Improving the Catalytic CO2 Reduction on Cs2AgBiBr6 by Halide Defect Engineering: A DFT Study
Abstract
1. Introduction
2. Computational Method
3. Results and Discussion
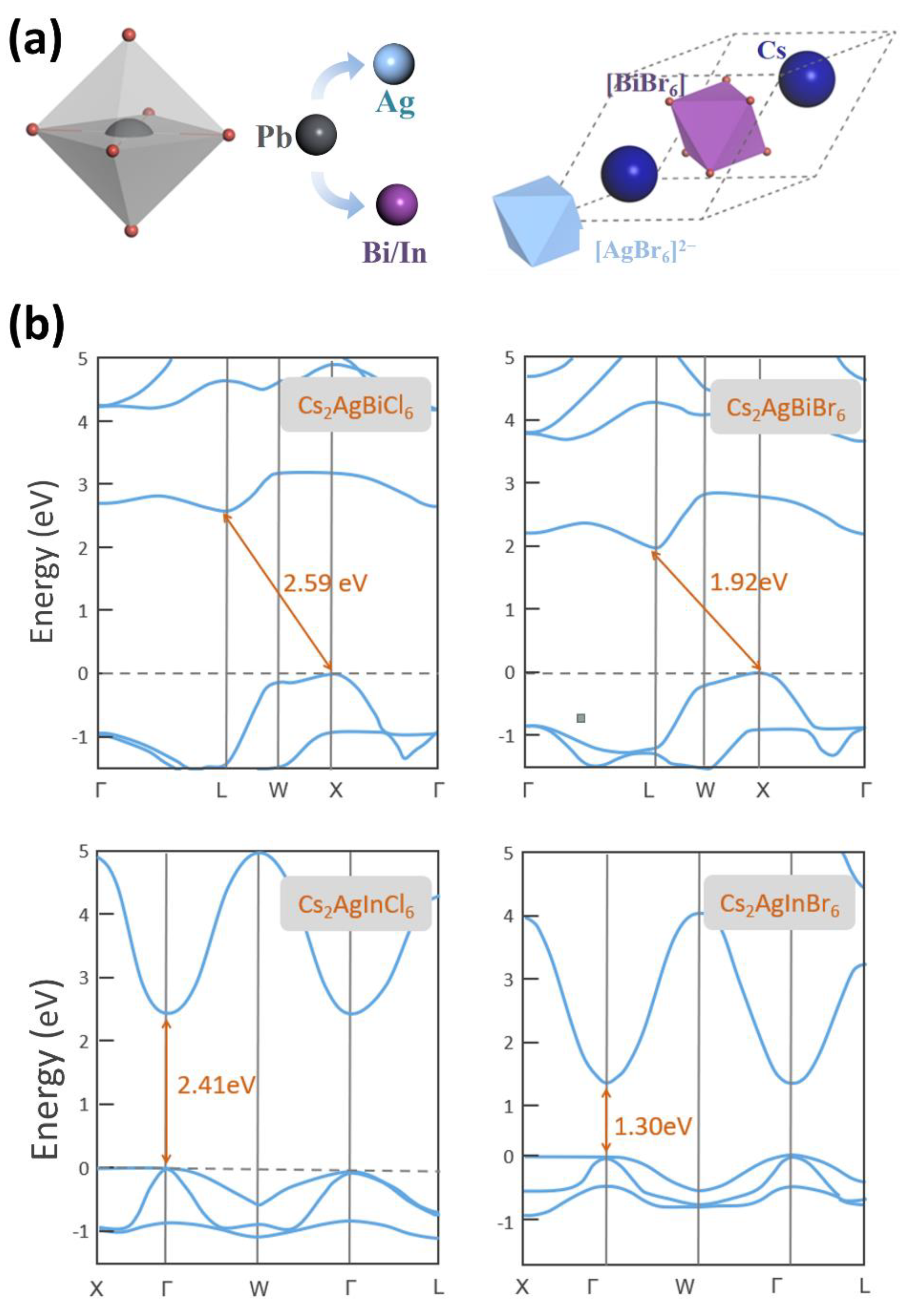
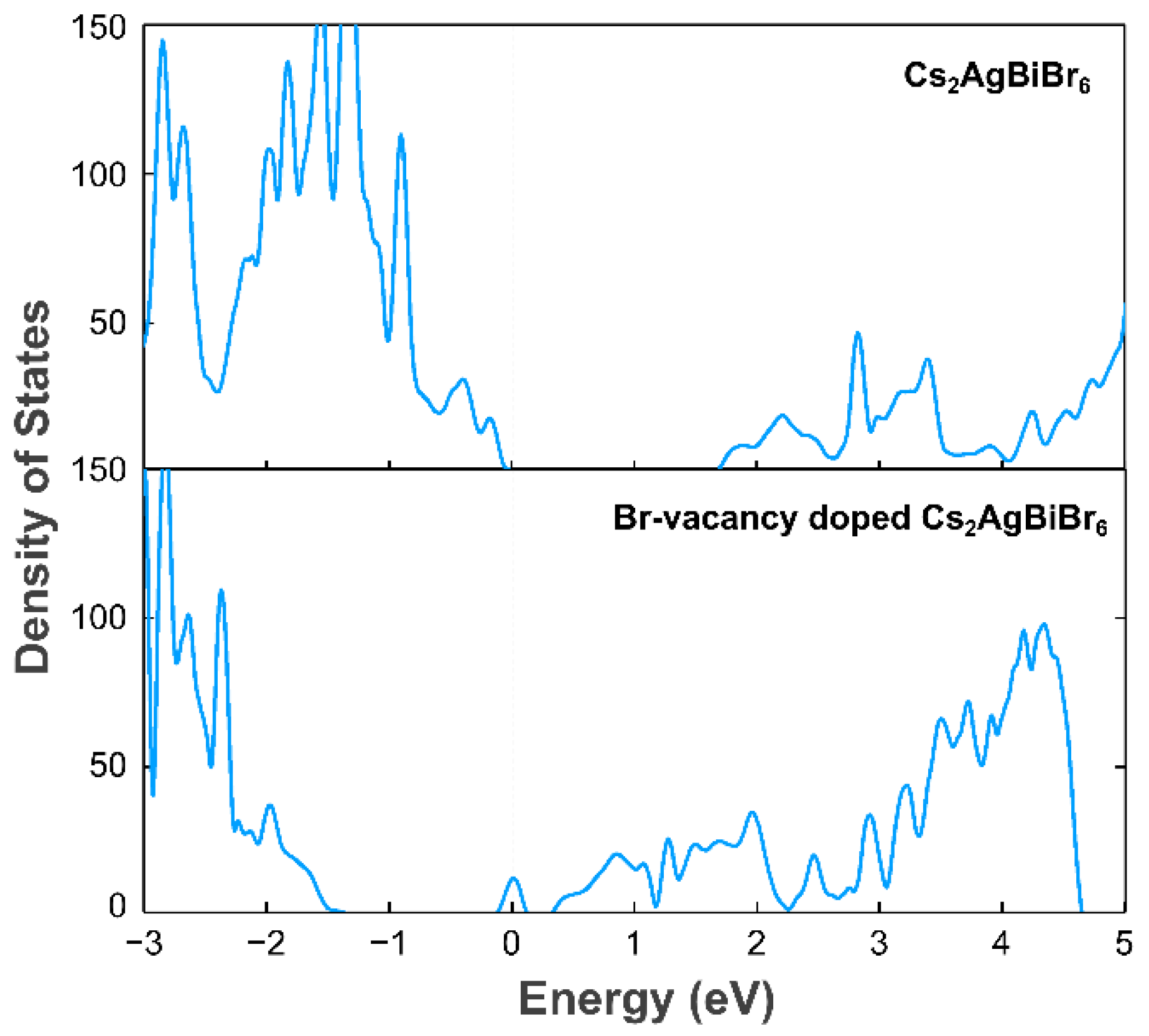
3.1. The Basic Crystal and Electronic Structure of Double Halide Perovskite
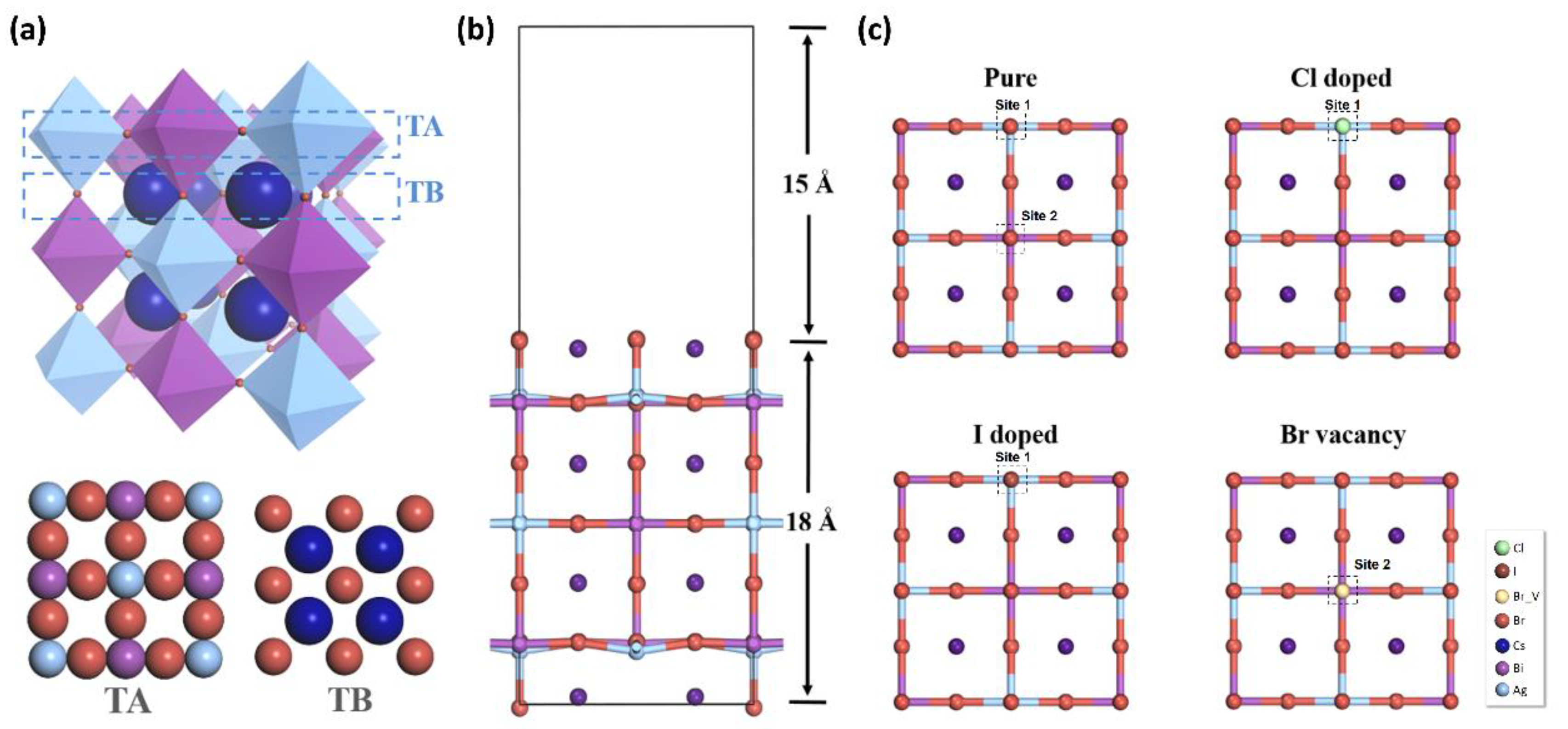
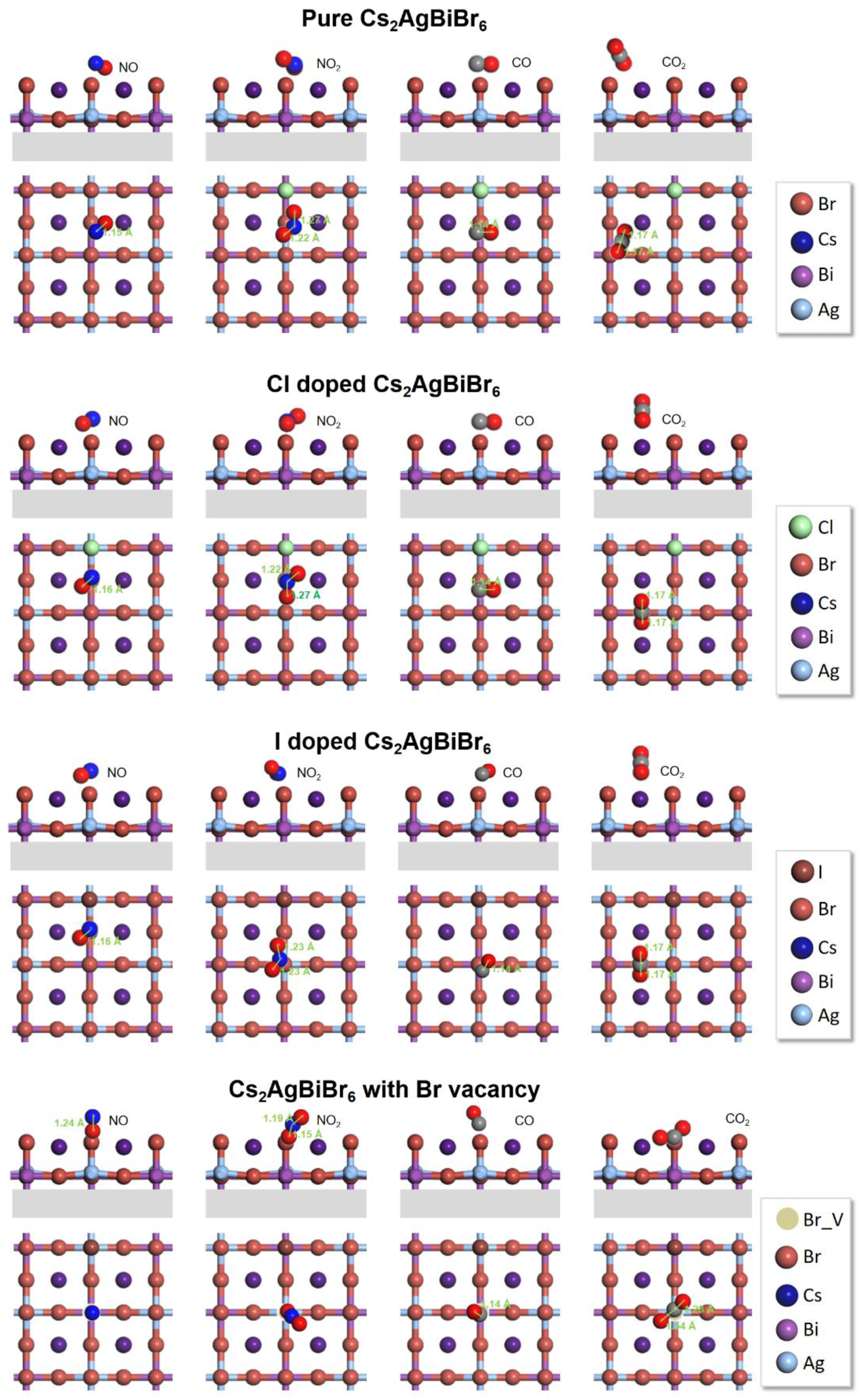
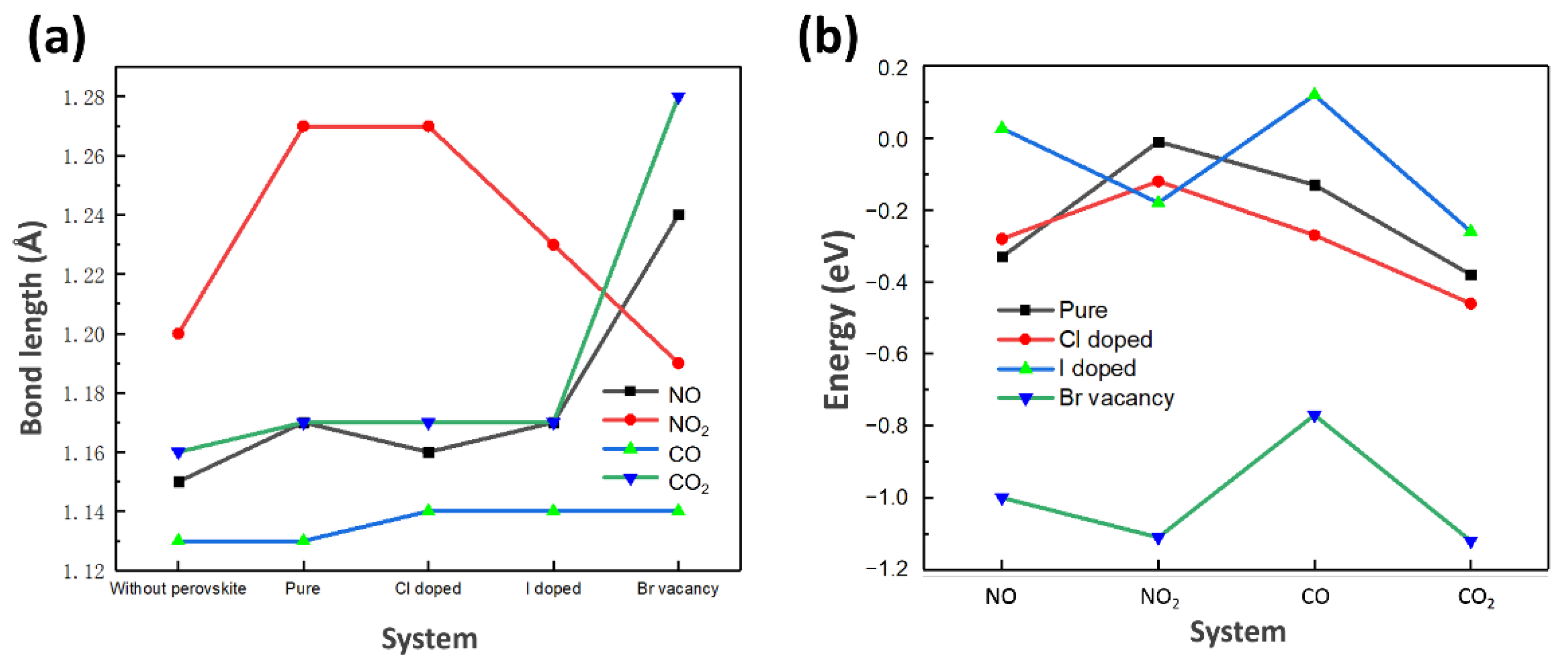
3.2. The Carbon Dioxide Capture Capacity on Modified Cs2AgBiBr6
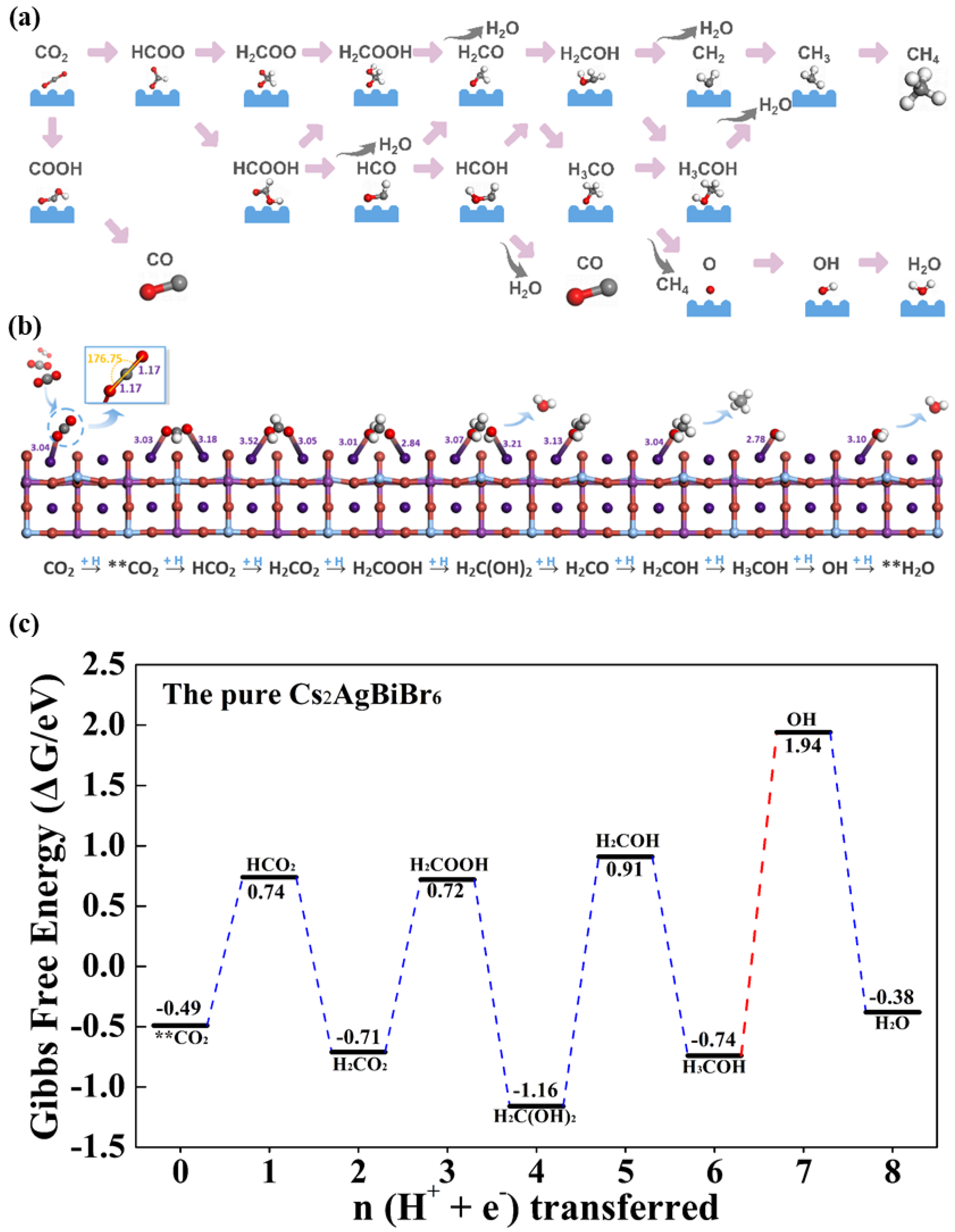
3.3. The Pure Cs2AgBiBr6 for CO2 Catalytic Performance
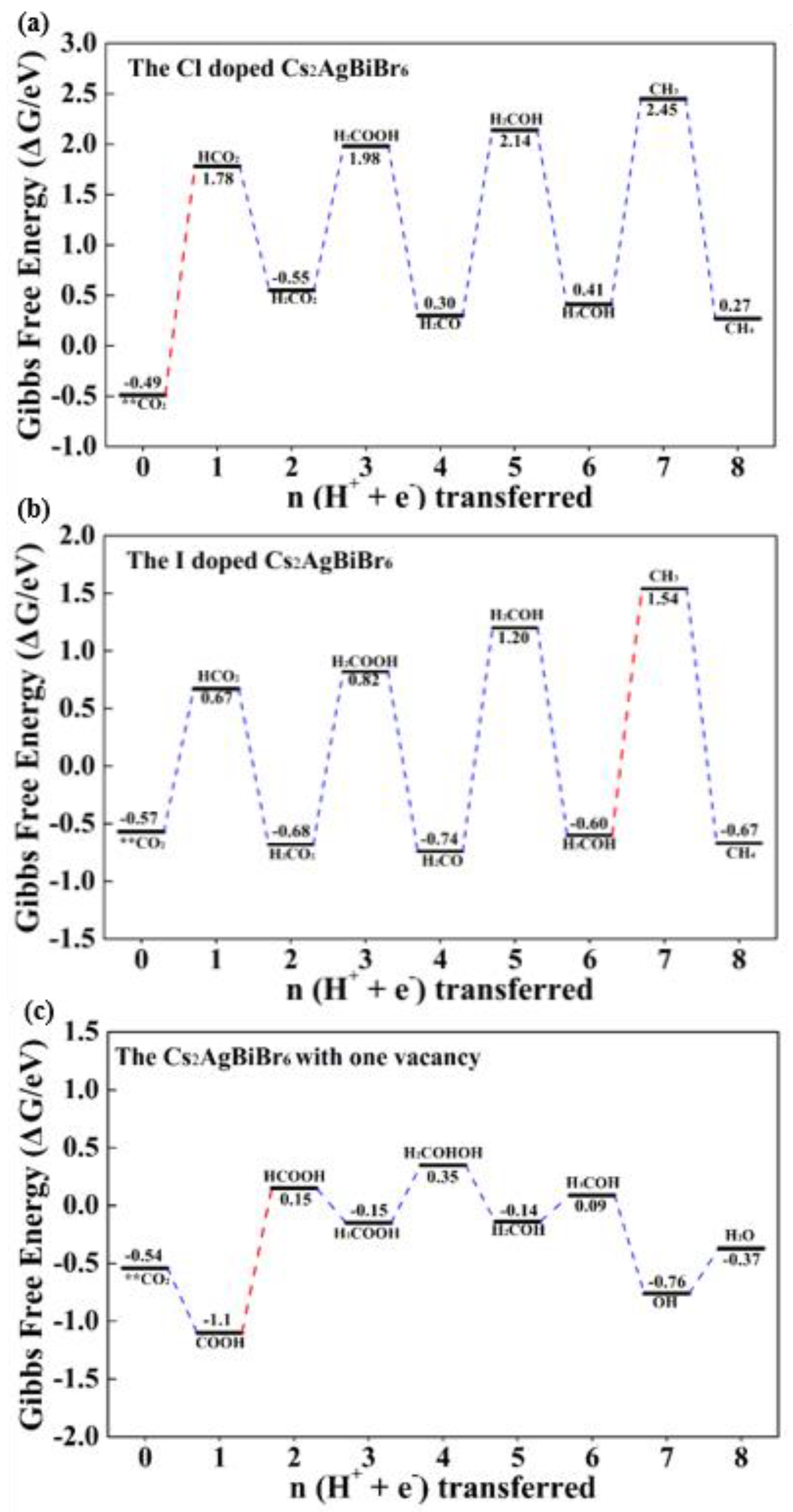
3.4. The Vacancy and Doping Engineering for the Improved CO2 Catalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Tade, M.O.; Shao, Z.P. Research Progress of Perovskite Materials in Photocatalysis- and Photovoltaics-Related Energy Conversion and Environmental Treatment. Chem. Soc. Rev. 2015, 44, 5371–5408. [Google Scholar] [CrossRef]
- Vautier, M.; Guillard, C.; Herrmann, J.-M. Photocatalytic Degradation of Dyes in Water: Case Study of Indigo and of Indigo Carmine. J. Catal. 2001, 201, 46–59. [Google Scholar] [CrossRef]
- He, H.; Zhong, M.; Yacatto, K.; Rappold, T.; Sugar, G.; David, N.E.; Gelb, J.; Kotwal, N.; Merkle, A.; Konkolewicz, D.; et al. Three-Dimensionally Ordered Macroporous Polymeric Materials by Colloidal Crystal Templating for Reversible CO2 Capture. Adv. Funct. Mater. 2013, 23, 4720–4728. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, M.; Ma, H.; Yu, C.; Li, K.; Yu, C.; Jiang, L. Morphology-Control Strategy of the Superhydrophobic Poly(Methyl Methacrylate) Surface for Efficient Bubble Adhesion and Wastewater Remediation. Adv. Funct. Mater. 2017, 27, 1702020. [Google Scholar] [CrossRef]
- Phuan, Y.W.; Ong, W.-J.; Chong, M.N.; Ocon, J.D. Prospects of Electrochemically Synthesized Hematite Photoanodes for Photoelectrochemical Water Splitting: A Review. J. Photochem. Photobiol. C Photochem. Rev. 2017, 33, 54–82. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, P.; Liu, S.; Yu, K. Three-Dimensionally Ordered Macroporous Perovskite Materials for Environmental Applications. Chin. J. Catal. 2019, 40, 1324–1338. [Google Scholar] [CrossRef]
- Abe, R.; Shinmei, K.; Hara, K.; Ohtani, B. Robust Dye-Sensitized Overall Water Splitting System With Two-Step Photoexcitation of Coumarin Dyes and Metal Oxide Semiconductors. Chem. Commun. 2009, 24, 3577. [Google Scholar] [CrossRef]
- Abe, R.; Takata, T.; Sugihara, H.; Domen, K. Photocatalytic Overall Water Splitting under Visible Light by TaON and WO3 With an IO3–/I– Shuttle Redox Mediator. Chem. Commun. 2005, 30, 3829–3831. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.P.; Hood, Z.D.; More, K.L.; Chen, V.W.; Lachgar, A. A Visible-Light-Active Heterojunction with Enhanced Photocatalytic Hydrogen Generation. ChemSusChem 2016, 9, 1869–1879. [Google Scholar] [CrossRef]
