Prolonged Post-Polymerization Biocompatibility of Polymethylmethacrylate-Tri-n-Butylborane (PMMA-TBB) Bone Cement
Abstract
1. Introduction
2. Materials and Methods
2.1. Bone Cement Preparation
2.2. Rat Femur-Derived Osteoblasts Cell Culture
2.3. Initial Cell Behavior
2.4. Morphological Observation of Osteoblasts
2.5. Alkaline Phosphatase (ALP) Activity and ALP Staining
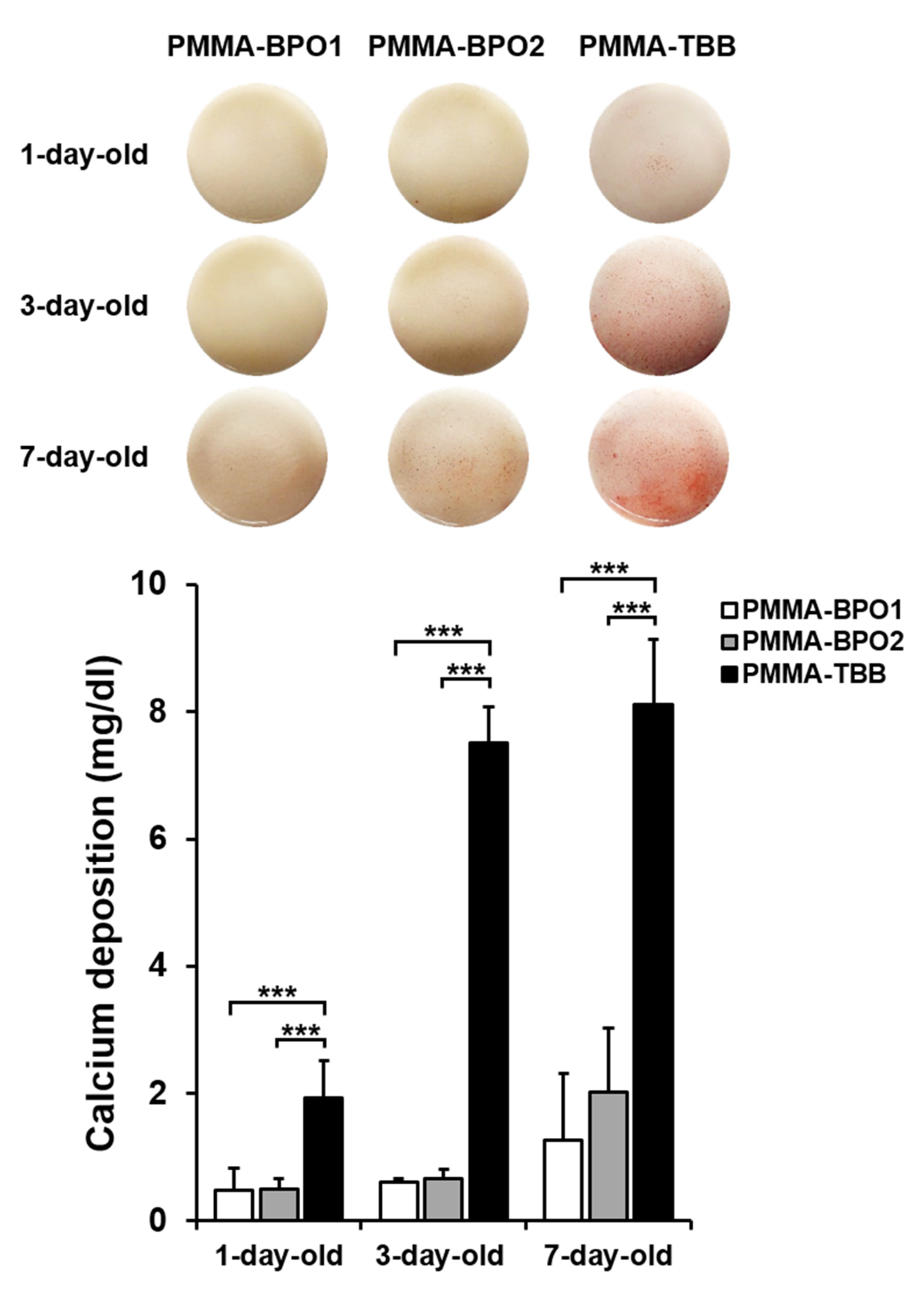
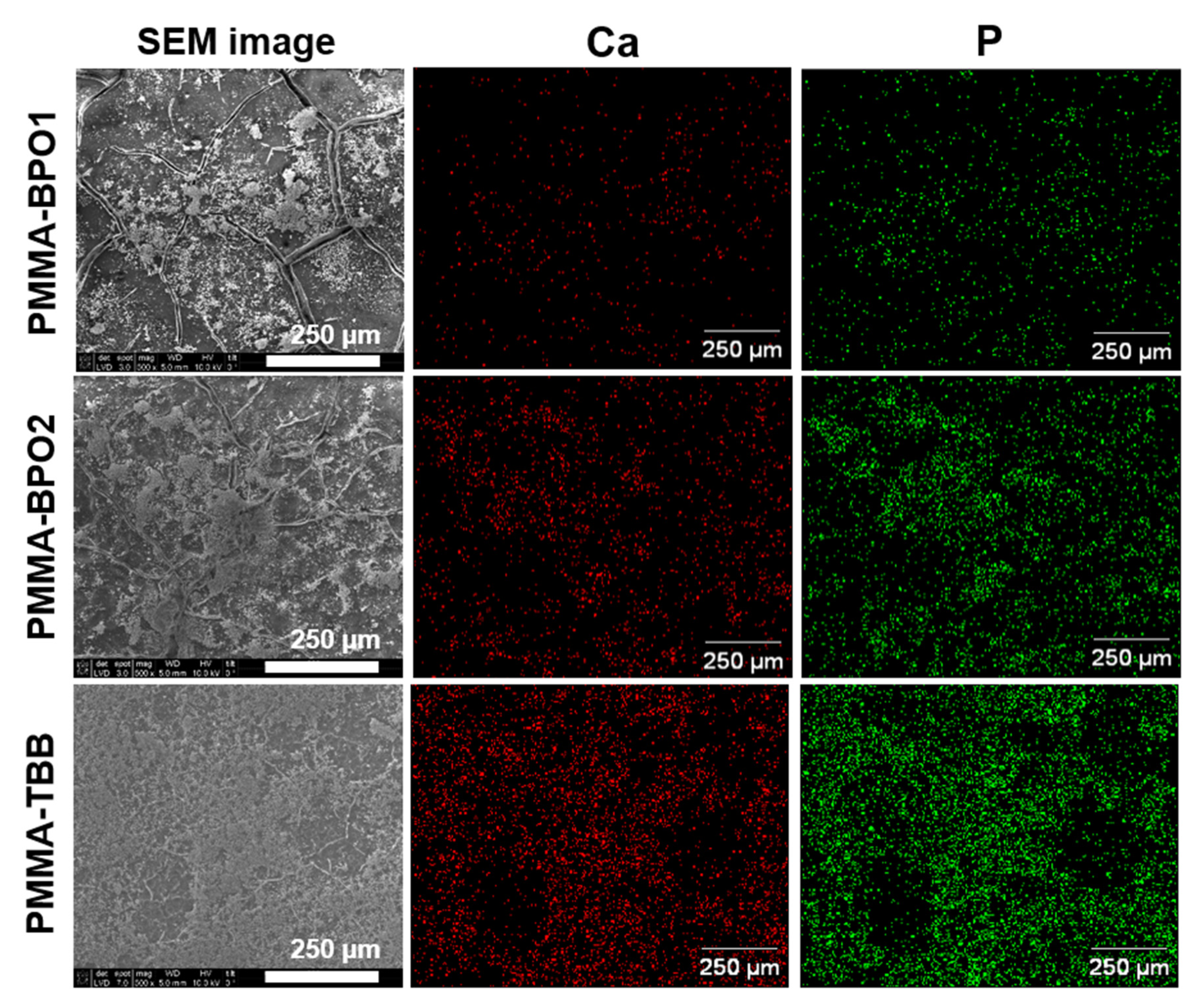
2.6. Mineralization Assay
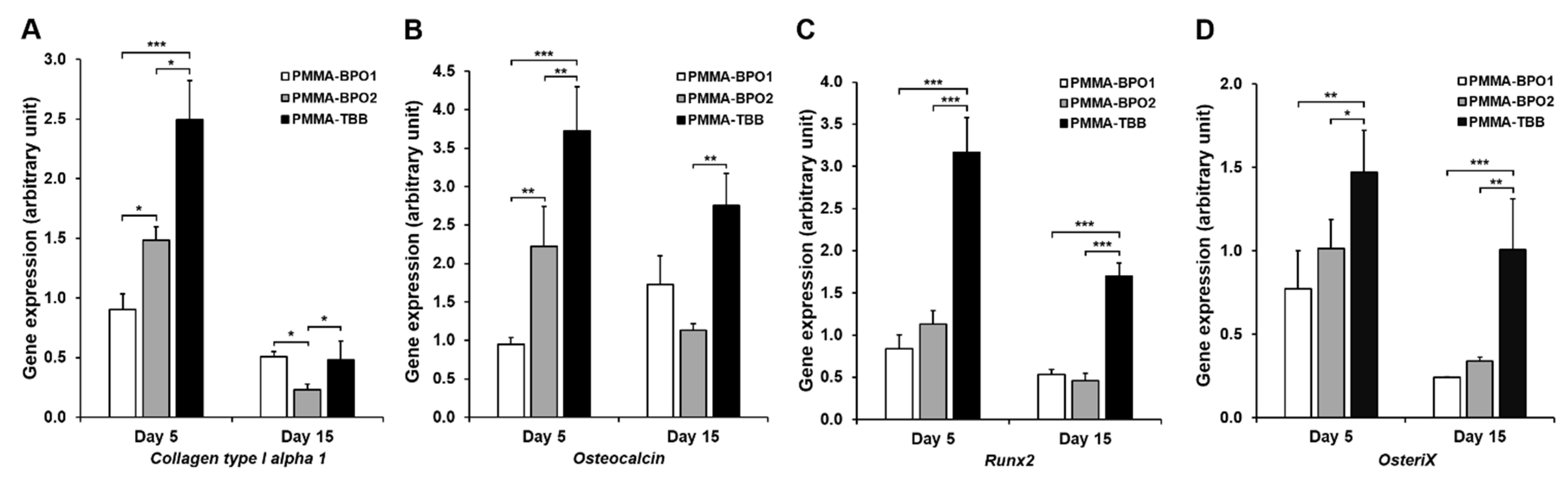
2.7. Bone-Related Genes Expression
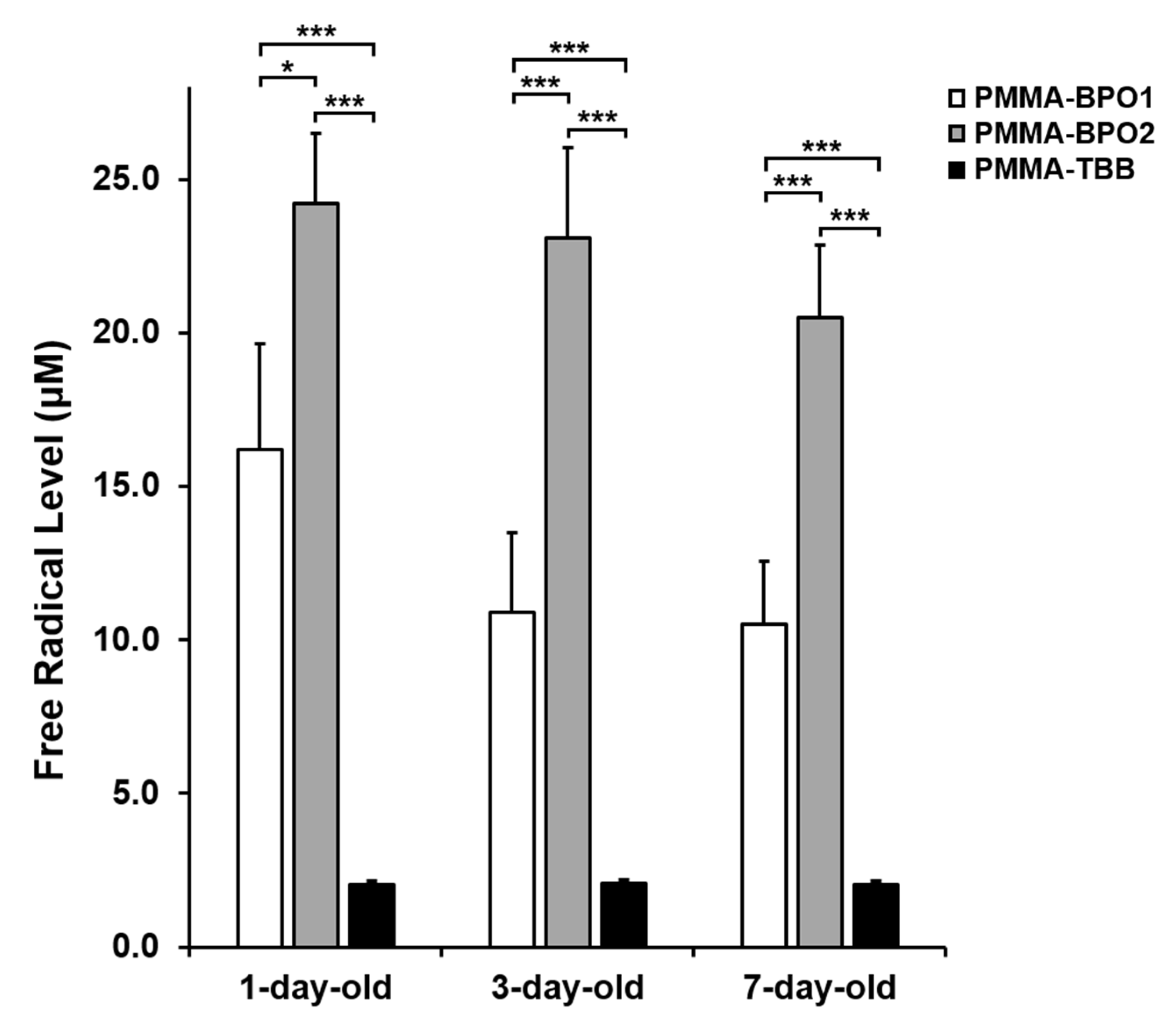
2.8. Polymerization Radical Detection by Electron Spin Resonance Spectroscopy (ESR)
2.9. Statistical Analysis
3. Results
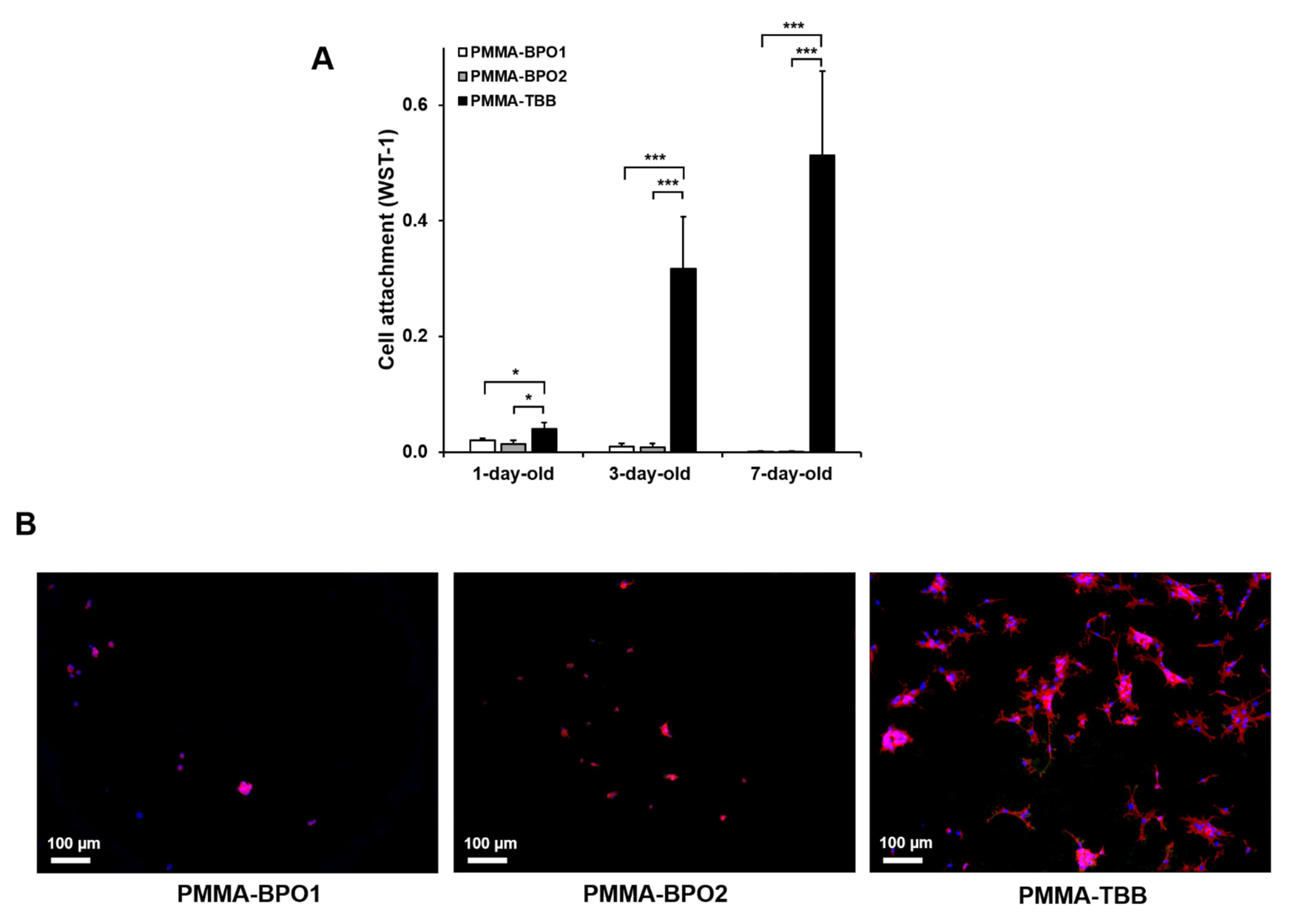
3.1. Initial Cell Attachment to Bone Cement
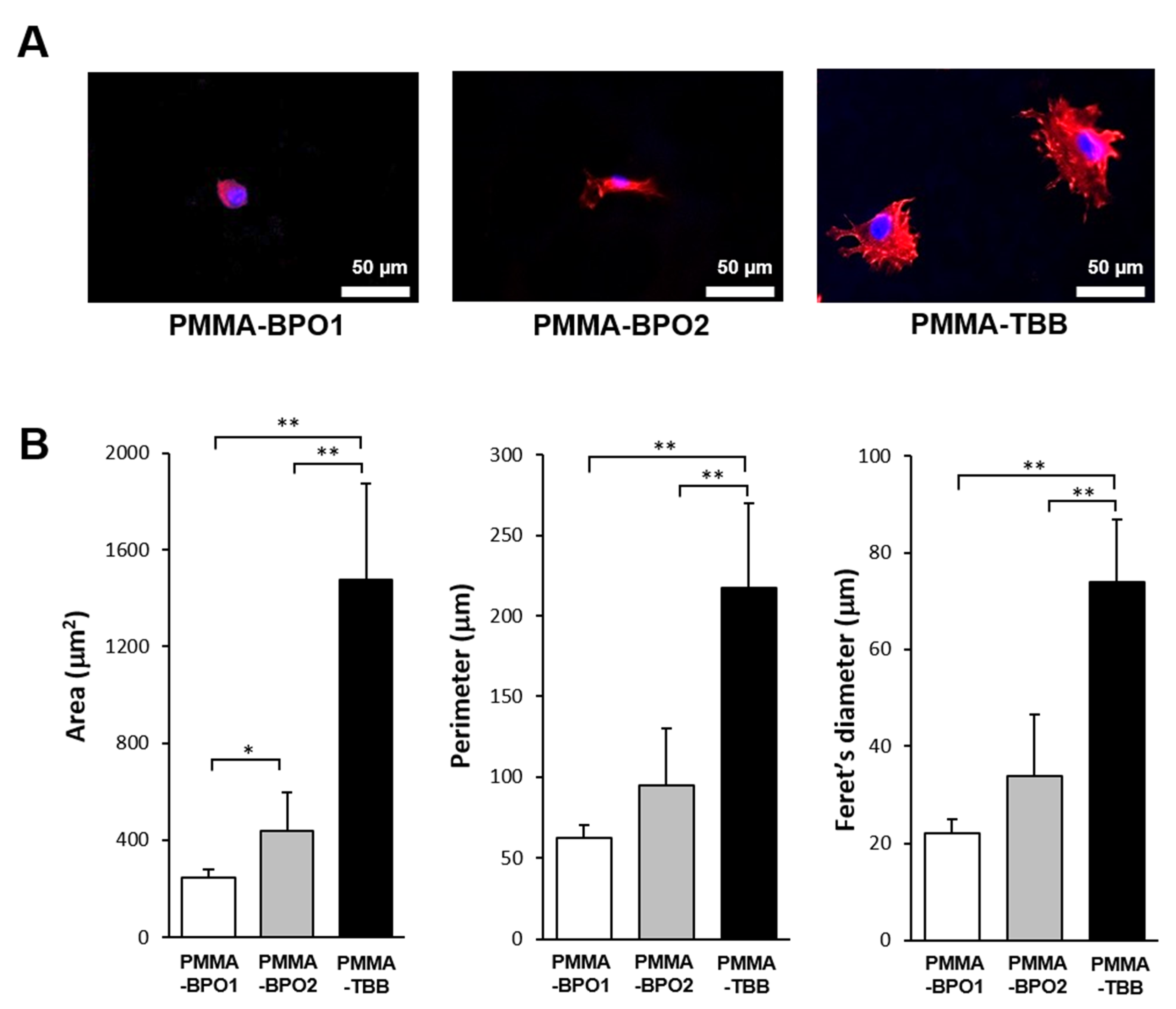
3.2. Cell Spreading Behavior
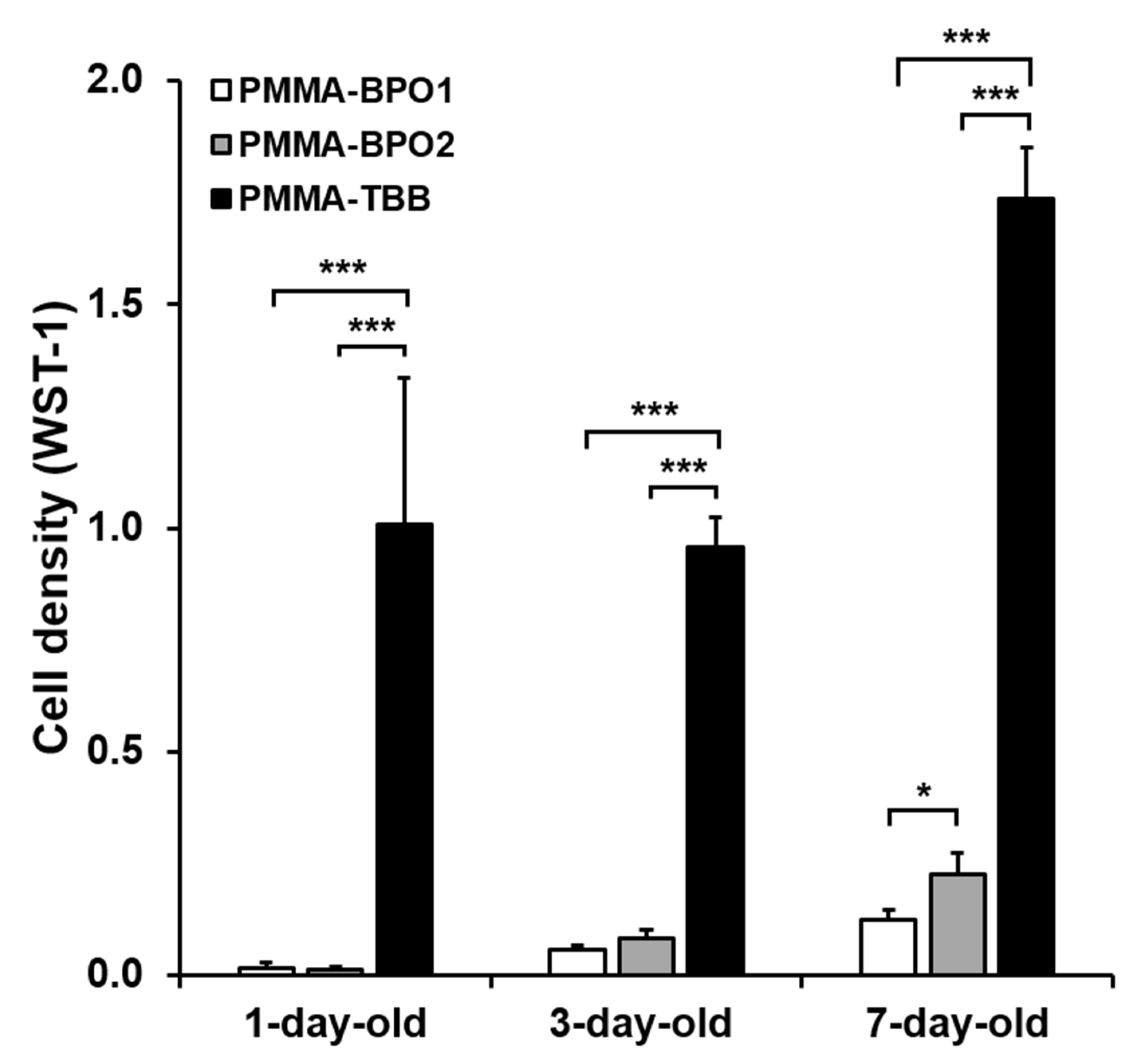
3.3. Cell Proliferation
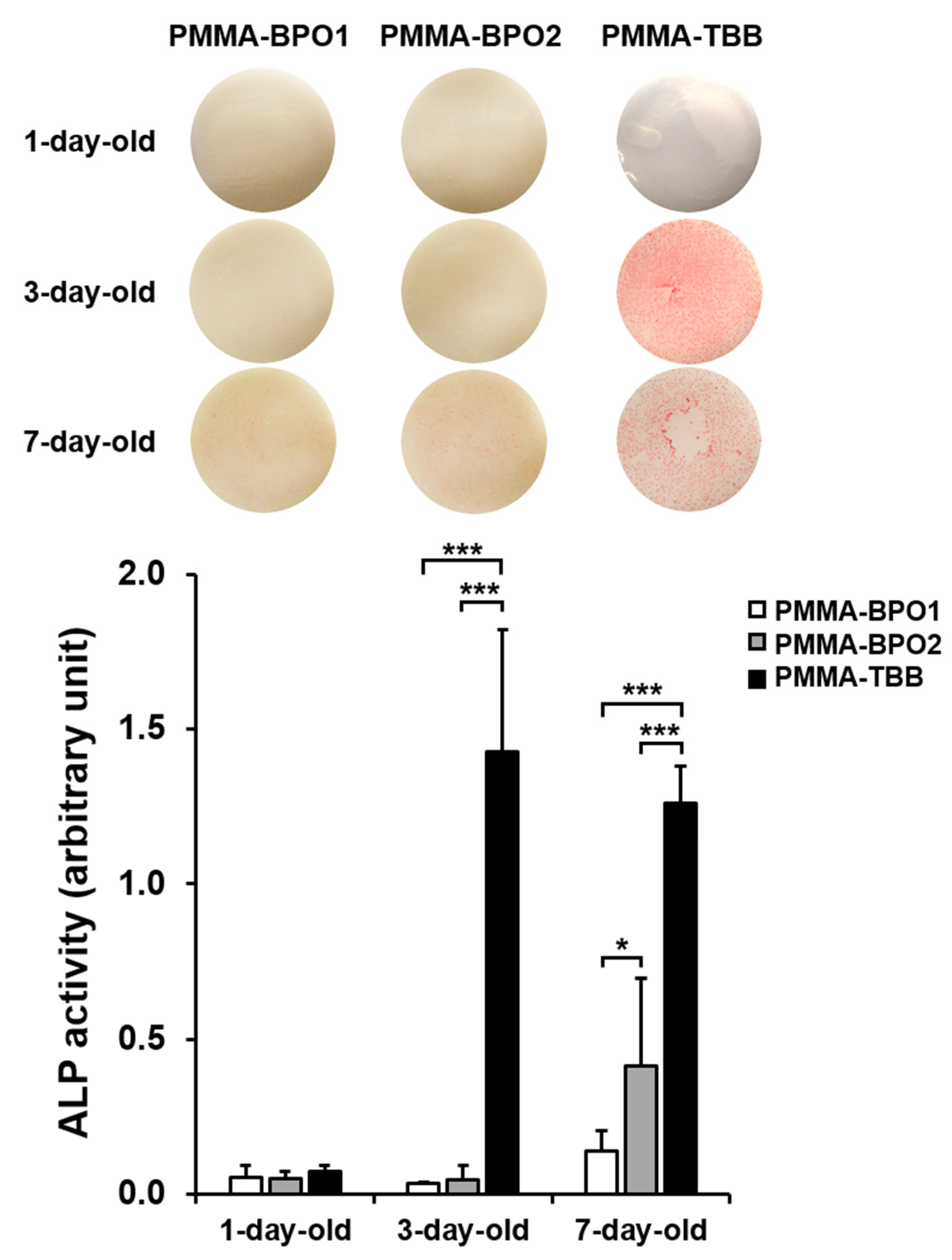
3.4. Osteoblastic Differentiation
3.5. Free Radical Production by Bone Cements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mertz, L. The Coming Gray Tide: Wanted: Health Innovations for an Increasingly Older Population. IEEE Pulse 2017, 8, 6–11. [Google Scholar] [CrossRef]
- Bonafede, M.; Shi, N.; Barron, R.; Li, X.; Crittenden, D.; Chandler, D. Predicting imminent risk for fracture in patients aged 50 or older with osteoporosis using US claims data. Arch. Osteoporos. 2016, 11, 1–7. [Google Scholar] [CrossRef]
- Li, X.-Z.; Zhang, S.-N. Recent advance in treatment of osteoarthritis by bioactive components from herbal medicine. Chin. Med. 2020, 15, 1–13. [Google Scholar] [CrossRef]
- Akkoc, N.; Khan, M.A. Is Axial Spondyloarthritis More Common Than Rheumatoid Arthritis? Curr. Rheumatol. Rep. 2020, 22, 1–10. [Google Scholar] [CrossRef]
- Jakubaszek, M.; Płaza, M.; Kwiatkowska, B. Color fraction as a useful method of imaging synovium vascularization in patients with high activity of rheumatoid arthritis. Reumatologia 2020, 58, 42–47. [Google Scholar] [CrossRef]
