Novel Hybrid Composites Based on PVA/SeTiO2 Nanoparticles and Natural Hydroxyapatite for Orthopedic Applications: Correlations between Structural, Morphological and Biocompatibility Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication and Structural Characterization of Se-Doped TiO2 Nanoparticles
2.2. Fabrication of PVA/HA/Se-Doped TiO2 Composites
2.3. Structural Characterization of PVA/HA/Se-TiO2 Composites
2.4. In Vitro Cells Culture
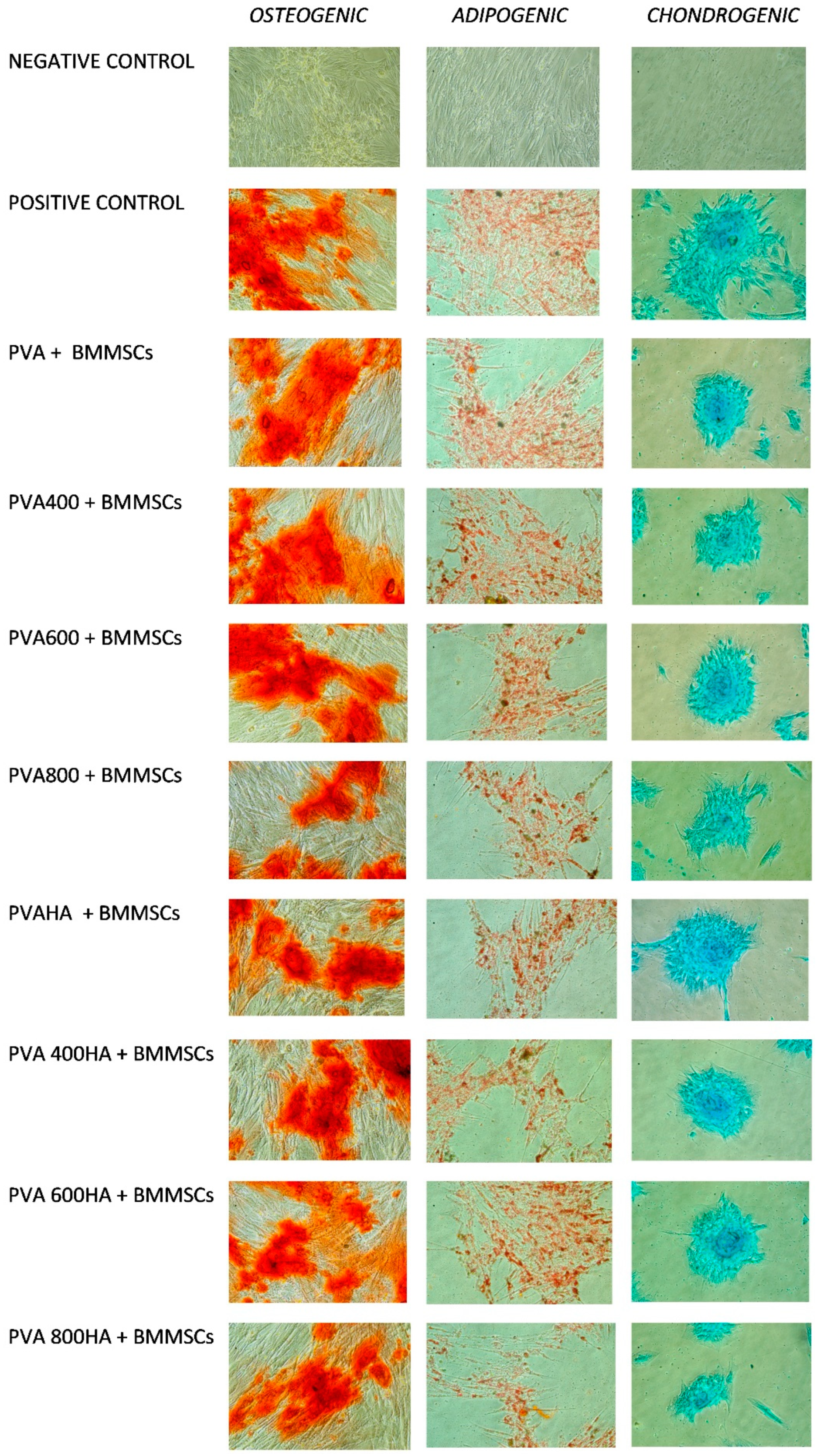
2.4.1. Differentiation in the Different Lineages
- (1)
- Osteogenic differentiation: 3 weeks of stimulation in αMEM containing 10% FBS, ascorbic acid 50 µg/mL, ß-glycerophosphate 10 mM and dexamethasone 10−7 M, the negative control being the same cells grown in αMEM containing only 10% FBS. After osteogenic differentiation, calcium deposits were detected by staining with Alizarin Red S (Carlo Erba, Val de Reuil, France). The cells were fixed for one hour at room temperature in a 70% ethanol solution. Subsequently, they were washed with distilled water and then coated with a 2% solution of Alizarin Red for 30 min, so the calcium deposits were marked in orange-red.
- (2)
- Adipogenic differentiation: 2 weeks of stimulation in αMEM containing 10% FBS, dexamethasone 1 µM, insulin 10 µg/mL, indomethacin 0.2 mM and 3-isobutyl-methyl-xanthine 0.5 mM. After adipogenic differentiation, the lipid vacuoles accumulated in the cytoplasm were highlighted by Oil Red O (Alfa Aesar) staining. The cells were fixed with a 10% formalin solution for 1 h at room temperature, then washed and stained with Oil Red O.
- (3)
- Chondrogenic differentiation: 3 weeks of stimulation with αMEM containing 1% FBS, 0.1 µM dexamethasone, 6.25 µg/mL insulin, 50 nM ascorbic acid, 10 ng/mL of TGFα. Alcian Blue (Sigma-Aldrich) staining was applied after chondrogenic differentiation, in order to highlight intra- and extracellular glycosaminoglycans. After fixation with a 10% formalin for 1 h, the cells were washed and stained with Alcian Blue for 15 min.