- Andrei, V.; Hoye, R.L.Z.; Crespo-Quesada, M.; Bajada, M.; Ahmad, S.; De Volder, M.; Friend, R.; Reisner, E. Scalable Triple Cation Mixed Halide Perovskite-BiVO4 Tandems for Bias-Free Water Splitting. Adv. Energy Mater. 2018, 8, 1801403. [Google Scholar] [CrossRef]
- Ni, G.; Chen, S.; Sunku, S.S.; Sternbach, A.; McLeod, A.S.; Xiong, L.; Fogler, M.M.; Chen, G.; Basov, D.N. Nanoscale Infrared Spectroscopy and Imaging of Catalytic Reactions in Cu2O Crystals. ACS Photon. 2020, 7, 576–580. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, H.; Yu, Z.; Song, R.; Qian, K.; Chen, X.; Tian, J.; Zhang, W.; Huang, W. Site-Resolved Cu2O Catalysis in the Oxidation of CO. Angew. Chem. Int. Ed. 2019, 58, 4276–4280. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Li, X.; Guo, H.; Li, J.; Shang, K.; Lu, N.; Wu, Y. Plasma-Assisted Catalysis Decomposition of BPA over Graphene-CdS Nanocomposites in Pulsed Gas-Liquid Hybrid Discharge: Photocorrosion Inhibition and Synergistic Mechanism Analysis. Chem. Eng. J. 2021, 412, 128627. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, Q.; Ma, K.; Ding, Y.; Li, C. Pyroelectric Effect in CdS Nanorods Decorated With a Molecular Co-Catalyst for Hydrogen Evolution. Nano Energy 2020, 73, 104810. [Google Scholar] [CrossRef]
- Nasir, M.S.; Yang, G.; Ayub, I.; Wang, S.; Yan, W. Tin Diselinide a Stable Co-Catalyst Coupled With Branched TiO2 Fiber and G-C3N4 Quantum Dots for Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2020, 270, 118900. [Google Scholar] [CrossRef]
- Zhuang, Z.; Li, Y.; Li, Z.; Lv, F.; Lang, Z.; Zhao, K.; Zhou, L.; Moskaleva, L.; Guo, S.; Mai, L. MoB/G-C3N4 Interface Materials As a Schottky Catalyst to Boost Hydrogen Evolution. Angew. Chem. Int. Ed. 2018, 57, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Marszewski, M.; Cao, S.; Yu, J.; Jaroniec, M. Semiconductor-Based Photocatalytic CO2 Conversion. Mater. Horiz. 2015, 2, 261–278. [Google Scholar] [CrossRef]
- Shen, J.; Kolb, M.J.; Göttle, A.J.; Koper, M.T.M. DFT Study on the Mechanism of the Electrochemical Reduction of CO2 Catalyzed by Cobalt Porphyrins. J. Phys. Chem. C 2016, 120, 15714–15721. [Google Scholar] [CrossRef]
- Qin, B.; Zhang, Q.; Li, Y.; Yang, G.; Yu, H.; Peng, F. Mechanistic Insights into the Electrochemical Reduction of CO2 on Cyclocarbon Using Density Functional Theory Calculations. ChemElectroChem 2020, 7, 1838–1842. [Google Scholar] [CrossRef]
- Vijay, S.; Gauthier, J.A.; Heenen, H.H.; Bukas, V.J.; Kristoffersen, H.H.; Chan, K. Dipole-Field Interactions Determine the CO2 Reduction Activity of 2D Fe–N–C Single-Atom Catalysts. ACS Catal. 2020, 10, 7826–7835. [Google Scholar] [CrossRef]
