Novel Yttria-Stabilized Zirconium Oxide and Lithium Disilicate Coatings on Titanium Alloy Substrate for Implant Abutments and Biomedical Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Surface Preparation
2.3. Reagents for Coatings
2.4. Preparation of Stabilized Zirconium Oxide (3YSZ) and Lithium Disilicate (LS2) Sols
2.5. Deposition of 3YSZ and LS2 Coatings
2.6. Surface Morphology
2.7. Water Contact Angle and Surface Free Energy
2.8. Cell Culture
2.9. Biocompatibility Evaluation
2.10. Cellular Adhesion
2.11. Statistical Analysis
3. Results
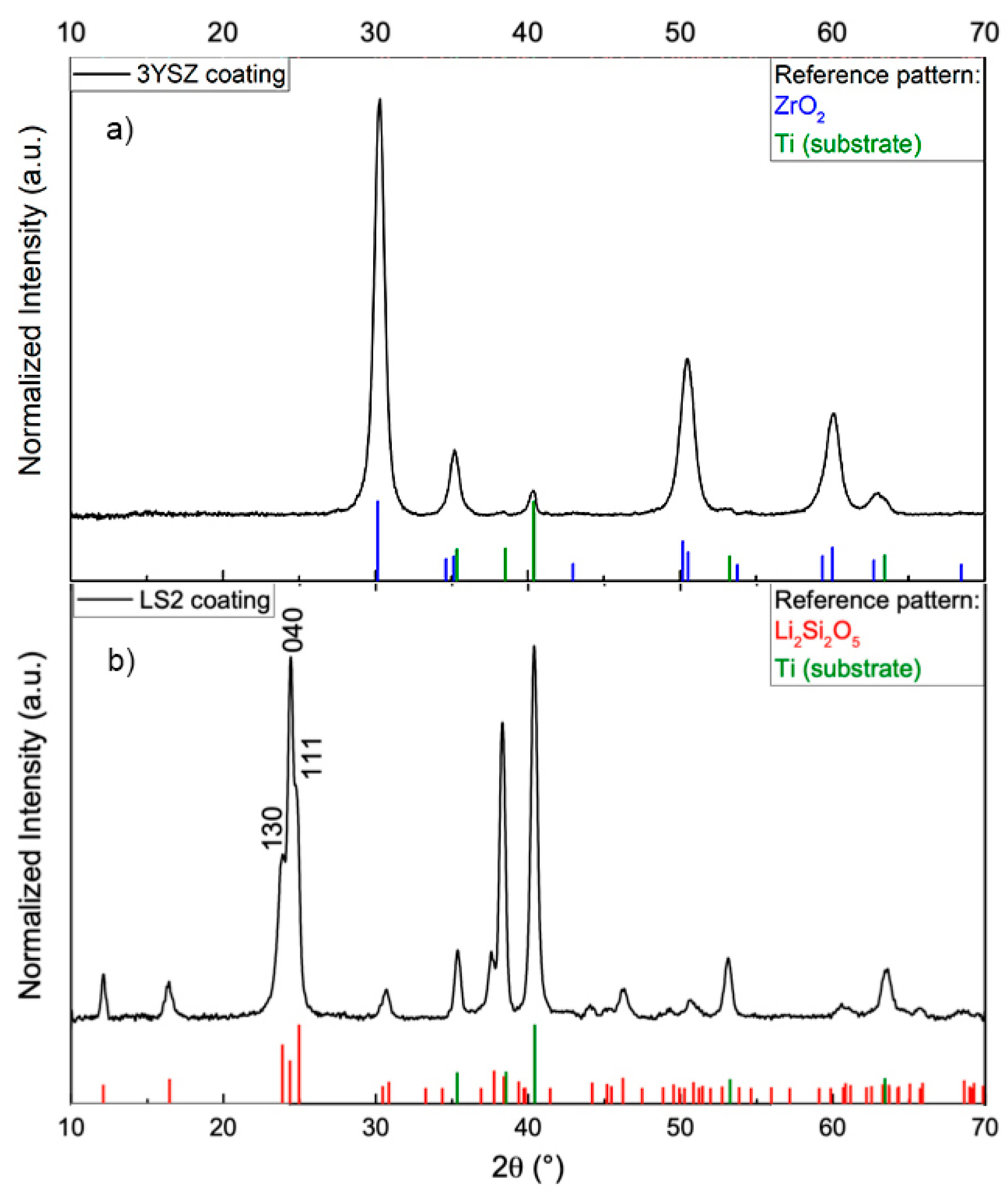
3.1. Phase Composition
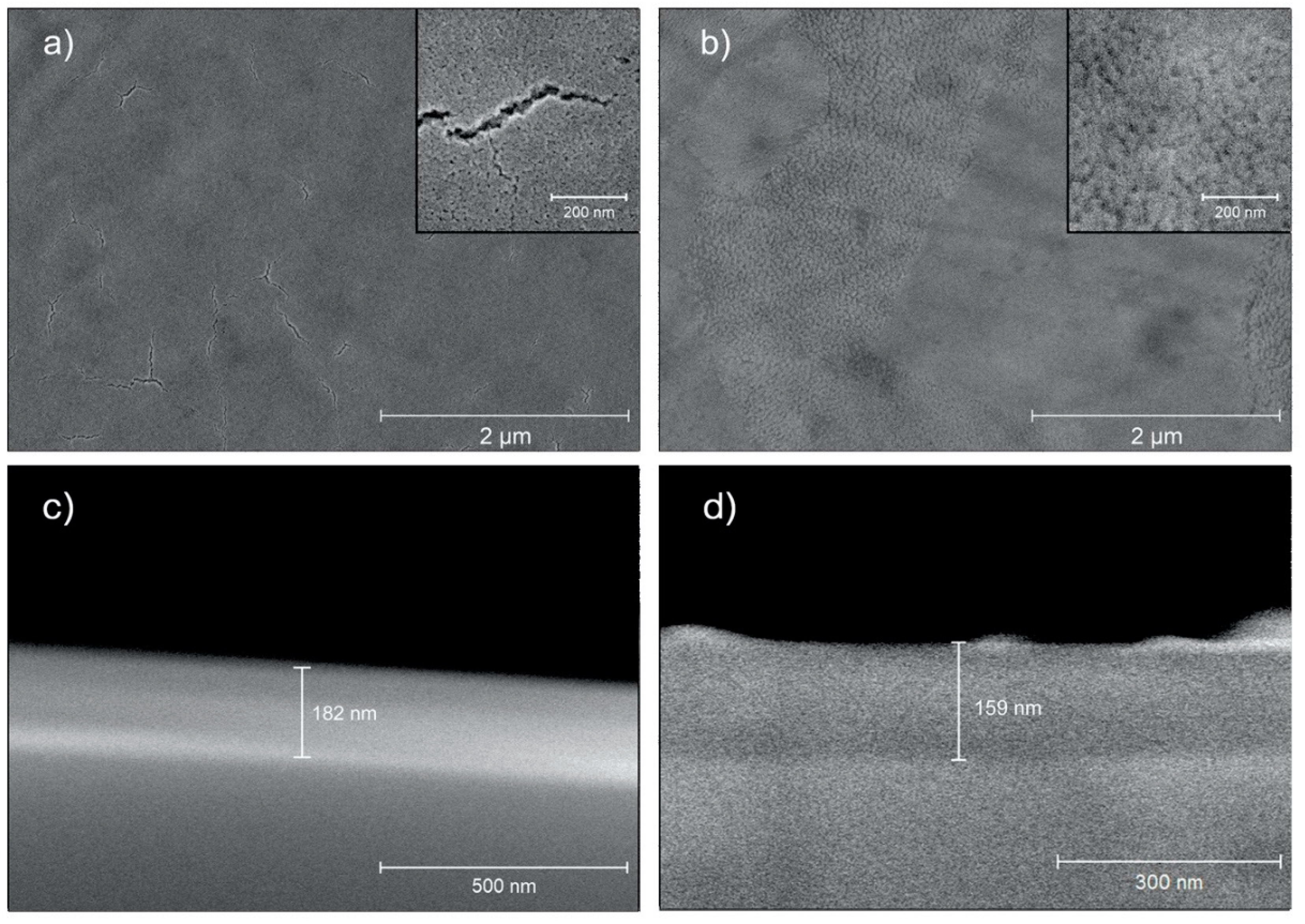
3.2. Surface Characterization
3.3. Water Contact Angle and Surface Free Energy
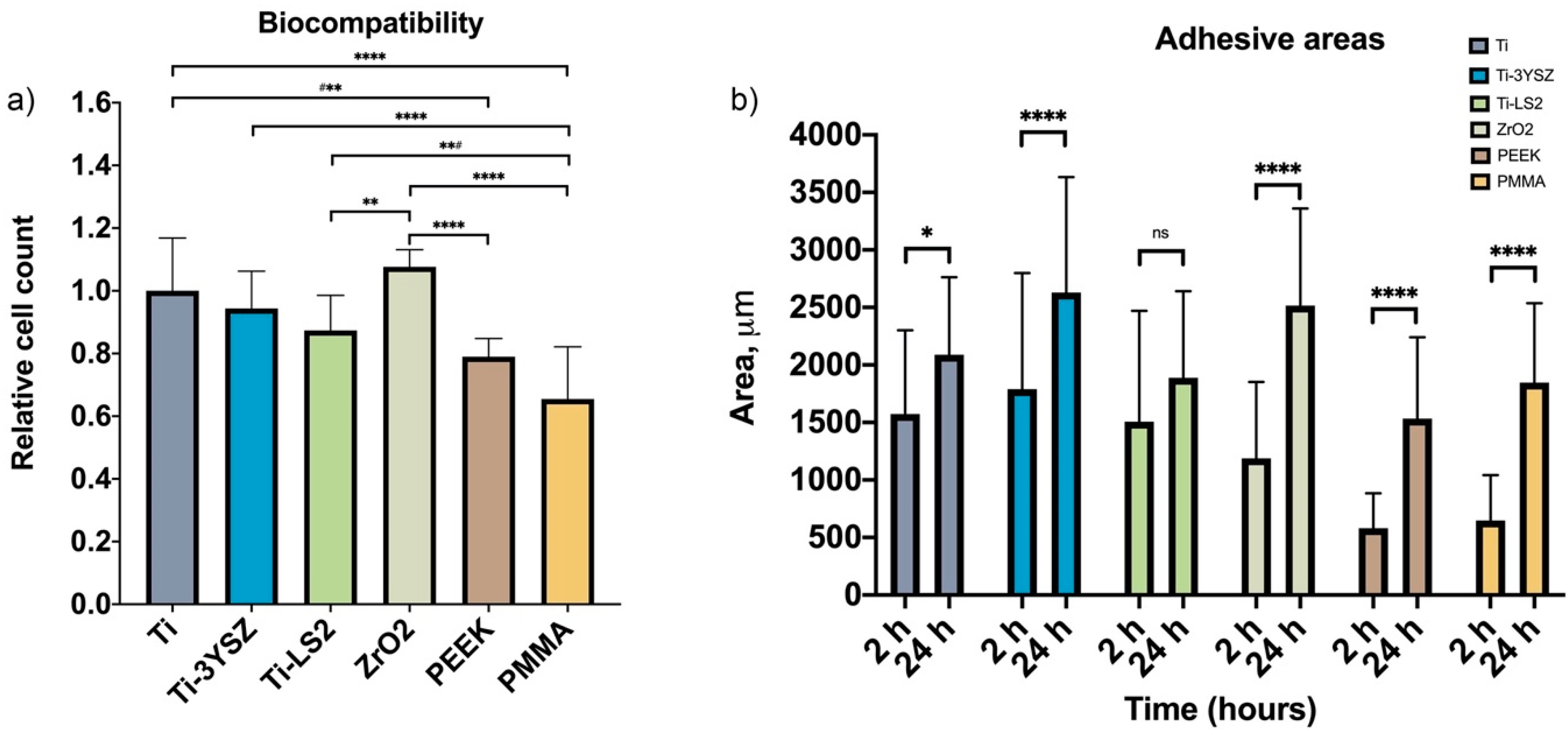
3.4. Biocompatibility Evaluation
3.5. Cell Adhesion Area
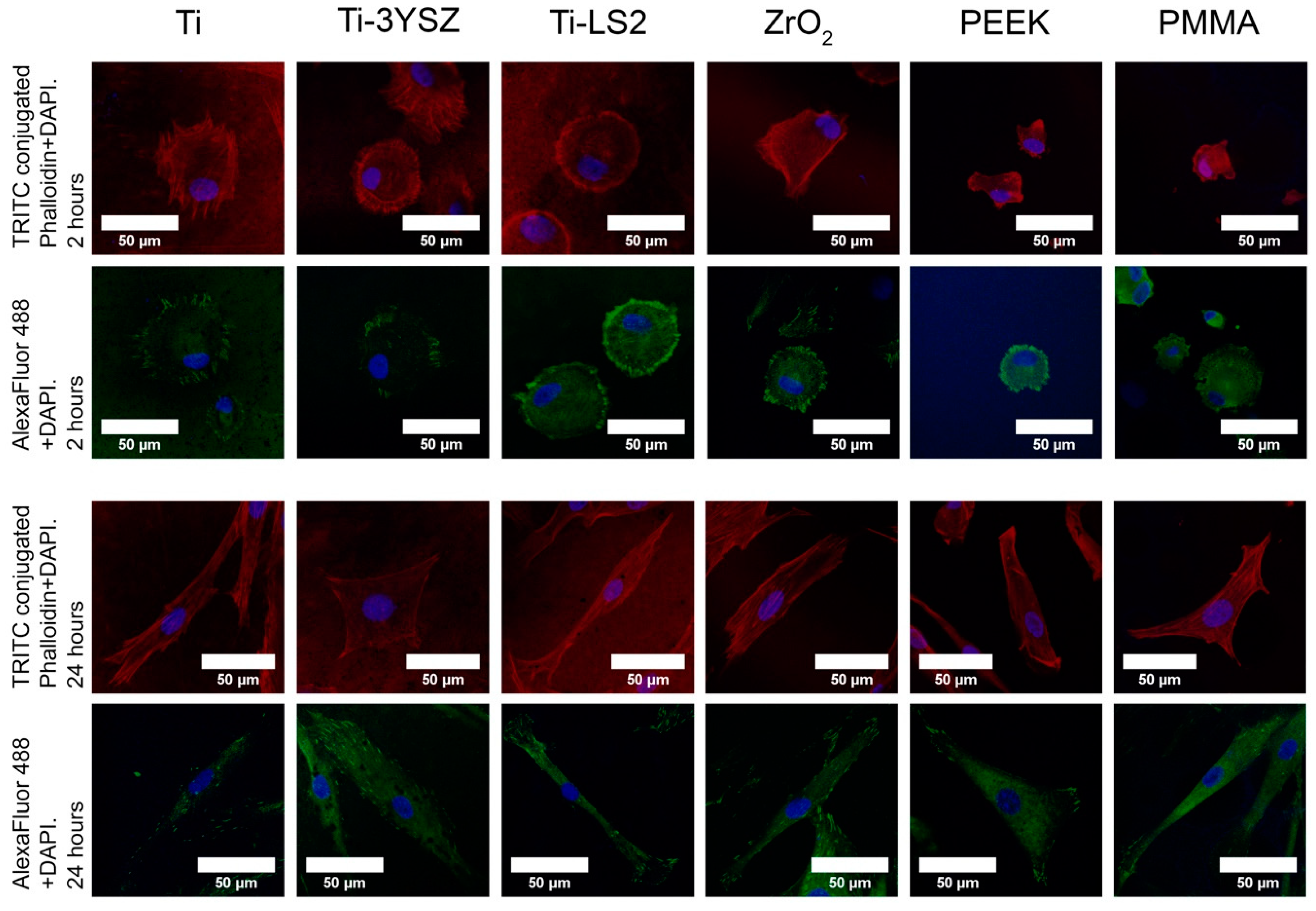
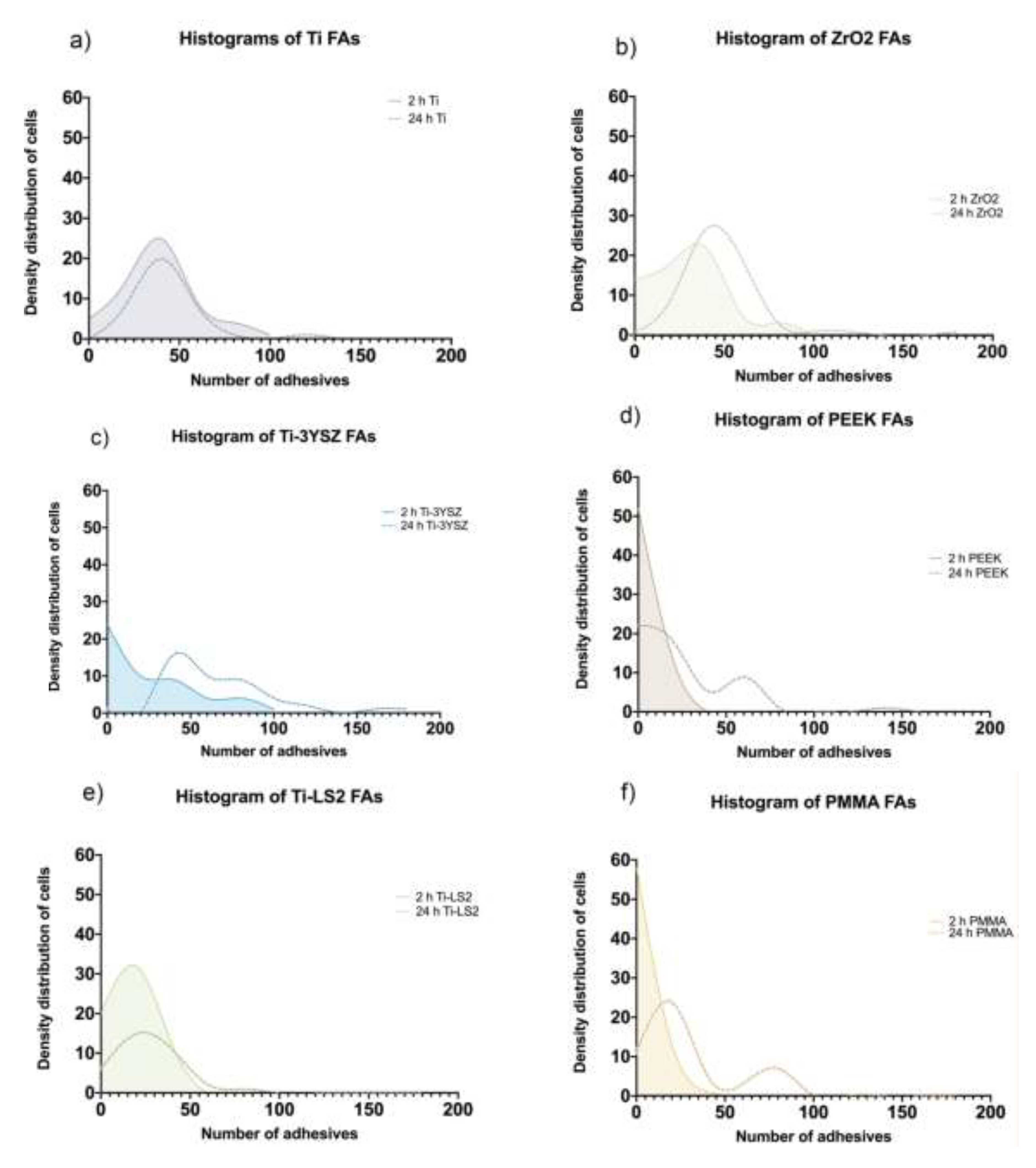
3.6. Cell Focal Adhesion (Density Distributions)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Papaspyridakos, P.; Chen, C.J.; Singh, M.; Weber, H.P.; Gallucci, G.O. Success criteria in implant dentistry: A systematic review. J. Dent. Res. 2012, 91, 242–248. [Google Scholar] [PubMed]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S278–S285. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Schenk, R.K.; Buser, D. Osseointegration: A reality. Periodontology 2000 1998, 17, 22–35. [Google Scholar] [CrossRef]
- Zhao, G.; Raines, A.L.; Wieland, M.; Schwartz, Z.; Boyan, B.D. Requirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials 2007, 28, 2821–2829. [Google Scholar] [CrossRef]
- Sculean, A.; Gruber, R.; Bosshardt, D.D. Soft tissue wound healing around teeth and dental implants. J. Clin. Periodontol. 2014, 41 (Suppl. S15), S6–S22. [Google Scholar] [CrossRef]
- Pranskunas, M.; Poskevicius, L.; Juodzbalys, G.; Kubilius, R.; Jimbo, R. Influence of Peri-Implant Soft Tissue Condition and Plaque Accumulation on Peri-Implantitis: A Systematic Review. J. Oral Maxillofac. Res. 2016, 7, e2. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Salvi, G.E. Peri-implant mucositis. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S237–S245. [Google Scholar] [CrossRef]
- Welander, M.; Abrahamsson, I.; Berglundh, T. The mucosal barrier at implant abutments of different materials. Clin. Oral Implant. Res. 2008, 19, 635–641. [Google Scholar]
- Verardi, S.; Quaranta, M.; Bordin, S. Peri-implantitis fibroblasts respond to host immune factor C1q. J. Periodontal Res. 2011, 46, 134–140. [Google Scholar] [CrossRef]
- Naruishi, K.; Nagata, T. Biological effects of interleukin-6 on Gingival Fibroblasts: Cytokine regulation in periodontitis. J. Cell. Physiol. 2018, 233, 6393–6400. [Google Scholar] [CrossRef] [PubMed]
