3.1. Phase Formation
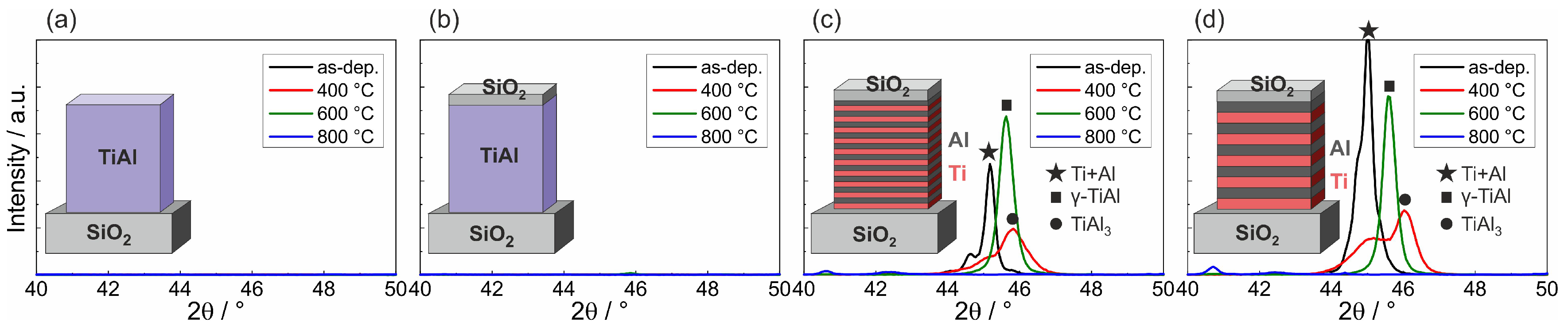
Figure 1 presents the results of the XRD measurements of the different samples in the as-prepared state and after annealing at 400, 600, and 800 °C in HV. It can be seen that for both co-sputtered films no peaks were visible for all sample states (
Figure 1a,b). In contrast to this, clear XRD peaks were present for the ML films. In the as-prepared state, a superposition of the Ti (002, theoretical peak position: 2
) and Al (111, theoretical peak position: 2
) appeared (
Figure 1c,d). The measured peaks were slightly shifted with respect to the theoretical position, which might be due to stresses in the thin films. Annealing at 400 °C led to a decrease of the peak intensities. However, there was still a superposition of two XRD peaks.
The phase formation in
m thick Ti/Al ML films was analyzed by various authors. For very thin individual layers (2 nm), a transition from (Ti) + (Al) → disordered TiAl + (Ti) →
-TiAl +
-Ti
Al was described by Ramos et al. [
18]. In contrast to this, for thicker individual layers (100 nm), the authors described the transition from Al and Ti to
-TiAl via a TiAl
intermediate phase. The occurrence of TiAl
in the phase sequence during annealing of Ti/Al ML samples was also described by Illekova et al. for an individual layer thickness of 20 nm or more [
5]. For Al rich Ti-Al ML samples (substrate/40 nm Ti/180 nm Al/40 nm Ti/180 nm Al), the formation of TiAl
was also observed after a heat treatment at 450 °C [
19,
20]. The two peaks measured in our work after annealing at 400 °C are therefore ascribed to Ti (002, now measured at a slightly higher
of 45.1°) and TiAl
(103 or 112, theoretical
, measured at 46.0°).
After annealing at 600 °C, one single peak was measured for both ML samples, which can be attributed to the -TiAl (101) peak (theoretical position: , measured at 45.6°). After annealing at 800 °C, the TiAl peak disappeared.