- Kremers, H.M.; Larson, D.R.; Crowson, C.S.; Kremers, W.K.; Washington, R.E.; Steiner, C.A.; Jiranek, W.A.; Berry, D.J. Prevalence of Total Hip and Knee Replacement in the United States. J. Bone Jt. Surg. Am. Vol. 2015, 97, 1386–1397. [Google Scholar] [CrossRef]
- Kurtz, S.; Mowat, F.; Ong, K.; Chan, N.; Lau, E.; Halpern, M. Prevalence of Primary and Revision Total Hip and Knee Arthroplasty in the United States From 1990 Through 2002. J. Bone Jt. Surg. Am. Vol. 2005, 87, 1487–1497. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. Vol. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Day, J.S.; Lau, E.; Ong, K.L.; Williams, G.R.; Ramsey, M.L.; Kurtz, S.M. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J. Shoulder Elb. Surg. 2010, 19, 1115–1120. [Google Scholar] [CrossRef]
- Bakhtiari, S.S.E.; Bakhsheshi-Rad, H.R.; Karbasi, S.; Tavakoli, M.; Razzaghi, M.; Ismail, A.F.; Ramakrishna, S.; Berto, F. Polymethyl Methacrylate-Based Bone Cements Containing Carbon Nanotubes and Graphene Oxide: An Overview of Physical, Mechanical, and Biological Properties. Polymers 2020, 12, 1469. [Google Scholar] [CrossRef] [PubMed]
- Melo-Fonseca, F.; Miranda, G.; Domingues, H.S.; Pinto, I.M.; Gasik, M.; Silva, F.S. Reengineering Bone-Implant Interfaces for Improved Mechanotransduction and Clinical Outcomes. Stem Cell Rev. Rep. 2020, 16, 1121–1138. [Google Scholar] [CrossRef]
- Bistolfi, A.; Ferracini, R.; Albanese, C.; Vernè, E.; Miola, M. PMMA-Based Bone Cements and the Problem of Joint Arthroplasty Infections: Status and New Perspectives. Materials 2019, 12, 4002. [Google Scholar] [CrossRef]
- Randall, D.J.; Anderson, M.B.; Gililland, J.M.; Peters, C.L.; Pelt, C.E. A potential need for surgeon consensus: Cementation techniques for total knee arthroplasty in orthopedic implant manufacturers’ guidelines lack consistency. J. Orthop. Surg. 2019, 27. [Google Scholar] [CrossRef]