- Moon, I.S.; Berglundh, T.; Abrahamsson, I.; Linder, E.; Lindhe, J. The barrier between the keratinized mucosa and the dental implant. J. Clin. Periodontol. 1999, 26, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Ritz, U.; Nusselt, T.; Sewing, A.; Ziebart, T.; Kaufmann, K.; Baranowski, A.; Rommens, P.M.; Hofmann, A. The effect of different collagen modifications for titanium and titanium nitrite surfaces on functions of gingival fibroblasts. Clin. Oral Investig. 2017, 21, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, C.; Tessarolo, F.; Caola, I.; Wennström, J.; Nollo, G.; Berglundh, T. Morphogenesis of peri-implant mucosa revisited: An experimental study in humans. Clin. Oral Implant. Res. 2014, 25, 997–1003. [Google Scholar] [CrossRef]
- Nothdurft, F.P.; Fontana, D.; Ruppenthal, S.; May, A.; Aktas, C.; Mehraein, Y.; Lipp, P.; Kaestner, L. Differential Behavior of Fibroblasts and Epithelial Cells on Structured Implant Abutment Materials: A Comparison of Materials and Surface Topographies. Clin. Implant. Dent. Relat. Res. 2015, 17, 1237–1249. [Google Scholar] [CrossRef]
- Schwarz, F.; Sculean, A.; Wieland, M.; Horn, N.; Nuesry, E.; Bube, C.; Becker, J. Effects of hydrophilicity and microtopography of titanium implant surfaces on initial supragingival plaque biofilm formation. A pilot study. Mund Kiefer Gesichtschir. 2007, 11, 333–338. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Engler, D. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677689. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Ringer, P.; Colo, G.; Fässler, R.; Grashoff, C. Sensing the mechano-chemical properties of the extracellular matrix. Matrix Biol. 2017, 64, 6–16. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Schwarz, U.S.; Riveline, D.; Goichberg, P.; Tzur, G.; Sabanay, I.; Mahalu, D.; Safran, S.; Bershadsky, A.; Addadi, L.; et al. Force and focal adhesion assembly: A close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001, 3, 466–472. [Google Scholar] [CrossRef]
- Narimatsu, I.; Atsuta, I.; Ayukawa, Y.; Oshiro, W.; Yasunami, N.; Furuhashi, A.; Koyano, K. Epithelial and Connective Tissue Sealing around Titanium Implants with Various Typical Surface Finishes. ACS Biomater. Sci. Eng. 2019, 5, 4976–4984. [Google Scholar] [CrossRef]
- Derchi, G.; Vano, M.; Barone, A.; Covani, U.; Diaspro, A.; Salerno, M. Bacterial adhesion on direct and indirect dental restorative composite resins: An in vitro study on a natural biofilm. J. Prosthet. Dent. 2017, 117, 669–676. [Google Scholar] [CrossRef]
- Lang, N.P.; Berglundh, T. Working Group 4 of Seventh European Workshop on Periodontology. Periimplant diseases: Where are we now?—Consensus of the Seventh European Workshop on Periodontology. J. Clin. Periodontol. 2011, 38 (Suppl. S11), 178–181. [Google Scholar] [CrossRef] [PubMed]
