Buccal Peri-Implant Soft Tissue Augmentation by Means of a Porcine Collagen Matrix: A Proof of Concept Technical Note
Abstract
1. Introduction
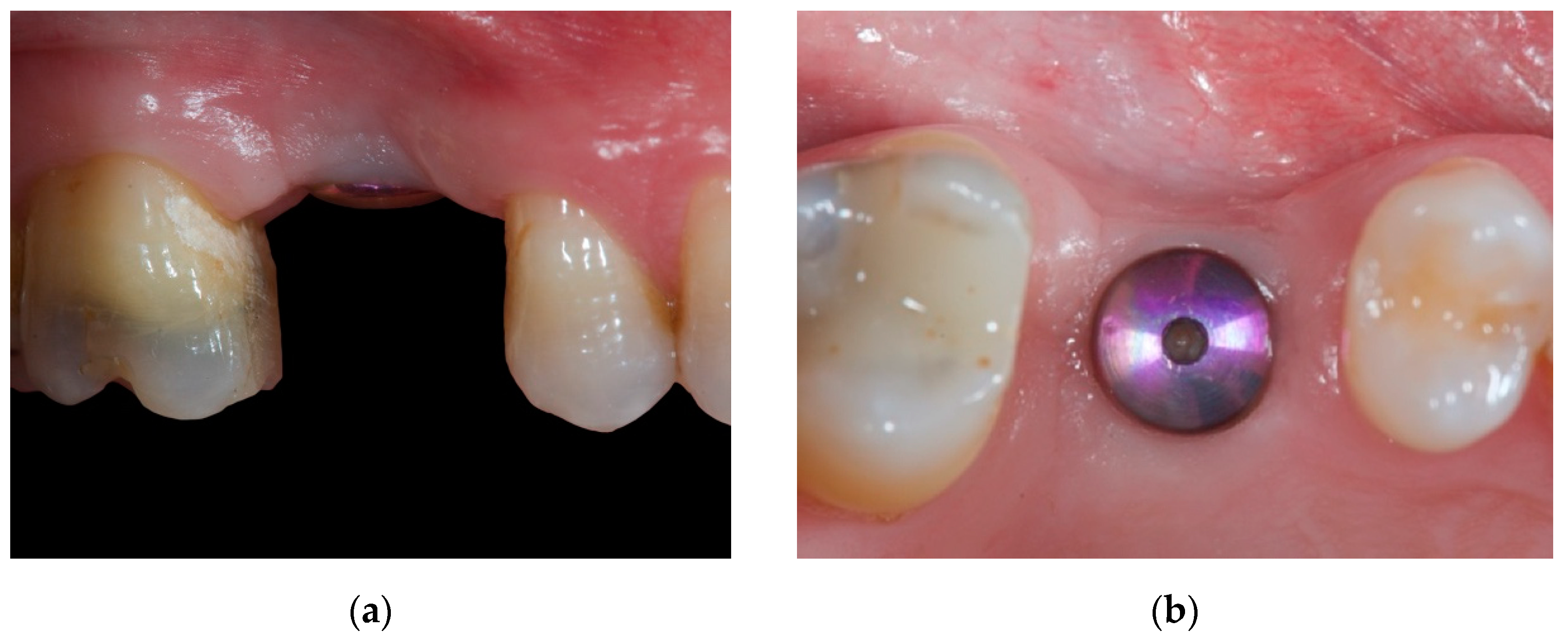
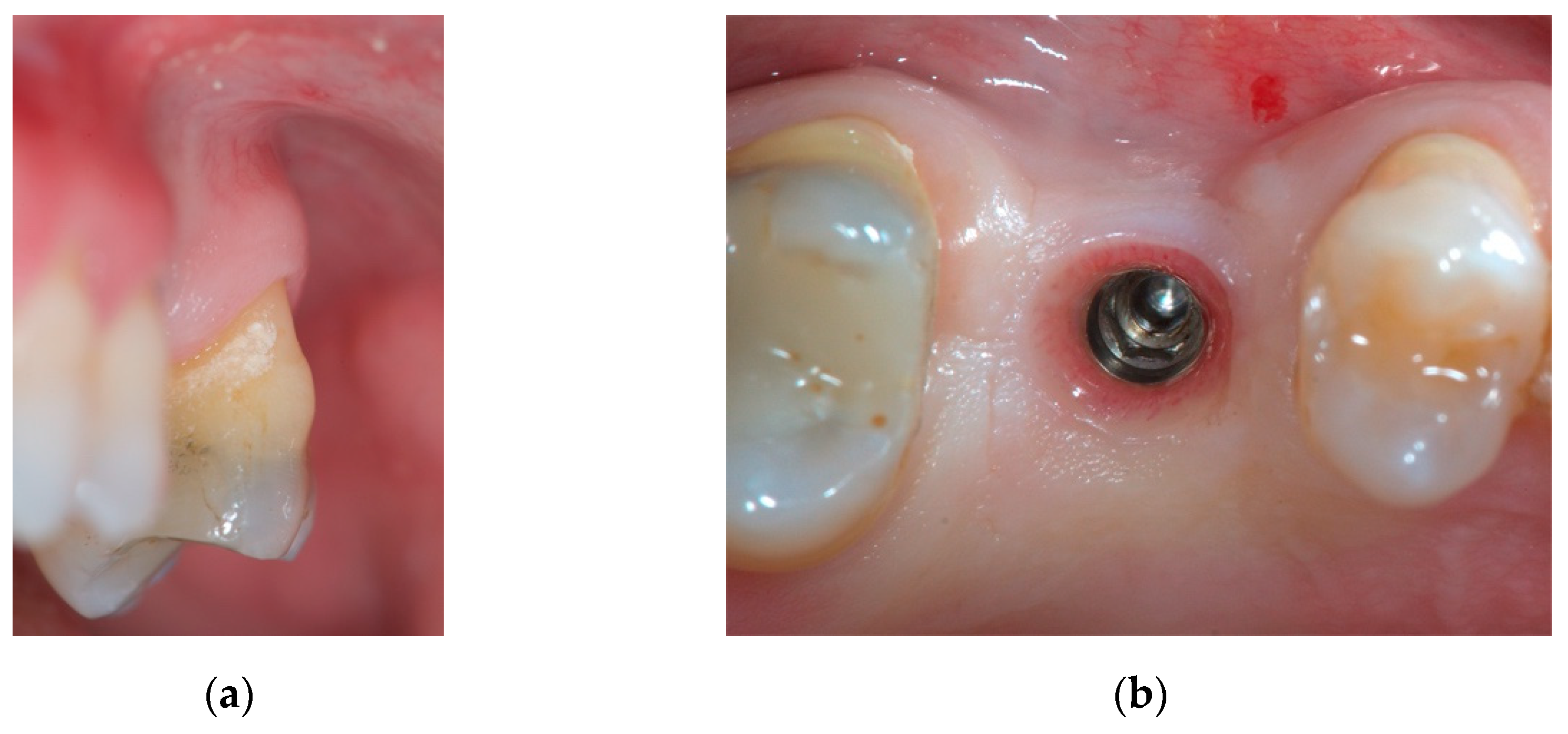
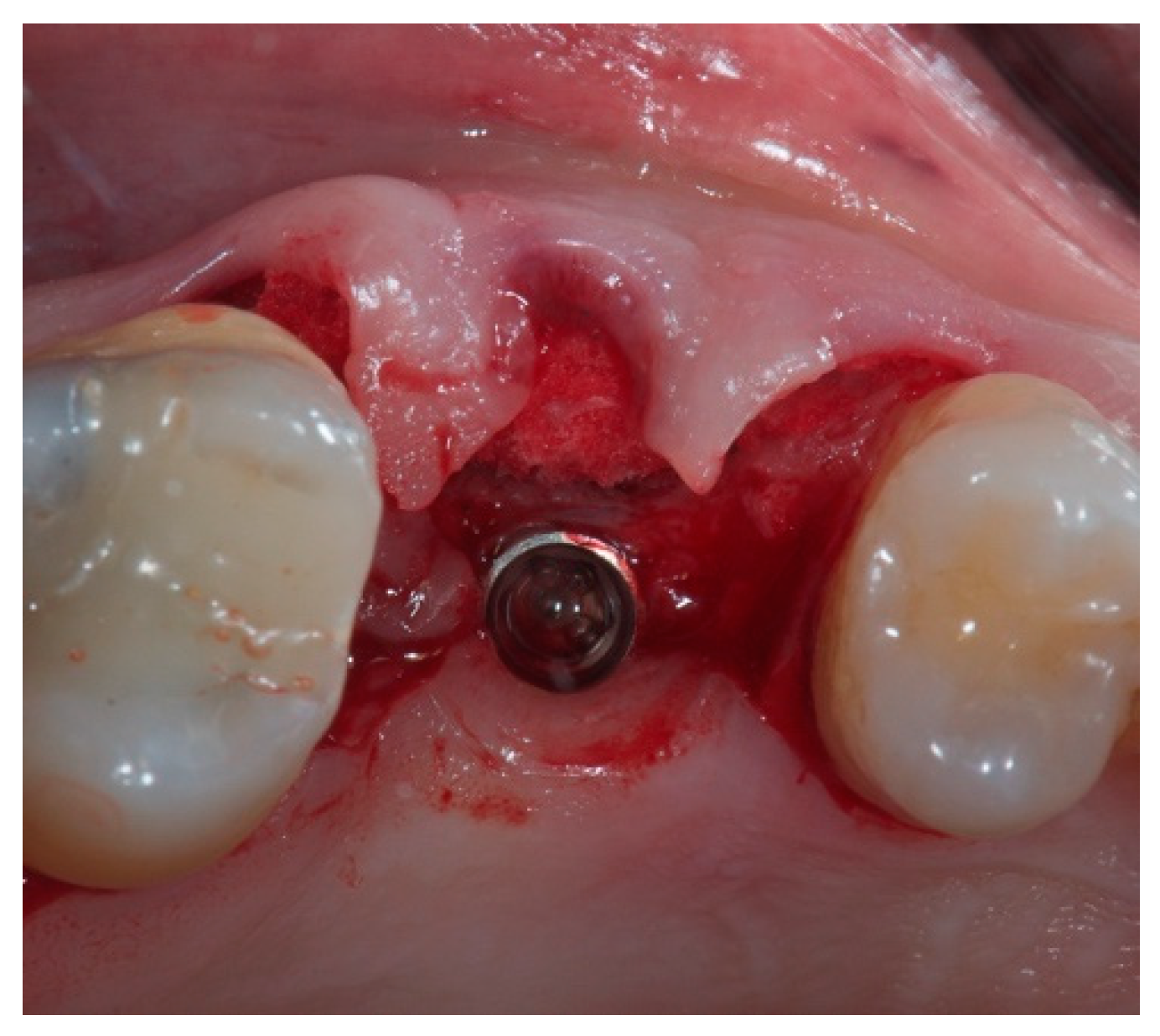
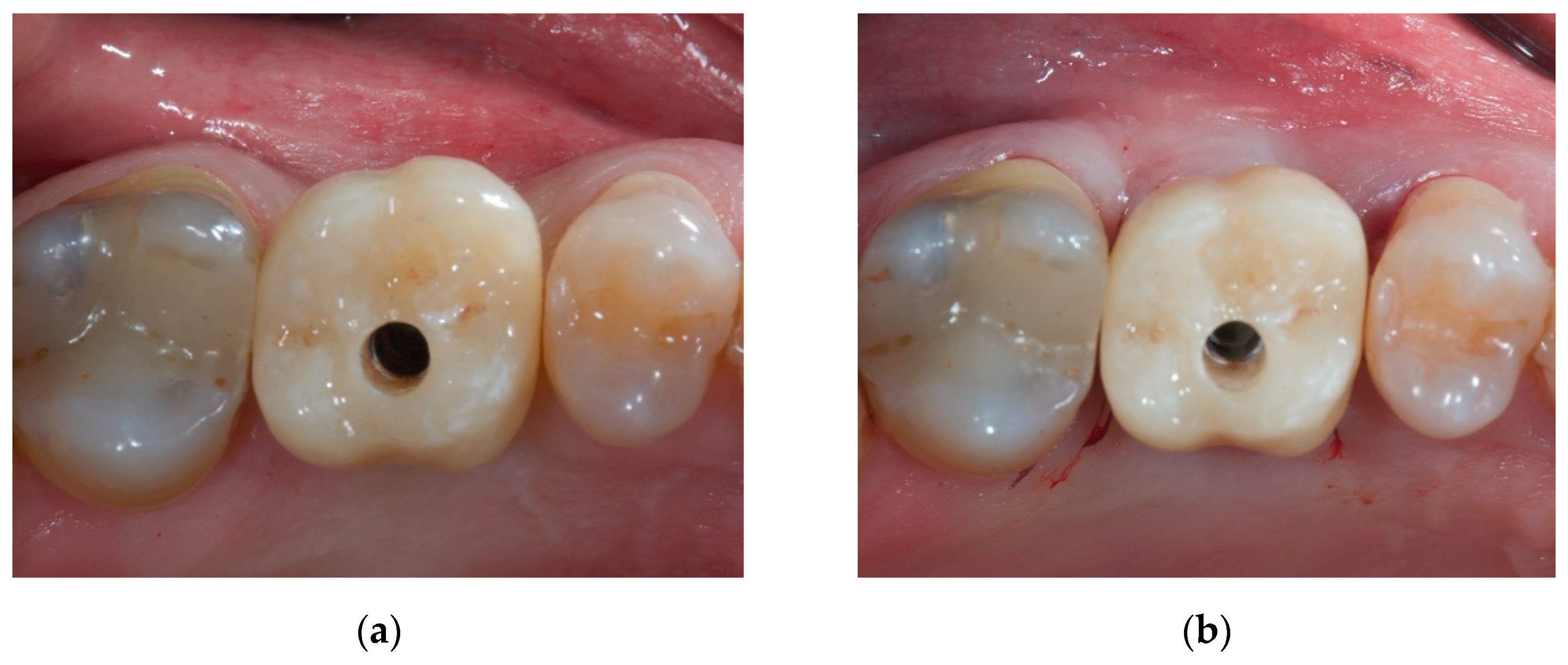
2. Case Report
2.1. Material
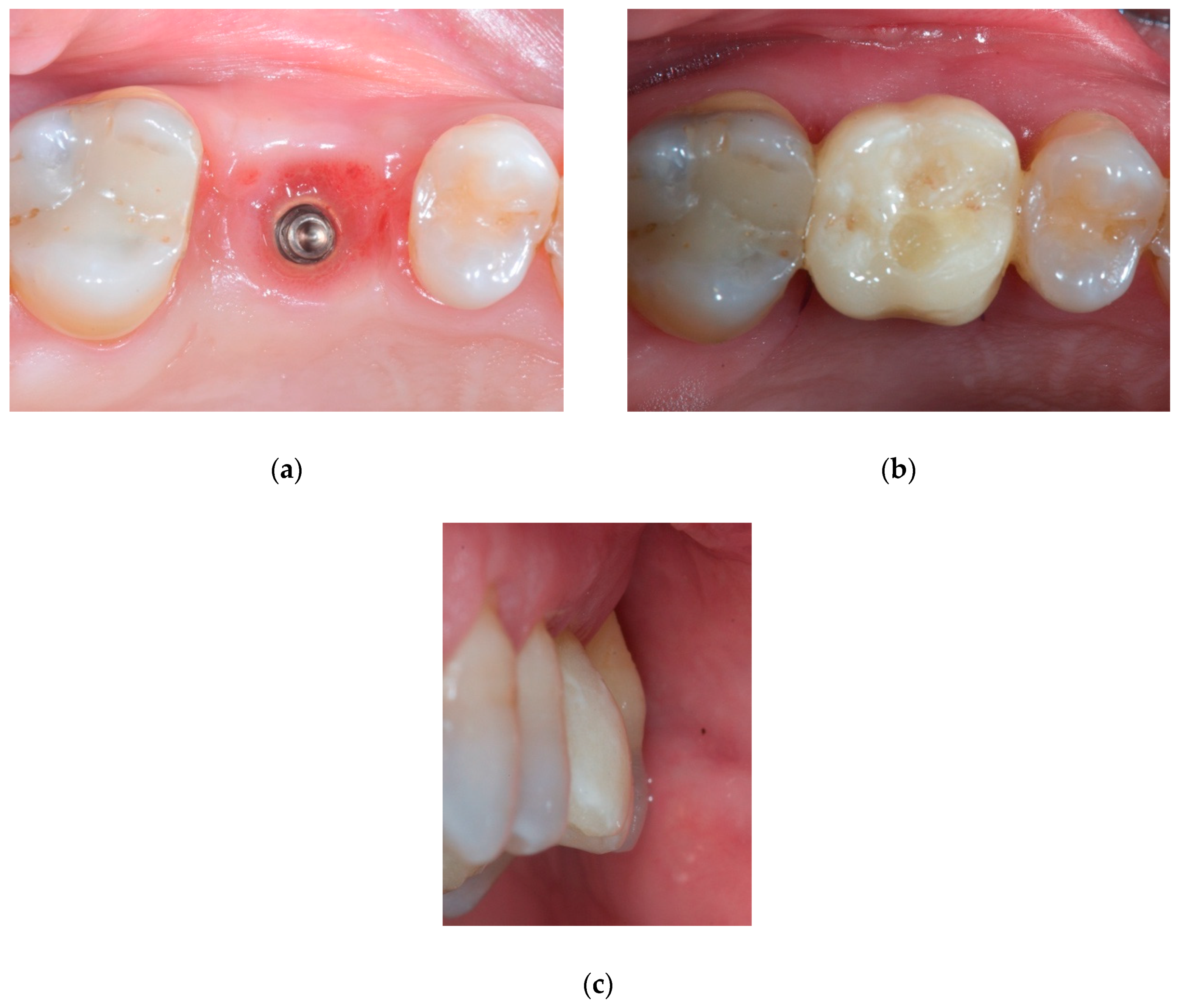
2.2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Warrer, K.; Buser, D.; Lang, N.P.; Karring, T. Plaque-induced peri-implantitis in the presence or absence of keratinized mucosa. An experimental study in monkeys. Clin. Oral Implant. Res. 1995, 6, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Mühlemann, S.; Jung, R.E. Critical soft-tissue dimensions with dental implants and treatment concepts. Periodontol. 2000 2014, 66, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Poli, P.P.; Maridati, P.C.; Stoffella, E.; Beretta, M.; Maiorana, C. Influence of Timing on the Horizontal Stability of Connective Tissue Grafts for Buccal Soft Tissue Augmentation at Single Implants: A Prospective Controlled Pilot Study. J. Oral Maxillofac. Surg. 2019, 77, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- van