Fluorescence Spectroscopy Study of Protoporphyrin IX in Optical Tissue Simulating Liquid Phantoms
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Liquid Phantom Samples Preparation
3. Results and Discussion
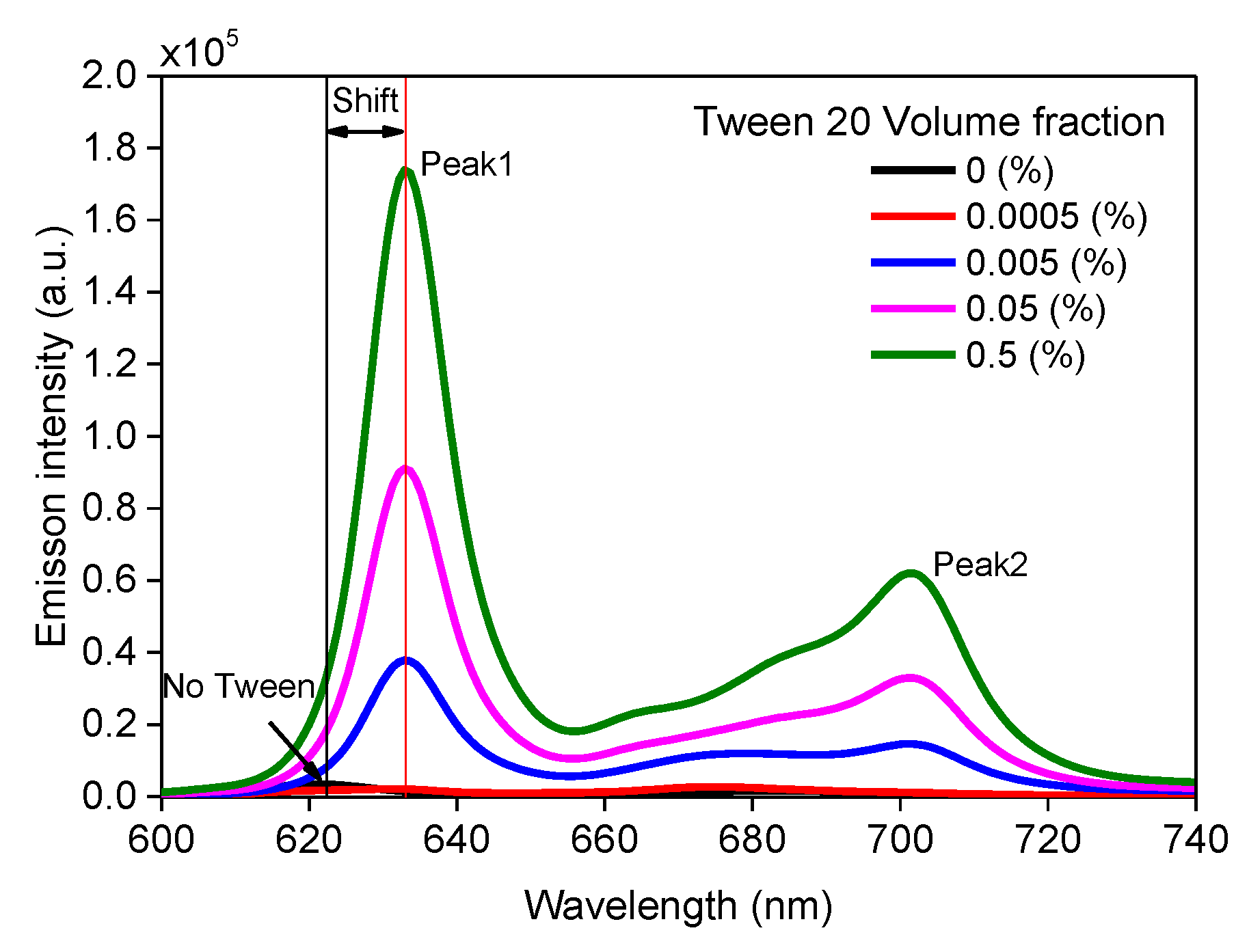
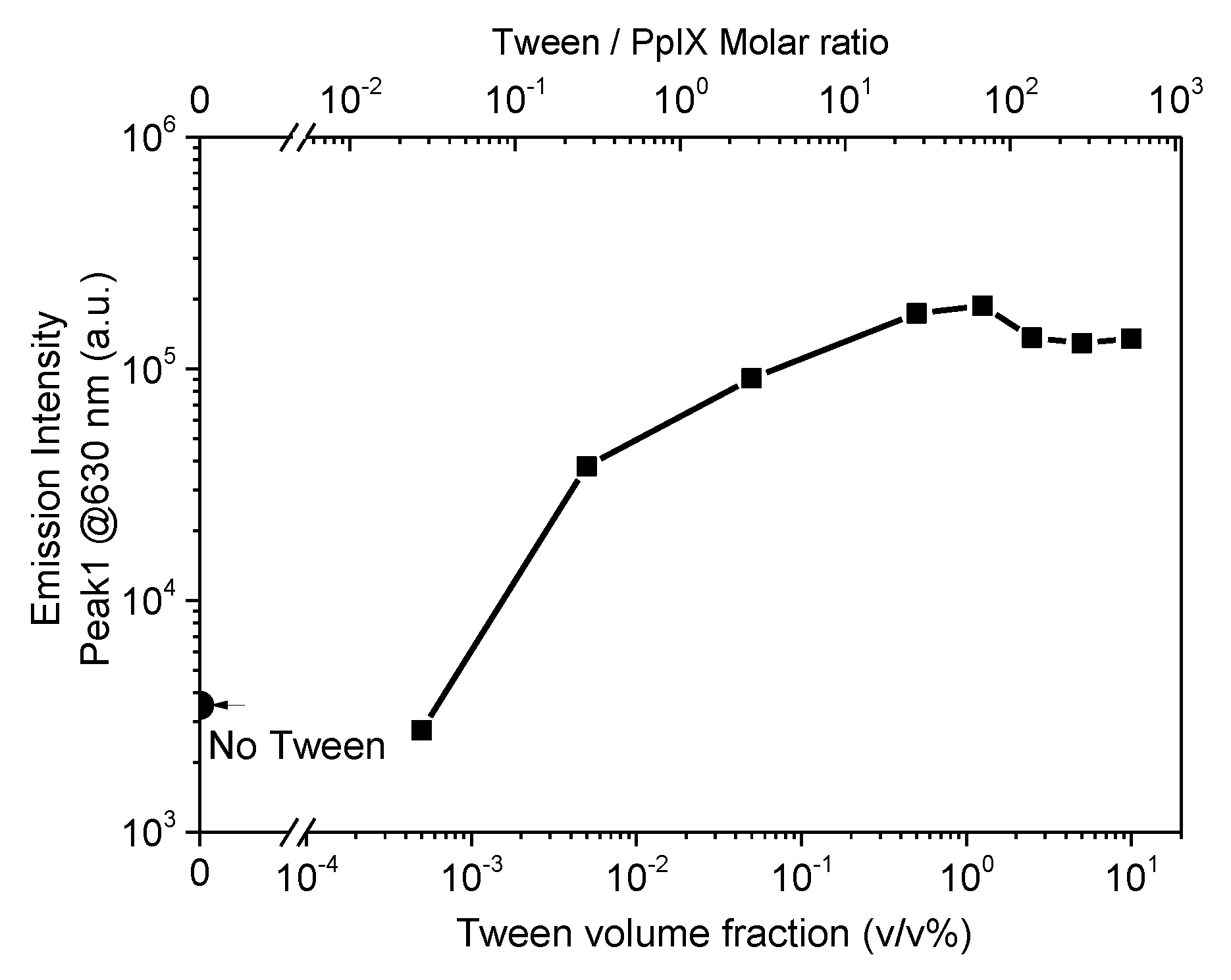
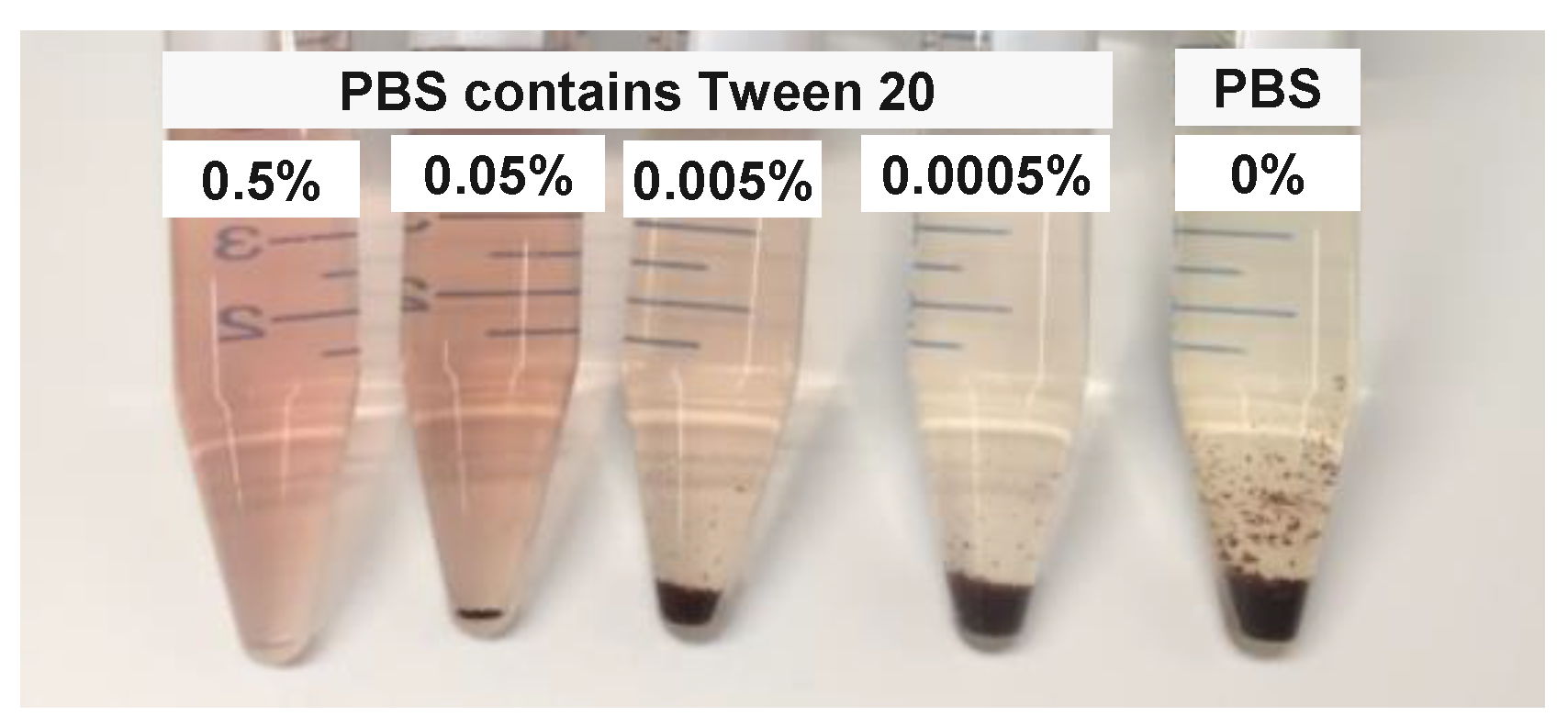
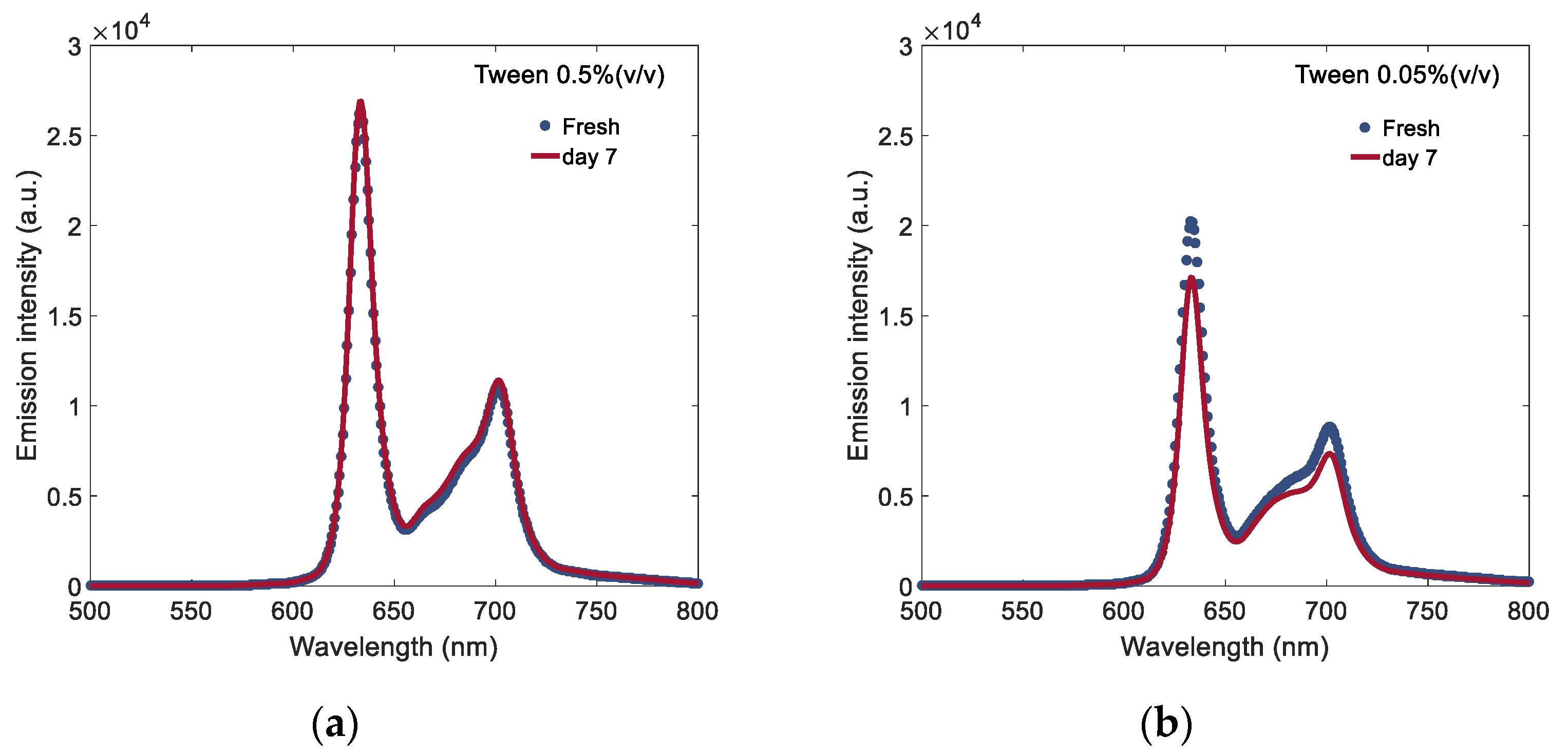
3.1. Effect of Surfactant on PpIX Fluorescence in Non-Turbid Phantom
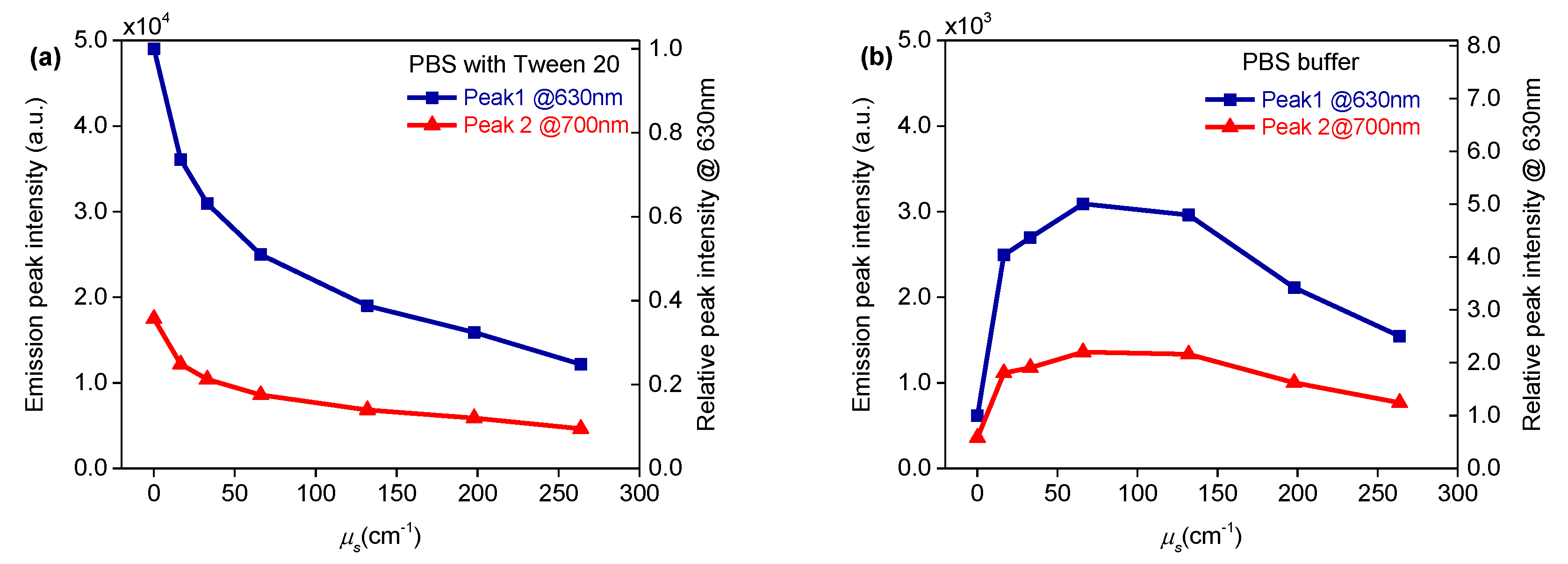
3.2. Effect of Scattering on PpIX Fluorescence
3.3. Effect of Combined Scattering and Absorption on PpIX Fluorescence
3.4. Temporal Stability Study of PpIX Fluorescence
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dsouza, A.V.; Lin, H.; Henderson, E.R.; Samkow, K.S.; Pogue, B.W. Review of fluorescence guided surgery systems: Identification of key performance capabilities beyond indocyanine green imaging. J. Biomed. Opt. 2016, 21, 080901. [Google Scholar] [CrossRef]
- Breslin, T.M.; Xu, F.; Palmer, G.M.; Zhu, C.; Gilchrist, K.W.; Ramanujam, N. Autofluorescence and diffuse reflectance properties of malignant and benign breast tissue. Ann. Surg. Oncol. 2003, 11, 65–70. [Google Scholar] [CrossRef]
