Insights on Substitution Preference of Pb Ions in Sulfoaluminate Cement Clinker Phases
Abstract
1. Introduction
2. Simulations and Experiments
2.1. Simulations
2.2. Experiments
3. Results and Discussion
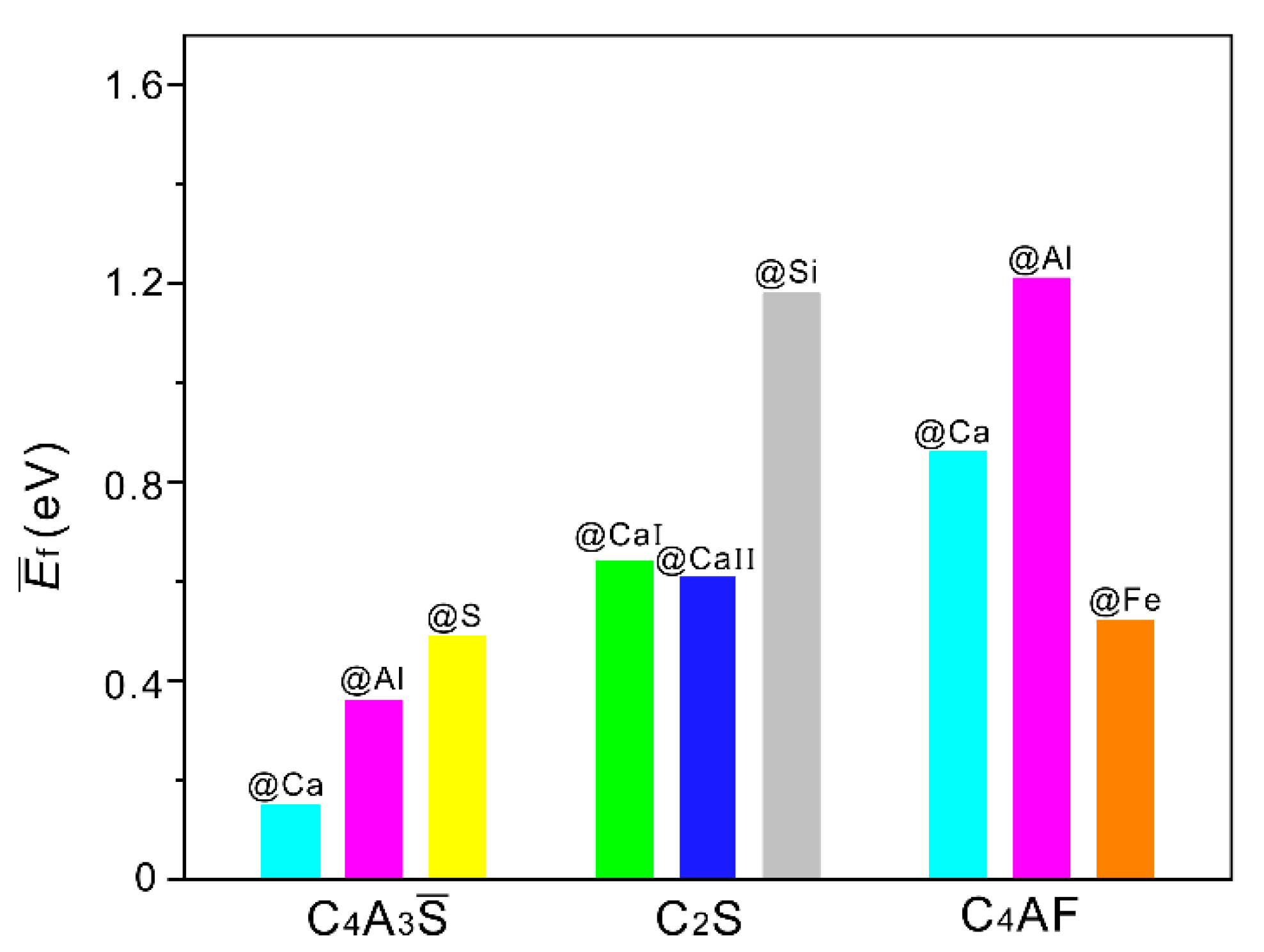
3.1. Defect Formation Energies
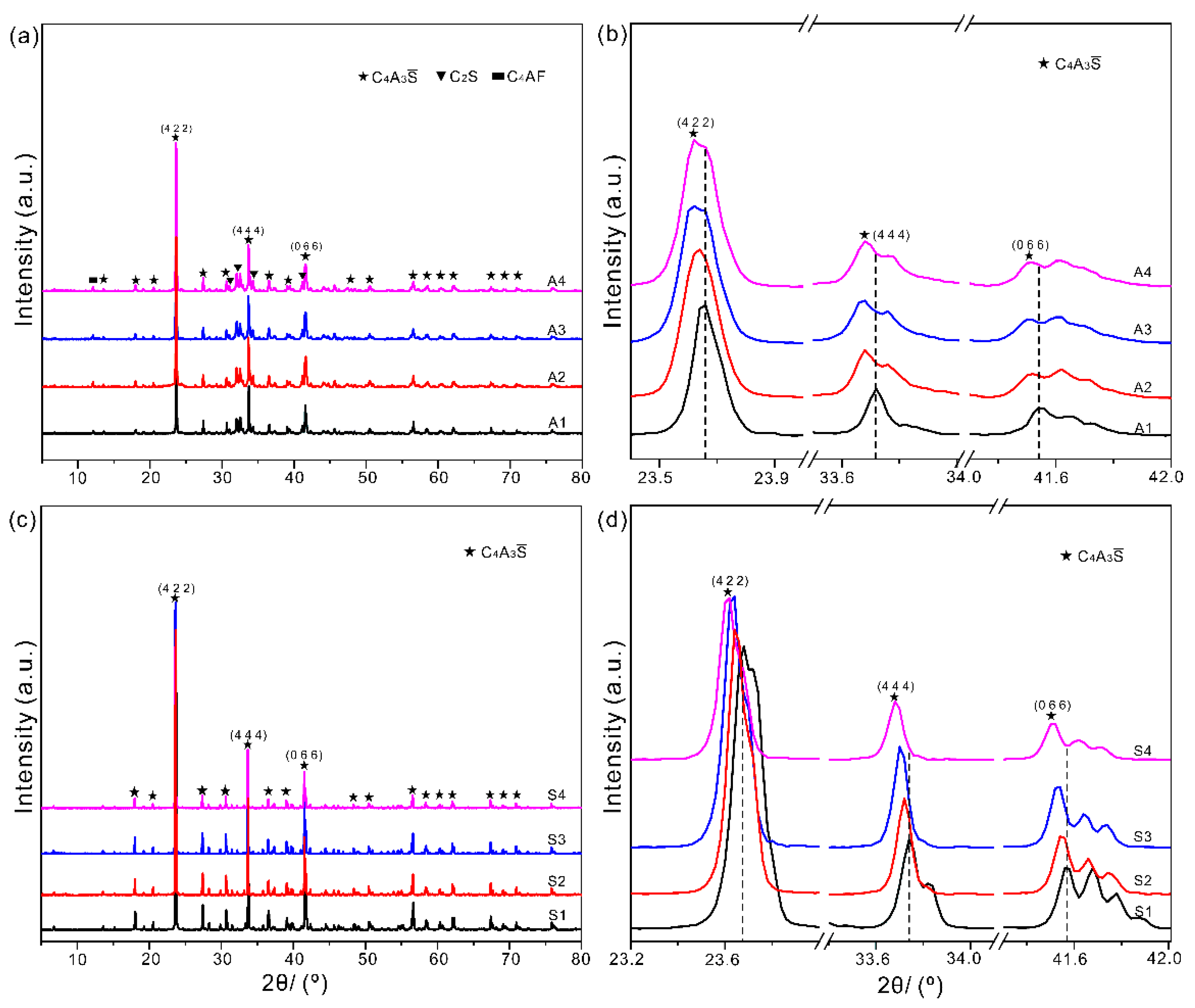
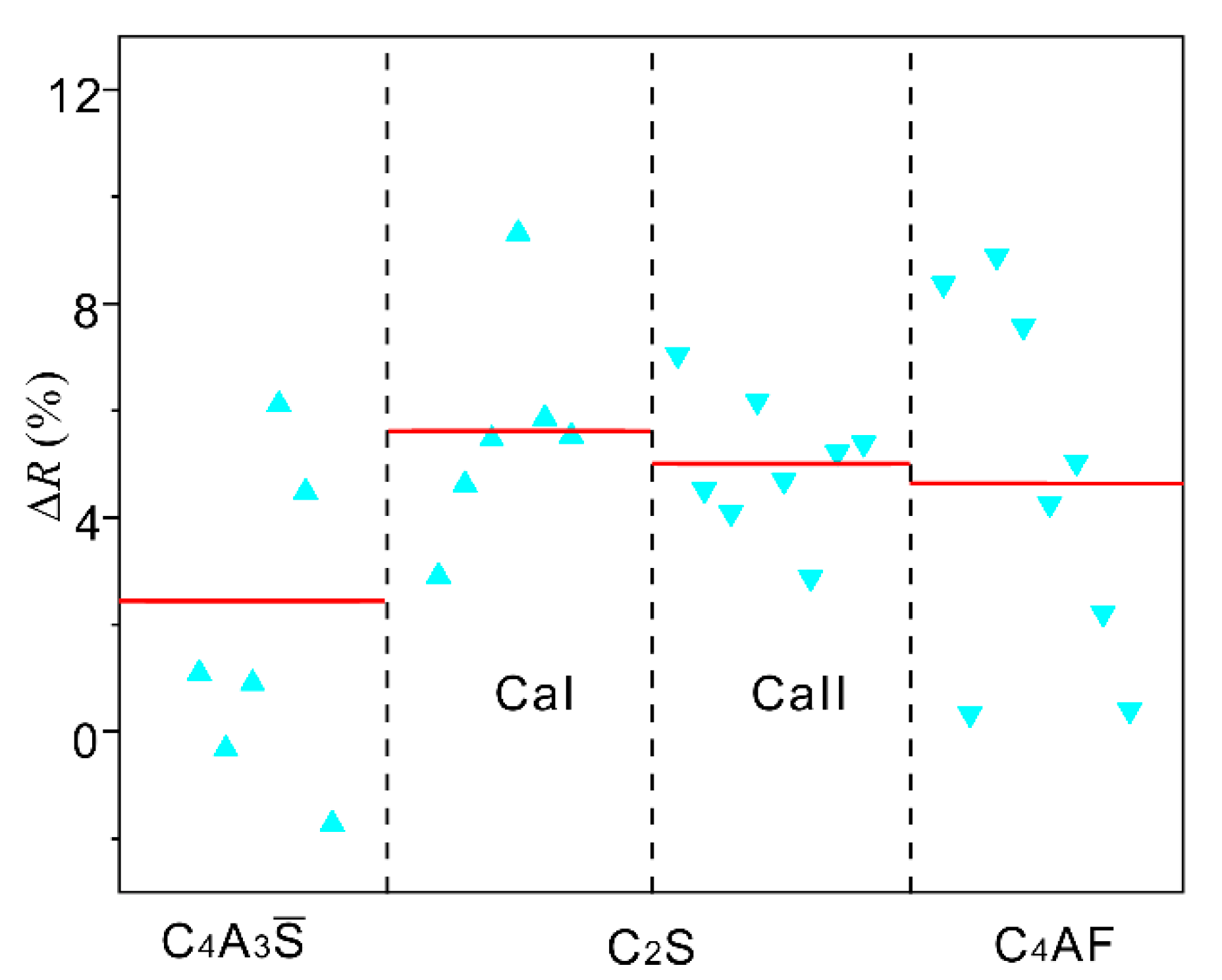
3.2. Experimental Analyses
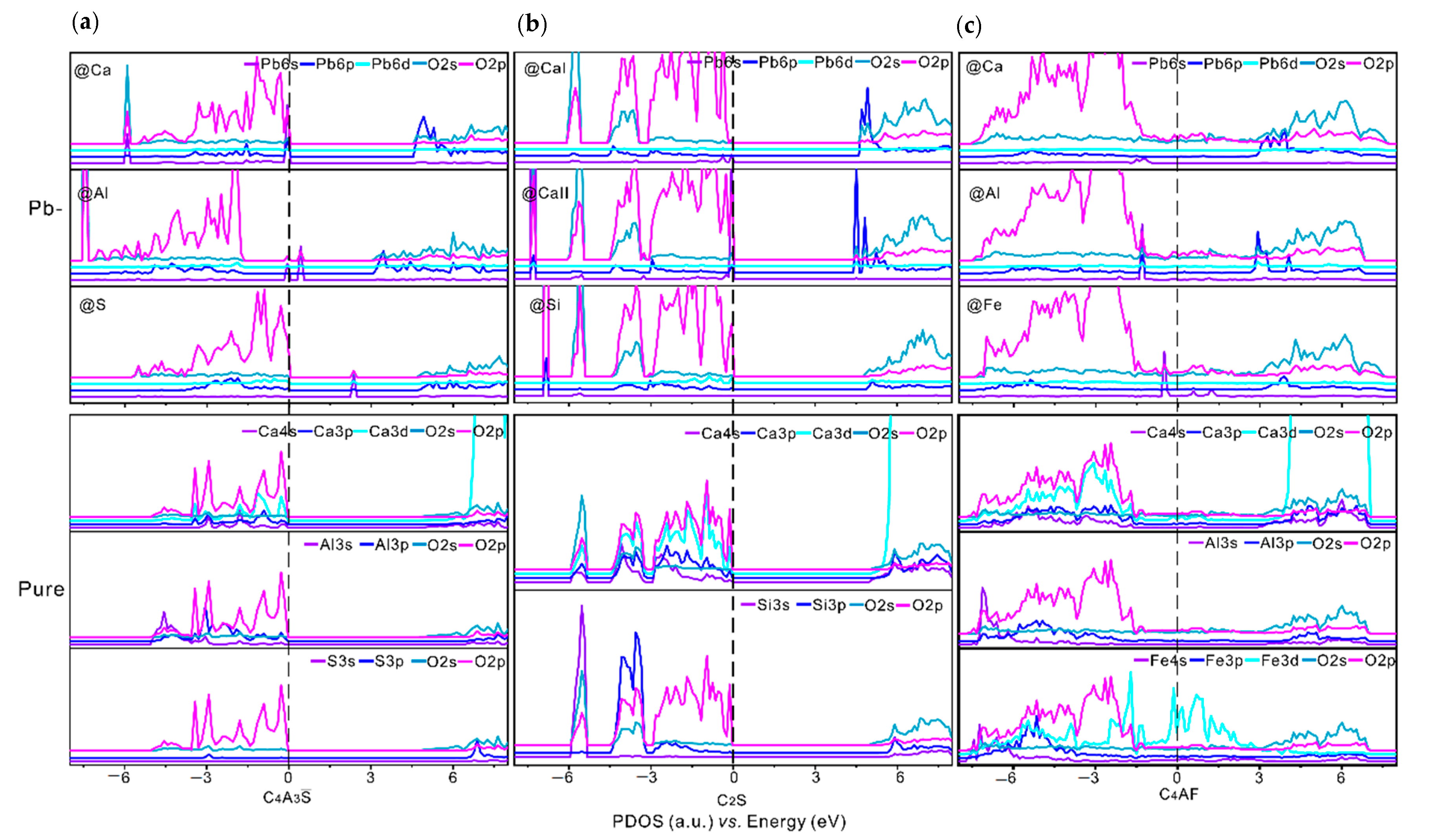
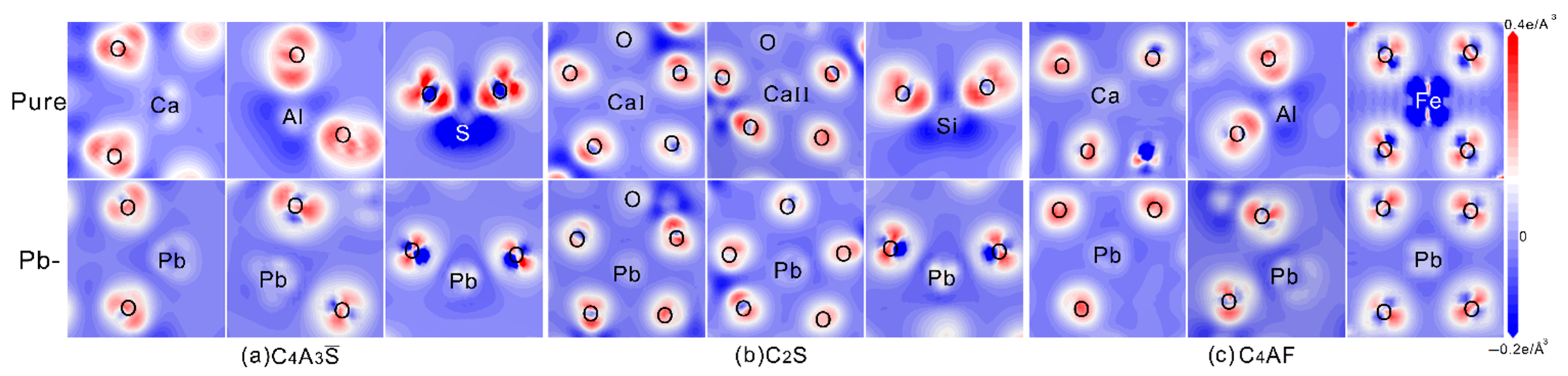
3.3. Partial Density of States and Electron Density Difference
3.4. Local Structural Distortions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Minerals | Supercells | Lattice Parameters/Å | Relax | PDOS |
|---|---|---|---|---|
| C4A3S [25] | 1 × 1 × 1 | a = b = c = 9.19 | 3 × 3 × 3 | 5 × 5 × 5 |
| β-C2S [26] | 2 × 2 × 1 | a = 11.01, b = 13.51, c = 9.31 | 2 × 2 × 3 | 5 × 4 × 5 |
| C4AF [28] | 2 × 1 × 2 | a = 11.17, b = 14.60, c = 10.75 | 2 × 2 × 2 | 5 × 3 × 5 |
| Phase | Space Group | ICSD IDs | Lattice Parameters | μ/eV | |||||
|---|---|---|---|---|---|---|---|---|---|
| a/Å | b/Å | c/Å | α/° | β/° | γ/° | ||||
| αPb | Fm3m | 648,343 | 0.50 | 0.50 | 0.50 | 90 | 90 | 90 | −3.56 |
| αCa | Fm3m | 426,932 | 5.59 | 5.59 | 5.59 | 90 | 90 | 90 | −1.92 |
| αAl | Fm3m | 53,774 | 4.05 | 4.05 | 4.05 | 90 | 90 | 90 | −3.75 |
