Whey as an Alternative Nutrient Medium for Growth of Sporosarcina pasteurii and Its Effect on CaCO3 Polymorphism and Fly Ash Bioconsolidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolation
2.2. Culture Media
2.3. Effect of Whey on CaCO3 Crystals and Bioconsolidation Treatment
2.4. Characterization of Biogenic CaCO3 Obtained Using Whey at Different Proportions in Nutrient Broth
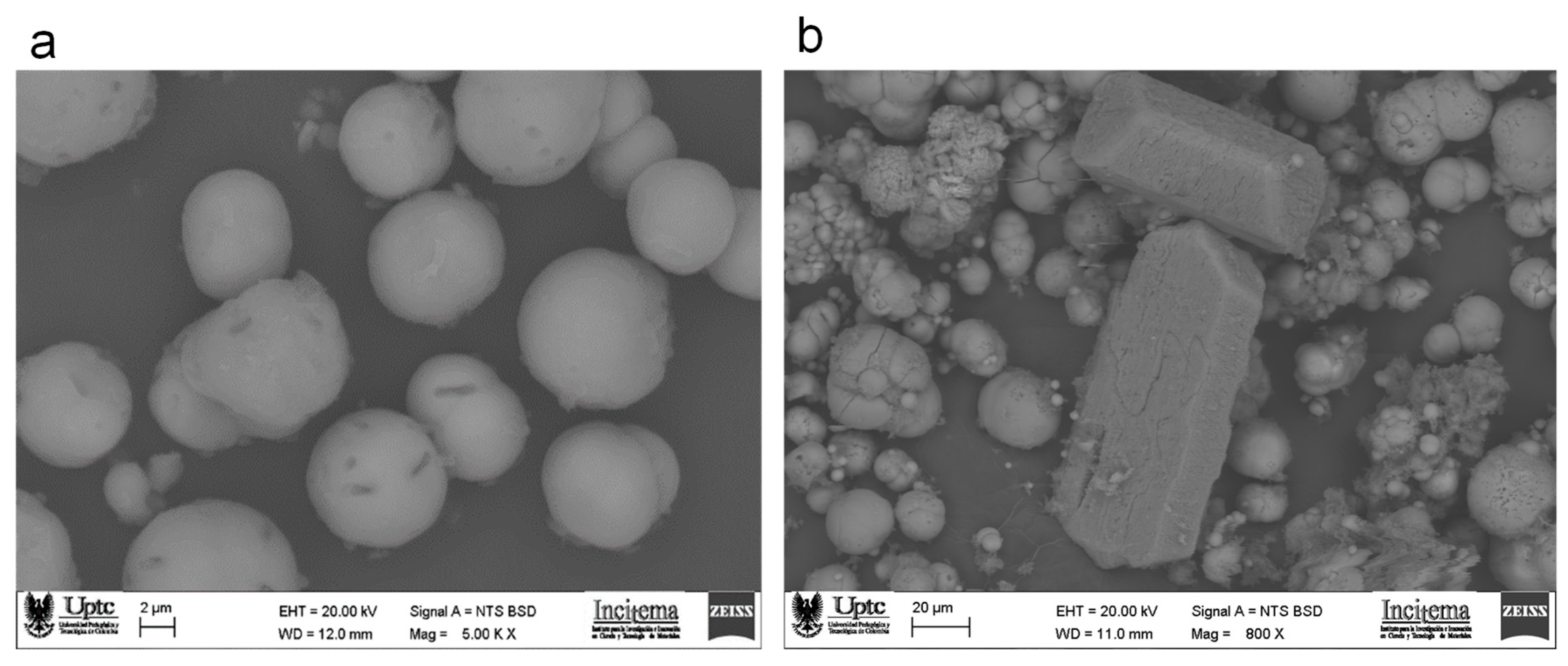
2.5. Tests of Sand and Fly Ash Bioconsolidation
2.6. Statistical Analysis
3. Results and Discussion
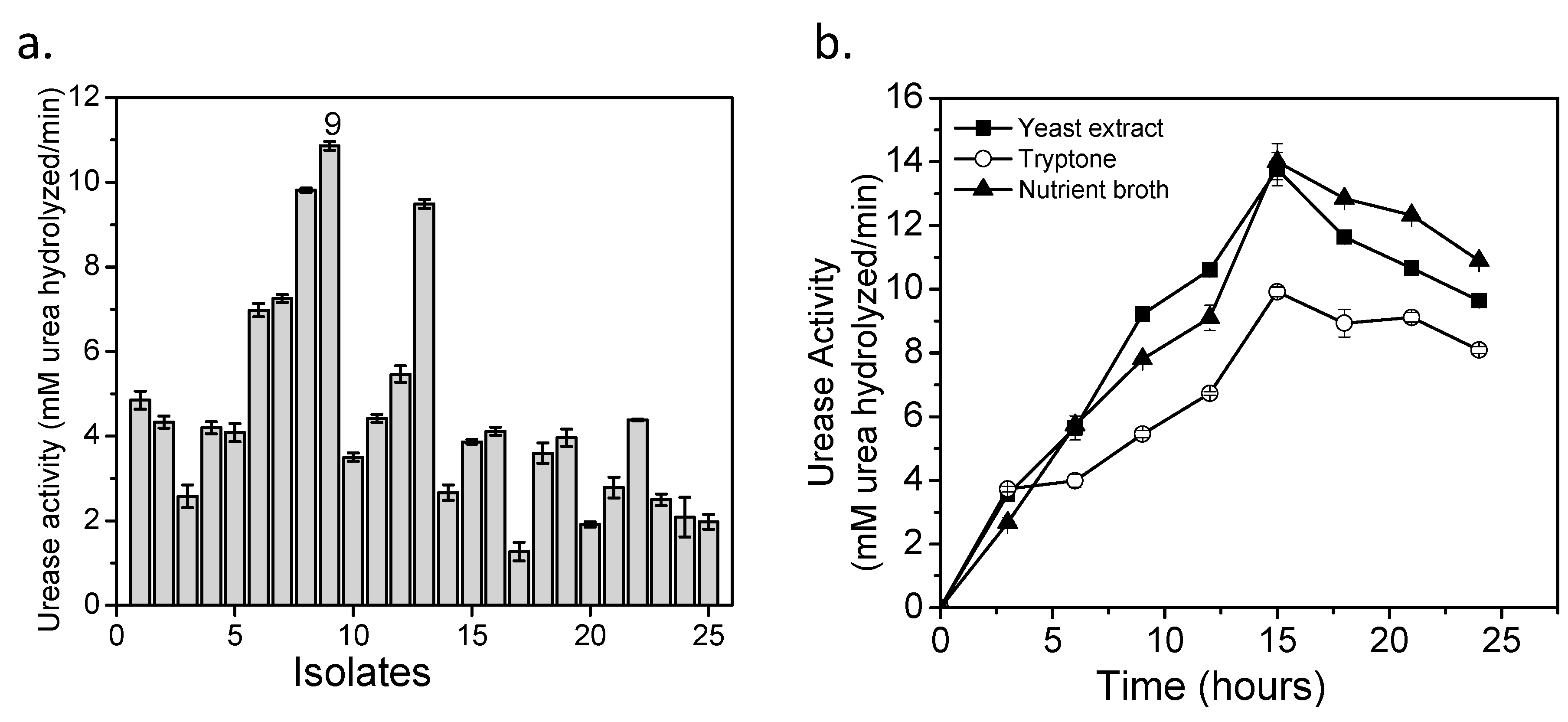
3.1. Bacterial Isolation
3.2. Culture Media
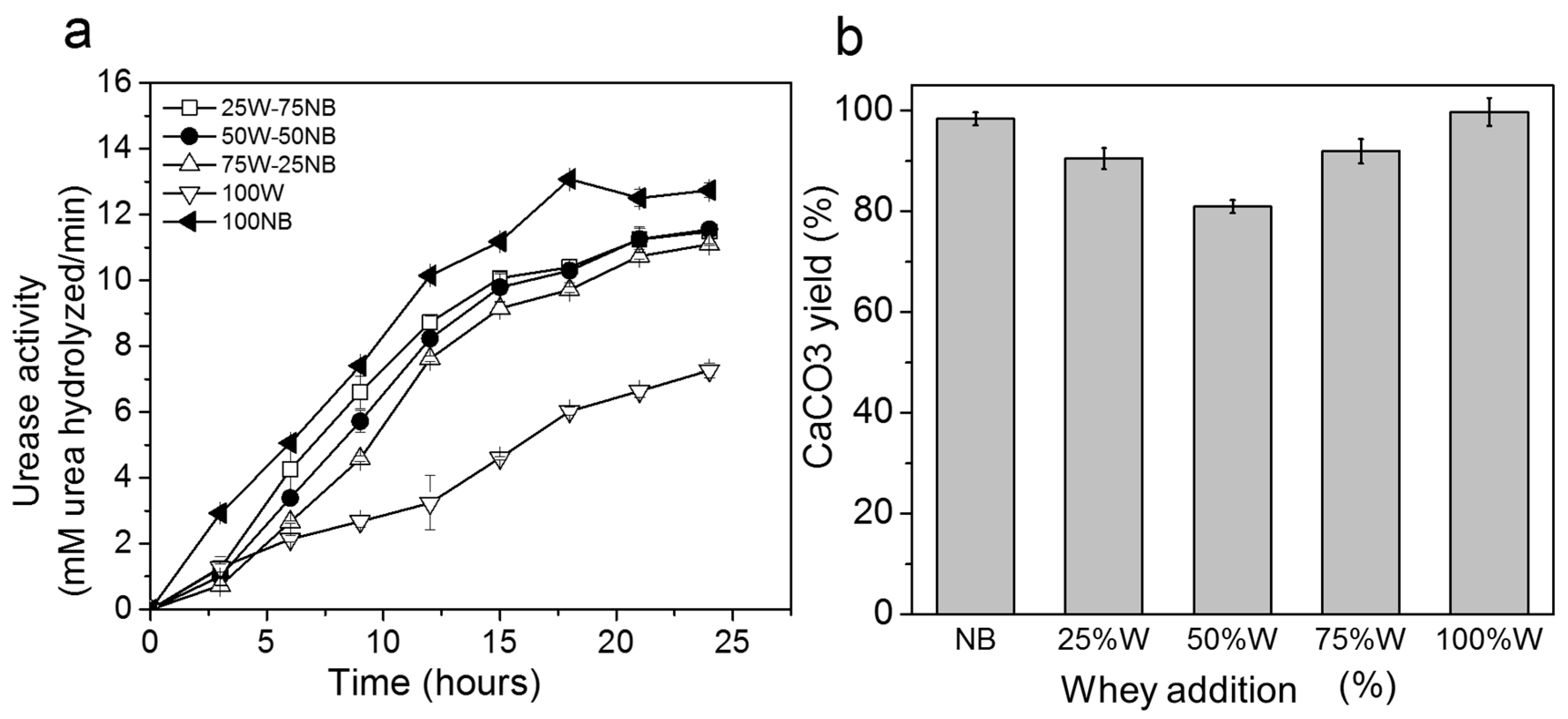
3.3. Effect of Whey in Nutrient Broth on Biogenic CaCO3
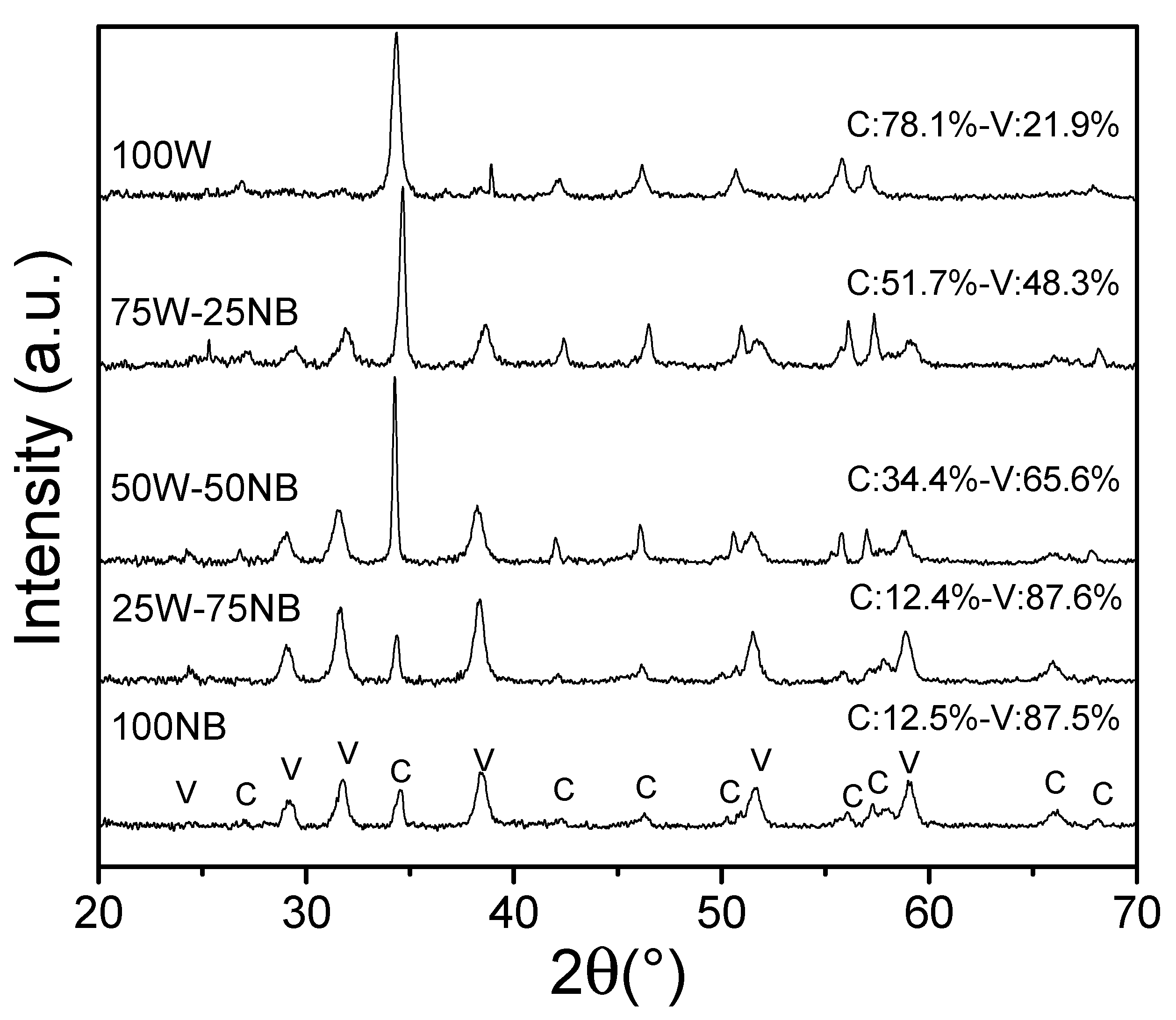
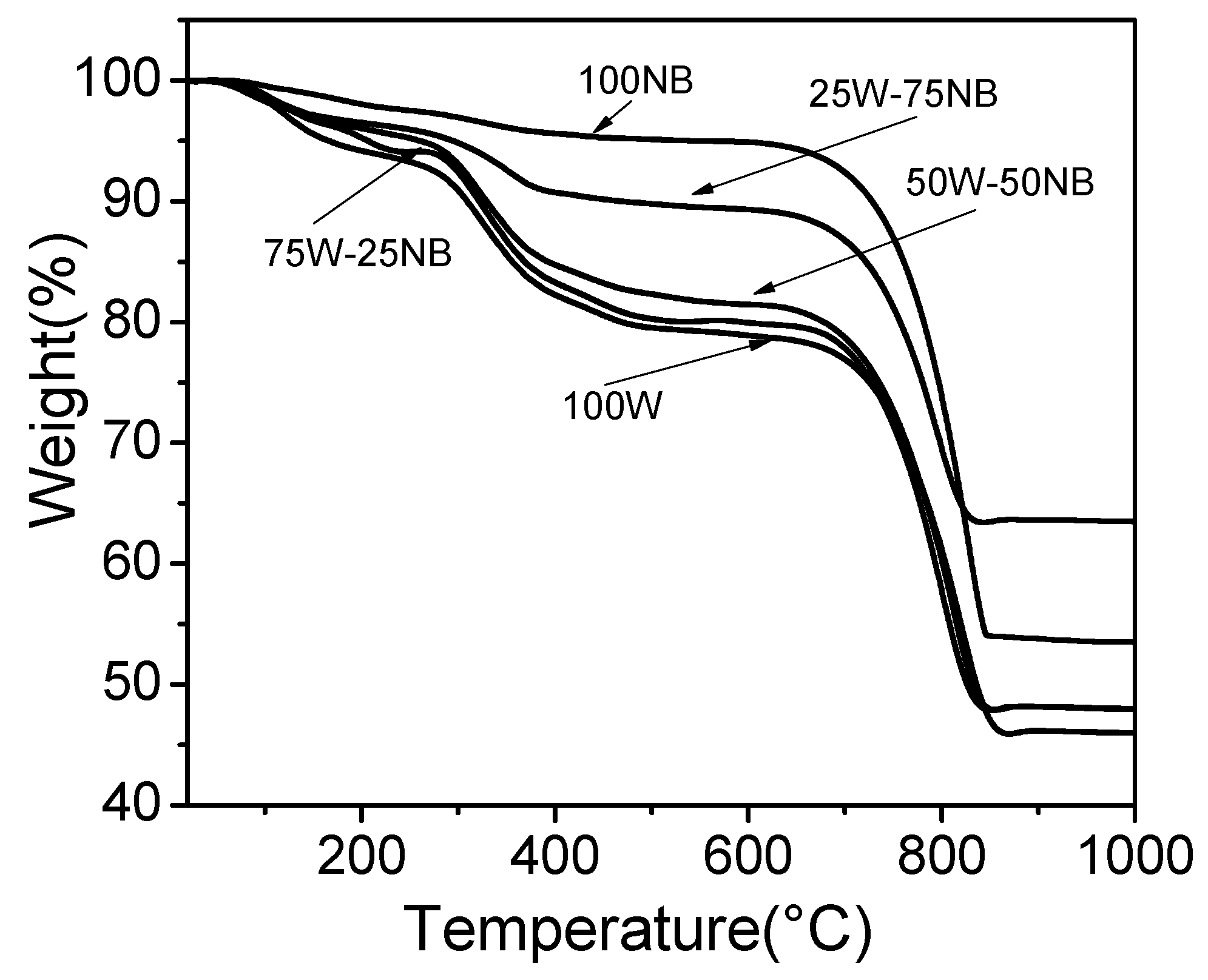
3.4. Characterization of Biogenic CaCO3 Obtained Using Whey at Different Proportions in Nutrient Broth
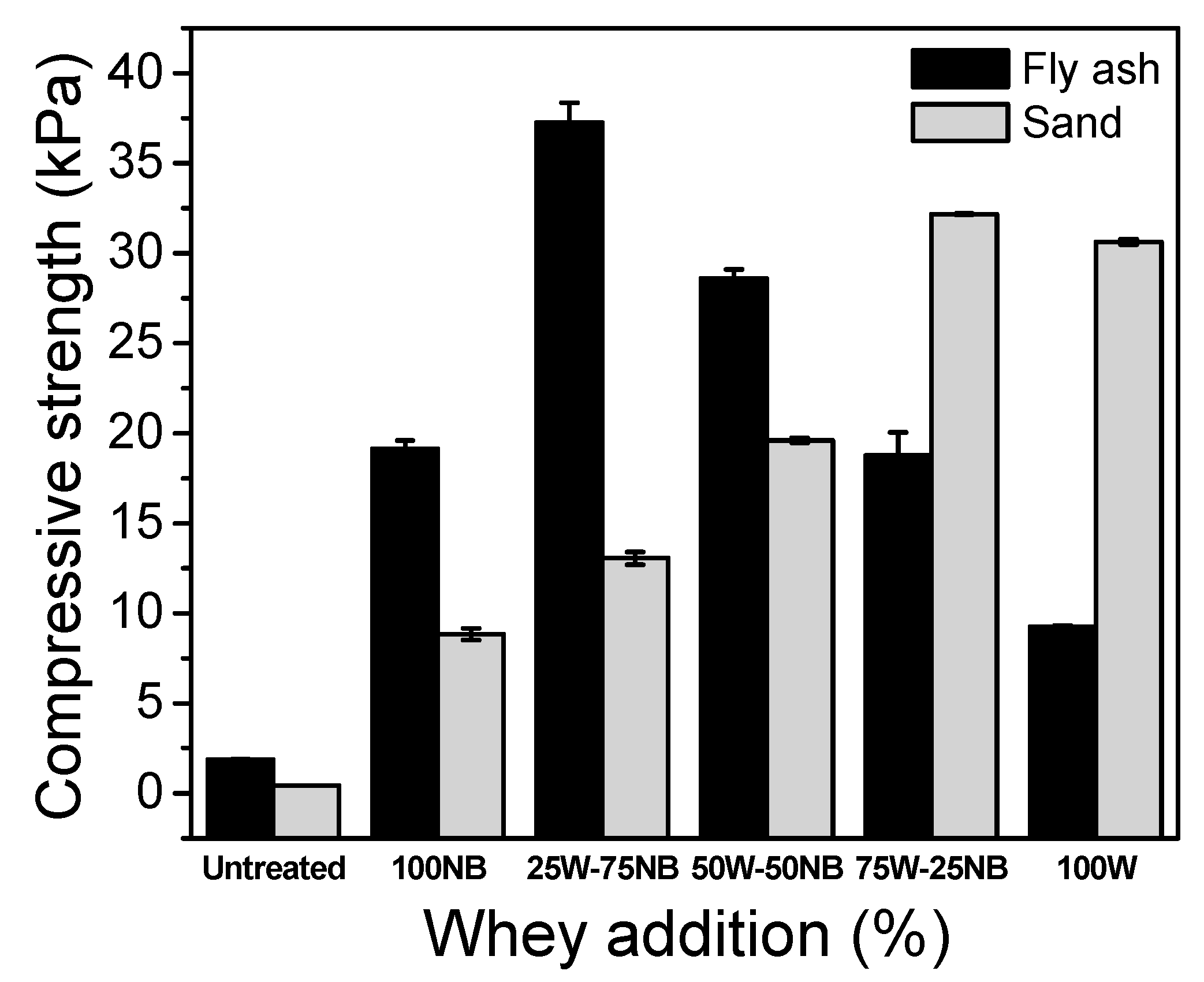
3.5. Bioconsolidation of Sand and Fly Ash
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bosak, T. Calcite Precipitation, Microbially Induced; Encyclopedia of Earth Sciences Series; Reitner, J., Thiel, V., Eds.; Springer: Berlin, Germany, 2011; pp. 223–227. [Google Scholar]