- Pacheco, K.A. Allergy to Surgical Implants. Clin. Rev. Allergy Immunol. 2019, 56, 72–85. [Google Scholar] [CrossRef]
- Duffau, P.; Beylot-Barry, M.; Palussière, J.; Ly, S.; Cogrel, O.; Doutre, M.-S. Necrotic livedo after vertebroplasty. Br. J. Dermatol. 2006, 156, 382–383. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amer, W.; Arra, M.; Clohisy, J.C.; Abu-Amer, Y.; Swarnkar, G. Targeting vascular endothelial growth factor ameliorates PMMA-particles induced inflammatory osteolysis in murine calvaria. Bone 2019, 123, 86–91. [Google Scholar] [CrossRef]
- Singh, V.; Bhakta, P.; Zietak, E.; Hussain, A.; Zietak, E. Bone cement implantation syndrome: A delayed postoperative presentation. J. Clin. Anesthesia 2016, 31, 274–277. [Google Scholar] [CrossRef]
- Paz, E.; Ballesteros, Y.; Abenojar, J.; Del Real, J.; Dunne, N. Graphene Oxide and Graphene Reinforced PMMA Bone Cements: Evaluation of Thermal Properties and Biocompatibility. Materials 2019, 12, 3146. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Saita, M.; Ikeda, T.; Hirota, M.; Park, W.; Lee, M.C.-I.; Ogawa, T. Biocompatibility of 4-META/MMA-TBB resin used as a dental luting agent. J. Prosthet. Dent. 2015, 114, 114–121. [Google Scholar] [CrossRef]
- Tsukimura, N.; Yamada, M.; Aita, H.; Hori, N.; Yoshino, F.; Lee, M.C.-I.; Kimoto, K.; Jewett, A.; Ogawa, T. N-acetyl cysteine (NAC)-mediated detoxification and functionalization of poly(methyl methacrylate) bone cement. Biomaterials 2009, 30, 3378–3389. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kojima, N.; Paranjpe, A.; Att, W.; Aita, H.; Jewett, A.; Ogawa, T. N-acetyl Cysteine (NAC)-assisted Detoxification of PMMA Resin. J. Dent. Res. 2008, 87, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Ciapetti, G.; Granchi, D.; Cenni, E.; Savarino, L.; Cavedagna, D.; Pizzoferrato, A. Cytotoxic effect of bone cements in HL-60 cells: Distinction between apoptosis and necrosis. J. Biomed. Mater. Res. 2000, 52, 338–345. [Google Scholar] [CrossRef]