| αS | P2/c | 82,372 | 13.64 | 9.25 | 10.90 | 57 | 90 | 90 | −4.12 |
| αSi | Fd3m | 181,356 | 5.43 | 5.43 | 5.43 | 90 | 90 | 90 | −5.43 |
| αFe | Im3m | 631,729 | 2.87 | 2.87 | 2.87 | 90 | 90 | 90 | −8.24 |
| Ions | Coordination | Electronegativity | Radius/Å |
|---|---|---|---|
| Pb4+ | 8 | 1.557 | 1.08 |
| Ca2+ | 6 | 1.032 | 1.00 |
| Al3+ | 4 | 1.499 | 0.39 |
| S6+ | 4 | 2.479 | 0.12 |
| Si4+ | 4 | 1.709 | 0.26 |
| Fe3+ | 6 | 1.687 | 0.645 |
References
- Zhan, L.; Jiang, L.; Zhang, Y.; Gao, B.; Xu, Z. Reduction, detoxification and recycling of solid waste by hydrothermal technology: A review. Chem. Eng. J. 2020, 390, 14111–14118. [Google Scholar] [CrossRef]
- Ahmed, M.J.K.; Ahmaruzzaman, M. A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. Water Process Eng. 2016, 10, 39–47. [Google Scholar] [CrossRef]
- Trauchessec, R.; Mechling, J.M.; Lecomte, A.; Roux, A.; Le Rolland, B. Hydration of ordinary portland cement and calcium sulfoaluminate cement blends. Cem. Concr. Res. 2015, 56, 106–114. [Google Scholar] [CrossRef]
- Tang, H.; Qian, J.; Ji, Z.; Dai, X.; Li, Z. The protective effect of magnesium phosphate cement on steel corrosion. Constr. Build. Mater. 2020, 255, 119422. [Google Scholar] [CrossRef]