- Shibli, J.A.; Melo, L.; Ferrari, D.S.; Figueiredo, L.C.; Faveri, M.; Feres, M. Composition of supra- and subgingival biofilm of subjects with healthy and diseased implants. Clin. Oral Implant. Res. 2008, 19, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Damek-Poprawa, M.; Korostoff, J.; Gill, R.; DiRienzo, J.M. Cell Junction Remodeling in Gingival Tissue Exposed to a Microbial Toxin. J. Dent. Res. 2013, 92, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Truffa Giachet, F.; Miola, M.; Bertone, E.; Varesano, A.; Vineis, C.; Cochis, A.; Sorrentino, R.; Rimondini, L.; Spriano, S. Nanogrooves and keratin nanofibers on titanium surfaces aimed at driving gingival fibroblasts alignment and proliferation without increasing bacterial adhesion. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 1–12. [Google Scholar] [CrossRef]
- Ferraris, S.; Warchomicka, F.; Ramskogler, C.; Tortello, M.; Cochis, A.; Scalia, A.; di Confiengo, G.G.; Keckes, J.; Rimondini, L.; Spriano, S. Surface structuring by Electron Beam for improved soft tissues adhesion and reduced bacterial contamination on Ti-grade 2. J. Mater. Process. Technol. 2019, 266, 518–529. [Google Scholar] [CrossRef]
- Anselme, K.; Davidson, P.; Popa, A.M.; Giazzon, M.; Liley, M.; Ploux, L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef]
- Jian, X.; Huang, W.; Wu, D.; You, D.; Lin, Z.; Chen, J. Effect of Fibronectin-Coated Micro-Grooved Titanium Surface on Alignment, Adhesion, and Proliferation of Human Gingival Fibroblasts. Med. Sci. Monit. 2017, 23, 4749–4759. [Google Scholar] [CrossRef][Green Version]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Osman, R.; Swain, M. A Critical Review of Dental Implant Materials with an Emphasis on Titanium versus Zirconia. Materials 2015, 8, 932–958. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant. Dent. 2019, 5, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Linkevicius, T.; Vaitelis, J. The effect of zirconia or titanium as abutment material on soft peri-implant tissues: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2015, 26 (Suppl. S11), 139–147. [Google Scholar] [CrossRef]
- Salerno, M.; Itri, A.; Frezzato, M.; Rebaudi, A. Surface microstructure of dental implants before and after insertion: An in vitro study by means of scanning probe microscopy. Implant. Dent. 2015, 24, 248–255. [Google Scholar] [CrossRef]
- Lang, M.S.; Cerutis, D.R.; Miyamoto, T.; Nunn, M.E. Cell Attachment Following Instrumentation with Titanium and Plastic Instruments, Diode Laser, and Titanium Brush on Titanium, Titanium-Zirconium, and Zirconia Surfaces. JOMI 2016, 31, 799–806. [Google Scholar] [CrossRef]
- Marchi, J.; Ussui, V.; Delfino, C.S.; Bressiani, A.H.A.; Marques, M.M. Analysis in vitro of the cytotoxicity of potential implant materials. I: Zirconia-titania sintered ceramics. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 305–311. [Google Scholar] [CrossRef]