- Jiang, Y.; Girard, E.J.; Pakiam, F.; Seibel, E.J. Calibration of fluorescence imaging for tumor surgical margin delineation: Multistep registration of fluorescence and histological images. J. Med. Imaging 2019, 6, 025005. [Google Scholar]
- Toms, S.A.; Konrad, P.E.; Lin, W.-C.; Weil, R.J. Neuro-oncological applications of optical spectroscopy. Technol. Cancer Rese. Treat. 2006, 5, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Zhang, Z.-W.; Yoo, K.M.; Ali, M.A.; Alfano, R.R. Effect of multiple light scattering and self-absorption on the fluorescence and excitation spectra of dyes in random media. Appl. Opt. 1994, 33, 2746–2750. [Google Scholar] [CrossRef] [PubMed]
- Anand, B.S.S.; Sujatha, N. Effect of intralipid-10% in fluorescence distortion studies on liquid-tissue phantoms in UV range. J. Biophotonics 2011, 4, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.G.; Georgakoudi, I.; Zhang, Q.; Wu, J.; Feld, M.S. Intrinsic fluorescence spectroscopy in turbid media: Disentangling effects of scattering and absorption. Appl. Opt. 2001, 40, 4633–4646. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Gayen, S.K.; Xu, M. Fluorescence spectroscopy using excitation and emission matrix for quantification of tissue native fluorophores and cancer diagnosis. SPIE BiOS 2014, 8926, 89261. [Google Scholar]
