Ultraviolet Light Treatment of Titanium Enhances Attachment, Adhesion, and Retention of Human Oral Epithelial Cells via Decarbonization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Titanium Disks and UV Treatment
2.2. Human Oral Epithelial Cells Culture
2.3. Initial Cell Attachment Assay
2.4. Cytomorphology and Cytomorphometry
2.5. Adhesion Protein Assay
2.6. Real-Time Quantitative Polymerase Chain Reaction (qPCR)
2.7. Cell Detachment Assay
2.8. Laminin Coating of Titanium Disks
2.9. Statistical Analysis
3. Results
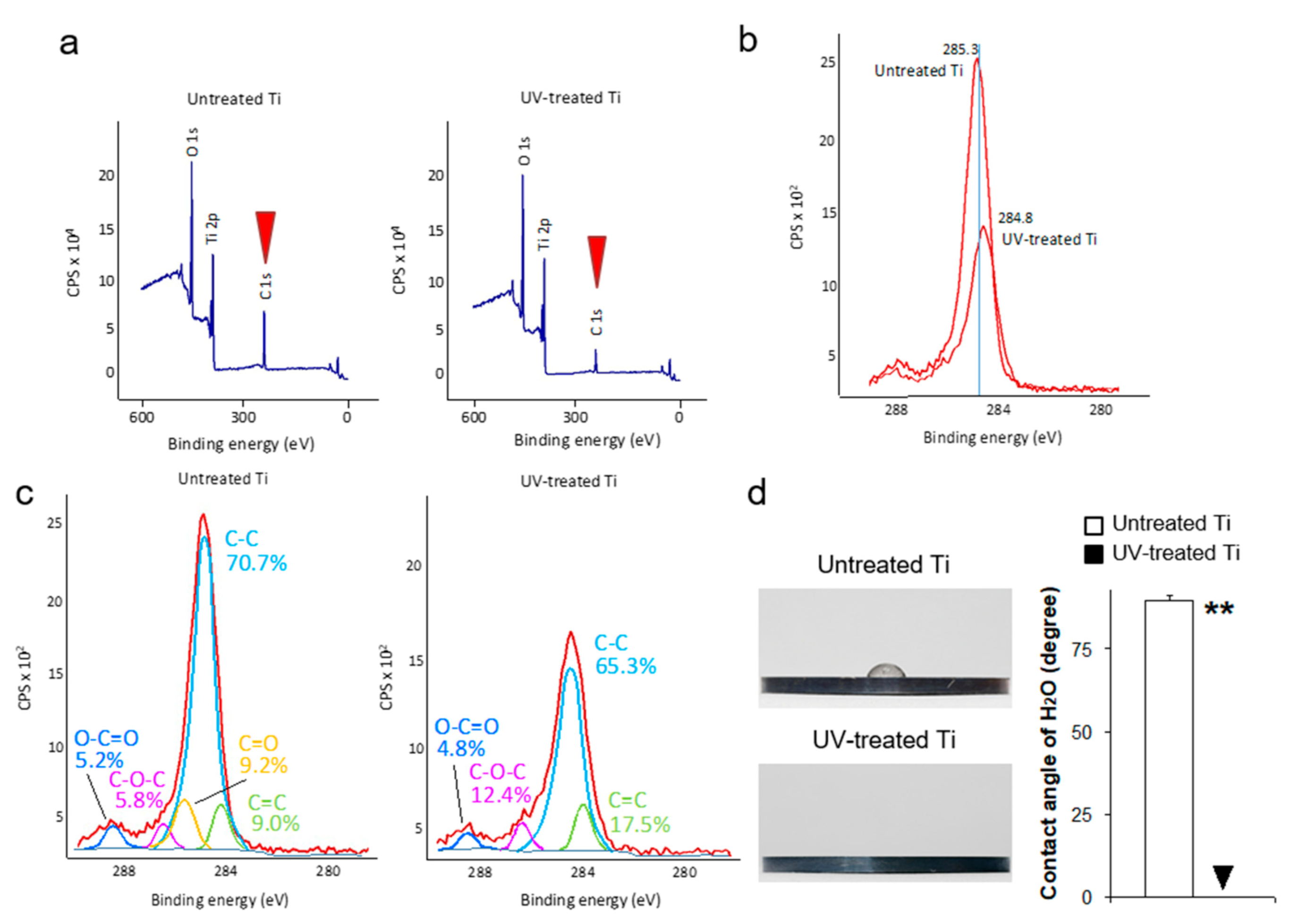
3.1. UV Treatment Induced Decarbonized and Super-Hydrophilic Surfaces
3.2. Enhanced Initial Cell Attachment to UV-Treated Surfaces
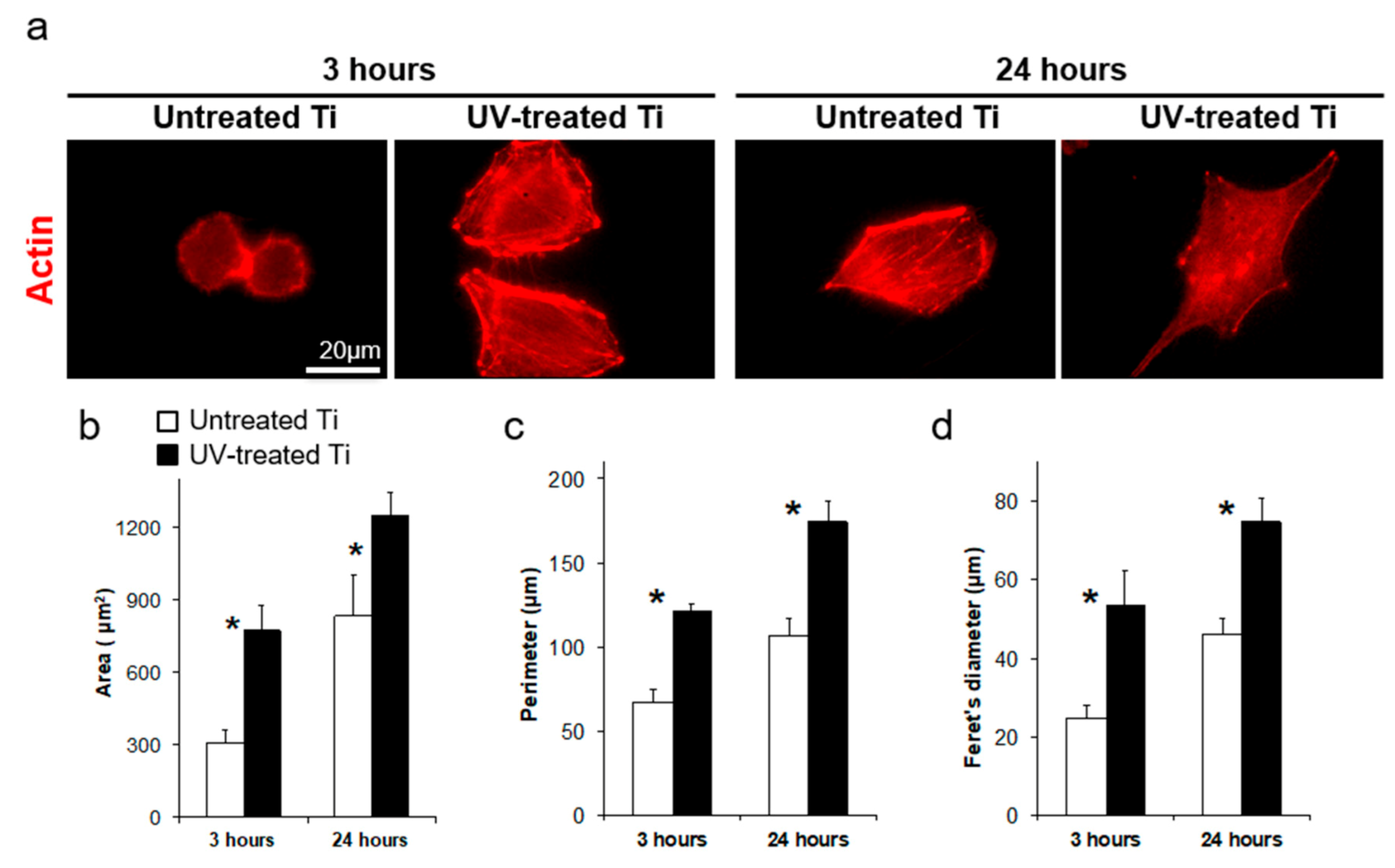
3.3. Accelerated Cell Spreading on UV-Treated Titanium
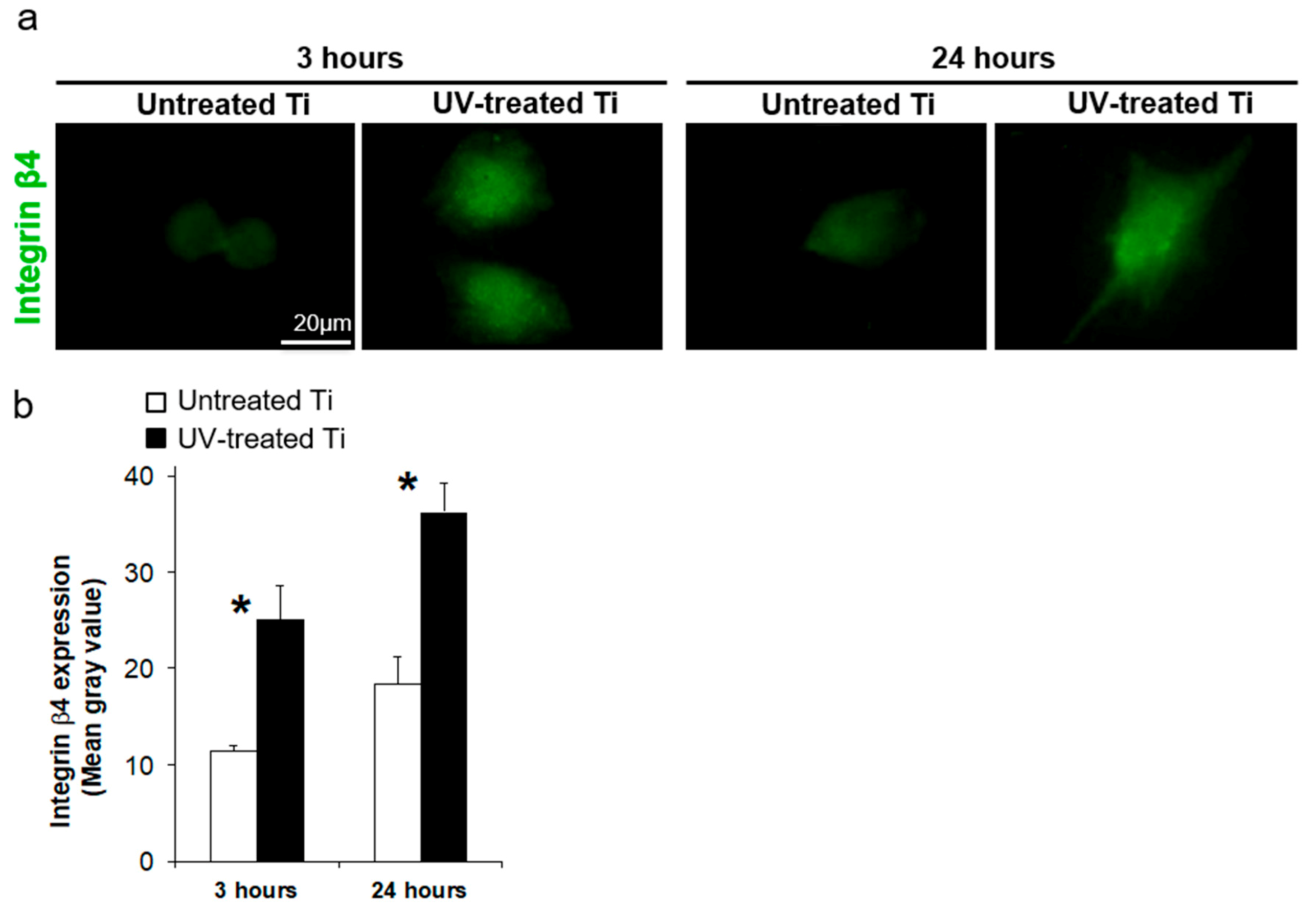
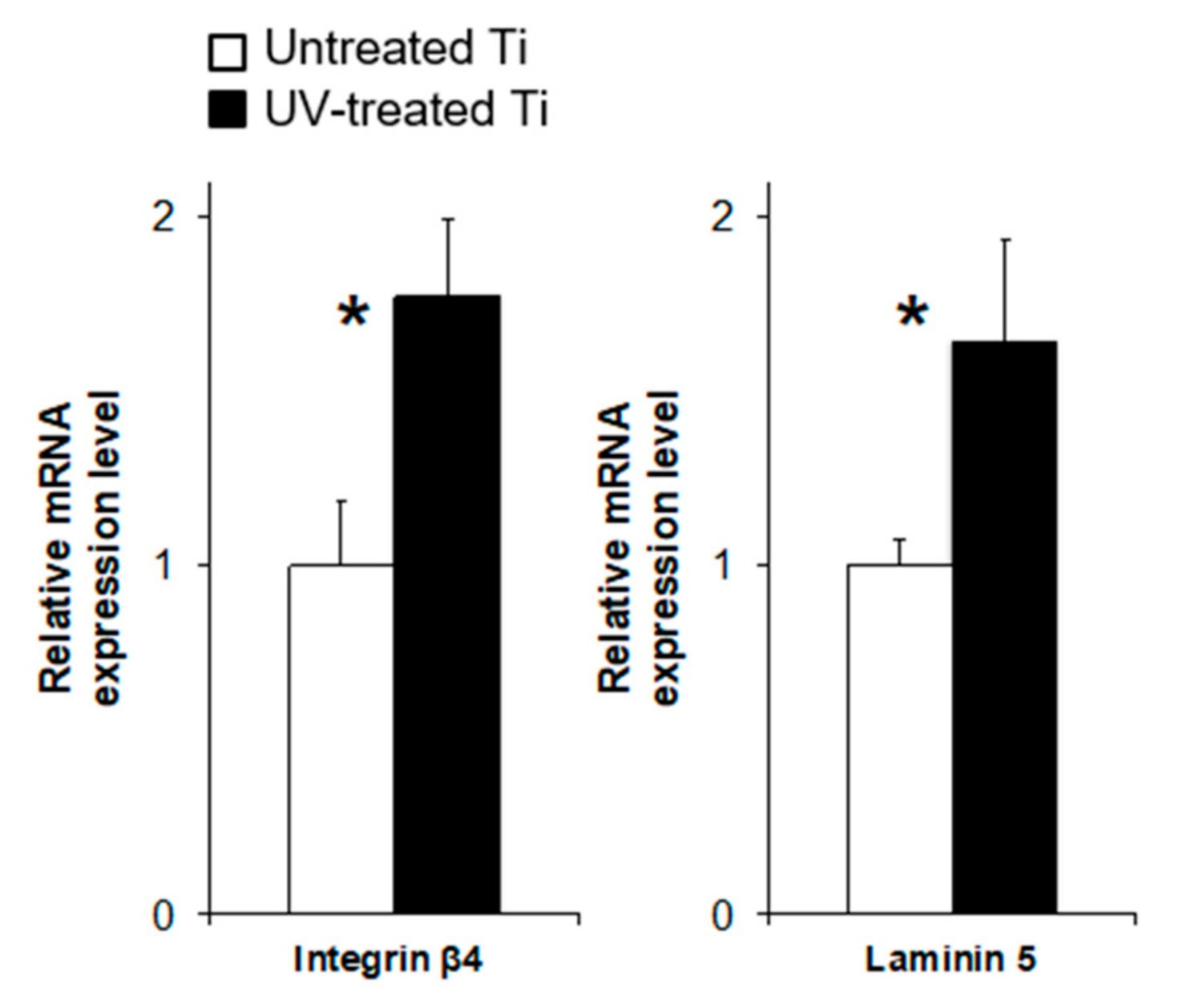
3.4. UV Treatment Enhanced the Expression of Adhesion Proteins
3.5. UV Treatment Enhanced Cell Retention
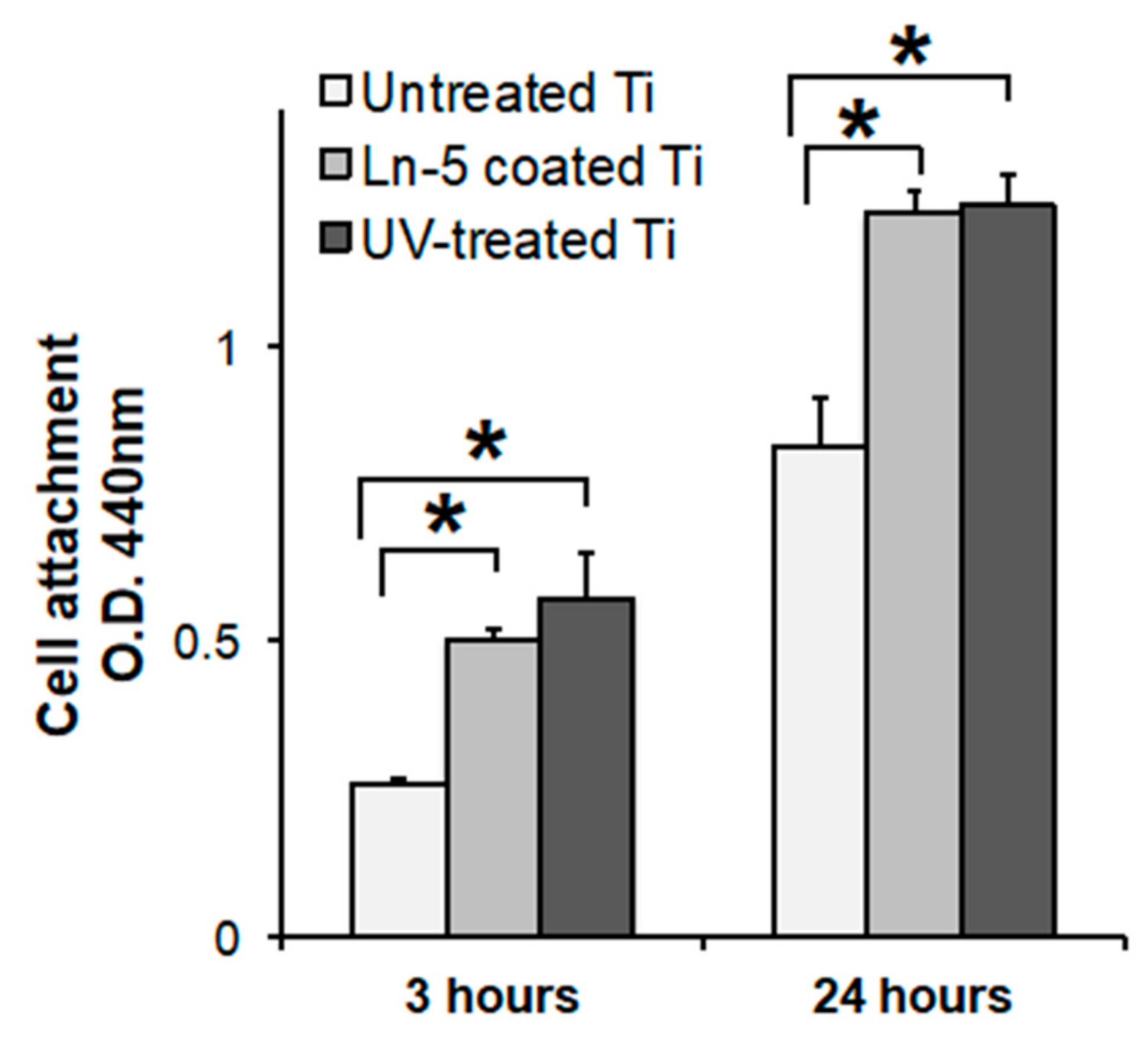
3.6. Laminin Coating
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brook, I. Microbiology and management of soft tissue and muscle infections. Int. J. Surg. 2008, 6, 328–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paster, B.J.; Boches, S.K.; Galvin, J.L.; Ericson, R.E.; Lau, C.N.; Levanos, V.A.; Sahasrabudhe, A.; Dewhirst, F.E. Bacterial diversity in human subgingival plaque. J. Bacteriol. 2001, 183, 3770–3783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paster, B.J.; Olsen, I.; Aas, J.A.; Dewhirst, F.E. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontology 2000 2006, 42, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Atieh, M.A.; Alsabeeha, N.H.; Faggion, C.M., Jr.; Duncan, W.J. The frequency of peri-implant diseases: A systematic review and meta-analysis. J. Periodontol. 2013, 84, 1586–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mombelli, A.; Muller, N.; Cionca, N. The epidemiology of peri-implantitis. Clin. Oral Implant. Res. 2012, 23, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.D. Transcutaneous implants: Reactions of the skin-implant interface. J. Biomed. Mater. Res. 1974, 8, 99–113. [Google Scholar] [CrossRef]
- Pendegrass, C.J.; Goodship, A.E.; Blunn, G.W. Development of a soft tissue seal around bone-anchored transcutaneous amputation prostheses. Biomaterials 2006, 27, 4183–4191. [Google Scholar] [CrossRef]
- Rompen, E.; Domken, O.; Degidi, M.; Pontes, A.E.; Piattelli, A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clin. Oral Implant. Res. 2006, 17, 55–67. [Google Scholar] [CrossRef]
- Klinge, B.; Meyle, J.; Working, G. Soft-tissue integration of implants. Consensus report of Working Group 2. Clin. Oral Implant. Res. 2006, 17, 93–96. [Google Scholar] [CrossRef]
- Atsuta, I.; Yamaza, T.; Yoshinari, M.; Goto, T.; Kido, M.A.; Kagiya, T.; Mino, S.; Shimono, M.; Tanaka, T. Ultrastructural localization of laminin-5 (gamma2 chain) in the rat peri-implant oral mucosa around a titanium-dental implant by immuno-electron microscopy. Biomaterials 2005, 26, 6280–6287. [Google Scholar] [CrossRef]