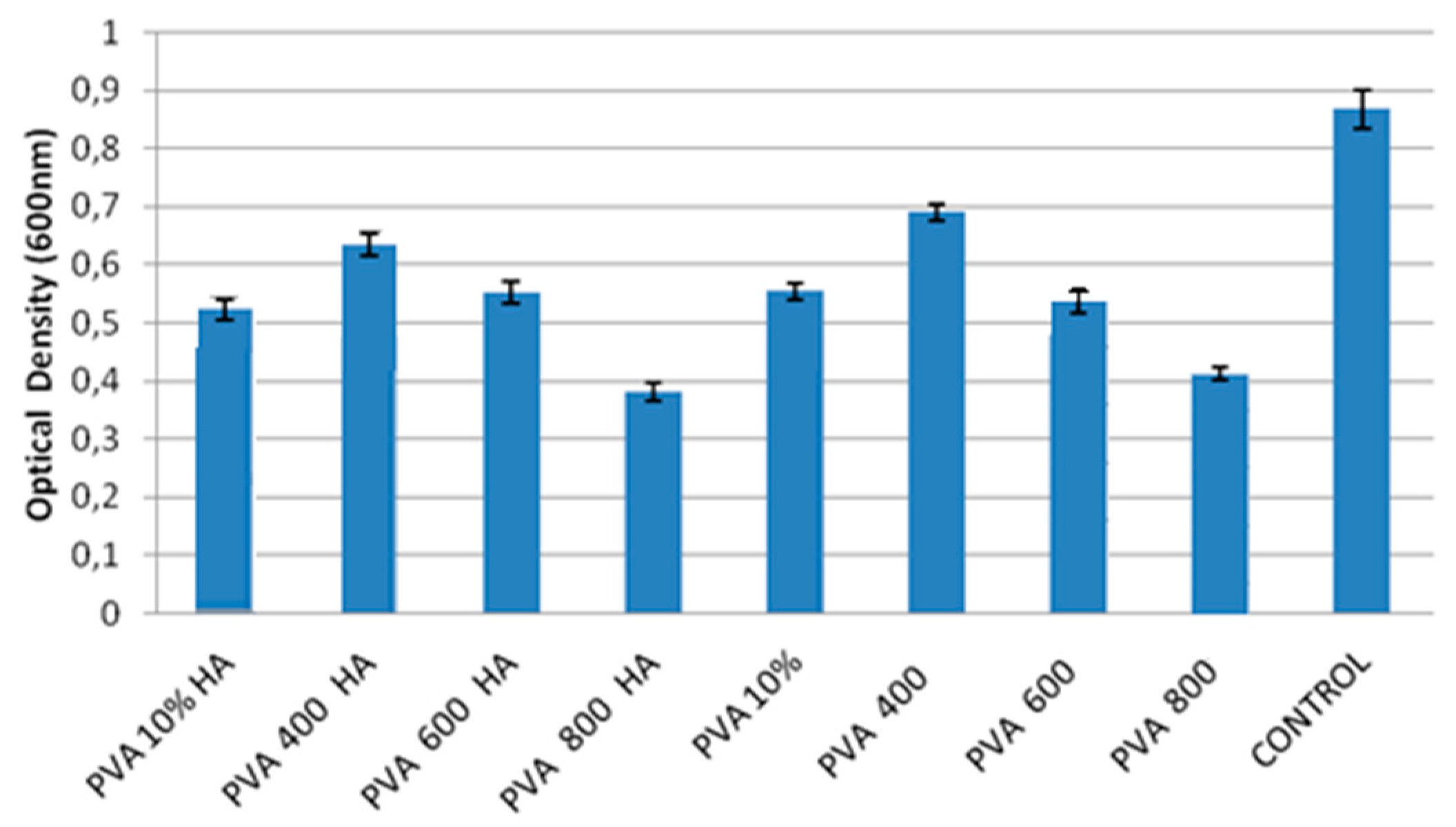
2.4.2. Viability Test by MTT Assay
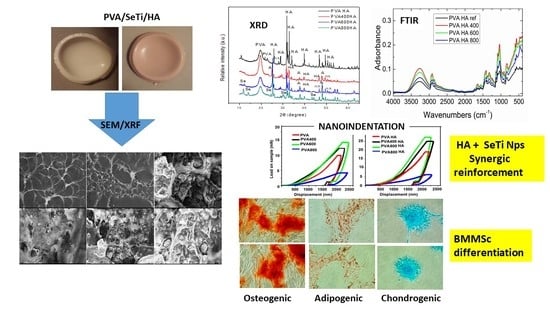
3. Results
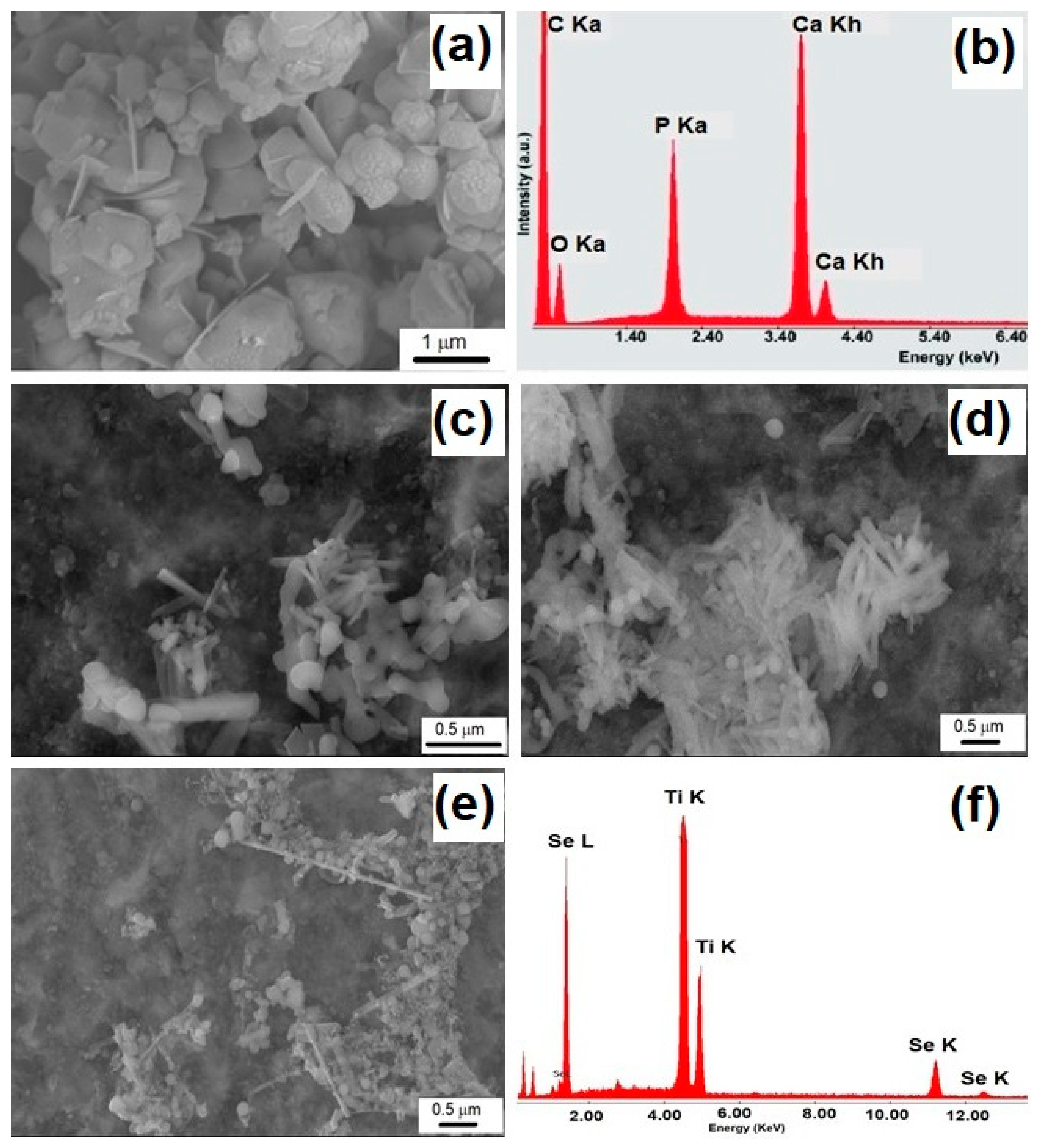
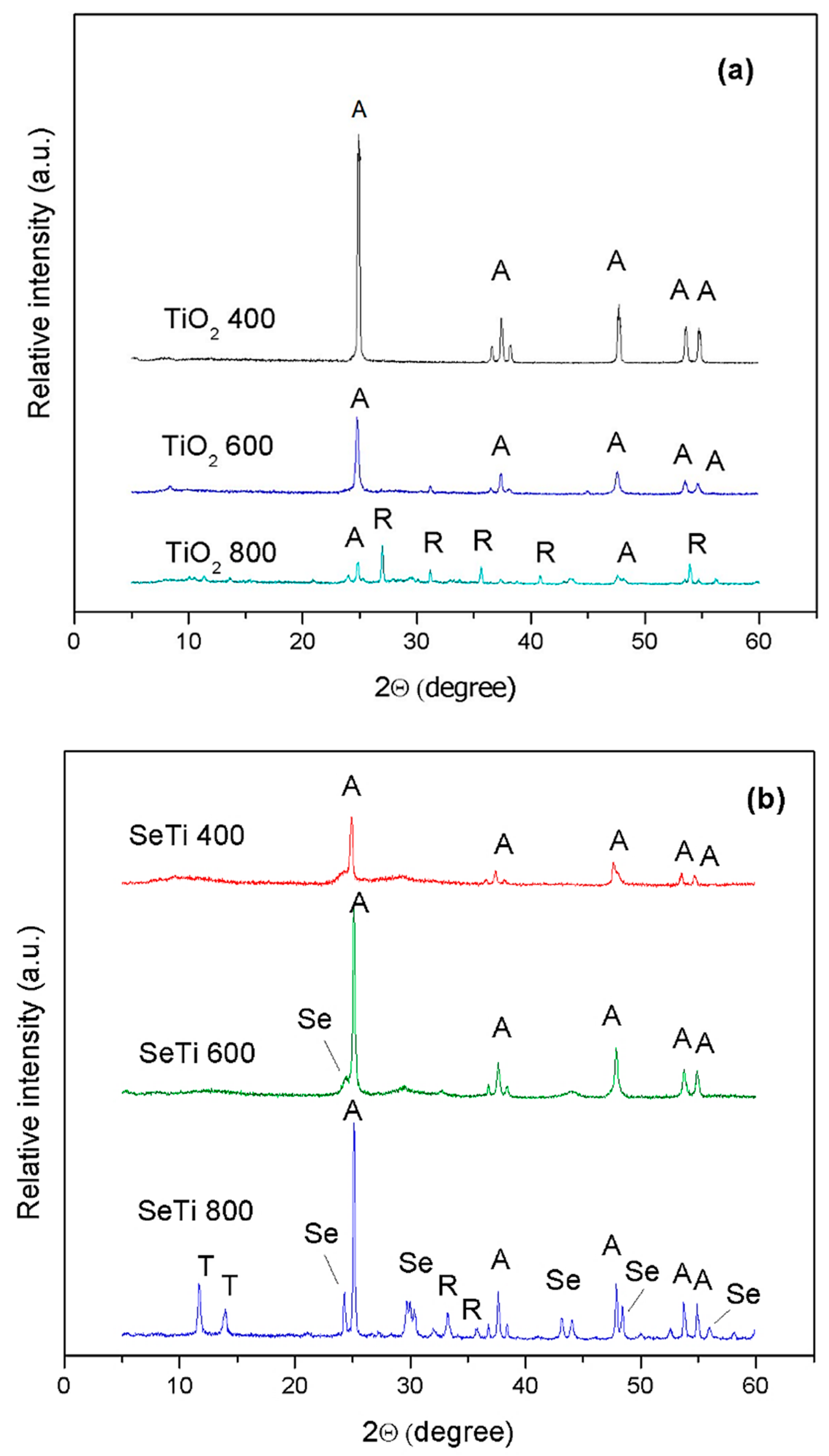
3.1. Characterization of Se-Doped TiO2 Particles
3.2. Structural and Morphological Characterization of PVA/Se-TiO2 and PVA/HA/SeTiO2 Composites
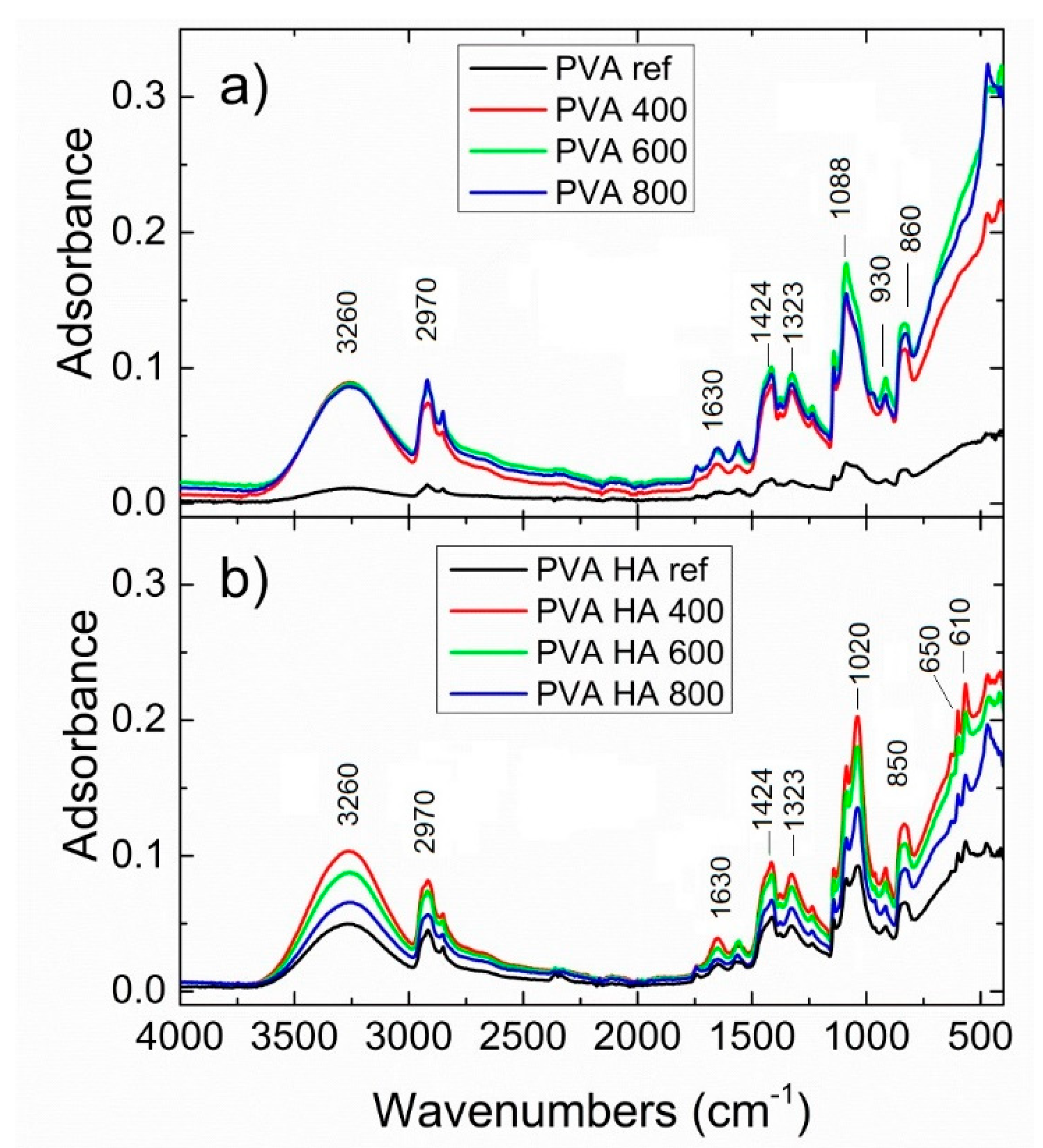
3.2.1. FTIR Spectroscopy of PVA Composites
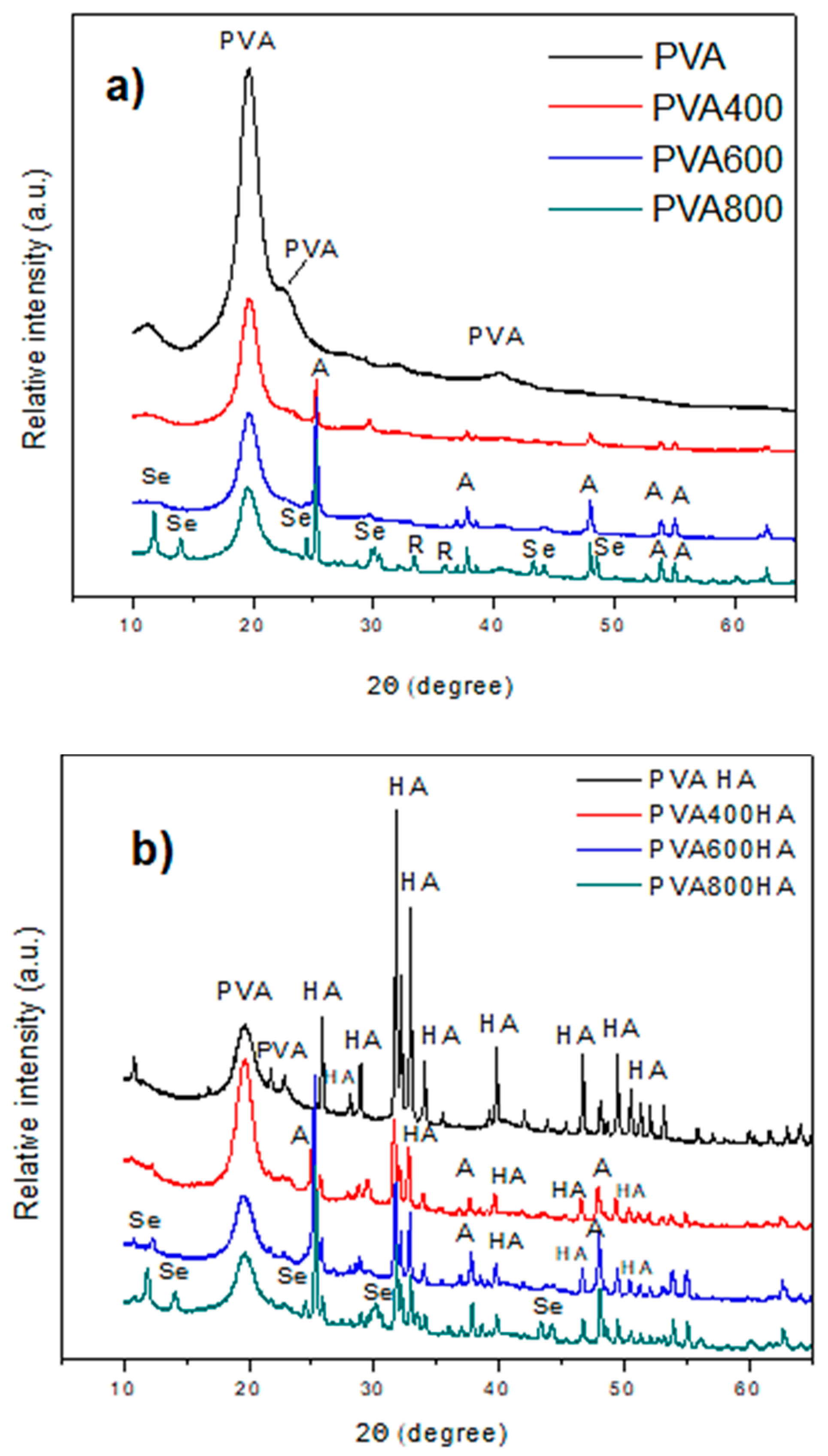
3.2.2. XRD Patterns of PVA Composites
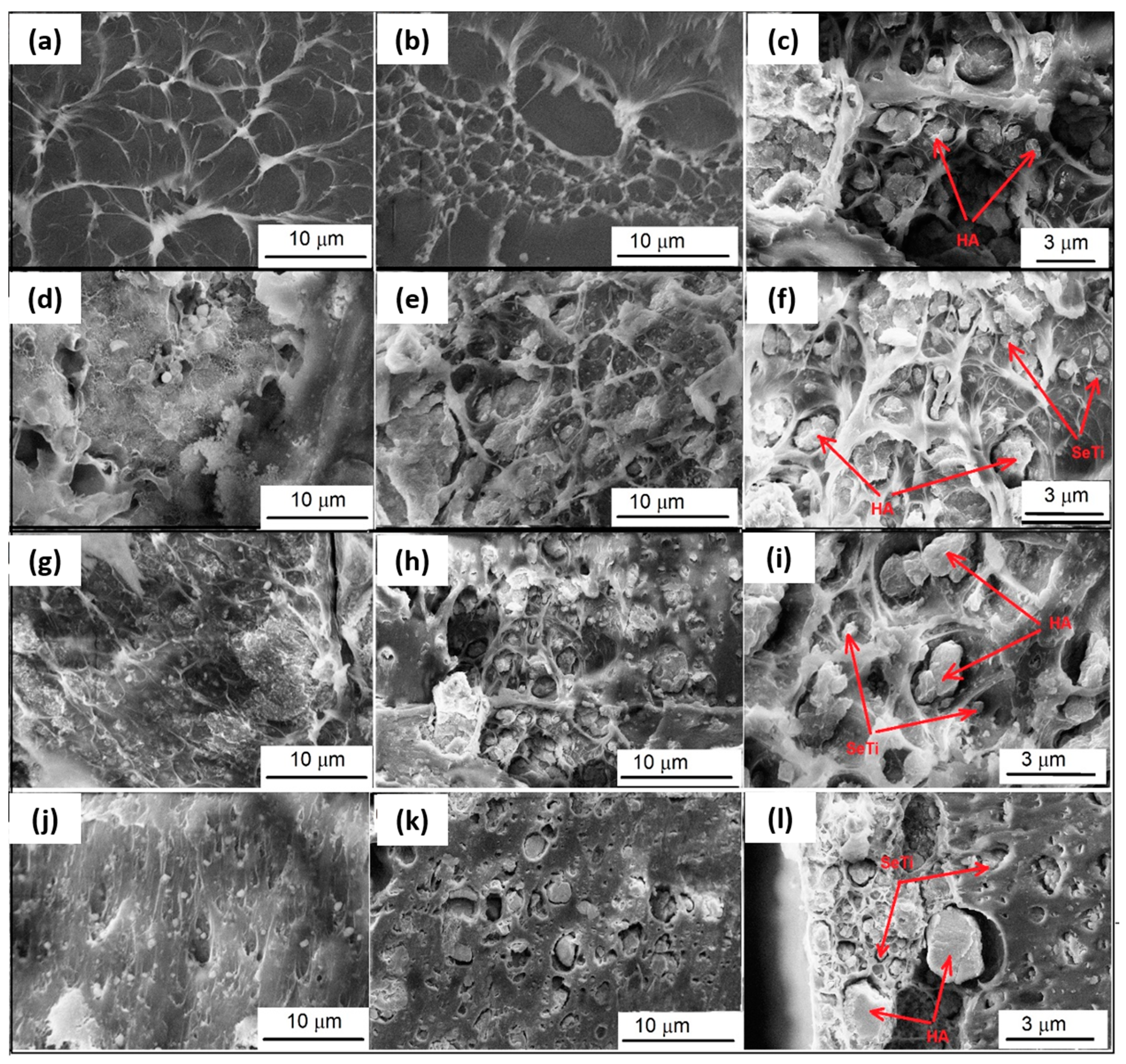
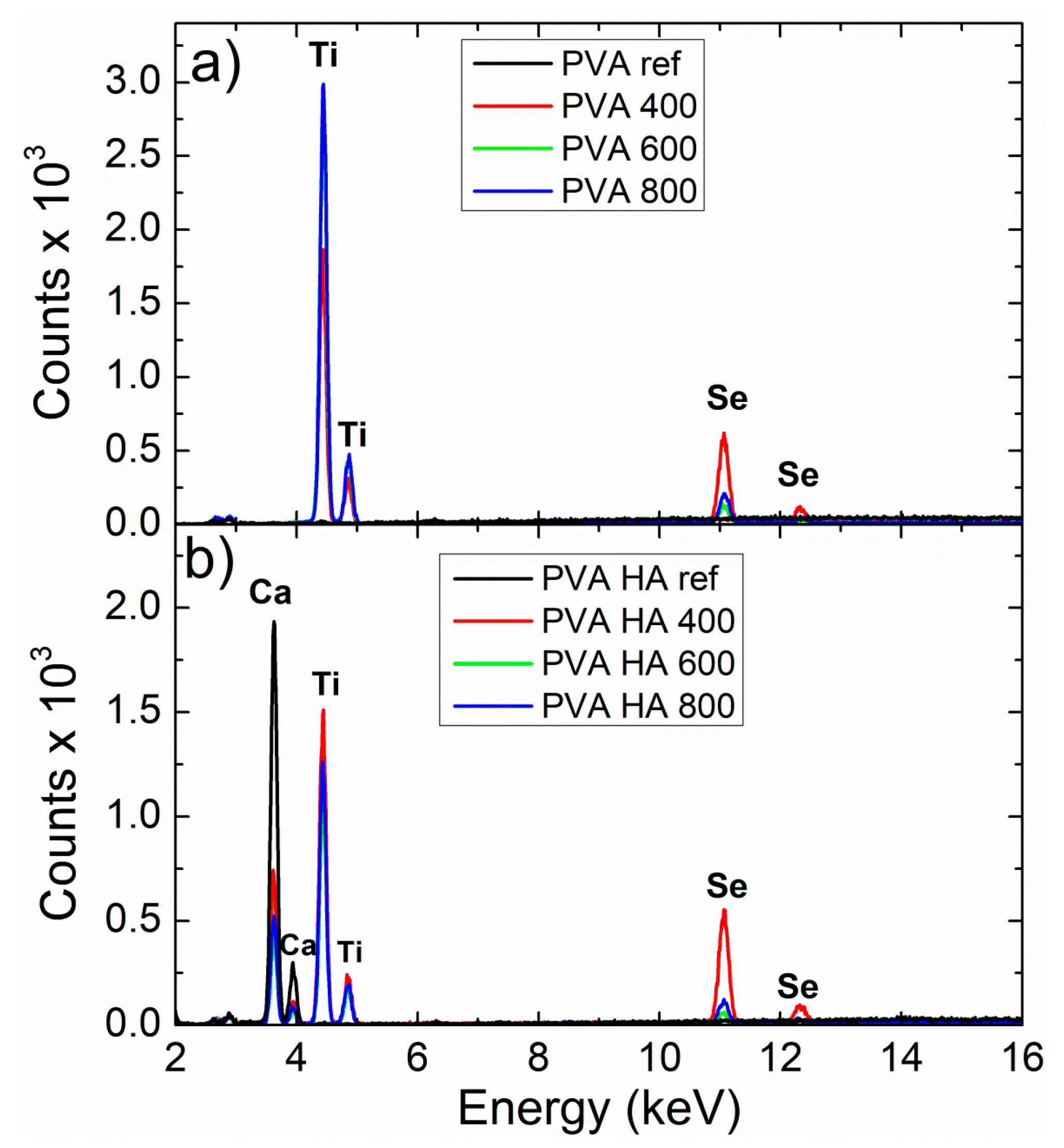
3.2.3. Morphological Characterization by SEM
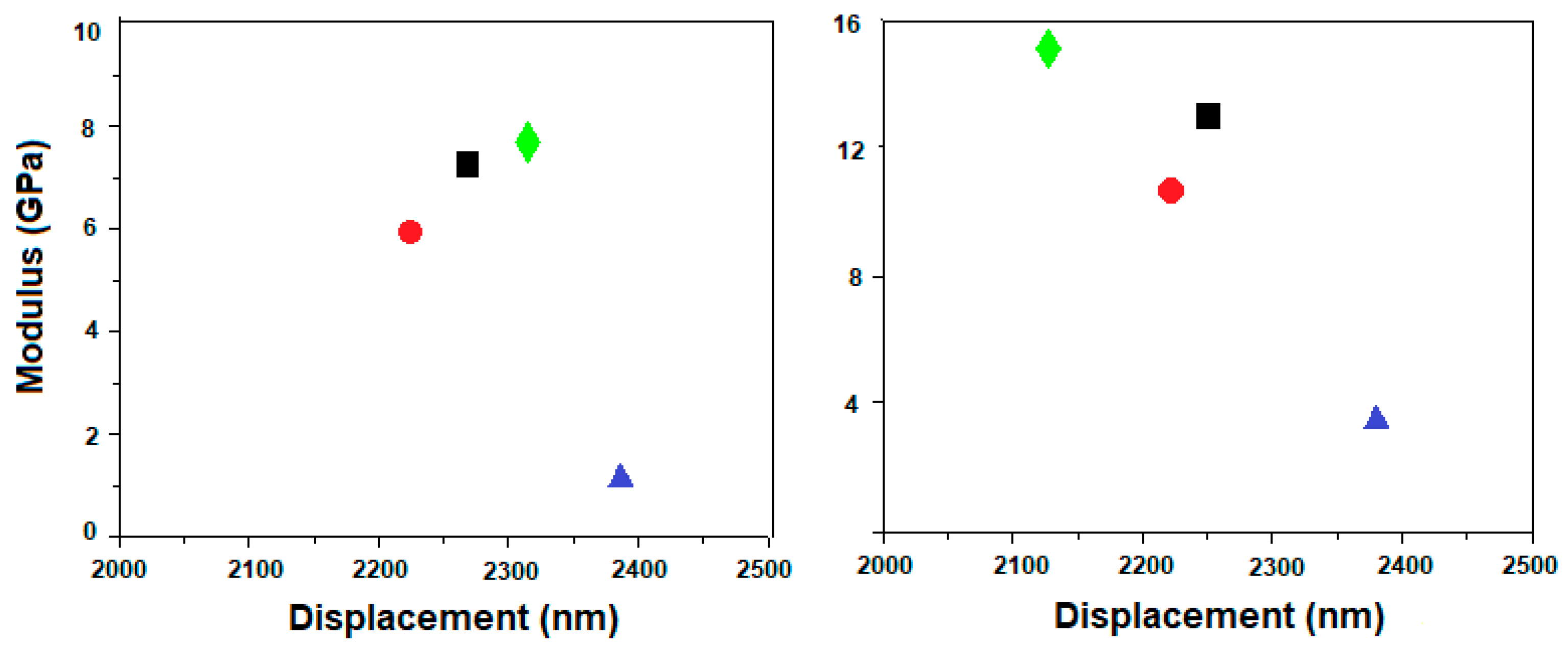
3.3. Nanoindentation Measurements
3.4. In Vitro Cells Culture, BMMSC Viability, Differentiation and Proliferation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of Poly(vinyl alcohol) and Natural Polymers. Polym. Rev. 2018, 58, 247–287. [Google Scholar] [CrossRef]
- Mohanapriya, S.; Mumjitha, M.; Babitha, S.; PurnaSai, K.; Raj, V. Fabrication and Characterization of Poly(vinyl alcohol)-TiO2 Nanocomposite films for Orthopaedic applications. J. Mech. Behav. Biomed. 2016, 63, 141–156. [Google Scholar] [CrossRef]
- Gu, Z.Q.; Xiao, J.M.; Zhang, X.H. The development of artificial articular cartilage–PVA-hydrogel. Bio-Med. Mater. Eng. 1998, 8, 75–81. [Google Scholar] [PubMed]
- Oka, M.; Ushio, K.; Kumar, P.; Ikeuchi, K.; Hyon, S.H.; Nakamura, T.; Fujita, H. Development of artificial articular cartilage. Proc. Inst. Mech. Eng. H 2000, 214, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Hyon, S.H.; Cha, W.; Ikada, Y.; Kita, M.; Ogura, Y.; Honda, Y. Poly(vinyl alcohol) hydrogels as soft contact lens material. J. Biomater. Sci. Polym. Ed. 1994, 5, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Musgrave, C.S.A.; Fang, F. Contact Lens Materials: A Materials Science Perspective. Materials 2019, 12, 261. [Google Scholar] [CrossRef]
- Amri, C.; Mudasir, M.; Siswanta, D.; Roto, R. In Vitro hemocompatibility of PVA-alginate ester as a candidate for hemodialysis membrane. Int. J. Biol. Macromol. 2016, 82, 48–53. [Google Scholar] [CrossRef]