- Yamada, M.; Ogawa, T. Chemodynamics underlying N-acetyl cysteine-mediated bone cement monomer detoxification. Acta Biomater. 2009, 5, 2963–2973. [Google Scholar] [CrossRef]
- Aita, H.; Tsukimura, N.; Yamada, M.; Hori, N.; Kubo, K.; Sato, N.; Maeda, H.; Kimoto, K.; Ogawa, T. N-acetyl cysteine prevents polymethyl methacrylate bone cement extract-induced cell death and functional suppression of rat primary osteoblasts. J. Biomed. Mater. Res. Part A 2010, 92, 285–296. [Google Scholar] [CrossRef]
- Goiato, M.C.; Freitas, E.; Dos Santos, D.; De Medeiros, R.; Sonego, M. Acrylic Resin Cytotoxicity for Denture Base: Literature Review. Adv. Clin. Exp. Med. 2015, 24, 679–686. [Google Scholar] [CrossRef]
- Oonishi, H.; Ohashi, H.; Kawahara, I. Total Hip Arthroplasty around the Inception of the Interface Bioactive Bone Cement Technique. Clin. Orthop. Surg. 2016, 8, 237–242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blom, E.J.; Klein-Nulend, J.; Klein, C.P.; Kurashina, K.; van Waas, M.A.; Burger, E.H. Transforming growth factor-beta1 incorporated during setting in calcium phosphate cement stimulates bone cell differentiation in vitro. J. Biomed. Mater. Res. 2000, 50, 67–74. [Google Scholar] [CrossRef]
- Elisabettacenni, E.; Granchi, D.; Ciapetti, G.; Savarino, L.; Vancini, M.; Di Leo, A. Effect of CMW 1 bone cement on transforming growth factor-beta 1 expression by endothelial cells. J. Biomater. Sci. Polym. Ed. 2001, 12, 1011–1025. [Google Scholar] [CrossRef]
- Bigi, A.; Torricelli, P.; Fini, M.; Bracci, B.; Panzavolta, S.; Sturba, L.; Giardino, R. A Biomimetic Gelatin-Calcium Phosphate Bone Cement. Int. J. Artif. Organs 2004, 27, 664–673. [Google Scholar] [CrossRef]
- Hamajima, K.; Ozawa, R.; Saruta, J.; Saita, M.; Kitajima, H.; Taleghani, S.R.; Usami, D.; Goharian, D.; Uno, M.; Miyazawa, K.; et al. The Effect of TBB, as an Initiator, on the Biological Compatibility of PMMA/MMA Bone Cement. Int. J. Mol. Sci. 2020, 21, 4016. [Google Scholar] [CrossRef]