- Hong, Z.; Chong, W.K.; Ng, A.Y.R.; Li, M.; Ganguly, R.; Sum, T.C.; Soo, H.S. Hydrophobic Metal Halide Perovskites for Visible-Light Photoredox C-C Bond Cleavage and Dehydrogenation Catalysis. Angew. Chem. Int. Ed. 2019, 58, 3456–3460. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, H.; Zhao, J.; Solís-Fernández, G.; Zhou, C.; Seo, J.W.; Hendrix, J.; Debroye, E.; Steele, J.A.; Hofkens, J.; et al. C(sp3)–H Bond Activation by Perovskite Solar Photocatalyst Cell. ACS Energy Lett. 2019, 4, 203–208. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, G.; Wang, G.; Irvine, J.T.S. Synthesis and Applications of Nanoporous Perovskite Metal Oxides. Chem. Sci. 2018, 9, 3623–3637. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Yang, T.; Rui, Z.; Ji, H. Perovskite-Based Photocatalysts for Organic Contaminants Removal: Current Status and Future Perspectives. Catal. Today 2019, 327, 47–63. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Protesescu, L.; Bodnarchuk, M.I. Properties and Potential Optoelectronic Applications of Lead Halide Perovskite Nanocrystals. Science 2017, 358, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Babayigit, A.; Ethirajan, A.; Muller, M.; Conings, B. Toxicity of Organometal Halide Perovskite Solar Cells. Nat. Mater. 2016, 15, 247–251. [Google Scholar] [CrossRef]
- Babayigit, A.; Thanh, D.D.; Ethirajan, A.; Manca, J.; Muller, M.; Boyen, H.G.; Conings, B. Assessing the Toxicity of Pb- and Sn-Based Perovskite Solar Cells in Model OrganismDanio Rerio. Sci. Rep. 2016, 6, 18721. [Google Scholar] [CrossRef]
- Feng, J.; Xiao, B. Effective Masses and Electronic and Optical Properties of Nontoxic MASnX3 (X = Cl, Br, and I) Perovskite Structures As Solar Cell Absorber: A Theoretical Study Using HSE06. J. Phys. Chem. C 2014, 118, 19655–19660. [Google Scholar] [CrossRef]
- Hsu, H.-Y.; Ji, L.; Du, M.; Zhao, J.; Yu, E.T.; Bard, A.J. Optimization of Lead-Free Organic–inorganic Tin(II) Halide Perovskite Semiconductors by Scanning Electrochemical Microscopy. Electrochim. Acta 2016, 220, 205–210. [Google Scholar] [CrossRef]
- Cao, G. Lead-Free Organic-Inorganic Halide Perovskites Grown With Nontoxic Solvents. Sci. Bull. 2017, 62, 901–902. [Google Scholar] [CrossRef]
- Bi, Z.; Rodríguez-Martínez, X.; Aranda, C.; Pascual-San-José, E.; Goñi, A.R.; Campoy-Quiles, M.; Xu, X.; Guerrero, A. Defect Tolerant Perovskite Solar Cells from Blade Coated Non-Toxic Solvents. J. Mater. Chem. A 2018, 6, 19085–19093. [Google Scholar] [CrossRef]