- Gineys, N.; Aouad, G.; Sorrentino, F.; Damidot, D. Incorporation of trace elements in Portland cement clinker: Thresholds limits for Cu, Ni, Sn or Zn. Cem. Concr. Res. 2011, 41, 1177–1184. [Google Scholar] [CrossRef]
- Pera, J.; Ambroise, J. New applications of calcium sulfoaluminate cement. Cem. Concr. Res. 2004, 34, 671–676. [Google Scholar] [CrossRef]
- Cau Dit Coumes, C.; Dhoury, M.; Champenois, J.-B.; Mercier, C.; Damidot, D. Physico-chemical mechanisms involved in the acceleration of the hydration of calcium sulfoaluminate cement by lithium ions. Cem. Concr. Res. 2017, 96, 42–51. [Google Scholar] [CrossRef]
- Julphunthong, P.; Joyklad, P. Utilization of Several Industrial Wastes as Raw Material for Calcium Sulfoaluminate Cement. Materials 2019, 12, 3319. [Google Scholar] [CrossRef]
- Li, P.; Ma, Z.; Zhang, Z.; Li, X.; Lu, X.; Hou, P.; Du, P. Effect of Gypsum on Hydration and Hardening Properties of Alite Modified Calcium Sulfoaluminate Cement. Materials 2019, 12, 3131. [Google Scholar] [CrossRef]
- Yang, L.; Zheng, M.; Zhao, Y.; Yang, Y.; Li, C.; Liu, G. Unintentional persistent organic pollutants in cement kilns co-processing solid wastes. Ecotox. Environ. Saf. 2019, 182, 109373. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Viczek, S.A.; Aldrian, A.; Pomberger, R.; Sarc, R. Origins and carriers of Sb, As, Cd, Cl, Cr, Co, Pb, Hg, and Ni in mixed solid waste—A literature-based evaluation. Waste Manag. 2020, 103, 87–112. [Google Scholar] [CrossRef] [PubMed]