- Paradossi, G.; Cavalieri, F.; Chiessi, E. Poly(vinyl alcohol) as versatile biomaterial for potential biomedical applications. J. Mater. Sci. Mater. Med. 2003, 14, 687–691. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. Part B 2012, 100, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Sonker, A.K.; Rathore, K.; Nagarale, R.K.; Verma, V. Crosslinking of Polyvinyl Alcohol (PVA) and Effect of Crosslinker Shape (Aliphatic and Aromatic) Thereof. J. Polym. Environ. 2018, 26, 1782–1794. [Google Scholar] [CrossRef]
- Nkhwa, S.; Lauriaga, K.F.; Kemal, E.; Deb, S. Poly(vinyl alcohol): Physical Approaches to Designing Biomaterials for Biomedical Applications. Conf. Pap. Sci. 2014, 403472. [Google Scholar] [CrossRef]
- Oviedo, I.R.; Mendez, N.A.N.; Gomez, M.P.G.; Rodriguez, H.C.; Martinez, A.R. Design of a physical and nontoxic crosslinked poly(vinyl alcohol) hydrogel. Int. J. Polym. Mater. 2008, 57, 1095–1103. [Google Scholar] [CrossRef]
- Ye, M.; Mohanty, P.; Ghosh, G. Morphology and properties of poly vinyl alcohol (PVA) scaffolds: Impact of process variables. Mat. Sci. Eng. C-Mater. 2014, 42, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Kempson, G.E.; Muir, H.; Pollard, C.; Tuke, M. The tensile properties of the cartilage of human femoral condyles related to the content of collagen and glycosaminoglycans. Biochim. Biophys. Acta 1973, 297, 456–472. [Google Scholar] [CrossRef]
- Almarza, A.J.; Athanasiou, K.A. Design characteristics for the tissue engineering of cartilaginous tissues. Ann. Biomed. Eng. 2004, 32, 2–17. [Google Scholar] [CrossRef]
- Rau, J.V.; Antoniac, I.; Cama, G.; Komlev, V.S.; Ravaglioli, A. Bioactive Materials for Bone Tissue Engineering. Special issue “Bioactive Materials for Bone Tissue Engineering”. BioMed Res. Int. 2016, 3741428. [Google Scholar] [CrossRef]
- Shuai, C.; Shuai, C.; Feng, P.; Gao, C.; Peng, S.; Yang, Y. Antibacterial Capability, Physicochemical Properties, and Biocompatibility of nTiO2 Incorporated Polymeric Scaffolds. Polymers 2018, 10, 328. [Google Scholar] [CrossRef]
- Gutwein, L.G.; Webster, T.J. Osteoblast and chondrocyte proliferation in the presence of alumina and titania nanoparticles. J. Nanopart. Res. 2002, 4, 231–238. [Google Scholar] [CrossRef]
- Davar, F.; Majedi, A.; Mirzaei, A. Polyvinyl alcohol thin film reinforced by green synthesized zirconia nanoparticles. Ceram. Int. 2018, 44, 19377–19382. [Google Scholar] [CrossRef]
- Kokabi, M.; Sirousazar, M.; Hassan, Z.M. PVA–clay nanocomposite hydrogels for wound dressing. Eur. Polym. J. 2007, 43, 773–781. [Google Scholar] [CrossRef]