- Rong, H.; Qian, C.-X.; Li, L.-Z. Study on microstructure and properties of sandstone cemented by microbe cement. Constr. Build. Mater. 2012, 36, 687–694. [Google Scholar] [CrossRef]
- Castanier, S.; Le Metayer-Levrel, G.; Perthuisot, J.C. Ca-carbonates precipitation and limestone genesis—The microbiogeologist point of view. Sediment. Geol. 1999, 126, 9–23. [Google Scholar] [CrossRef]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial carbonate precipitation in construction materials: A review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Biomineralization of calcium carbonates and their engineered applications: A review. Front. Microbiol. 2013, 4, 314. [Google Scholar] [CrossRef] [PubMed]
- Warthmann, R.; Van Lith, Y.; Vasconcelos, C.; McKenzie, J.; Karpoff, A. Bacterially induced dolomite precipitation in anoxic culture experiments. Geology 2000, 28, 1091–1094. [Google Scholar] [CrossRef]
- Arias, J.L.; Fernández, M.S. Polysaccharides and proteoglycans in calcium carbonate-based biomineralization. Chem. Rev. 2008, 108, 4475–4482. [Google Scholar] [CrossRef]
- Hammes, F.; Boon, N.; De Villiers, J.; Verstraete, W.; Siciliano, S.D.; Villiers, J. Strain-specific ureolytic microbial calcium carbonate precipitation strain-specific ureolytic microbial calcium carbonate precipitation. Appl. Environ. Microbiol. 2003, 69, 4901–4909. [Google Scholar] [CrossRef]
