RILEM TC 262-SCI. Mater. Struct. 2019, 52, 88. [Google Scholar] [CrossRef]
- Stefanoni, M.; Angst, U.M.; Elsener, B. Kinetics of electrochemical dissolution of metals in porous media. Nat. Mater. 2019, 18, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Kovalov, D.; Ghanbari, E.; Mao, F.; Kursten, B.; Macdonald, D.D. Investigation of artificial pit growth in carbon steel in highly alkaline solutions containing 0.5 M NaCl under oxic and anoxic conditions. Electrochim. Acta 2019, 320, 134554. [Google Scholar] [CrossRef]
- Vennesland, Ø.; Raupach, M.; Andrade, C. Recommendation of Rilem TC 154-EMC: “Electrochemical techniques for measuring corrosion in concrete”—measurements with embedded probes. Mater. Struct. 2007, 40, 745–758. [Google Scholar] [CrossRef]
- Stern, M.; Geaby, A.L. Electrochemical Polarization: I. A Theoretical Analysis of the Shape of Polarization Curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Elsener, B. Corrosion rate of steel in concrete—Measurements beyond the Tafel law. Corros. Sci. 2005, 47, 3019–3033. [Google Scholar] [CrossRef]
- Joiret, S.; Keddam, M.; Nóvoa, X.; Pérez, M.; Rangel, C.; Takenouti, H. Use of EIS, ring-disk electrode, EQCM and Raman spectroscopy to study the film of oxides formed on iron in 1 M NaOH. Cem. Concr. Compos. 2002, 24, 7–15. [Google Scholar] [CrossRef]
- Keddam, M.; Takenouti, H.; Nóvoa, X.; Andrade, C.; Alonso, C. Impedance measurements on cement paste. Cem. Concr. Res. 1997, 27, 1191–1201. [Google Scholar] [CrossRef]
- Haeri, M.; Goldberg, S.; Gilbert, J.L. The voltage-dependent electrochemical impedance spectroscopy of CoCrMo medical alloy using time-domain techniques: Generalized Cauchy–Lorentz, and KWW–Randles functions describing non-ideal inter-facial behaviour. Corros. Sci. 2011, 53, 582–588. [Google Scholar] [CrossRef]
- Song, H.-W.; Saraswathy, V. Corrosion monitoring of reinforced concrete structures-A. Int. J. Electrochem. Sci. 2007, 2, 1–28. [Google Scholar]
- Andrade, C.; Sanchez, J.; Fullea, J.; Rebolledo, N.; Tavares, F. On-site corrosion rate measurements: 3D simulation and representative values. Mater. Corros. 2012, 63, 1154–1164. [Google Scholar] [CrossRef]
- Caneda-Martínez, L.; Sánchez, J.; Medina, C.; De Rojas, M.I.S.; Torres, J.; Frías, M. Reuse of coal mining waste to lengthen the service life of cementitious matrices. Cem. Concr. Compos. 2019, 99, 72–79. [Google Scholar] [CrossRef]
- Walsh, M.T.; Sagüés, A.A. Steel Corrosion in Submerged Concrete Structures—Part 2: Modeling of Corrosion Evolution and Control. Corrosion 2016, 72, 665–678. [Google Scholar] [CrossRef]
- Taniguchi, N.; Naitou, M.; Kawasaki, M. Experimental Study on Corrosion Behavior of Carbon Steel in Buffer Material-I. Behavior of Corrosion Propagation Based on the Results of Immersion Tests for 10 Years; Japan Atomic Energy Agency: Ibaraki Prefecture, Japan, 2008.