- Lee, J.; Jin Lee, K.; Jang, J. Effect of silica nanofillers on isothermal crystallization of poly(vinyl alcohol): In-Situ ATR-FTIR study. Polym. Test. 2008, 27, 360–367. [Google Scholar] [CrossRef]
- Mallakpour, S.; Barati, A. Efficient preparation of hybrid nanocomposite coatings based on poly(vinyl alcohol) and silane coupling agent modified TiO2 nanoparticles. Prog. Org. Coat 2011, 71, 391–398. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M. Enhancement in thermal properties of poly(vinyl alcohol) nanocomposites reinforced with Al2O3 nanoparticles. J. Reinf. Plast. Compos. 2013, 32, 217–224. [Google Scholar] [CrossRef]
- Hong, K.H.; Park, J.L.; Sul, I.H.; Youk, J.H.; Kang, T.J. Preparation of antimicrobial poly(vinyl alcohol) nanofibers containing silver nanoparticles. J. Polym. Sci. Polym. Phys. 2006, 44, 2468–2474. [Google Scholar] [CrossRef]
- Pan, Y.; Xiong, D.; Gao, F. Viscoelastic behavior of nano-hydroxyapatite reinforced poly(vinyl alcohol) gel biocomposites as an articular cartilage. J. Mater. Sci. Mater. Med. 2008, 19, 1963–1969. [Google Scholar] [CrossRef]
- Cheng, H.K.F.; Sahoo, N.G.; Tan, Y.P.; Pan, Y.; Bao, H.; Li, L.; Chan, S.H.; Zhao, J. Poly(vinyl alcohol) nanocomposites filled with poly(vinyl alcohol)-grafted graphene oxide. ACS Appl. Mater. Interfaces 2012, 4, 2387–2394. [Google Scholar] [CrossRef]
- Bray, J.C.; Merrill, E.W. Poly(vinyl alcohol) hydrogels for synthetic articular cartilage material. J. Biomed. Mater. Res. 1973, 7, 431–443. [Google Scholar] [CrossRef]
- Cavalu, S.; Kamel, E.; Laslo, V.; Fritea, L.; Costea, T.; Antoniac, I.V.; Eugeniu, V.; Antoniac, A.; Semenescu, A.; Mohan, A.; et al. Eco-friendly, facile and rapid way for synthesis of selenium nanoparticles production, structural and morphological characterization. Rev. Chim-Buchar. 2017, 68, 2963–2966. [Google Scholar] [CrossRef]
- Cavalu, S.; Vicas, S.; Costea, T.; Fritea, L.; Copolovici, D.; Laslo, V. Preparation, Physico-chemical Characterization and Photocatalytic Properties of Se-Doped TiO2 Nanoparticles. Rev. Chim. Buchar. 2020, 71, 22–27. [Google Scholar] [CrossRef]
- Turkmen, A.K.; Cavalu, S.; Goller, G. Development of Chitosan-Hydroxyapatite-Fibrinogen 3D Scaffolds for Bone Tissue Regeneration. J. Aust. Ceram. Soc. 2016, 52, 34–41. [Google Scholar]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Ratiu, C.; Brocks, M.; Costea, T.; Moldovan, L.; Cavalu, S. PRGF-Modified Collagen Membranes for Guided Bone Regeneration: Spectroscopic, Microscopic and Nano-Mechanical Investigations. Appl. Sci. 2019, 9, 1035. [Google Scholar] [CrossRef]