- Borradori, L.; Sonnenberg, A. Structure and function of hemidesmosomes: More than simple adhesion complexes. J. Investig. Dermatol. 1999, 112, 411–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepp, M.A.; Spurr-Michaud, S.; Tisdale, A.; Elwell, J.; Gipson, I.K. Alpha 6 beta 4 integrin heterodimer is a component of hemidesmosomes. Proc. Natl. Acad. Sci. USA 1990, 87, 8970–8974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonnenberg, A.; Calafat, J.; Janssen, H.; Daams, H.; van der Raaij-Helmer, L.M.; Falcioni, R.; Kennel, S.J.; Aplin, J.D.; Baker, J.; Loizidou, M.; et al. Integrin alpha 6/beta 4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J. Cell Biol. 1991, 113, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Hori, N.; Ueno, T.; Minamikawa, H.; Iwasa, F.; Yoshino, F.; Kimoto, K.; Lee, M.C.; Ogawa, T. Electrostatic control of protein adsorption on UV-photofunctionalized titanium. Acta Biomater. 2010, 6, 4175–4180. [Google Scholar] [CrossRef]
- Iwasa, F.; Hori, N.; Ueno, T.; Minamikawa, H.; Yamada, M.; Ogawa, T. Enhancement of osteoblast adhesion to UV-photofunctionalized titanium via an electrostatic mechanism. Biomaterials 2010, 31, 2717–2727. [Google Scholar] [CrossRef]
- Aita, H.; Att, W.; Ueno, T.; Yamada, M.; Hori, N.; Iwasa, F.; Tsukimura, N.; Ogawa, T. Ultraviolet light-mediated photofunctionalization of titanium to promote human mesenchymal stem cell migration, attachment, proliferation and differentiation. Acta Biomater. 2009, 5, 3247–3257. [Google Scholar] [CrossRef]
- Aita, H.; Hori, N.; Takeuchi, M.; Suzuki, T.; Yamada, M.; Anpo, M.; Ogawa, T. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials 2009, 30, 1015–1025. [Google Scholar] [CrossRef]
- Ogawa, T. Ultraviolet photofunctionalization of titanium implants. Int. J. Oral Maxillofac. Implant. 2014, 29, e95–e102. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Kobayashi, H.; Ogawa, T. Implant stability change and osseointegration speed of immediately loaded photofunctionalized implants. Implant Dent. 2013, 22, 481–490. [Google Scholar] [CrossRef] [Green Version]