- Hargis, C.W.; Lothenbach, B.; Müller, C.J.; Winnefeld, F. Carbonation of calcium sulfoaluminate mortars. Cement Concr. Com. 2017, 80, 123–134. [Google Scholar] [CrossRef]
- Ren, C.; Wang, W.; Yao, Y.; Wu, S.; Qamar; Yao, X. Complementary use of industrial solid wastes to produce green materials and their role in CO2 reduction. J. Clean. Prod. 2020, 252, 119840. [Google Scholar] [CrossRef]
- Rahla, K.M.; Mateus, R.; Bragança, L. Comparative sustainability assessment of binary blended concretes using Supplementary Cementitious Materials (SCMs) and Ordinary Portland Cement (OPC). J. Clean. Prod. 2019, 220, 445–459. [Google Scholar] [CrossRef]
- Berrio, A.; Rodriguez, C.; Tobon, J. Effect of Al2O3/SiO2 ratio on ye’elimite production on CSA cement. Constr. Build. Mater. 2018, 168, 512–521. [Google Scholar] [CrossRef]
- Mao, Y.; Wu, H.; Wang, W.; Jia, M.; Che, X. Pretreatment of Municipal Solid Waste Incineration Fly Ash and Preparation of Solid Waste Source Sulphoaluminate Cementitious Material. J. Hazard. Mater. 2020, 385, 121580.1–121580.9. [Google Scholar] [CrossRef]