- Hammes, F.; Verstraete, W. Key roles of pH and calcium metabolism in microbial carbonate precipitation. Rev. Environ. Sci. Biotechnol. 2002, 1, 3–7. [Google Scholar] [CrossRef]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-mediated soil improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Charpe, A.U.; Latkar, M.V.; Chakrabarti, T. Microbially assisted cementation—A biotechnological approach to improve mechanical properties of cement. Constr. Build. Mater. 2017, 135, 472–476. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A.; Kumari, D.; Zhang, Q. Biomineralization for sustainable construction-A review of processes and applications. Earth-Sci. Rev. 2015, 148, 1–17. [Google Scholar] [CrossRef]
- Bang, S.S.; Galinat, J.K.; Ramakrishnan, V. Calcite precipitation induced by polyurethane-immobilized Bacillus pasteurii. Enzyme Microb. Technol. 2001, 28, 404–409. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Ferris, F.G. The influence of Bacillus pasteurii on the nucleation and growth of calcium carbonate. Geomicrobiol. J. 2006, 23, 213–226. [Google Scholar] [CrossRef]
- Qian, C.; Wang, R.; Cheng, L.; Wang, J. Theory of microbial carbonate precipitation and its application in restoration of cement-based materials defects. Chin. J. Chem. 2010, 28, 847–857. [Google Scholar] [CrossRef]