- Kitajima, H.; Ogawa, T. The Use of Photofunctionalized Implants for Low or Extremely Low Primary Stability Cases. Int. J. Oral Maxillofac. Implant. 2016, 31, 439–447. [Google Scholar] [CrossRef]
- Hirota, M.; Ozawa, T.; Iwai, T.; Ogawa, T.; Tohnai, I. Implant Stability Development of Photofunctionalized Implants Placed in Regular and Complex Cases: A Case-Control Study. Int. J. Oral Maxillofac. Implant. 2016, 31, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Funato, A.; Yamada, M.; Ogawa, T. Success rate, healing time, and implant stability of photofunctionalized dental implants. Int. J. Oral Maxillofac. Implant. 2013, 28, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Funato, A.; Ogawa, T. Photofunctionalized dental implants: A case series in compromised bone. Int. J. Oral Maxillofac. Implant. 2013, 28, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Hori, N.; Att, W.; Ueno, T.; Sato, N.; Yamada, M.; Saruwatari, L.; Suzuki, T.; Ogawa, T. Age-dependent degradation of the protein adsorption capacity of titanium. J. Dent. Res. 2009, 88, 663–667. [Google Scholar] [CrossRef]
- Att, W.; Ogawa, T. Biological aging of implant surfaces and their restoration with ultraviolet light treatment: A novel understanding of osseointegration. Int. J. Oral Maxillofac. Implant. 2012, 27, 753–761. [Google Scholar] [CrossRef]
- Att, W.; Hori, N.; Takeuchi, M.; Ouyang, J.; Yang, Y.; Anpo, M.; Ogawa, T. Time-dependent degradation of titanium osteoconductivity: An implication of biological aging of implant materials. Biomaterials 2009, 30, 5352–5363. [Google Scholar] [CrossRef]