- Sugita, Y.; Saruta, J.; Taniyama, T.; Kitajima, H.; Hirota, M.; Ikeda, T.; Ogawa, T. UV-Pre-Treated and Protein-Adsorbed Titanium Implants Exhibit Enhanced Osteoconductivity. Int. J. Mol. Sci. 2020, 21, 4194. [Google Scholar] [CrossRef]
- Saruta, J.; Sato, N.; Ishijima, M.; Okubo, T.; Hirota, M.; Ogawa, T. Disproportionate Effect of Sub-Micron Topography on Osteoconductive Capability of Titanium. Int. J. Mol. Sci. 2019, 20, 4027. [Google Scholar] [CrossRef]
- Hasegawa, M.; Saruta, J.; Hirota, M.; Taniyama, T.; Sugita, Y.; Kubo, K.; Ishijima, M.; Ikeda, T.; Maeda, H.; Ogawa, T. A Newly Created Meso-, Micro-, and Nano-Scale Rough Titanium Surface Promotes Bone-Implant Integration. Int. J. Mol. Sci. 2020, 21, 783. [Google Scholar] [CrossRef]
- Ghassemi, A.; Ishijima, M.; Hasegawa, M.; Rezaei, N.M.; Nakhaei, K.; Sekiya, T.; Torii, Y.; Hirota, M.; Park, W.; Miley, D.D.; et al. Biological and Physicochemical Characteristics of 2 Different Hydrophilic Surfaces Created by Saline-Storage and Ultraviolet Treatment. Implant. Dent. 2018, 27, 405–414. [Google Scholar] [CrossRef]
- Taniyama, T.; Saruta, J.; Rezaei, N.M.; Nakhaei, K.; Ghassemi, A.; Hirota, M.; Okubo, T.; Ikeda, T.; Sugita, Y.; Hasegawa, M.; et al. UV-Photofunctionalization of Titanium Promotes Mechanical Anchorage in A Rat Osteoporosis Model. Int. J. Mol. Sci. 2020, 21, 1235. [Google Scholar] [CrossRef]
- Sakaguchi, W.; Kubota, N.; Shimizu, T.; Saruta, J.; Fuchida, S.; Kawata, A.; Yamamoto, Y.; Sugimoto, M.; Yakeishi, M.; Tsukinoki, K. Existence of SARS-CoV-2 Entry Molecules in the Oral Cavity. Int. J. Mol. Sci. 2020, 21, 6000. [Google Scholar] [CrossRef] [PubMed]
- Shiotsu-Ogura, Y.; Yoshida, A.; Kan, P.; Sasaki, H.; Toyama, T.; Izukuri, K.; Hamada, N.; Yoshino, F.; Shiotusu-Ogura, Y. Antimicrobial photodynamic therapy using a plaque disclosing solution on Streptococcus mutans. Photodiagnosis Photodyn. Ther. 2019, 26, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, R.; Saita, M.; Sakaue, S.; Okada, R.; Sato, T.; Kawamata, R.; Sakurai, T.; Hamada, N.; Kimoto, K.; Nagasaki, Y. Redox injectable gel protects osteoblastic function against oxidative stress and suppresses alveolar bone loss in a rat peri-implantitis model. Acta Biomater. 