- Quinn, G.D.; Hoffman, K.; Scherrer, S.; Lohbauer, U.; Amberger, G.; Karl, M.; Kelly, J.R. Fractographic Analysis of Broken Ceramic Dental Restorations. In Fractography of Glasses and Ceramics VI; Ceramic Transactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; Volume 21, pp. 161–174. ISBN 9781118273739. [Google Scholar]
- Monaco, C.; Tucci, A.; Esposito, L.; Scotti, R. Microstructural changes produced by abrading Y-TZP in presintered and sintered conditions. J. Dent. 2013, 41, 121–126. [Google Scholar] [CrossRef]
- Traini, T.; Gherlone, E.; Parabita, S.F.; Caputi, S.; Piattelli, A. Fracture toughness and hardness of a Y-TZP dental ceramic after mechanical surface treatments. Clin. Oral Investig. 2014, 18, 707–714. [Google Scholar] [CrossRef]
- Antanasova, M.; Jevnikar, P. Bonding of Dental Ceramics to Titanium: Processing and Conditioning Aspects. Curr. Oral Health Rep. 2016, 3, 234–243. [Google Scholar] [CrossRef]
- Parchańska-Kowalik, M.; Wołowiec-Korecka, E.; Klimek, L. Effect of chemical surface treatment of titanium on its bond with dental ceramics. J. Prosthet. Dent. 2018, 120, 470–475. [Google Scholar] [CrossRef]
- Antanasova, M.; Kocjan, A.; Kovač, J.; Žužek, B.; Jevnikar, P. Influence of thermo-mechanical cycling on porcelain bonding to cobalt-chromium and titanium dental alloys fabricated by casting, milling, and selective laser melting. J. Prosthodont. Res. 2018, 62, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Besinis, A.; Hadi, S.D.; Le, H.R.; Tredwin, C.; Handy, R.D. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology 2017, 11, 327–338. [Google Scholar] [CrossRef] [PubMed]
- De Wilde, E.A.W.J.; Jimbo, R.; Wennerberg, A.; Naito, Y.; Coucke, P.; Bryington, M.S.; Vandeweghe, S.; De Bruyn, H. The soft tissue immunologic response to hydroxyapatite-coated transmucosal implant surfaces: A study in humans. Clin. Implant. Dent. Relat. Res. 2015, 17 (Suppl. S1), e65–e74. [Google Scholar] [CrossRef]
- Brunot-Gohin, C.; Duval, J.-L.; Verbeke, S.; Belanger, K.; Pezron, I.; Kugel, G.; Laurent-Maquin, D.; Gangloff, S.; Egles, C. Biocompatibility study of lithium disilicate and zirconium oxide ceramics for esthetic dental abutments. J. Periodontal Implant. Sci. 2016, 46, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Usinskas, P.; Stankeviciute, Z.; Niaura, G.; Maminskas, J.; Juodzbalys, G.; Kareiva, A. Sol–gel processing of calcium hydroxyapatite thin films on silicon nitride (Si3N4) substrate. J. Sol. Gel. Sci. Technol. 2017, 83, 268–274. [Google Scholar] [CrossRef]
- Okubo, T.; Nagamoto, H. Low-temperature preparation of nanostructured zirconia and YSZ by sol-gel processing. J. Mater. Sci. 1995, 30, 749–757. [Google Scholar] [CrossRef]
- Wen, T.L.; Hebert, V.; Vilminot, S.; Bernier, J.C. Preparation of nanosized yttria-stabilized zirconia powders and their characterization. J. Mater. Sci. 1991, 26, 3787–3791. [Google Scholar] [CrossRef]
- Wang, F.; Li, K.; Ning, C. Sintering properties of sol–gel derived lithium disilicate glass ceramics. J. Sol. Gel. Sci. Technol. 2018, 87, 372–379. [Google Scholar] [CrossRef]