- Ju, M.-G.; Chen, M.; Zhou, Y.; Garces, H.F.; Dai, J.; Ma, L.; Padture, N.P.; Zeng, X.C. Earth-Abundant Nontoxic Titanium(IV)-Based Vacancy-Ordered Double Perovskite Halides With Tunable 1.0 to 1.8 EV Bandgaps for Photovoltaic Applications. ACS Energy Lett. 2018, 3, 297–304. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Shao, Z. Double Perovskites in Catalysis, Electrocatalysis, and Photo(electro)catalysis. Trends Chem. 2019, 1, 410–424. [Google Scholar] [CrossRef]
- Slavney, A.H.; Hu, T.; Lindenberg, A.M.; Karunadasa, H.I. A Bismuth-Halide Double Perovskite With Long Carrier Recombination Lifetime for Photovoltaic Applications. J. Am. Chem. Soc. 2016, 138, 2138–2141. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, Y.-F.; Chen, B.-X.; Kuang, D.-B.; Su, C.-Y. Synthesis and Photocatalytic Application of Stable Lead-Free Cs2 AgBiBr6 Perovskite Nanocrystals. Small 2018, 14, e1703762. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Ong, W.; Shi, Z.; Zhao, X.; Li, N. Pb-Based Halide Perovskites: Recent Advances in Photo(electro)catalytic Applications and Looking Beyond. Adv. Funct. Mater. 2020, 30, 1909667. [Google Scholar] [CrossRef]
- Greul, E.; Petrus, M.L.; Binek, A.; Docampo, P.; Bein, T. Highly Stable, Phase Pure Cs2AgBiBr6 Double Perovskite Thin Films for Optoelectronic Applications. J. Mater. Chem. A 2017, 5, 19972–19981. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, P.; Wei, S.-H. Band Structure Engineering of Cs2AgBiBr6 Perovskite through Order–Disordered Transition: A First-Principle Study. J. Phys. Chem. Lett. 2018, 9, 31–35. [Google Scholar] [CrossRef]
- Xiao, Z.; Meng, W.; Wang, J.; Mitzi, D.B.; Yan, Y. Searching for Promising New Perovskite-Based Photovoltaic Absorbers: The Importance of Electronic Dimensionality. Mater. Horiz. 2016, 4, 206–216. [Google Scholar] [CrossRef]
- Li, L. Probe for Electronic Dimensionality. Nat. Phys. 2010, 6, 7–8. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Engel, E.; Chevary, J.A.; Macdonald, L.D.; Vosko, S.H. Asymptotic Properties of the Exchange Energy Density and the Exchange Potential of Finite Systems: Relevance for Generalized Gradient Approximations. Eur. Phys. J. D 1992, 23, 7–14. [Google Scholar] [CrossRef]
- Azofra, L.M.; Li, N.; Macfarlane, D.R.; Sun, C. Promising Prospects for 2D d2–d4M3C2transition Metal Carbides (MXenes) in N2capture and Conversion into Ammonia. Energy Environ. Sci. 2016, 9, 2545–2549. [Google Scholar] [CrossRef]
- Li, T.; Zhao, X.; Yang, D.; Du, M.-H.; Zhang, L. Intrinsic Defect Properties in Halide Double Perovskites for Optoelectronic Applications. Phys. Rev. Appl. 2018, 10, 041001. [Google Scholar] [CrossRef]
- Vasala, S.; Karppinen, M. A2B′B″O6 Perovskites: A Review. Prog. Solid State Chem. 2015, 43, 1–36. [Google Scholar] [CrossRef]
- Volonakis, G.; Filip, M.R.; Haghighirad, A.A.; Sakai, N.; Wenger, B.; Snaith, H.J.; Giustino, F. Lead-Free Halide Double Perovskites via Heterovalent Substitution of Noble Metals. J. Phys. Chem. Lett. 2016, 7, 1254–1259. [Google Scholar] [CrossRef]