- Ferris, F.G.; Stehmeier, L.G.; Kantzas, A.; Mourits, F.M. Bacteriogenic mineral plugging. J. Can. Pet. Technol. 1996, 35, 56–61. [Google Scholar] [CrossRef]
- Ramachandran, S.K.; Ramakrishnan, V.; Bang, S.S. Remediation of concrete using micro-organisms. ACI Mater. J. 2001, 98, 3–9. [Google Scholar]
- Wiffin, V. Microbial CaCO3 Precipitation for the Production of Biocement. Ph.D. Thesis, Murdoch University, St Ives Murdoch, Australia, 2004. [Google Scholar]
- Eryürük, K.; Yang, S.; Suzuki, D.; Sakaguchi, I.; Katayama, A. Effects of bentonite and yeast extract as nutrient on decrease in hydraulic conductivity of porous media due to CaCO3 precipitation induced by Sporosarcina pasteurii. J. Biosci. Bioeng. 2015, 120, 411–418. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Ferris, F.G. The coprecipitation of Sr into calcite precipitates induced by bacterial ureolysis in artificial groundwater: Temperature and kinetic dependence. Geochim. Cosmochim. Acta 2005, 69, 4199–4210. [Google Scholar] [CrossRef]
- Nosouhian, F.; Mostofinejad, D.; Hasheminejad, H. Influence of biodeposition treatment on concrete durability in a sulphate environment. Biosyst. Eng. 2015, 133, 141–152. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A. A review of microbial precipitation for sustainable construction. Constr. Build. Mater. 2015, 93, 1224–1235. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A.; Reddy, M.S. Effect of calcifying bacteria on permeation properties of concrete structures. J. Ind. Microbiol. Biotechnol. 2011, 38, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Vaisnav, N.; Mathur, A.; Gupta, R.; Tuli, D.K. Whey waste as potential feedstock for biohydrogen production. Renew. Energy 2016, 98, 221–225. [Google Scholar] [CrossRef]
- Fernandes, M.; Fornari, R.C.; Mazutti, M.A.; De Oliveira, D.; Ferreira, F.; Cichoski, A.J.; Cansian, R.L.; Di Lucio, M.; Treichel, H. Production and characterization of xantham gum by Xanthomonas campestris using cheese whey as sole carbon source. J. Food Eng. 2009, 90, 119–123. [Google Scholar]
- Aider, M.; De Halleux, D.; Melnikova, I. Skim acidic milk whey cryoconcentration and assessment of its functional properties: Impact of processing conditions. Innov. Food Sci. Emerg. Technol. 2009, 10, 334–341. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A.; Basu, P.C.; Reddy, M.S. Lactose mother liquor as an alternative nutrient source for microbial concrete production by Sporosarcina pasteurii. J. Ind. Microbiol. Biotecnol. 2009, 36, 433–438. [Google Scholar] [CrossRef]