- Luo, Z.; Ma, B.; Yu, Z.; Li, X.; Wu, B. The effect of heavy metal lead on sulphoaluminate cement hydration and leaching toxicity. J. Qingdao Technol. Univ. 2009, 30, 124–126. [Google Scholar]
- Wang, L.; Jamro, I.A.; Chen, Q.; Li, S.; Luan, J.; Yang, T. Immobilization of trace elements in municipal solid waste incinerator (MSWI) fly ash by producing calcium sulphoaluminate cement after carbonation and washing. Waste Manag. Res. 2015, 34, 184–194. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, K.; Chen, Y.; Fan, G.; Zhang, L.; Guo, B.; Guan, X.; Zhao, R. Revealing the substitution preference of zinc in ordinary Portland cement clinker phases: A study from experiments and DFT calculations. J. Hazard. Mater. 2020. [Google Scholar] [CrossRef]
- Zhao, R.; Zhang, L.; Fan, G.; Chen, Y.; Huang, G.; Zhang, H.; Zhu, J.; Guan, X. Probing the exact form and doping preference of magnesium in ordinary Portland cement clinker phases. Cem. Concr. Res. 2020. submitted. [Google Scholar]
- Zhu, J.; Chen, Y.; Yang, K.; Zhang, L.; Guo, B.; Guan, X.; Zhao, R. Revealing the possibility of producing sulfoaluminate cement clinker with Ba-bearing industrial solid waste: A study from experiments and theoretical simulations. J. Clean. Prod. 2020. submitted. [Google Scholar]