2020, 110, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kojima, N.; Att, W.; Hori, N.; Suzuki, T.; Ogawa, T. N-Acetyl cysteine restores viability and function of rat odontoblast-like cells impaired by polymethylmethacrylate dental resin extract. Redox Rep. 2009, 14, 13–22. [Google Scholar] [CrossRef]
- Kojima, N.; Yamada, M.; Paranjpe, A.; Tsukimura, N.; Kubo, K.; Jewett, A.; Ogawa, T. Restored viability and function of dental pulp cells on poly-methylmethacrylate (PMMA)-based dental resin supplemented with N-acetyl cysteine (NAC). Dent. Mater. 2008, 24, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.-M.; Wei, Y.; Wang, W.-Q.; Wang, Y.; Xu, Y.-D.; Yang, Y.-Q. Simultaneous application of BrdU and WST-1 measurements for detection of the proliferation and viability of airway smooth muscle cells. Biol. Res. 2014, 47, 75. [Google Scholar] [CrossRef]
- Joo, K.-M.; Kim, S.; Koo, Y.J.; Lee, M.; Lee, S.-H.; Choi, D.; Lim, K.-M. Development and validation of UPLC method for WST-1 cell viability assay and its application to MCTT HCE™ eye irritation test for colorful substances. Toxicol. Vitr. 2019, 60, 412–419. [Google Scholar] [CrossRef]
- Lutter, A.-H.; Scholka, J.; Richter, H.; Anderer, U. Applying XTT, WST-1, and WST-8 to human chondrocytes: A comparison of membrane-impermeable tetrazolium salts in 2D and 3D cultures. Clin. Hemorheol. Microcirc. 2017, 67, 327–342. [Google Scholar] [CrossRef]
- Lian, J.B.; Javed, A.; Zaidi, S.K.; Lengner, C.; Montecino, M.; Van Wijnen, A.J.; Stein, J.L.; Stein, G.S. Regulatory controls for osteoblast growth and differentiation: Role of Runx/Cbfa/AML factors. Crit. Rev. Eukaryot. Gene Expr. 2004, 14, 1–41. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Sugita, Y.; Okubo, T.; Saita, M.; Ishijima, M.; Torii, Y.; Tanaka, M.; Iwasaki, C.; Sekiya, T.; Tabuchi, M.; Rezaei, N.M.; et al. Novel Osteogenic Behaviors around Hydrophilic and Radical-Free 4-META/MMA-TBB: Implications of an Osseointegrating Bone Cement. Int. J. Mol. Sci. 2020, 21, 2405. [Google Scholar] [CrossRef]