- Saruta, J.; Sato, N.; Ishijima, M.; Okubo, T.; Hirota, M.; Ogawa, T. Disproportionate Effect of Sub-Micron Topography on Osteoconductive Capability of Titanium. Int. J. Mol. Sci. 2019, 20, 4027. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, R.; Ueno, T.; Migita, S.; Tsutsumi, Y.; Doi, H.; Ogawa, T.; Hanawa, T.; Wakabayashi, N. Hydrocarbon Deposition Attenuates Osteoblast Activity on Titanium. J. Dent. Res. 2014, 93, 698–703. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, Y.; Miyamura, M.; Hirano, Y.; Goto, K.; Matsuda, T. Preparation of poly(ethylene glycol)-polystyrene block copolymers using photochemistry of dithiocarbamate as a reduced cell-adhesive coating material. Biomaterials 1999, 20, 963–970. [Google Scholar] [CrossRef]
- Murakami, D.; Yamato, M.; Nishida, K.; Ohki, T.; Takagi, R.; Yang, J.; Namiki, H.; Okano, T. The effect of micropores in the surface of temperature-responsive culture inserts on the fabrication of transplantable canine oral mucosal epithelial cell sheets. Biomaterials 2006, 27, 5518–5523. [Google Scholar] [CrossRef]
- Ide, T.; Nishida, K.; Yamato, M.; Sumide, T.; Utsumi, M.; Nozaki, T.; Kikuchi, A.; Okano, T.; Tano, Y. Structural characterization of bioengineered human corneal endothelial cell sheets fabricated on temperature-responsive culture dishes. Biomaterials 2006, 27, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.H.; Kikuchi, A.; Yamato, M.; Okano, T. Accelerated cell sheet recovery by co-grafting of PEG with PIPAAm onto porous cell culture membranes. Biomaterials 2003, 24, 1223–1232. [Google Scholar] [CrossRef]