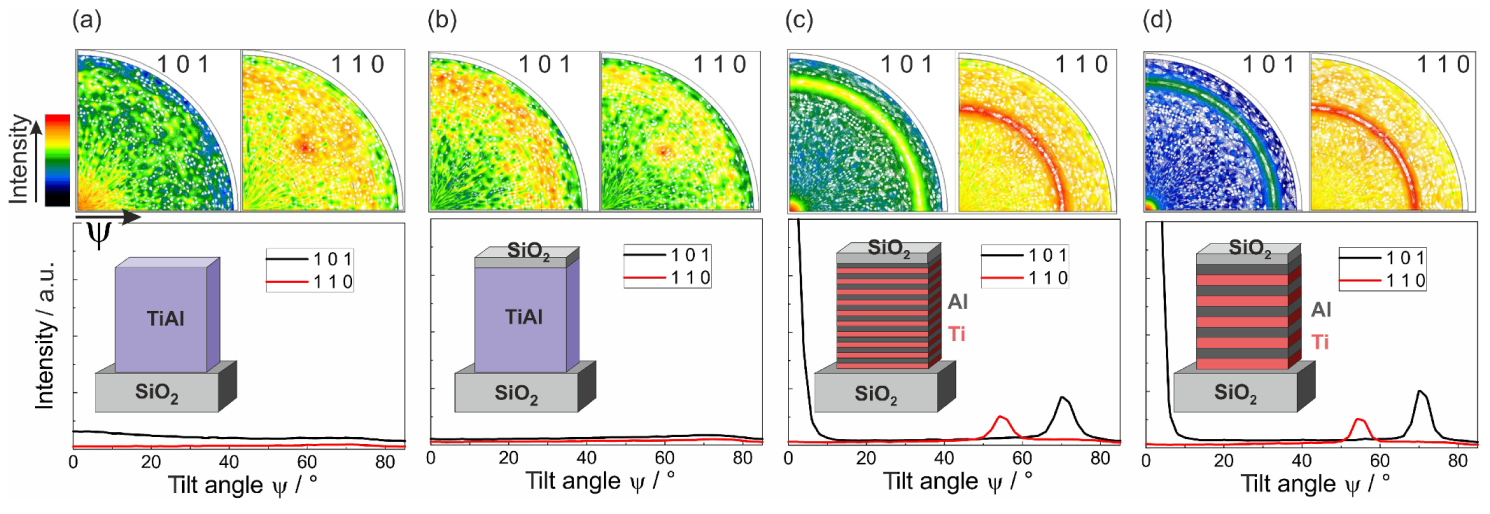
Pole figure measurements were performed to confirm the formation of the
-TiAl phase in the ML films. In addition, these measurements allowed to exclude that in the co-sputtered films the
-TiAl phase has formed with a tilted texture, which cannot be detected by XRD in Bragg Brentano geometry.
Figure 2 summarizes the pole figures of the (110) and (101)
-TiAl poles of the four different samples annealed at 600 °C for 10 h. Since the pole figures are scaled to their respective maximum, in addition, the azimuthally averaged intensity versus the tilt angle
is presented to allow a comparison between the different samples. It can be seen that both co-sputtered samples with or without SiO
cover layer did not show any phase formation (see
Figure 2a,b). The pole, which was visible in the TiAl (110) pole figure at
was caused by the Si (220) lattice planes. Their 2
value of 47.3° (Cu K
) is close to that of the TiAl (110), so that it is also measured. Besides this, no significant intensity was detected.
In contrast to this, both ML samples showed a strong intensity in the center of the (101) pole figure and a clear ring at
(see
Figure 2c,d). Corresponding to this, the TiAl (110) pole figure contained a ring at
. These two measurements confirmed the formation of the
-TiAl phase.
It is known from the literature that Ti-Al films co-sputtered at room temperature are in general an amorphous mixture of Ti and Al [
4,
21,
22]. The formation of crystalline TiAl phases in amorphous Ti-Al or multilayer Ti/Al thick films in the range of 1.6
m to 150
m was investigated by several authors. Illekova et al. studied the activation energy of the phase formation in Ti/Al multilayers with various thicknesses of the individual layer (from 4 to 1000 nm). For a thickness of the individual layer of 20 nm, they derived a value of
kJ/mol [
5]. They also described that for very thin individual layers (4 nm) at first the material becomes amorphous, so that the start of the crystallization is delayed. Senkov et al. analyzed amorphous Ti-Al films and determined an activation energy for the transition from the amorphous state to the first crystalline phase of
kJ/mol [
23]. Although these values were extracted for films with a much larger thickness, the higher activation energy for the formation of the crystalline TiAl phase in amorphous films as compared to ML films can explain the observed difference in the behaviour of the thin films studied in this work.