- Revell, P.; Braden, M.; Freeman, M. Review of the biological response to a novel bone cement containing poly(ethyl methacrylate) and n-butyl methacrylate. Biomaterials 1998, 19, 1579–1586. [Google Scholar] [CrossRef]
- Johnston, R.C. Acrylic bone cement: Clinical development and current status in North America. Orthop. Clin. N. Am. 2005, 36, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Granchi, N.; Cenni, E.; Savarino, L.; Ciapetti, G.; Forbicini, G.; Vancini, M.; Maini, C.; Baldini, N.; Giunti, A. Bone cement extracts modulate the osteoprotegerin/osteoprotegerin-ligand expression in MG63 osteoblast-like cells. Biomaterials 2002, 23, 2359–2365. [Google Scholar] [CrossRef]
- Cenni, E.; Granchi, D.; Ciapetti, G.; Savarino, L.; Corradini, A.; Vancini, M.; Giunti, A. Gene expression of bone-associated cytokines in MG63 osteoblast-like cells incubated with acrylic bone cement extracts in minimum essential medium. J. Biomater. Sci. Polym. Ed. 2002, 13, 1283–1294. [Google Scholar] [CrossRef] [PubMed]
- Granchi, D.; Ciapetti, G.; Filippini, F.; Stea, S.; Elisabettacenni, E.; Pizzoferrato, A.; Toni, A. Modulation of pro- and anti-apoptotic genes in lymphocytes exposed to bone cements. J. Biomater. Sci. Polym. Ed. 2000, 11, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Tsukeoka, T.; Suzuki, M.; Ohtsuki, C.; Sugino, A.; Tsuneizumi, Y.; Miyagi, J.; Kuramoto, K.; Moriya, H. Mechanical and histological evaluation of a PMMA-based bone cement modified with gamma-methacryloxypropyltrimethoxysilane and calcium acetate. Biomaterials 2006, 27, 3897–3903. [Google Scholar] [CrossRef] [PubMed]
| Bone Cement Materials | Constituents |
|---|---|