- Tereshchenko, A.; Smyntyna, V.; Ramanavicius, A. Interaction Mechanism between TiO2 Nanostructures and Bovine Leukemia Virus Proteins in Photoluminescence-based Immunosensors. RSC Adv. 2018, 8, 37740–37748. [Google Scholar] [CrossRef] [Green Version]
- Ramanavicius, S.; Ramanavicius, A. Insights in the Application of Stoichiometric and Non-Stoichiometric Titanium Oxides for the Design of Sensors for the Determination of Gases and VOCs (TiO2−x and TinO2n−1 vs. TiO2). Sensors 2020, 20, 6833. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Y.; Wu, D.; Wang, Q.L.; Yan, J.; Buekens, A.G.; Cen, K.F. Photocatalytic decomposition on nano-TiO2: Destruction of chloroaromatic compounds. Chemosphere 2011, 82, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Atsuta, I.; Ayukawa, Y.; Furuhashi, A.; Yamaza, T.; Tsukiyama, Y.; Koyano, K. Promotive effect of insulin-like growth factor-1 for epithelial sealing to titanium implants. J. Biomed. Mater. Res. A 2013, 101, 2896–2904. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.; Fan, C.; Wang, D.A. A novel gelatin-based micro-cavitary hydrogel for potential application in delivery of anchorage dependent cells: A study with vasculogenesis model. Colloids Surf. B 2016, 146, 334–342. [Google Scholar] [CrossRef]
- Wang, C.; Gong, Y.; Zhong, Y.; Yao, Y.; Su, K.; Wang, D.A. The control of anchorage-dependent cell behavior within a hydrogel/microcarrier system in an osteogenic model. Biomaterials 2009, 30, 2259–2269. [Google Scholar] [CrossRef]
- Werner, S.; Huck, O.; Frisch, B.; Vautier, D.; Elkaim, R.; Voegel, J.C.; Brunel, G.; Tenenbaum, H. The effect of microstructured surfaces and laminin-derived peptide coatings on soft tissue interactions with titanium dental implants. Biomaterials 2009, 30, 2291–2301. [Google Scholar] [CrossRef]