- Rau, J.V.; Fadeeva, I.V.; Fomin, A.S.; Barbaro, K.; Galvano, E.; Ryzhov, A.P.; Murzakhanov, F.; Gafurov, M.; Orlinskii, S.; Antoniac, I.; et al. Sic Parvis Magna: Manganese-substituted tricalcium phosphate and its biophysical properties. ACS Biomater. Sci. Eng. 2019, 5, 6632–6644. [Google Scholar] [CrossRef]
- Zavala, M.A.L.; Morales, S.A.L.; Avila-Santos, M. Synthesis of stable TiO2 nanotubes: Effect of hydrothermal treatment, acid washing and annealing temperature. Helyon 2017, 3, e00456. [Google Scholar] [CrossRef]
- Nolan, N.; Pillai, S.; Seery, M. Spectroscopic Investigation of the Anatase-to-Rutile Transformation of Sol-Gel Synthesised TiO2 Photocatalysts. J. Phys. Chem. C 2009, 113, 16151–16157. [Google Scholar] [CrossRef]
- Fresneda, M.A.R.; Martín, J.D.; Bolívar, J.G.; Cantos, M.V.F.; Germán Bosch-Estévez, G.; Moreno, M.F.M.; Merroun, M.L. Green synthesis and biotransformation of amorphous Se nanospheres to trigonal 1D Se nanostructures: Impact on Se mobility within the concept of radioactive waste disposal. Environm. Sci. Nano 2018, 5, 1. [Google Scholar] [CrossRef]
- Cavalu, S.; Prokisch, J.; Laslo, V.; Vicas, S. Preparation, structural characterisation and release study of novel hybrid microspheres entrapping nanoselenium, produced by green synthesis. IET Nanobiotechnol. 2017, 11, 426–432. [Google Scholar] [CrossRef]
- Aphairaj, D.; Wirunmongkol, T.; Pavasupree, S.; Limsuwana, P. Effect of calcination temperatures on structures of TiO2 powders prepared by hydrothermal method using Thai leucoxene mineral. Energy Proced. 2011, 9, 539–544. [Google Scholar] [CrossRef]
- Praveen, P.; Viruthagiri, G.; Mugundan, S.; Shanmugam, N. Structural, optical and morphological analyses of pristine titanium di-oxide nanoparticles--synthesized via sol-gel route. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 117, 622–629. [Google Scholar] [CrossRef]
- Balgová, Z.; Palou, M.; Wasserbauer, J.; Kozánková, J. Synthesis of poly(vinyl alcohol)–hydroxyapatite composites and characterization of their bioactivity. Cent. Eur. J. Chem. 2013, 11, 1403–1411. [Google Scholar] [CrossRef]