| PMMA-BPO1 (Surgical Simplex P, Stryker, Kalamazoo, MI) | Powder: Polymethyl methacrylate (PMMA) (15.00%) Methyl methacrylate-styrene copolymer (73.70%) Benzoyl peroxide (BPO) initiator (1.30%) Barium sulfate (10.00%) Liquid: Methyl methacrylate (MMA) (97.40%) N,N-dimethyl p-toluidine (DmpT) (2.60%) Hydroquinone (HQ) (75 ppm) |
| PMMA-BPO2 (Endurance MV, DePuy Synthes, Warsaw, IN) | Powder: Polymethyl methacrylate (PMMA) (67.05%) Methyl methacrylate/styrene copolymer (21.10%) Benzoyl peroxide (BPO) initiator (1.85%) Barium sulfate (10.00%) Liquid: Methyl methacrylate (MMA) (98.00%) N,N-dimethyl-p-toluidine (DmpT)(< 2.00%) Hydroquinone (HQ) (75 ppm) |
| PMMA-TBB (Experimental) | Powder: Polymethyl methacrylate (PMMA) (90.00%) Barium sulfate (10.00%) Liquid: Methyl methacrylate (MMA) (91.00%) tri-n-butyl borane (TBB) initiator (9.00%) Hydroquinone (HQ) (50 ppm) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saruta, J.; Ozawa, R.; Hamajima, K.; Saita, M.; Sato, N.; Ishijima, M.; Kitajima, H.; Ogawa, T. Prolonged Post-Polymerization Biocompatibility of Polymethylmethacrylate-Tri-n-Butylborane (PMMA-TBB) Bone Cement. Materials 2021, 14, 1289. https://doi.org/10.3390/ma14051289
Saruta J, Ozawa R, Hamajima K, Saita M, Sato N, Ishijima M, Kitajima H, Ogawa T. Prolonged Post-Polymerization Biocompatibility of Polymethylmethacrylate-Tri-n-Butylborane (PMMA-TBB) Bone Cement. Materials. 2021; 14(5):1289. https://doi.org/10.3390/ma14051289
Chicago/Turabian StyleSaruta, Juri, Ryotaro Ozawa, Kosuke Hamajima, Makiko Saita, Nobuaki Sato, Manabu Ishijima, Hiroaki Kitajima, and Takahiro Ogawa. 2021. "Prolonged Post-Polymerization Biocompatibility of Polymethylmethacrylate-Tri-n-Butylborane (PMMA-TBB) Bone Cement" Materials 14, no. 5: 1289. https://doi.org/10.3390/ma14051289
APA StyleSaruta, J., Ozawa, R., Hamajima, K., Saita, M., Sato, N., Ishijima, M., Kitajima, H., & Ogawa, T. (2021). Prolonged Post-Polymerization Biocompatibility of Polymethylmethacrylate-Tri-n-Butylborane (PMMA-TBB) Bone Cement. Materials, 14(5), 1289. https://doi.org/10.3390/ma14051289