- El-Ghannam, A.; Starr, L.; Jones, J. Laminin-5 coating enhances epithelial cell attachment, spreading, and hemidesmosome assembly on Ti-6A1-4V implant material in vitro. J. Biomed. Mater. Res. 1998, 41, 30–40. [Google Scholar] [CrossRef]
- Okawachi, H.; Ayukawa, Y.; Atsuta, I.; Furuhashi, A.; Sakaguchi, M.; Yamane, K.; Koyano, K. Effect of titanium surface calcium and magnesium on adhesive activity of epithelial-like cells and fibroblasts. Biointerphases 2012, 7, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Hagiwara, Y.; Hirota, M.; Tabuchi, M.; Yamada, M.; Sugita, Y.; Ogawa, T. Effect of photofunctionalization on fluoride-treated nanofeatured titanium. J. Biomater. Appl. 2014, 28, 1200–1212. [Google Scholar] [CrossRef]
- Tsukimura, N.; Yamada, M.; Iwasa, F.; Minamikawa, H.; Att, W.; Ueno, T.; Saruwatari, L.; Aita, H.; Chiou, W.A.; Ogawa, T. Synergistic effects of UV photofunctionalization and micro-nano hybrid topography on the biological properties of titanium. Biomaterials 2011, 32, 4358–4368. [Google Scholar] [CrossRef] [PubMed]
- de Avila, E.D.; Lima, B.P.; Sekiya, T.; Torii, Y.; Ogawa, T.; Shi, W.; Lux, R. Effect of UV-photofunctionalization on oral bacterial attachment and biofilm formation to titanium implant material. Biomaterials 2015, 67, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, Y.; Yamada, M.; Ueda, T.; Sakurai, K. Reduction of biofilm formation on titanium surface with ultraviolet-C pre-irradiation. J. Biomater. Appl. 2014, 29, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Grossner-Schreiber, B.; Griepentrog, M.; Haustein, I.; Muller, W.D.; Lange, K.P.; Briedigkeit, H.; Gobel, U.B. Plaque formation on surface modified dental implants. An in vitro study. Clin. Oral Implant. Res. 2001, 12, 543–551. [Google Scholar] [CrossRef]
- Groessner-Schreiber, B.; Neubert, A.; Muller, W.D.; Hopp, M.; Griepentrog, M.; Lange, K.P. Fibroblast growth on surface-modified dental implants: An in vitro study. J. Biomed. Mater. Res. A 2003, 64, 591–599. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakhaei, K.; Ishijima, M.; Ikeda, T.; Ghassemi, A.; Saruta, J.; Ogawa, T. Ultraviolet Light Treatment of Titanium Enhances Attachment, Adhesion, and Retention of Human Oral Epithelial Cells via Decarbonization. Materials 2021, 14, 151. https://doi.org/10.3390/ma14010151
Nakhaei K, Ishijima M, Ikeda T, Ghassemi A, Saruta J, Ogawa T. Ultraviolet Light Treatment of Titanium Enhances Attachment, Adhesion, and Retention of Human Oral Epithelial Cells via Decarbonization. Materials. 2021; 14(1):151. https://doi.org/10.3390/ma14010151
Chicago/Turabian StyleNakhaei, Kourosh, Manabu Ishijima, Takayuki Ikeda, Amirreza Ghassemi, Juri Saruta, and Takahiro Ogawa. 2021. "Ultraviolet Light Treatment of Titanium Enhances Attachment, Adhesion, and Retention of Human Oral Epithelial Cells via Decarbonization" Materials 14, no. 1: 151. https://doi.org/10.3390/ma14010151
APA StyleNakhaei, K., Ishijima, M., Ikeda, T., Ghassemi, A., Saruta, J., & Ogawa, T. (2021). Ultraviolet Light Treatment of Titanium Enhances Attachment, Adhesion, and Retention of Human Oral Epithelial Cells via Decarbonization. Materials, 14(1), 151. https://doi.org/10.3390/ma14010151