- Cuzman, O.A.; Ritcher, K.; Wittig, L.; Tiano, P. Alternative nutrient sources for biotechnological use of Sporosarcina pasteurii. World J. Microbiol. Biotechnol. 2015, 31, 897–906. [Google Scholar] [CrossRef]
- Grabiec, A.M.; Klama, J.; Zawal, D.; Krupa, D. Modification of recycled concrete aggregate by calcium carbonate biodeposition. Constr. Build. Mater. 2012, 34, 145–150. [Google Scholar] [CrossRef]
- Al-Thawadi, S.; Cord-Ruwisch, R. Calcium carbonate crystals formation by ureolytic bacteria isolated from Australian soil and sludge. J. Adv. Sci. Eng. Res. 2012, 2, 12–26. [Google Scholar]
- Li, H.; Song, Y.; Li, Q.; He, J.; Song, Y. Effective microbial calcite precipitation by a new mutant and precipitating regulation of extracellular urease. Bioresour. Technol. 2014, 167, 269–275. [Google Scholar] [CrossRef]
- De Muynck, W.; Verbeken, K.; De Belie, N.; Verstraete, W. Influence of urea and calcium dosage on the effectiveness of bacterially induced carbonate precipitation on limestone. Ecol. Eng. 2010, 36, 99–111. [Google Scholar] [CrossRef]
- Downs, R.T.; Hall-Wallace, M. The American mineralogist crystal structure database. Am. Mineral. 2003, 88, 247–250. Available online: https://goo.gl/LwfD48 (accessed on 20 August 2020).