- Sachar, M.; Anderson, K.E.; Ma, X. Protoporphyrin IX: The good, the bad, and the ugly. J. Pharmacol. Exp. Ther. 2016, 356, 267–275. [Google Scholar] [CrossRef]
- Valdés, P.A.; Roberts, D.W.; Lu, F.-K.; Golby, A. Optical technologies for intraoperative neurosurgical guidance. Neurosurg. Focus. 2016, 40, E8. [Google Scholar] [CrossRef]
- Xie, H.; Xie, Z.; Mousavi, M.; Bendsoe, N.; Brydegarrd, M.; Axeisson, J.; Andersson-Engels, S. Design and validation of a fiber optic point probe instrument for therapy guidance and monitoring. J. Biomed. Opt. 2014, 19, 071408. [Google Scholar] [CrossRef]
- Haj-Hosseini, N.; Richter, J.; Andersson-Engels, S.; Wårdell, K. Optical touch pointer for fluorescence guided glioblastoma resection using 5-aminolevulinic acid. Laser Surg. Med. 2010, 42, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Marois, M.; Bravo, J.; Davis, S.C.; Kanick, S.C. Characterization and standardization of tissue-simulating protoporphyrin IX optical phantoms. J. Biomed. Opt. 2016, 21, 035003. [Google Scholar] [CrossRef] [PubMed]