Brakel, R.; Noordmans, H.J.; Frenken, J.; de Roode, R.; de Wit, G.C.; Cune, M.S. The effect of zirconia and titanium implant abutments on light reflection of the supporting soft tissues. Clin. Oral Implant. Res. 2011, 22, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Sailer, I.; Hämmerle, C.H.; Attin, T.; Schmidlin, P. In vitro color changes of soft tissues caused by restorative materials. Int. J. Periodontics Restor. Dent. 2007, 27, 251–257. [Google Scholar]
- Thoma, D.S.; Ioannidis, A.; Cathomen, E.; Hämmerle, C.H.; Hüsler, J.; Jung, R.E. Discoloration of the Peri-implant Mucosa Caused by Zirconia and Titanium Implants. Int. J. Periodontics Restor. Dent. 2016, 36, 39–45. [Google Scholar] [CrossRef]
- Linkevicius, T.; Puisys, A.; Steigmann, M.; Vindasiute, E.; Linkeviciene, L. Influence of Vertical Soft Tissue Thickness on Crestal Bone Changes Around Implants with Platform Switching: A Comparative Clinical Study. Clin. Implant Dent. Relat. Res. 2015, 17, 1228–1236. [Google Scholar] [CrossRef]
- Schneider, D.; Grunder, U.; Ender, A.; Hämmerle, C.H.; Jung, R.E. Volume gain and stability of peri-implant tissue following bone and soft tissue augmentation: 1-year results from a prospective cohort study. Clin. Oral Implant. Res. 2011, 22, 28–37. [Google Scholar] [CrossRef]