- American Society for Testing Materials. Standard Test Method for Compressive Strength of Hydraulic Cement Mortars; ASTM C109M; ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar]
- Tourney, J.; Ngwenya, B.T. Bacterial extracellular polymeric substances (EPS) mediate CaCO3 morphology and polymorphism. Chem. Geol. 2009, 262, 138–146. [Google Scholar] [CrossRef]
- Tiano, P.; Biagiotti, L.; Mastromei, G. Bacterial bio-mediated calcite precipitation for monumental stones conservation: Methods of evaluation. J. Microbiol. Methods. 1999, 36, 139–145. [Google Scholar] [CrossRef]
- Kang, C.H.; So, J.S. Heavy metal and antibiotic resistance of ureolytic bacteria and their immobilization of heavy metals. Ecol. Eng. 2016, 97, 304–312. [Google Scholar] [CrossRef]
- Abo-El-Enein, S.A.; Ali, A.H.; Talkhan, F.N.; Abdel-Gawwad, H.A. Utilization of microbial induced calcite precipitation for sand consolidation and mortar crack remediation. HBRC J. 2012, 8, 185–192. [Google Scholar] [CrossRef]
- Mccusker, L.B.; Von Dreele, R.B.; Cox, D.E.; Loue, D.; Scardi, P. Rietveld refinement guidelines. J. Appl. Crystallogr. 1999, 32, 36–50. [Google Scholar] [CrossRef]
- Wolf, G.; Königsberger, E.; Schmidt, H.; Königsberger, C.; Gamsjäger, H. Thermodynamic aspects of the vaterite calcite phase transition. J. Therm. Anal. Calorim. 2000, 60, 463–472. [Google Scholar] [CrossRef]
- Aquilano, D.; Otálora, F.; Pastero, L.; García-Ruiz, J.M. Three study cases of growth morphology in minerals: Halite, calcite and gypsum. Prog. Cryst. Growth Charact. Mater. 2016, 62, 227–251. [Google Scholar] [CrossRef]
- Tegethoff, F.W.; Rohleder, J.; Kroker, E. Calcium Carbonate: From the Cretaceous Period Into the 21st Century; Springer: Basel, Switzerland, 2001. [Google Scholar]
- Preisig, D.; Haid, D.; Varum, F.J.; Bravo, R.; Alles, R.; Huwyler, J.; Puchkov, M. Drug loading into porous calcium carbonate microparticles by solvent evaporation. Eur. J. Pharm. Biopharm. 2014, 87, 548–558. [Google Scholar] [CrossRef]
- Bang, J.H.; Jang, Y.N.; Kim, W.; Song, K.S.; Jeon, C.W.; Chae, S.C.; Lee, S.; Park, S.; Lee, M. Specific surface area and particle size of calcium carbonate precipitated by carbon dioxide microbubbles. Chem. Eng. J. 2012, 198–199, 254–260. [Google Scholar] [CrossRef]
- Al, M.M.; Rashid, I.S.; Qinna, N.A.; Jaber, A.M.; Badwan, A.A. Calcium Carbonate. In Profiles of Drug Substances, Excipients and Related Methodology, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 41, pp. 31–132. [Google Scholar]
- Andersen, F.A.; Brečević, L. Infrared spectra of amorphous and crystalline calcium carbonate. Acta Chem. Scan. 1991, 45, 1018–1024. [Google Scholar] [CrossRef]
- Aouad, S.; Saab, E.; Nassrallah-Aboukaïs, N.; Abi-Aad, E.; Aboukaïs, A. The use of vaterite as a trap for metallic ions in water study of copper/vaterite and manganese/vaterite systems. Leban. Sci. J. 2009, 10, 101–111. [Google Scholar]
- Jimenez, C.; Jroundi, F.; Rodríguez, M.; Arias, J.M.; González-Muñoz, M.T. Biomineralization induced by Myxobacteria. Commun. Curr. Res. Educ. Top. Trends. Appl. Microbiol. 2007, 1, 143–154. [Google Scholar] [CrossRef]
- De Muynck, W.; Debrouwer, D.; De Belie, N.; Verstraete, W. Bacterial carbonate precipitation improves the durability of cementitious materials. Cem. Concr. Res. 2008, 38, 1005–1014. [Google Scholar] [CrossRef]