- Tang, C.; Chen, C.; Xu, W.; Xu, L. Design of Doped Cesium Lead Halide Perovskite as a Photo-Catalytic CO2 Reduction Catalyst. J. Mater. Chem. A 2019, 7, 6911–6919. [Google Scholar] [CrossRef]
- Zhao, X.-G.; Yang, D.; Ren, J.-C.; Sun, Y.; Xiao, Z.; Zhang, L. Rational Design of Halide Double Perovskites for Optoelectronic Applications. Joule 2018, 2, 1662–1673. [Google Scholar] [CrossRef]
- McClure, E.T.; Ball, M.R.; Windl, W.; Woodward, P.M. Cs2AgBiX6 (X = Br, Cl): New Visible Light Absorbing, Lead-Free Halide Perovskite Semiconductors. Chem. Mater. 2016, 28, 1348–1354. [Google Scholar] [CrossRef]
- Meng, W.; Wang, X.; Xiao, Z.; Wang, J.; Mitzi, D.B.; Yan, Y. Parity-Forbidden Transitions and Their Impact on the Optical Absorption Properties of Lead-Free Metal Halide Perovskites and Double Perovskites. J. Phys. Chem. Lett. 2017, 8, 2999–3007. [Google Scholar] [CrossRef]
- Xiao, Z.; Lei, H.; Zhang, X.; Zhou, Y.; Hosono, H.; Kamiya, T. Ligand-Hole in [SnI6] Unit and Origin of Band Gap in Photovoltaic Perovskite Variant Cs2SnI6. Bull. Chem. Soc. Jpn. 2015, 88, 1250–1255. [Google Scholar] [CrossRef]
- Saparov, B.; Sun, J.-P.; Meng, W.; Xiao, Z.; Duan, H.-S.; Gunawan, O.; Shin, D.; Hill, I.G.; Yan, Y.; Mitzi, D.B. Thin-Film Deposition and Characterization of a Sn-Deficient Perovskite Derivative Cs2SnI6. Chem. Mater. 2016, 28, 2315–2322. [Google Scholar] [CrossRef]
- Wang, M.; Zeng, P.; Wang, Z.; Liu, M. Vapor-Deposited Cs 2 AgBiCl 6 Double Perovskite Films Toward Highly Selective and Stable Ultraviolet Photodetector. Adv. Sci. 2020, 7, 1903662. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.; Liu, P.; Xiang, H.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Simultaneous Power Conversion Efficiency and Stability Enhancement of Cs 2 AgBiBr 6 Lead-Free Inorganic Perovskite Solar Cell through Adopting a Multifunctional Dye Interlayer. Adv. Funct. Mater. 2020, 30, 2001557. [Google Scholar] [CrossRef]
- Luo, J.; Li, S.; Wu, H.; Zhou, Y.; Li, Y.; Liu, J.; Li, J.; Li, K.; Yi, F.; Niu, G.; et al. Cs2AgInCl6 Double Perovskite Single Crystals: Parity Forbidden Transitions and Their Application For Sensitive and Fast UV Photodetectors. ACS Photon. 2018, 5, 398–405. [Google Scholar] [CrossRef]
- Liu, F.; Marongiu, D.; Pau, R.; Sarritzu, V.; Wang, Q.; Lai, S.; Lehmann, A.G.; Quochi, F.; Saba, M.; Mura, A.; et al. Ag/In Lead-Free Double Perovskites. EcoMat 2020, 2, e12017. [Google Scholar] [CrossRef]
- Lee, B.; Stoumpos, C.C.; Zhou, N.J.; Hao, F.; Malliakas, C.; Yeh, C.Y.; Marks, T.J.; Kanatzidis, M.G.; Chang, R.P.H. Air-Stable Molecular Semiconducting Lodosalts for Solar Cell Applications: Cs(2)Snl(6) As a Hole Conductor. J. Am. Chem. Soc. 2014, 136, 15379–15385. [Google Scholar] [CrossRef]