- Pogue, B.W.; Patterson, M.S. Review of tissue simulating phantoms for optical spectroscopy, imaging and dosimetry. J. Biomed. Opt. 2006, 11, 041102. [Google Scholar] [CrossRef] [PubMed]
- Inamura, I.; Isshiki, M.; Araki, T. Bull. Solubilization of hemi in neutral and acidic aqueous solution by forming complexes with water-soluble macromolecules. Chem. Soc. Jpn. 1989, 62, 2413–2415. [Google Scholar] [CrossRef]
- Scolaro, L.M.; Castriciano, M.; Romeo, A.; Patane, S.; Cefalı, E.; Allegrini, M. Aggregation behavior of protoporphyrin IX in aqueous solutions: Clear evidence of vesicle formation. J. Phys. Chem. B 2002, 106, 2453–2459. [Google Scholar] [CrossRef]
- Maiti, N.C.; Krishna, M.M.G.; Britto, P.J.; Periasamy, N. Fluorescence dynamics of dye probes in micelles. J. Phys. Chem. B 1997, 101, 11051–11060. [Google Scholar] [CrossRef]
- Markwardt, N.K.; Hai-Hosseini, N.; Hollnburger, B.; Stepp, H.; Zelenkov, P.; Rühm, A. 405nm versus 633 nm for protoporphyrin IX excitation in fluorescence-guided sterootactic biopsy of brain tumors. J. Biophotonics 2016, 9, 901–912. [Google Scholar] [CrossRef]