- Kaneko, M.; Miura, N.; Fujiwara, A.; Yamamoto, M. Evaluation of gas generation rate by metal corrosion in the reducing environment. Radioact. Waste Manag. Funding Res. Cent. 2004. Technical Report, RWMC-TRE-03003. [Google Scholar]
- Pergola, A.D.; Lollini, F.; Redaelli, E.; Bertolini, L. Numerical modeling of initiation and propagation of corrosion in hol-low submerged marine concrete structures. Corrosion 2013, 69, 1158–1170. [Google Scholar] [CrossRef]
- Sanchez, J.; Andrade, C.; Torres, J.; Rebolledo, N.; Fullea, J. Determination of reinforced concrete durability with on-site resistivity measurements. Mater. Struct. 2016, 50, 50. [Google Scholar] [CrossRef]
- Garzon, A.; Sanchez, J.P.; Andrade, C.; Rebolledo, N.; Menendez, E.; Fullea, J. Modification of four point method to measure the concrete electrical resistivity in presence of reinforcing bars. Cem. Concr. Compos. 2014, 53, 249–257. [Google Scholar] [CrossRef]
- Wenner, F. A method for measuring earth resistivity. J. Frankl. Inst. 1915, 180, 373–375. [Google Scholar] [CrossRef]
- Garzon, A.J.; Andrade, C.; Rebolledo, N.; Fullea, J.; Sanchez, J.; Menéndez, E. Shape factors of four point resistivity method in presence of rebars. In Proceedings of the Concrete Repair, Rehabilitation and Retrofitting III—Proceedings of the 3rd International Conference on Concrete Repair, Rehabilitation and Retrofitting, ICCRRR 2012, Cape Town, South Africa, 3–5 September 2012; pp. 695–700. [Google Scholar]
- Gulikers, J. Theoretical considerations on the supposed linear relationship between concrete resistivity and corrosion rate of steel reinforcement. Mater. Corros. 2005, 56, 393–403. [Google Scholar] [CrossRef]



| Parameter | Value | Unit | Reference |
|---|---|---|---|
| Anodic Tafel slope | 0.073–0.136 | V/dec | [14] |
| 0.067–0.080 | V/dec | [15] | |
| 0.013–0.017 | V/dec | [16] | |
| 0.320–1.570 | V/dec | [17] | |
| 0.077–0.089 | V/dec | [18] | |
| 0.01–0.369 | V/dec | [19] | |
| 0.09 | V/dec | [20] | |
| 0.075 | V/dec | [21] | |
| 1.00 × 10−12 | V/dec | [22] | |
| Cathodic Tafel slope | 0.112–0.242 | V/dec | [14] |
| 0.222–0.239 | V/dec | [15] | |
| 0.021–0.065 | V/dec | [16] | |
| 0.380–1.080 | V/dec | [17] | |
| 0.449–1.215 | V/dec | [18] | |
| 0.01–0.233 | V/dec | [19] | |
| 0.176 | V/dec | [20] | |
| 0.3–100 | V/dec | [21] | |
| 0.2 | V/dec | [22] | |
| Anodic exchange current density | 1.00 × 10−12–2.75 × 10−10 | A/mm2 | [7,23] |
| 3.07 × 10−8–1.46 × 10−7 | A/mm2 | [15] | |
| 1.73 × 10−8–1.69 × 10−7 | A/mm2 | [15] | |
| 1.00 × 10−12–1.00 × 10−9 | A/mm2 | [19] | |
| 1.00 × 10−12 | A/mm2 | [20] | |
| 3.75 × 10−10 | A/mm2 | [24] | |
| 1.88 × 10−10 | A/mm2 | [7] | |
| 1.00 × 10−12 | A/mm2 | [24] | |
| Cathodic exchange current density | 6.00 × 10−12–1.00 × 10−11 | A/mm2 | [7,23] |
| 6.00 × 10−11–1.60 × 10−10 | A/mm2 | [15] | |
| 1.00 × 10−12–8.50 × 10−10 | A/mm2 | [15] | |
| 1.00 × 10−12–1.00 × 10−9 | A/mm2 | [19] | |
| 1.00 x 10−13 | A/mm2 | [20] | |
| 1.25 x 10−11 | A/mm2 | [24,25] | |
| 6.25 × 10−12 | A/mm2 | [7] | |
| 1.00 × 10−10 | A/mm2 | [24] | |
| 1.00 × 10−10 | A/mm2 | [26] |
| Dosage | Material | Quantity (kg/m3) | |
| Cement | 350 | ||
| Aggregate | AF-0/4 | 766.0 | |
| AG-4/12 | 823.6 | ||
| AG-12/20 | 325.6 | ||
| water/cement ratio | 0.45 | ||
| Chloride content | Specimen | % cem | |
| E01–E02 | 0 | ||
| E11–E12 | 1 | ||
| E21–E22 | 2 | ||
| Electrochemical techniques | Chronoamperometry | Initial test | |
| Linear polarization resistance Electrochemical impedance spectroscopy Chronopotentiometry | During 232 days every 2–4 days | ||
| Cyclic polarisation curves | Final test | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, E.; Torres, J.; Rebolledo, N.; Arrabal, R.; Sanchez, J. Corrosion of Steel Rebars in Anoxic Environments. Part I: Electrochemical Measurements. Materials 2021, 14, 2491. https://doi.org/10.3390/ma14102491
Garcia E, Torres J, Rebolledo N, Arrabal R, Sanchez J. Corrosion of Steel Rebars in Anoxic Environments. Part I: Electrochemical Measurements. Materials. 2021; 14(10):2491. https://doi.org/10.3390/ma14102491
Chicago/Turabian StyleGarcia, Elena, Julio Torres, Nuria Rebolledo, Raul Arrabal, and Javier Sanchez. 2021. "Corrosion of Steel Rebars in Anoxic Environments. Part I: Electrochemical Measurements" Materials 14, no. 10: 2491. https://doi.org/10.3390/ma14102491
APA StyleGarcia, E., Torres, J., Rebolledo, N., Arrabal, R., & Sanchez, J. (2021). Corrosion of Steel Rebars in Anoxic Environments. Part I: Electrochemical Measurements. Materials, 14(10), 2491. https://doi.org/10.3390/ma14102491