- Wang, A.; Yan, X.; Zhang, M.; Sun, S.; Yang, M.; Shen, W.; Pan, X.; Wang, P.; Deng, Z. Controlled Synthesis of Lead-Free and Stable Perovskite Derivative Cs2SnI6 Nanocrystals via a Facile Hot-Injection Process. Chem. Mater. 2016, 28, 8132–8140. [Google Scholar] [CrossRef]
- Chen, M.; Ju, M.-G.; Carl, A.D.; Zong, Y.; Grimm, R.L.; Gu, J.; Zeng, X.C.; Zhou, Y.; Padture, N.P. Cesium Titanium(IV) Bromide Thin Films Based Stable Lead-Free Perovskite Solar Cells. Joule 2018, 2, 558–570. [Google Scholar] [CrossRef]
- Gou, G.; Young, J.; Liu, X.; Rondinelli, J.M. Interplay of Cation Ordering and Ferroelectricity in Perovskite Tin Iodides: Designing a Polar Halide Perovskite for Photovoltaic Applications. Inorg. Chem. 2016, 56, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Deng, Z.; Sun, S.; Zhang, F.; Evans, D.M.; Kieslich, G.; Tominaka, S.; Carpenter, M.A.; Zhang, J.; Bristowe, P.D.; et al. Synthesis and Properties of a Lead-Free Hybrid Double Perovskite: (CH3NH3)2AgBiBr6. Chem. Mater. 2017, 29, 1089–1094. [Google Scholar] [CrossRef]
- Wei, F.; Deng, Z.; Sun, S.; Xie, F.; Kieslich, G.; Evans, D.M.; Carpenter, M.A.; Bristowe, P.D.; Cheetham, A.K. The Synthesis, Structure and Electronic Properties of a Lead-Free Hybrid inorganic–organic Double Perovskite (MA)2KBiCl6 (MA = Methylammonium). Mater. Horiz. 2016, 3, 328–332. [Google Scholar] [CrossRef]
- Haruyama, J.; Sodeyama, K.; Han, L.; Tateyama, Y. Termination Dependence of Tetragonal CH3NH3PbI3 Surfaces for Perovskite Solar Cells. J. Phys. Chem. Lett. 2014, 5, 2903–2909. [Google Scholar] [CrossRef]
- Volonakis, G.; Giustino, F. Ferroelectric Graphene–Perovskite Interfaces. J. Phys. Chem. Lett. 2015, 6, 2496–2502. [Google Scholar] [CrossRef]
- Volonakis, G.; Giustino, F. Surface Properties of Lead-Free Halide Double Perovskites: Possible Visible-Light Photo-Catalysts for Water Splitting. Appl. Phys. Lett. 2018, 112, 243901. [Google Scholar] [CrossRef]
- Živković, A.; Somers, M.; Camprubi, E.; King, H.; Wolthers, M.; de Leeuw, N. Changes in CO2 Adsorption Affinity Related to Ni Doping in FeS Surfaces: A DFT-D3 Study. Catalysts 2021, 11, 486. [Google Scholar] [CrossRef]
- Xie, C.; Zhu, B.; Sun, Y.; Song, W.; Xu, M. Effect of Doping Cr on NH3 Adsorption and NO Oxidation over the FexOy/AC Surface: A DFT-D Study. J. Hazard. Mater. 2021, 416, 125798. [Google Scholar] [CrossRef]
- Ortiz-Medina, J.; Wang, Z.; Cruz-Silva, R.; Morelos-Gomez, A.; Wang, F.; Yao, X.; Terrones, M.; Endo, M. Defect Engineering and Surface Functionalization of Nanocarbons for Metal-Free Catalysis. Adv. Mater. 2019, 31, e1805717. [Google Scholar] [CrossRef]
- Wang, S.; Jin, S.; Yang, S.; Chen, S.; Song, Y.; Zhang, J.; Zhu, M. Total Structure Determination of Surface Doping Nanocluster and Its Structure-Related Catalytic Property. Sci. Adv. 2015, 1, e1500441. [Google Scholar] [CrossRef]