- Tao, Y.; Zhang, W.; Shang, D.; Xia, Z.; Li, N.; Ching, W.-Y.; Wang, F.; Hu, S. Comprehending the occupying preference of manganese substitution in crystalline cement clinker phases: A theoretical study. Cement Concrete Res. 2018, 109, 19–29. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, W.; Li, N.; Shang, D.; Xia, Z.; Wang, F. Fundamental principles that govern the copper doping behavior in complex clinker system. J. Am. Ceram. Soc. 2018, 101, 2527–2536. [Google Scholar] [CrossRef]
- Álvarez-Pinazo, G.; Cuesta, A.; García-Maté, M.; Santacruz, I.; Losilla, E.R.; la Torre, A.G.D.; León-Reina, L.; Aranda, M.A.G. Rietveld quantitative phase analysis of Yeelimite-containing cements. Cem. Concr. Res. 2012, 42, 960–971. [Google Scholar] [CrossRef]
- Chen, Y.L.; Shih, P.H.; Chiang, L.C.; Chang, Y.K.; Lu, H.C.; Chang, J.E. The influence of heavy metals on the polymorphs of dicalcium silicate in the belite-rich clinkers produced from electroplating sludge. J. Hazard. Mater. 2009, 170, 443–448. [Google Scholar] [CrossRef]
- Jost, K.H.; Ziemer, B.; Seydel, R. Redetermination of the structure of β-dicalcium silicate. Acta Crystallogr. B 1977, 33, 1696–1700. [Google Scholar] [CrossRef]