- Zhang, T.B.; Easteal, A.J.; Edmonds, N.R.; Bhattacharyya, D. Sol-Gel Preparation and Characterization of Lithium Disilicate Glass-Ceramic. J. Am. Ceram. Soc. 2007, 90, 1592–1596. [Google Scholar] [CrossRef]
- Maçon, A.L.B.; Jacquemin, M.; Page, S.J.; Li, S.; Bertazzo, S.; Stevens, M.M.; Hanna, J.V.; Jones, J.R. Lithium-silicate sol-gel bioactive glass and the effect of lithium precursor on structure-property relationships. J. Sol. Gel. Sci. Technol. 2017, 81, 84–94. [Google Scholar] [CrossRef]
- Jeong, B.-H.; Park, S.-M.; Hwang, W.-S.; Hyun, K.-H.; Park, Y.-O.; Jung, T.-K.; Hyun, S.-K. Thermal Properties of Plasma-Sprayed Multilayer Al₂O₃/Yttria-Stabilized Zirconia Coating. J. Nanosci. Nanotechnol. 2020, 20, 524–529. [Google Scholar] [CrossRef]
- Salerno, M.; Giacomelli, L.; Derchi, G.; Patra, N.; Diaspro, A. Atomic force microscopy in vitro study of surface roughness and fractal character of a dental restoration composite after air-polishing. Biomed. Eng. Online 2010, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Löberg, J.; Mattisson, I.; Hansson, S.; Ahlberg, E. Characterisation of Titanium Dental Implants I: Critical Assessment of Surface Roughness Parameters. Open Biomater. J. 2010, 2, 18–35. [Google Scholar] [CrossRef]
- Steinmassl, P.-A.; Wiedemair, V.; Huck, C.; Klaunzer, F.; Steinmassl, O.; Grunert, I.; Dumfahrt, H. Do CAD/CAM dentures really release less monomer than conventional dentures? Clin. Oral Investig. 2017, 21, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Caneva-Soumetz, F.; Pastorino, L.; Patra, N.; Diaspro, A.; Ruggiero, C. Adhesion and proliferation of osteoblast-like cells on anodic porous alumina substrates with different morphology. IEEE Trans. Nanobiosci. 2013, 12, 106–111. [Google Scholar] [CrossRef]
- Veerachamy, S.; Hameed, P.; Sen, D.; Dash, S.; Manivasagam, G. Studies on Mechanical, Biocompatibility and Antibacterial Activity of Plasma Sprayed Nano/Micron Ceramic Bilayered Coatings on Ti-6Al-4V Alloy for Biomedical Application. J. Nanosci. Nanotechnol. 2018, 18, 4515–4523. [Google Scholar] [CrossRef] [PubMed]
- Khalili, A.A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. IJMS 2015, 16, 18149–18184. [Google Scholar] [CrossRef]
- Tetè, S.; Zizzari, V.L.; Borelli, B.; De Colli, M.; Zara, S.; Sorrentino, R.; Scarano, A.; Gherlone, E.; Cataldi, A.; Zarone, F. Proliferation and adhesion capability of human gingival fibroblasts onto zirconia, lithium disilicate and feldspathic veneering ceramic in vitro. Dent. Mater. J. 2014, 33, 7–15. [Google Scholar] [CrossRef]