- Wagnieres, G.A.; Willem, M.; Wilson, B.C. ln vivo fluorescence spectroscopy and imaging for neuro oncological applications. Photochem. Photobiol. 1998, 68, 603–632. [Google Scholar] [CrossRef]
- Johansson, A.; Faber, F.; Kniebühler, G.; Stepp, H.; Sroka, R.; Egensperger, R.; Beyer, W.; Kreth, F.-W. Protoporphyrin IX fluorescence and photobleaching during interstitial photodynamic therapy of malignant gliomas for early treatment prognosis. Laser Surg. Med. 2013, 45, 225–234. [Google Scholar] [CrossRef]
- Lu, H.; Floris, F.; Rensing, M.; Andersson-Engels, S. Fluorescence spectroscopy study of protoporphyrin IX in tissue-like phantoms. SPIE-Intl. Soc. Opt. Eng. 2019, 11190, 111901S. [Google Scholar]
- Wilson, B.C.; Jacques, S.L. Optical reflectance and transmittance of tissues: Principles and applications. IEEE J. Quantum Electron. 1990, 26, 2186–2199. [Google Scholar] [CrossRef]
- Michels, R.; Foschum, F.; Kienle, A. Optical properties of fat emulsions. Opt. Express 2008, 16, 5907–5925. [Google Scholar] [CrossRef]