- Colville, A.A.; Geller, S. The crystal structure of brownmillerite, Ca2FeAlO5. Acta Crystallogr. B 1971, 27, 2311–2315. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initiomolecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initiomolecular dynamics for open-shell transition metals. Phys. Rev. B 1993, 48, 13115–13118. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Kohan, A.F.; Ceder, G.; Morgan, D.; Van de Walle, C.G. First-principles study of native point defects in ZnO. Phys. Rev. B 2000, 61, 15019–15027. [Google Scholar] [CrossRef]
- Zhao, R.; Gao, J.; Liu, Z.; Ding, F. The reconstructed edges of the hexagonal BN. Nanoscale 2015, 7, 9723–9730. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Persson, K.A. Commentary: The materials project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Iacobescu, R.I.; Pontikes, Y.; Koumpouri, D.; Angelopoulos, G.N. Synthesis, characterization and properties of calcium ferroaluminate belite cements produced with electric arc furnace steel slag as raw material. Cement Concr. Comp. 2013, 44, 1–8. [Google Scholar] [CrossRef]
- Shannon, R.D.J. Revised effective ionic radii and systematic study of inter atomic distances in halides and chalcogenides. Acta Crys. 1976, 32, 751–767. [Google Scholar] [CrossRef]


| Samples | Ratio of Materials (wt%) | ||||||
|---|---|---|---|---|---|---|---|
| CaCO3 | SiO2 | Al2O3 | CaSO4 | Fe2O3 | PbO2 * | ||
| SAC | A1 | 54.56 | 7.66 | 23.58 | 10.46 | 3.74 | 0 |