- Mansor, M.; Winkler, C.; Hochella, M.F.J.; Xu, J. Nanoparticulate Nickel-Hosting Phases in Sulfidic Environments: Effects of Ferrous Iron and Bacterial Presence on Mineral Formation Mechanism and Solid-Phase Nickel Distribution. Front. Earth Sci. 2019, 7, 151. [Google Scholar] [CrossRef]
- Brinck, S.T.; Zaccaria, F.; Infante, I. Defects in Lead Halide Perovskite Nanocrystals: Analogies and (Many) Differences with the Bulk. ACS Energy Lett. 2019, 4, 2739–2747. [Google Scholar] [CrossRef]
- Emery, A.A.; Wolverton, C. High-Throughput DFT Calculations of Formation Energy, Stability and Oxygen Vacancy Formation Energy of ABO3 Perovskites. Sci. Data 2017, 4, 170153. [Google Scholar] [CrossRef]
- Curnan, M.T.; Kitchin, J.R. Effects of Concentration, Crystal Structure, Magnetism, and Electronic Structure Method on First-Principles Oxygen Vacancy Formation Energy Trends in Perovskites. J. Phys. Chem. C 2014, 118, 28776–28790. [Google Scholar] [CrossRef]
- Gergen, B.; Nienhaus, H.; Weinberg, W.H.; McFarland, E.W. Chemically Induced Electronic Excitations at Metal Surfaces. Science 2001, 294, 2521–2523. [Google Scholar] [CrossRef]
- Foster, A.S.; Gejo, F.L.; Shluger, A.L.; Nieminen, R.M. Vacancy and Interstitial Defects in Hafnia. Phys. Rev. B 2002, 65, 174117. [Google Scholar] [CrossRef]
- Dong, C.; Fu, J.; Liu, H.; Ling, T.; Yang, J.; Qiao, S.Z.; Du, X.-W. Tuning the Selectivity and Activity of Au Catalysts for Carbon Dioxide Electroreduction via Grain Boundary Engineering: A DFT Study. J. Mater. Chem. A 2017, 5, 7184–7190. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, Y.; Yan, L.; Su, Z. DFT Study on Sulfur-Doped G-C3N4 Nanosheets as a Photocatalyst for CO2 Reduction Reaction. J. Phys. Chem. C 2018, 122, 7712–7719. [Google Scholar] [CrossRef]
- Chrétien, S.; Metiu, H. DFT Study of the Electronic Properties of LaOCl Surfaces. J. Phys. Chem. C 2011, 116, 681–691. [Google Scholar] [CrossRef]
- Heydari, H.; Elahifard, M.; Behjatmanesh-Ardakani, R. Role of Oxygen Vacancy in the Adsorption and Dissociation of the Water Molecule on the Surfaces of Pure and Ni-Doped Rutile (110): A Periodic Full-Potential DFT Study. Surf. Sci. 2019, 679, 218–224. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, L.; Lan, C.; Pandey, S.S.; Hayase, S.; Ma, T. Oxygen Vacancy Formation and Migration in Double Perovskite Sr2CrMoO6: A First-Principles Study. RSC Adv. 2016, 6, 43034–43040. [Google Scholar] [CrossRef]
- Liu, Y.; Palotas, K.; Yuan, X.; Hou, T.; Lin, H.; Li, Y.; Lee, S.-T. Atomistic Origins of Surface Defects in CH3NH3PbBr3 Perovskite and Their Electronic Structures. ACS Nano 2017, 11, 2060–2065. [Google Scholar] [CrossRef] [PubMed]