- Nguyen, L.T.B.; Lee, K.H.; Lee, B.T. Fabrication of photocatalytic PVA-TiO2 nano-fibrous hybrid membrane using electro-spinning method. J. Mater. Sci. 2011, 46, 5615–5620. [Google Scholar] [CrossRef]
- Stipniece, L.; Salma-Ancane, K.; Rjabovs, V.; Juhnevica, I.; Turks, M.; Narkevica, I.; Berzina-Cimdina, L. Development of functionalized hydroxyapatite/poly(vinyl alcohol) composites. J. Cryst. Growth. 2016, 444, 14–20. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Ramahdita, G.; Puspita, D.M.; Albab, M.F.; Alfata, R.; Sofyan, N.; Yuwono, A.H. The effect of hydroxyapatite addition on the mechanical properties of polyvinyl alcohol/chitosan biomaterials for bone scaffolds application. AIP Conf. Proc. 2018, 1933, 020006. [Google Scholar] [CrossRef]
- Tao Guo, T.; Tian, X.; Li, B.; Yang, T.; Li, Y. Repair of articular cartilage and subchondral defects in rabbit knee joints with a polyvinyl alcohol/nanohydroxyapatite/polyamide biological composite material. J. Orthop. Surg. Res. 2017, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Duan, J.; Wang, D.; Ge, S. Effect of Preparation Methods on Mechanical Properties of PVA/HA Composite Hydrogel. J. Bionic Eng. 2010, 7, 235–243. [Google Scholar] [CrossRef]
- Gonzalez, J.S.; Alvarez, V.A. The effect of the annealing on the poly (vinyl alcohol) obtained by freezing–thawing. Thermochim. Acta 2011, 521, 184–190. [Google Scholar] [CrossRef]
- Nanda, G.S.; Kuo, F.C.H.; Li, L.; Siew, H.C.; Judeh, Z.; Zhao, J. Specific Functionalization of carbon nanotubes for advanced polymer nanocomposites. Adv. Funct. Mater. 2009, 19, 3962–3971. [Google Scholar] [CrossRef]
- Liu, W.; Deng, X.; Hickey, D.; Ziemer, K.S.; Li, H.; Webster, T.J. Selenium nanoparticles incorporated into titania nanotubes inhibit bacterial growth and macrophage proliferation. Nanoscale 2016, 8, 15783–15794. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, H.; Li, Y.; Zhang, S. Selenium-substituted hydroxyapatite nanoparticles and their in vivo antitumor effect on hepatocellular carcinoma. Colloid. Surf. B 2016, 140, 297–306. [Google Scholar] [CrossRef]
- Minaev, V.S.; Timoshenkov, S.P.; Kalugin, V.V. Structural and Phase Transformation in Condensed Selenium. J. Optoelectron. Adv. Mater. 2005, 7, 1717–1741. [Google Scholar]
- Wu, G.; Su, B.; Zhang, W.; Wang, C. In Vitro behaviors of hydroxyapatite reinforced polyvinyl alcohol hydrogel composite. Mater. Chem. Phys. 2008, 107, 364–369. [Google Scholar] [CrossRef]