- Ericsson, I.; Persson, L.G.; Berglundh, T.; Marinello, C.P.; Lindhe, J.; Klinge, B. Different types of inflammatory reactions in peri-implant soft tissues. J. Clin. Periodontol. 1995, 22, 255–261. [Google Scholar] [CrossRef]
- Hermann, J.S.; Buser, D.; Schenk, R.K.; Cochran, D.L. Crestal bone changes around titanium implants. A histometric evaluation of unloaded non-submerged and submerged implants in the canine mandible. J. Periodontol. 2000, 71, 1412–1424. [Google Scholar] [CrossRef]
- Caballé-Serrano, J.; Zhang, S.; Ferrantino, L.; Simion, M.; Chappuis, V.; Bosshardt, D.D. Tissue Response to a Porous Collagen Matrix Used for Soft Tissue Augmentation. Materials 2019, 12, 3721. [Google Scholar] [CrossRef] [PubMed]
- Cairo, F.; Barbato, L.; Tonelli, P.; Batalocco, G.; Pagavino, G.; Nieri, M. Xenogeneic collagen matrix versus connective tissue graft for buccal soft tissue augmentation at implant site. A randomized, controlled clinical trial. J. Clin. Periodontol. 2017, 44, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Zucchelli, G.; Mele, M.; Stefanini, M.; Mazzotti, C.; Marzadori, M.; Montebugnoli, L.; de Sanctis, M. Patient morbidity and root coverage outcome after subepithelial connective tissue and de-epithelialized grafts: A comparative randomized-controlled clinical trial. J. Clin. Periodontol. 2010, 37, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; Barootchi, S.; Namazi, S.S.; Chan, H.L.; Brzezinski, D.; Danciu, T.; Wang, H.L. The influence of palatal harvesting technique on the donor site vascular injury: A split-mouth comparative cadaver study. J. Periodontol. 2020, 91, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Griffin, T.J.; Cheung, W.S.; Zavras, A.I.; Damoulis, P.D. Postoperative complications following gingival augmentation procedures. J. Periodontol. 2006, 77, 2070–2079. [Google Scholar] [CrossRef]
- Thoma, D.S.; Zeltner, M.; Hilbe, M.; Hämmerle, C.H.; Hüsler, J.; Jung, R.E. Randomized controlled clinical study evaluating effectiveness and safety of a volume-stable collagen matrix compared to autogenous connective tissue grafts for soft tissue augmentation at implant sites. J. Clin. Periodontol. 2016, 43, 874–885. [Google Scholar] [CrossRef]