- Wang, X.; Lu, T.; Wen, J.; Xu, L.; Zeng, D.; Wu, Q.; Cao, L.; Lin, S.; Liu, X.; Jiang, X. Selective responses of human gingival fibroblasts and bacteria on carbon fiber reinforced polyetheretherketone with multilevel nanostructured TiO2. Biomaterials 2016, 83, 207–218. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, X.; Li, S.; Lai, R.; Zhou, Z.; Zhang, Y.; Zhou, L. Effects of titania nanotubes with or without bovine serum albumin loaded on human gingival fibroblasts. Int. J. Nanomed. 2014, 9, 1185–1198. [Google Scholar] [CrossRef]
- Schwarz, F.; Ferrari, D.; Herten, M.; Mihatovic, I.; Wieland, M.; Sager, M.; Becker, J. Effects of surface hydrophilicity and microtopography on early stages of soft and hard tissue integration at non-submerged titanium implants: An immunohistochemical study in dogs. J. Periodontolog. 2007, 78, 2171–2184. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.-H.; Yoon, M.-K.; Moon, J.-S.; Kang, J.-H.; Kim, S.-H.; Yang, H.-S.; Kim, M.-S. Focal adhesion linker proteins expression of fibroblast related to adhesion in response to different transmucosal abutment surfaces. J. Adv. Prosthodont. 2013, 5, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Baharloo, B.; Textor, M.; Brunette, D.M. Substratum roughness alters the growth, area, and focal adhesions of epithelial cells, and their proximity to titanium surfaces. J. Biomed. Mater. Res. A 2005, 74, 12–22. [Google Scholar] [CrossRef]
- Takamori, E.R.; Cruz, R.; Gonçalvez, F.; Artificial, R.Z. Effect of roughness of zirconia and titanium on fibroblast adhesion. Artif. Organs 2008, 32, 305–309. [Google Scholar] [CrossRef]
- Merhie El, A.; Salerno, M.; Toccafondi, C.; Dante, S. Neuronal-like response of N2a living cells to nanoporous patterns of thin supported anodic alumina. Colloids Surf. B Biointerfaces 2019, 178, 32–37. [Google Scholar] [CrossRef]
- Größner-Schreiber, B.; Herzog, M.; Hedderich, J.; Dück, A.; Hannig, M.; Griepentrog, M. Focal adhesion contact formation by fibroblasts cultured on surface-modified dental implants: An in vitro study. Clin. Oral Implant. Res. 2006, 17, 736–745. [Google Scholar] [CrossRef]
- Brunette, D.M. Principles of Cell Behavior on Titanium Surfaces and Their Application to Implanted Devices. In Titanium in Medicine; Engineering Materials; Springer: Berlin/Heidelberg, Germany, 2001; Volume 27, pp. 485–512. ISBN 978-3-642-63119-1. [Google Scholar]
- Allen, L.T.; Tosetto, M.; Miller, I.S.; O’Connor, D.P.; Penney, S.C.; Lynch, I.; Keenan, A.K.; Pennington, S.R.; Dawson, K.A.; Gallagher, W.M. Surface-induced changes in protein adsorption and implications for cellular phenotypic responses to surface interaction. Biomaterials 2006, 27, 3096–3108. [Google Scholar] [CrossRef]
- Ying, P.; Yu, Y.; Jin, G.; Tao, Z. Competitive protein adsorption studied with atomic force microscopy and imaging ellipsometry. Colloids Surf. B Biointerfaces 2003, 32, 1–10. [Google Scholar] [CrossRef]
- Mengel, R.; Meer, C.; Flores-de-Jacoby, L. The treatment of uncoated and titanium nitride-coated abutments with different instruments. JOMI 2004, 19, 232–238. [Google Scholar]