- Ninni, P.D.; Martelli, F.; Zaccanti, G. The use of India ink in tissue-simulating phantoms. Opt. Express 2010, 18, 26854–26865. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Gunther, J.; Andersson-Engles, S. Determination optical properties of tissue-like phantoms using diffuse reflectance and transmittance spectroscopy. Opt. Health Care Biomed. Opt. 2018, 10820, 108202X. [Google Scholar]
- Gunther, J.; Lu, H.; Andersson-Engles, S. Combination of diffuse reflectance and transmittance spectroscopy to obtain optical properties of liquid phantoms. Opt. Eng. 2020, 59, 024109. [Google Scholar] [CrossRef]
- Zehentbauer, F.M.; Moretto, C.; Stephen, R.; Thevar, T.; Gilchrist, J.R.; Pokrajac, D.; Richard, K.L.; Kiefer, J. Fluorescence spectroscopy of Rhodamine 6G: Concentration and solvent effects. Spectrochim. Acta A 2014, 121, 147–151. [Google Scholar] [CrossRef]
- Suah, F.B.M.; Ahmad, M.; Mehamod, F.S. Effect of non-ionic surfactants to the Al (III)-morin complex and its application in determination of Al (III) ions: A preliminary study. Malays. J. Anal. Sci. 2017, 21, 793–800. [Google Scholar]
- Yang, R.-H.; Wang, K.-M.; Xiao, D.; Yang, X.-H.; Li, H.-M. A selective optical chemical sensor for the determination of Tween-60 based on fluorescence enhancement of tetraphenyporphyrin. Anal. Chim. Acta 2000, 404, 205–211. [Google Scholar] [CrossRef]