| A2 | 54.56 | 7.66 | 23.58 | 10.46 | 3.74 | 11.82 | |
| A3 | 54.56 | 7.66 | 23.58 | 10.46 | 3.74 | 17.74 | |
| A4 | 54.56 | 7.66 | 23.58 | 10.46 | 3.74 | 23.65 | |
| C4A3S | S1 | 40.47 | - | 40.80 | 18.73 | - | 0 |
| S2 | 40.47 | - | 40.80 | 18.73 | - | 16.86 | |
| S3 | 40.47 | - | 40.80 | 18.73 | - | 25.29 | |
| S4 | 40.47 | - | 40.80 | 18.73 | - | 33.71 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Chen, Y.; Zhang, L.; Yang, K.; Guan, X.; Zhao, R. Insights on Substitution Preference of Pb Ions in Sulfoaluminate Cement Clinker Phases. Materials 2021, 14, 44. https://doi.org/10.3390/ma14010044
Zhu J, Chen Y, Zhang L, Yang K, Guan X, Zhao R. Insights on Substitution Preference of Pb Ions in Sulfoaluminate Cement Clinker Phases. Materials. 2021; 14(1):44. https://doi.org/10.3390/ma14010044
Chicago/Turabian StyleZhu, Jianping, Yang Chen, Li Zhang, Kuo Yang, Xuemao Guan, and Ruiqi Zhao. 2021. "Insights on Substitution Preference of Pb Ions in Sulfoaluminate Cement Clinker Phases" Materials 14, no. 1: 44. https://doi.org/10.3390/ma14010044
APA StyleZhu, J., Chen, Y., Zhang, L., Yang, K., Guan, X., & Zhao, R. (2021). Insights on Substitution Preference of Pb Ions in Sulfoaluminate Cement Clinker Phases. Materials, 14(1), 44. https://doi.org/10.3390/ma14010044