3.2. Film Morphology
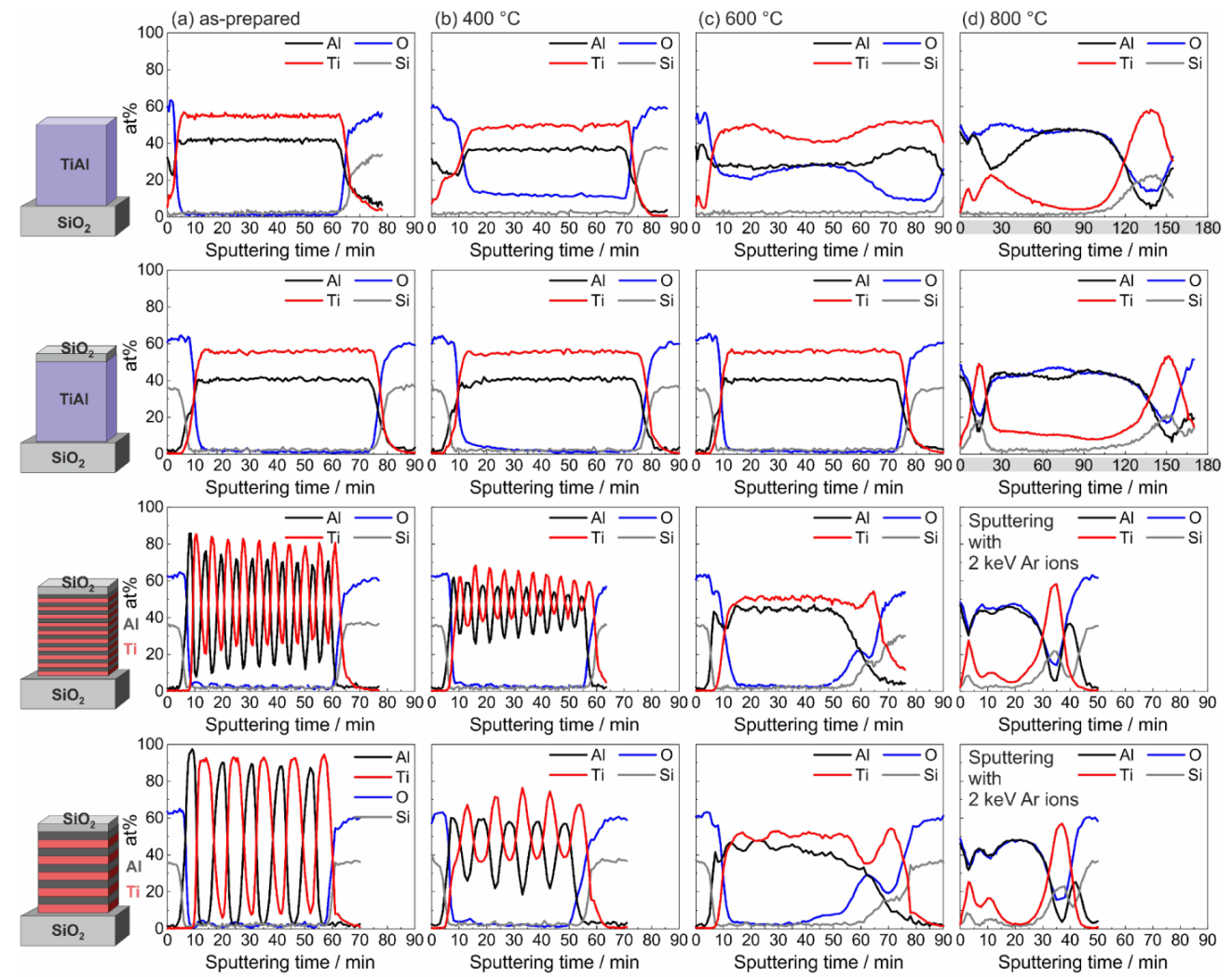
Figure 3 shows the results of the evaluation of the measured AES depth profiles for the amorphous and ML Ti-Al samples in the as-prepared state and after annealing at 400, 600, and 800 °C in HV. The composition was calculated from the measured AES spectra using the standard single element relative sensitivity factors (RSF). As can be seen for the co-sputtered films in the as-prepared state (
Figure 3a,b), the calculation led to a higher atomic concentration of Ti. In contrast to this, an EDX analysis of a thin lamella of such a sample in the TEM resulted in an atomic composition with slightly more Al than Ti. This deviation of the results can be explained by different actual values of the RSF for the Ti-Al alloy and by a preferential sputtering. The AES results therefore mainly allow a relative comparison of the different samples and annealing states. Besides this, from the analysis of the peak shape and position, the oxidation state of Ti, Al, and Si was derived.