- Thoma, D.S.; Naenni, N.; Benic, G.I.; Hämmerle, C.H.; Jung, R.E. Soft tissue volume augmentation at dental implant sites using a volume stable three-dimensional collagen matrix—Histological outcomes of a preclinical study. J. Clin. Periodontol. 2017, 44, 185–194. [Google Scholar] [CrossRef]
- Ferrantino, L.; Bosshardt, D.; Nevins, M.; Santoro, G.; Simion, M.; Kim, D. Tissue Integration of a Volume-Stable Collagen Matrix in an Experimental Soft Tissue Augmentation Model. Int. J. Periodontics Restor. Dent. 2016, 36, 807–815. [Google Scholar] [CrossRef]
- Thoma, D.S.; Gasser, T.J.W.; Jung, R.E.; Hämmerle, C.H.F. Randomized controlled clinical trial comparing implant sites augmented with a volume-stable collagen matrix or an autogenous connective tissue graft: 3-year data after insertion of reconstructions. J. Clin. Periodontol. 2020, 47, 630–639. [Google Scholar] [CrossRef]
- Naenni, N.; Bienz, S.P.; Benic, G.I.; Jung, R.E.; Hämmerle, C.H.F.; Thoma, D.S. Volumetric and linear changes at dental implants following grafting with volume-stable three-dimensional collagen matrices or autogenous connective tissue grafts: 6-month data. Clin. Oral Investig. 2018, 22, 1185–1195. [Google Scholar] [CrossRef]