- Stocker, S.; Foschun, F.; Krauter, P.; Bergmann, F.; Hohmann, A.; Happ, C.S.; Kienle, A. Broadband optical properties of milk. Appl. Spectrosc. 2017, 71, 951–962. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Floris, F.; Rensing, M.; Andersson-Engels, S. Fluorescence Spectroscopy Study of Protoporphyrin IX in Optical Tissue Simulating Liquid Phantoms. Materials 2020, 13, 2105. https://doi.org/10.3390/ma13092105
Lu H, Floris F, Rensing M, Andersson-Engels S. Fluorescence Spectroscopy Study of Protoporphyrin IX in Optical Tissue Simulating Liquid Phantoms. Materials. 2020; 13(9):2105. https://doi.org/10.3390/ma13092105
Chicago/Turabian StyleLu, Huihui, Francesco Floris, Marc Rensing, and Stefan Andersson-Engels. 2020. "Fluorescence Spectroscopy Study of Protoporphyrin IX in Optical Tissue Simulating Liquid Phantoms" Materials 13, no. 9: 2105. https://doi.org/10.3390/ma13092105
APA StyleLu, H., Floris, F., Rensing, M., & Andersson-Engels, S. (2020). Fluorescence Spectroscopy Study of Protoporphyrin IX in Optical Tissue Simulating Liquid Phantoms. Materials, 13(9), 2105. https://doi.org/10.3390/ma13092105