- Boschetti, F.; Pennati, G.; Gervaso, F.; Peretti, G.M.; Dubini, G. Biomechanical properties of human articular cartilage under compressive loads. Biorheology 2004, 41, 159–166. [Google Scholar]
- Ferara, T.L.; Boughton, P.; Slavich, E.; Wroe, S. A novel method for single multi-axial nanoindentation of hydrated heterogeneous tissues based on testing great white shark jaws. PLoS ONE. 2013, 8, e81196. [Google Scholar] [CrossRef]
- Wahab, A.H.A.; Kadir, M.R.A.; Harun, M.N.; Ramlee, M.H.; Syahrom, A.; Sulong, M.A.; Saad, A.P.M. Developing Functionally Graded PVA Hydrogel using Simple Freeze-Thaw Method for Artificial Glenoid Labrum. J. Mech. Behav. Biomed. 2019, 91, 406–415. [Google Scholar] [CrossRef]
- Broitman, E. Indentation hardness measurements at macro-, micro-, and nanoscale: A critical overview. Tribol. Lett. 2019, 65, 23. [Google Scholar] [CrossRef]
- Leach, J.K.; Whitehead, J. Materials-Directed Differentiation of Mesenchymal Stem Cells for Tissue Engineering and Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 1115–1127. [Google Scholar] [CrossRef]
- Carrion, B.; Souzanchi, M.F.; Wang, V.T.; Tiruchinapally, G.; Shikanov, A.; Putnam, A.J.; Coleman, R.M. The synergistic effects of matrix stiffness and composition on the response of chondroprogenitor cells in a 3D precondensation microenvironment. Adv. Health Mater. 2016, 5, 1192–1202. [Google Scholar] [CrossRef]
- Ahmed, H.H.; Aglan, H.A.; Mabrouk, M.; Abd-Rabou, A.A.; Beherei, H.H. Enhanced mesenchymal stem cell proliferation through complexation of selenium/titanium nanocomposites. J. Mater. Sci. Mater. M 2019, 30, 24. [Google Scholar] [CrossRef]
- Mazaheri, M.; Eslahi, N.; Ordikhani, F.; Tamjid, E.; Simchi, A. Nanomedicine applications in orthopedic medicine: State of the art. Int. J. Nanomed. 2015, 10, 6039–6054. [Google Scholar]
- Wang, Y.; Ma, J.; Zhou, L.; Chen, J.; Liu, Y.; Qiu, Z.; Zhang, S. Dual functional selenium-substituted Hydroxyapatite. Interface Focus. 2012, 2, 378–386. [Google Scholar] [CrossRef]
- Chen, X.; Cai, K.; Lai, M.; Zhao, L.; Tang, L. Mesenchymal stem cell differentiation on hierarchically micro/nano-structured titanium substrates. Adv. Eng. Mater. 2012, 14, B216–B223. [Google Scholar] [CrossRef]