- Ghosh, T.; Bhaduri, S.; Montemagno, C.; Kumar, A. Sporosarcina pasteurii can form nanoscale calcium carbonate crystals on cell surface. PLoS ONE 2019, 14, 1–15. [Google Scholar] [CrossRef]
- Choi, H.; Choi, H.; Inoue, M.; Sengoku, R. Control of the polymorphism of calcium carbonate produced by self-healing in the cracked part of cementitious materials. Appl. Sci. 2017, 7, 546. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Özyurt, N. Improved strength and durability of fly ash-amended concrete by microbial calcite precipitation. Ecol. Eng. 2011, 37, 554–559. [Google Scholar] [CrossRef]
- Udrea, I.; Capat, C.; Olaru, E.A.; Isopescu, R.; Mihai, M.; Mateescu, C.D.; Bradu, C. Vaterite synthesis via gas-liquid route under controlled pH conditions. Ind. Eng. Chem. Res. 2012, 51, 8185–8193. [Google Scholar] [CrossRef]
- Anbu, P.; Kang, C.; Shin, Y.; So, J. Formations of calcium carbonate minerals by bacteria and its multiple applications. SpringerPlus 2016, 5, 250. [Google Scholar] [CrossRef]
- Park, S.J.; Park, Y.M.; Chun, W.Y.; Kim, W.J.; Ghim, S.Y. Calcite-forming bacteria for compressive strength improvement in mortar. J. Microbiol. Biotechn. 2010, 20, 782–788. [Google Scholar]
- Chaparro, S.P.; Becerrra, M.L.; Martínez, J.J.; Rojas, H.A. Soil bacteria that precipitate calcium carbonate: Mechanism and applications of the process. Act. Agron. 2018, 67, 277–288. [Google Scholar] [CrossRef]

| Treatment | Crystal Size Vaterite (nm) * | Crystal Size Calcite (nm) * | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | Purity (%) ** |
|---|---|---|---|---|---|---|
| 100 NB | 3.6 | 3.2 | 25 | 0.11 | 22.5 | 96 |
| 25 W-75 NB | 3.6 | 3.2 | 23 | 0.04 | 8.6 | 91 |
| 50 W-50 NB | 3.2 | 3.6 | 19 | 0.05 | 9.3 | 83 |
| 75 W-25 NB | 3.2 | 3.2 | 6 | 0.01 | 24.8 | 81 |
| 100 W | 3.8 | 2.7 | 2 | 0.01 | 24.8 | 80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaparro, S.; Rojas, H.A.; Caicedo, G.; Romanelli, G.; Pineda, A.; Luque, R.; Martínez, J.J. Whey as an Alternative Nutrient Medium for Growth of Sporosarcina pasteurii and Its Effect on CaCO3 Polymorphism and Fly Ash Bioconsolidation. Materials 2021, 14, 2470. https://doi.org/10.3390/ma14102470
Chaparro S, Rojas HA, Caicedo G, Romanelli G, Pineda A, Luque R, Martínez JJ. Whey as an Alternative Nutrient Medium for Growth of Sporosarcina pasteurii and Its Effect on CaCO3 Polymorphism and Fly Ash Bioconsolidation. Materials. 2021; 14(10):2470. https://doi.org/10.3390/ma14102470
Chicago/Turabian StyleChaparro, Sandra, Hugo A. Rojas, Gerardo Caicedo, Gustavo Romanelli, Antonio Pineda, Rafael Luque, and José J. Martínez. 2021. "Whey as an Alternative Nutrient Medium for Growth of Sporosarcina pasteurii and Its Effect on CaCO3 Polymorphism and Fly Ash Bioconsolidation" Materials 14, no. 10: 2470. https://doi.org/10.3390/ma14102470
APA StyleChaparro, S., Rojas, H. A., Caicedo, G., Romanelli, G., Pineda, A., Luque, R., & Martínez, J. J. (2021). Whey as an Alternative Nutrient Medium for Growth of Sporosarcina pasteurii and Its Effect on CaCO3 Polymorphism and Fly Ash Bioconsolidation. Materials, 14(10), 2470. https://doi.org/10.3390/ma14102470