| Perovskites | Space Group | Lattice Parameters (Å) | Band Gap (eV) | Ref. | |
|---|---|---|---|---|---|
| Theory | Experiment | ||||
| Cs2AgBiCl6 | Fm3-m | 10.51 (This work) | 2.59 (This work) | 2.41 (Ref. [55]) | |
| Cs2AgBiBr6 | Fm3-m | 11.48 (This work) | 1.92 (This work) | 2.02 (Ref. [56]) | |
| Cs2AgInCl6 | Fm3-m | 10.53 (This work) | 2.41 (This work) | 2.1 (Ref. [57]) | |
| Cs2AgInBr6 | Fm3-m | 10.12 (This work) | 1.30 (This work) | 1.17 (Ref. [58]) | |
| Cs2SnI6 | Fm3-m | 11.6276 | 1.3 | 1.26 | [59] |
| 11.6276 | 1.6 | 1.62 | [54] | ||
| 11.65 | - | - | [60] | ||
| Cs2TiBr6 Cs2TiBr6(@C60) | Fm3-m | 10.92 | 0.89 1.01 | - - | [61] |
| Cs2TiI6 | Fm3-m | 11.67 | 0.79 | - | [32] |
| CsRbSnI6 | Pmn21 | a = 8.2608 b = 12.1507 c = 8.7913 | 1.58 | - | [62] |
| (CH3NH3)2AgBiBr6 | Fm3m | 11.6370 | 2.02 | 2.02 | [63] |
| (CH3NH3)2KBiCl6 | R3m | a = 7.8372 c =20.9938 | 3.02 | 3.04 | [64] |
| Pervskite | Gas | Eb (eV) | Bond Length of Gas Molecular (Å) | Bond Angle of Gas Molecular (º) | ||
|---|---|---|---|---|---|---|
| Original | Adsorbed | Original | Adsorbed | |||
| Pure | NO | −0.33 | 1.15 | 1.17 | / | / |
| NO2 | −0.01 | 1.20 | 1.27, 1.22 | 134.3 | 127.47 | |
| CO | −0.13 | 1.13 | 1.13 | / | / | |
| CO2 | −0.38 | 1.16 | 1.17, 1.17 | 180.0 | 176.75 | |
| Cl doped | NO | −0.28 | 1.15 | 1.16 | / | / |
| NO2 | −0.12 | 1.20 | 1.27, 1.22 | 134.3 | 127.47 | |
| CO | −0.27 | 1.13 | 1.14 | / | / | |
| CO2 | −0.46 | 1.16 | 1.17, 1.17 | 180.0 | 179.55 | |
| I doped | NO | 0.027 | 1.15 | 1.17 | / | / |
| NO2 | −0.18 | 1.20 | 1.23, 1.23 | 134.3 | 126.63 | |
| CO | 0.12 | 1.13 | 1.14 | / | / | |
| CO2 | −0.26 | 1.16 | 1.17, 1.17 | 180.0 | ||
| Br vacancy | NO | −1.00 | 1.15 | 1.24 | / | / |
| NO2 | −1.11 | 1.20 | 1.19, 1.15 | 134.3 | 149.71 | |
| CO | −0.77 | 1.13 | 1.14 | / | / | |
| CO2 | −1.12 | 1.16 | 1.28, 1.24 | 180.0 | 143.15 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Huang, Y.; Shi, Z.; Chen, X.; Li, N. Improving the Catalytic CO2 Reduction on Cs2AgBiBr6 by Halide Defect Engineering: A DFT Study. Materials 2021, 14, 2469. https://doi.org/10.3390/ma14102469
Chen P, Huang Y, Shi Z, Chen X, Li N. Improving the Catalytic CO2 Reduction on Cs2AgBiBr6 by Halide Defect Engineering: A DFT Study. Materials. 2021; 14(10):2469. https://doi.org/10.3390/ma14102469
Chicago/Turabian StyleChen, Pengfei, Yiao Huang, Zuhao Shi, Xingzhu Chen, and Neng Li. 2021. "Improving the Catalytic CO2 Reduction on Cs2AgBiBr6 by Halide Defect Engineering: A DFT Study" Materials 14, no. 10: 2469. https://doi.org/10.3390/ma14102469
APA StyleChen, P., Huang, Y., Shi, Z., Chen, X., & Li, N. (2021). Improving the Catalytic CO2 Reduction on Cs2AgBiBr6 by Halide Defect Engineering: A DFT Study. Materials, 14(10), 2469. https://doi.org/10.3390/ma14102469