- Perla, V.; Webster, T.J. Better osteoblast adhesion on nanoparticulate selenium-a promising orthopedic implant material. J. Biomed. Mater. Res. A 2005, 75, 356–364. [Google Scholar] [CrossRef]
- Hassan, C.E.; Webster, T.J. The effect of red-allotrope selenium nanoparticles on head and neck squamous cell viability and growth. Int. J. Nanomed. 2016, 11, 3641–3654. [Google Scholar]
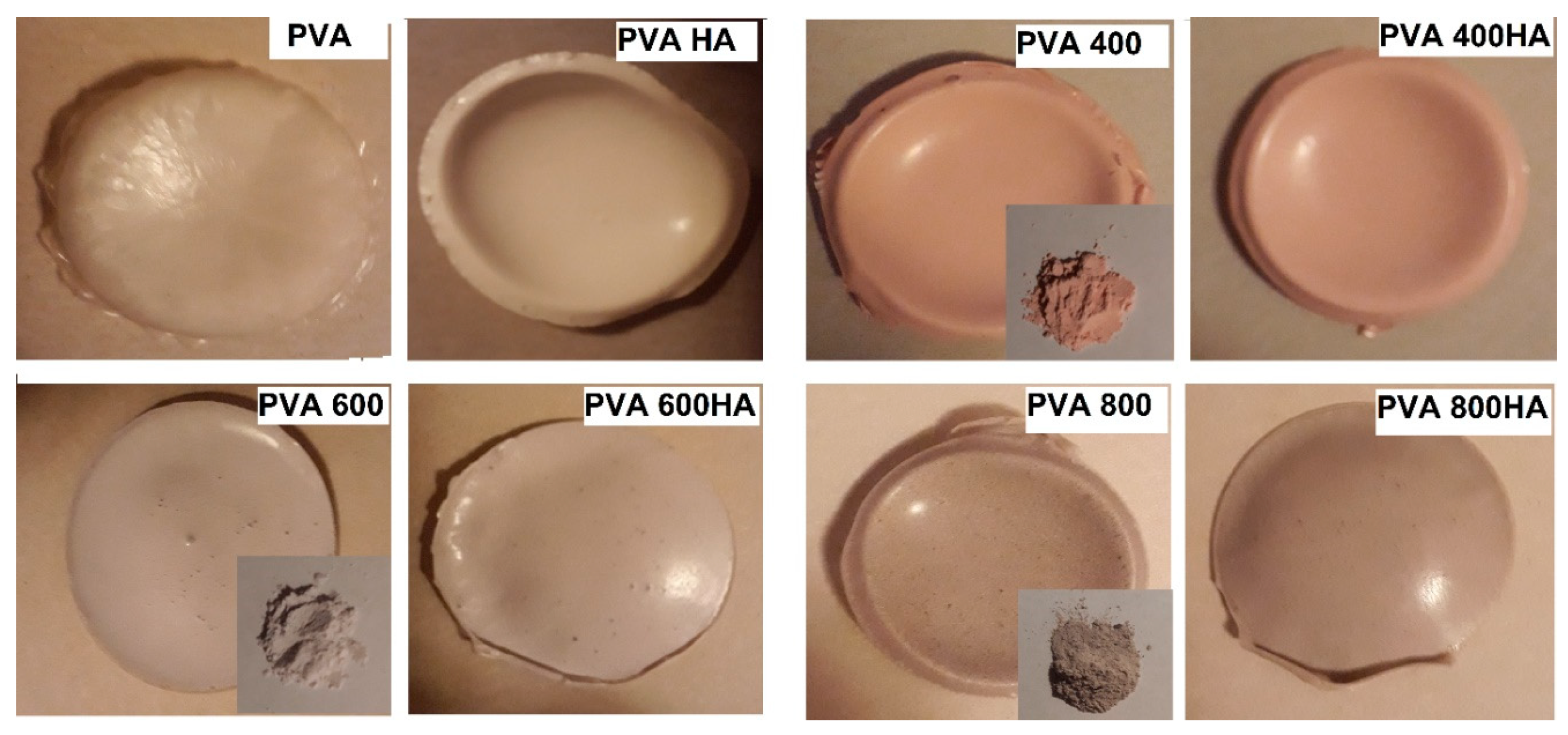
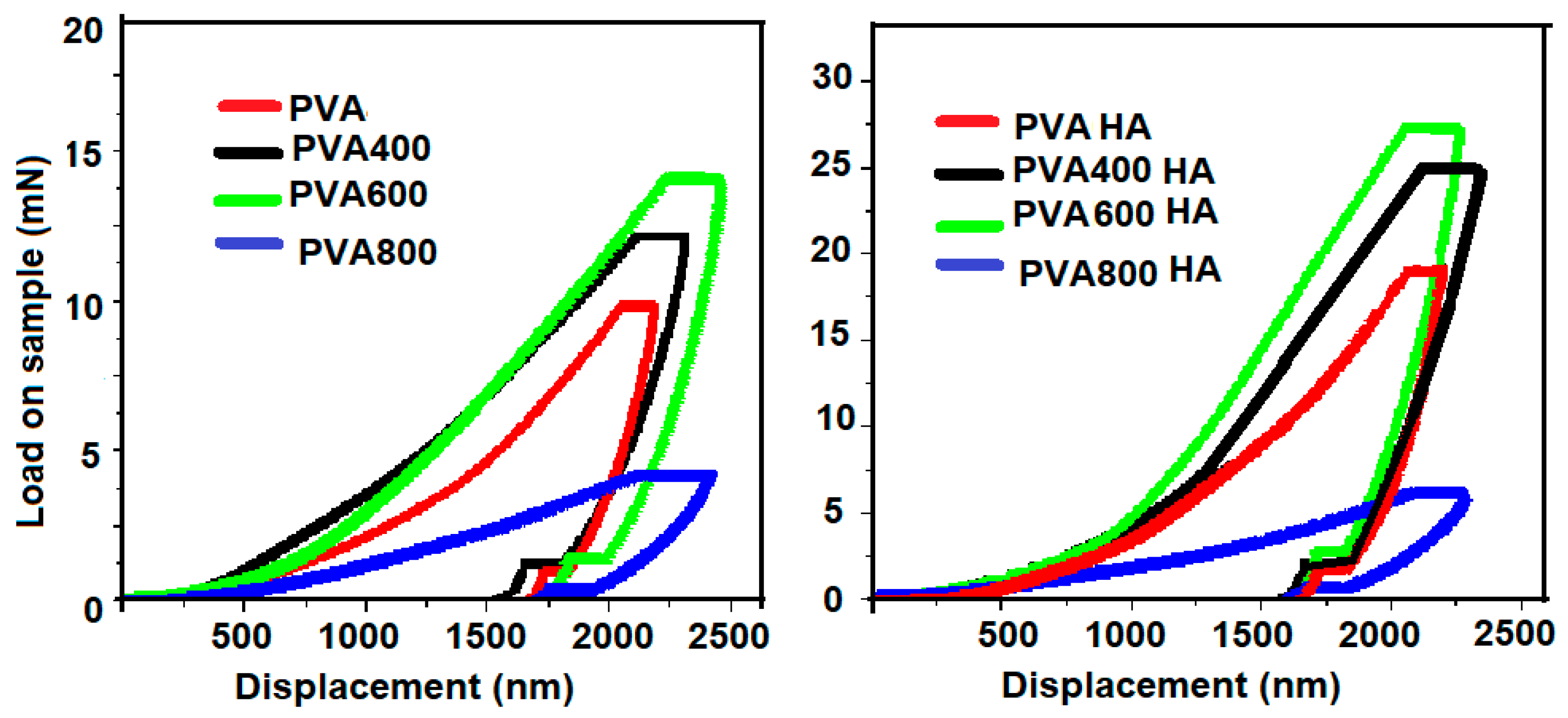
 ; PVA400 and PVA400HA
; PVA400 and PVA400HA  ; PVA600 and PVA600HA
; PVA600 and PVA600HA  ; PVA800 and PVA800HA
; PVA800 and PVA800HA  .
.
 ; PVA400 and PVA400HA
; PVA400 and PVA400HA  ; PVA600 and PVA600HA
; PVA600 and PVA600HA  ; PVA800 and PVA800HA
; PVA800 and PVA800HA  .
.
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalu, S.; Fritea, L.; Brocks, M.; Barbaro, K.; Murvai, G.; Costea, T.O.; Antoniac, I.; Verona, C.; Romani, M.; Latini, A.; et al. Novel Hybrid Composites Based on PVA/SeTiO2 Nanoparticles and Natural Hydroxyapatite for Orthopedic Applications: Correlations between Structural, Morphological and Biocompatibility Properties. Materials 2020, 13, 2077. https://doi.org/10.3390/ma13092077
Cavalu S, Fritea L, Brocks M, Barbaro K, Murvai G, Costea TO, Antoniac I, Verona C, Romani M, Latini A, et al. Novel Hybrid Composites Based on PVA/SeTiO2 Nanoparticles and Natural Hydroxyapatite for Orthopedic Applications: Correlations between Structural, Morphological and Biocompatibility Properties. Materials. 2020; 13(9):2077. https://doi.org/10.3390/ma13092077
Chicago/Turabian StyleCavalu, Simona, Luminita Fritea, Marcel Brocks, Katia Barbaro, Gelu Murvai, Traian Octavian Costea, Iulian Antoniac, Claudio Verona, Martina Romani, Alessandro Latini, and et al. 2020. "Novel Hybrid Composites Based on PVA/SeTiO2 Nanoparticles and Natural Hydroxyapatite for Orthopedic Applications: Correlations between Structural, Morphological and Biocompatibility Properties" Materials 13, no. 9: 2077. https://doi.org/10.3390/ma13092077
APA StyleCavalu, S., Fritea, L., Brocks, M., Barbaro, K., Murvai, G., Costea, T. O., Antoniac, I., Verona, C., Romani, M., Latini, A., Zilli, R., & Rau, J. V. (2020). Novel Hybrid Composites Based on PVA/SeTiO2 Nanoparticles and Natural Hydroxyapatite for Orthopedic Applications: Correlations between Structural, Morphological and Biocompatibility Properties. Materials, 13(9), 2077. https://doi.org/10.3390/ma13092077