- Berglundh, T.; Abrahamsson, I.; Welander, M.; Lang, N.P.; Lindhe, J. Morphogenesis of the peri-implant mucosa: An experimental study in dogs. Clin. Oral Implant. Res. 2007, 18, 1–8. [Google Scholar] [CrossRef]
- Salvi, G.E.; Bosshardt, D.D.; Lang, N.P.; Abrahamsson, I.; Berglundh, T.; Lindhe, J.; Ivanovski, S.; Donos, N. Temporal sequence of hard and soft tissue healing around titanium dental implants. Periodontology 2000 2015, 68, 135–152. [Google Scholar] [CrossRef] [PubMed]
| Descriptive Statistics | Ti | Ti-3YSZ | Ti-LS2 | ZrO2 | PEEK | PMMA |
|---|---|---|---|---|---|---|
| Number of values | 10 | 10 | 10 | 10 | 10 | 10 |
| Roughness | 17.67 ± 0.35 | 16.61 ± 0.52 | 9.61 ± 0.66 | 5.53 ± 0.21 | 38.00 ± 0.78 | 65.23 ± 2.41 |
| WCA | 60.21 ± 1.58 | 32.58 ± 1.94 | 25.71 ± 1.33 | 57.32 ± 1.86 | 75.62 ± 2.46 | 68.84 ± 2.65 |
| SFE_dispersive | 34.44 ± 1.56 | 36.89 ± 0.33 | 32.31 ± 0.59 | 30.38 ± 0.55 | 39.32 ± 0.38 | 36.98 ± 0.59 |
| SFE_polar | 7.37 ± 1.62 | 25.14 ± 0.41 | 33.39 ± 0.59 | 14.72 ± 0.36 | 4.14 ± 0.20 | 7.36 ± 0.32 |
| SFE_total | 41.81 ± 2.01 | 62.03 ± 0.57 | 65.70 ± 1.00 | 45.10 ± 0.34 | 43.46 ± 0.54 | 44.34 ± 0.73 |
| Tukey’s Multiple Comparisons Test | Mean 1 | Mean 2 | Mean Diff. | Adjusted p Value |
|---|---|---|---|---|
| Ti vs. Ti-3YSZ | 17.67 | 16.61 | 1.062 | 0.2759 ns |
| Ti vs. Ti-LS2 | 17.67 | 9.61 | 8.062 | <0.0001 **** |
| Ti vs. ZrO2 | 17.67 | 5.53 | 12.14 | <0.0001 **** |
| Ti vs. PEEK | 17.67 | 38.00 | 20.33 | <0.0001 **** |
| Ti vs. PMMA | 17.67 | 65.23 | 47.55 | <0.0001 **** |
| Ti-3YSZ vs. Ti-LS2 | 16.61 | 9.61 | 7.00 | <0.0001 **** |
| Ti-3YSZ vs. ZrO2 | 16.61 | 5.53 | 11.08 | <0.0001 **** |
| Ti-3YSZ vs. PEEK | 16.61 | 38.00 | 21.39 | <0.0001 **** |
| Ti-3YSZ vs. PMMA | 16.61 | 65.23 | 48.61 | <0.0001 **** |
| Ti-LS2 vs. ZrO2 | 9.61 | 5.53 | 4.081 | <0.0001 **** |
| Ti-LS2 vs. PEEK | 9.61 | 38.00 | 28.39 | <0.0001 **** |
| Ti-LS2 vs. PMMA | 9.61 | 65.23 | 55.61 | <0.0001 **** |
| ZrO2 vs. PEEK | 5.53 | 38.00 | 32.47 | <0.0001 **** |
| ZrO2 vs. PMMA | 5.53 | 65.23 | 59.70 | <0.0001 **** |
| PEEK vs. PMMA | 38.00 | 65.23 | 27.22 | <0.0001 **** |
| Tukey’s Multiple Comparisons Test | Mean 1 | Mean 2 | Mean Diff. | Adjusted pValue |
|---|---|---|---|---|
| Ti vs. Ti-3YSZ | 60.21 | 32.58 | 27.63 | <0.0001 **** |
| Ti vs. Ti-LS2 | 60.21 | 25.71 | 34.50 | <0.0001 **** |
| Ti vs. ZrO2 | 60.21 | 57.32 | 2.89 | 0.0264 * |
| Ti vs. PEEK | 60.21 | 75.62 | 15.41 | <0.0001 **** |
| Ti vs. PMMA | 60.21 | 68.84 | 8.63 | <0.0001**** |
| Ti-3YSZ vs. Ti-LS2 | 32.58 | 25.71 | 6.87 | <0.0001 **** |
| Ti-3YSZ vs. ZrO2 | 32.58 | 57.32 | 24.74 | <0.0001 **** |
| Ti-3YSZ vs. PEEK | 32.58 | 75.62 | 43.04 | <0.0001 **** |
| Ti-3YSZ vs. PMMA | 32.58 | 68.84 | 36.26 | <0.0001 **** |
| Ti-LS2 vs. ZrO2 | 25.71 | 57.32 | 31.61 | <0.0001 **** |
| Ti-LS2 vs. PEEK | 25.71 | 75.62 | 49.91 | <0.0001 **** |