- Zeltner, M.; Jung, R.E.; Hämmerle, C.H.; Hüsler, J.; Thoma, D.S. Randomized controlled clinical study comparing a volume-stable collagen matrix to autogenous connective tissue grafts for soft tissue augmentation at implant sites: Linear volumetric soft tissue changes up to 3 months. J. Clin. Periodontol. 2017, 44, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Späte, U.; Lee, C.T. Modified Vestibular Incision Subperiosteal Tunnel Access Procedure with Volume-Stable Collagen Matrix for Root Coverage: Report of Three Cases. Int. J. Periodontics Restor. Dent. 2019, 39, e181–e187. [Google Scholar] [CrossRef] [PubMed]
- Farronato, D.; Pasini, P.M.; Manfredini, M.; Scognamiglio, C.; Orsina, A.A.; Farronato, M. Influence of the implant-abutment connection on the ratio between height and thickness of tissues at the buccal zenith: A randomized controlled trial on 188 implants placed in 104 patients. BMC Oral Health 2020, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- Beretta, M.; Poli, P.P.; Pieriboni, S.; Tansella, S.; Manfredini, M.; Cicciù, M.; Maiorana, C. Peri-Implant Soft Tissue Conditioning by Means of Customized Healing Abutment: A Randomized Controlled Clinical Trial. Materials 2019, 12, 3041. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beretta, M.; Maiorana, C.; Manfredini, M.; Ferrario, S.; Poli, P.P. Buccal Peri-Implant Soft Tissue Augmentation by Means of a Porcine Collagen Matrix: A Proof of Concept Technical Note. Materials 2021, 14, 93. https://doi.org/10.3390/ma14010093
Beretta M, Maiorana C, Manfredini M, Ferrario S, Poli PP. Buccal Peri-Implant Soft Tissue Augmentation by Means of a Porcine Collagen Matrix: A Proof of Concept Technical Note. Materials. 2021; 14(1):93. https://doi.org/10.3390/ma14010093
Chicago/Turabian StyleBeretta, Mario, Carlo Maiorana, Mattia Manfredini, Susanna Ferrario, and Pier Paolo Poli. 2021. "Buccal Peri-Implant Soft Tissue Augmentation by Means of a Porcine Collagen Matrix: A Proof of Concept Technical Note" Materials 14, no. 1: 93. https://doi.org/10.3390/ma14010093
APA StyleBeretta, M., Maiorana, C., Manfredini, M., Ferrario, S., & Poli, P. P. (2021). Buccal Peri-Implant Soft Tissue Augmentation by Means of a Porcine Collagen Matrix: A Proof of Concept Technical Note. Materials, 14(1), 93. https://doi.org/10.3390/ma14010093