| Ti-LS2 vs. PMMA | 25.71 | 68.84 | 43.13 | <0.0001 **** |
| ZrO2 vs. PEEK | 57.32 | 75.62 | 18.30 | <0.0001 **** |
| ZrO2 vs. PMMA | 57.32 | 68.84 | 11.52 | <0.0001 **** |
| PEEK vs. PMMA | 75.62 | 68.84 | 6.778 | <0.0001 **** |
| Tukey’s Multiple Comparisons Tests | Mean 1 | Mean 2 | Mean Diff. | Adjusted p Value |
|---|---|---|---|---|
| Ti vs. Ti-3YSZ | 41.81 | 62.03 | 20.21 | <0.0001 **** |
| Ti vs. Ti-LS2 | 41.81 | 65.70 | 23.89 | <0.0001 **** |
| Ti vs. ZrO2 | 41.81 | 45.10 | 3.29 | <0.0001 **** |
| Ti vs. PEEK | 41.81 | 43.46 | 1.64 | 0.0071 **# |
| Ti vs. PMMA | 41.81 | 44.34 | 2.53 | <0.0001 **** |
| Ti-3YSZ vs. Ti-LS2 | 62.03 | 65.70 | 3.67 | <0.0001 **** |
| Ti-3YSZ vs. ZrO2 | 62.03 | 45.10 | 16.93 | <0.0001 **** |
| Ti-3YSZ vs. PEEK | 62.03 | 43.46 | 18.57 | <0.0001 **** |
| Ti-3YSZ vs. PMMA | 62.03 | 44.34 | 17.69 | <0.0001 **** |
| Ti-LS2 vs. ZrO2 | 65.70 | 45.10 | 20.60 | <0.0001 **** |
| Ti-LS2 vs. PEEK | 65.70 | 43.46 | 22.24 | <0.0001 **** |
| Ti-LS2 vs. PMMA | 65.70 | 44.34 | 21.36 | <0.0001 **** |
| ZrO2 vs. PEEK | 45.10 | 43.46 | 1.64 | 0.007 ** |
| ZrO2 vs. PMMA | 45.10 | 44.34 | 0.76 | 0.5408 ns |
| PEEK vs. PMMA | 43.46 | 44.34 | 0.88 | 0.37 ns |
| Descriptive Statistics | Ti | Ti-3YSZ | Ti-LS2 | ZrO2 | PEEK | PMMA |
|---|---|---|---|---|---|---|
| FAs per cell (2 h) | 36.16 ± 2.79 | 28.00 ± 3.76 | 16.94 ± 1.57 | 29.02 ± 2.42 | 5.15 ± 0.74 | 5.76 ± 0.93 |
| FAs per cell (24 h) | 43.47 ± 3.14 | 66.75 ± 4.91 | 26.03 ± 2.98 | 47.69 ± 3.27 | 23.45 ± 3.71 | 28.66 ± 3.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maminskas, J.; Pilipavicius, J.; Staisiunas, E.; Baranovas, G.; Alksne, M.; Daugela, P.; Juodzbalys, G. Novel Yttria-Stabilized Zirconium Oxide and Lithium Disilicate Coatings on Titanium Alloy Substrate for Implant Abutments and Biomedical Application. Materials 2020, 13, 2070. https://doi.org/10.3390/ma13092070
Maminskas J, Pilipavicius J, Staisiunas E, Baranovas G, Alksne M, Daugela P, Juodzbalys G. Novel Yttria-Stabilized Zirconium Oxide and Lithium Disilicate Coatings on Titanium Alloy Substrate for Implant Abutments and Biomedical Application. Materials. 2020; 13(9):2070. https://doi.org/10.3390/ma13092070
Chicago/Turabian StyleMaminskas, Julius, Jurgis Pilipavicius, Edvinas Staisiunas, Gytis Baranovas, Milda Alksne, Povilas Daugela, and Gintaras Juodzbalys. 2020. "Novel Yttria-Stabilized Zirconium Oxide and Lithium Disilicate Coatings on Titanium Alloy Substrate for Implant Abutments and Biomedical Application" Materials 13, no. 9: 2070. https://doi.org/10.3390/ma13092070
APA StyleMaminskas, J., Pilipavicius, J., Staisiunas, E., Baranovas, G., Alksne, M., Daugela, P., & Juodzbalys, G. (2020). Novel Yttria-Stabilized Zirconium Oxide and Lithium Disilicate Coatings on Titanium Alloy Substrate for Implant Abutments and Biomedical Application. Materials, 13(9), 2070. https://doi.org/10.3390/ma13092070