In
Figure 3a, the results of the measurements of the samples in the as-prepared state are presented. In the uncovered co-sputtered film, a thin Al
O
layer formed at the sample surface, which can be explained by the lower free energy of the formation of Al oxide as compared to that of TiO
for pure elements [
24]. (It is reported in the literature that in Ti-Al materials the oxide formation depends on the composition and the phases which are present in the material. Rahmel and Spencer determined that Al oxide is more stable than TiO
in Al rich Ti-Al materials [
25].) Across the whole sample thickness, the concentration of Al and Ti was constant.
In the SiO
covered alloy film as well, a thin Al
O
layer developed at the interface between the cover layer and the Ti-Al film. For both ML samples, the sequence of the Ti and Al layers was clearly visible. The concentration of both elements did not reach 100 at% in the separate layers and, especially for the film with 10 nm individual layer thickness, a decrease of the amplitudes of the element concentration with increasing sputtering time was seen. These findings can be explained by a partial intermixing of the elements at the interfaces during the sputtering process and by a roughening of the sample surface due to the Ar sputtering, which increases with increasing sputtering time. This roughening led to a simultaneous measurement of different depths, which resulted in an apparent mixture of Ti and Al. In addition, some intermixing can result from the high ion energy during the depositions of the layers. In these ML samples as well, Al
O
was present below the SiO
cover layer. The SiO
layer was deposited at 180 °C. Obviously, this temperature is sufficient to initiate a reduction reaction of the SiO
to Si by Al which forms Al
O
. This effect, which has been widely studied (see, e.g., [
26]), was proven for these samples by XPS measurements.
The AES profiles of the samples after annealing at 400 °C in HV are shown in
Figure 3b. For the uncovered co-sputtered sample, a strong oxide peak was visible at the sample surface, and a constant O signal was measured across the whole sample thickness. However, except for the sample surface, the AES showed Al and Ti only in the elemental and not in the oxidized state, which was derived from the AES peak position. This indicated that the O was solved within the amorphous Ti-Al layer. In contrast to this, in the co-sputtered film with SiO
cover layer as well as in the ML films no O was measured within the sample. In the ML films, a partly interdiffusion of Ti and Al occurred. In addition, at the Ti-SiO
interface at the bottom of the film some O was detected in the Ti layer.
After annealing at 600 °C, in the co-sputtered film without cover layer the O signal strongly increased. Within the first 5 min of sputtering, Ti was present in the oxidized and for the rest of the measurement in the elemental state. Oxidized Al was found at the sample surface. In the regime between 10 and 20 min of sputtering, Al was present in the elemental state, and the O which was detected there was again solved in the TiAl without forming an oxide. With increasing sputtering time, the amount of oxidized Al increased again, and all Al was present in the oxidized state for the sputtering time between about 35 and 50 min. Afterwards, the part of elemental Al increased again, and in the time regime between about 70 and 85 min only elemental Al was found. Again, O was just solved without forming an oxide. At this temperature, the SiO cover layer still successfully acted as a diffusion barrier in the case of the co-sputtered Ti-Al film, which was seen from the constant thickness of the AlO layer below it. However, in the case of the ML samples, the thickness of the AlO interlayer between the SiO cover and the TiAl layer increased. At this interfacial region as well, XPS proved the presence of elemental Si. The interdiffusion of the Al and Ti layers in the ML samples was almost completed and only a small variation of the composition remained visible. The annealing at 600 °C, however, led to a strong reaction in the Ti/Al ML samples with the SiO at the substrate surface. The measurements revealed a layer consisting of elemental Ti and Si on top of the substrate, followed by an AlO layer.
The annealing at 800 °C (
Figure 3d) led to a degradation of all films and all Al was oxidized. The formed Al
O
was mainly present in the center of the film. For all samples, at the interface to the substrate a strong Ti and Si signal was measured. In the samples with SiO
cover layer, Al
O
was found at the surface of the film. In the case of the co-sputtered film, below this Al
O
, there was a defined layer consisting of Ti and Si. In the ML samples, Ti and Si were distributed across a broader thickness in the upper region of the film, and no oxidized Ti was found at all.
The time necessary to sputter the film increased significantly as compared to the previous measurements. One reason was the low sputter rate of AlO. In addition, due to the insulating material, electric charges were accumulated, leading to electric fields which further decreased the sputter rate. Therefore, for the ML samples an increased energy of the Ar ions (2 keV instead of 1 keV) was applied to achieve higher sputter rates.
The AES results showed that the chemical reaction between the Ti-Al film and the SiO cover layer and substrate strongly depended on the initial distribution of the elements Ti and Al in the film. In the ML samples, stronger reactions took place at the respective interfaces between the pure Al layer on top and the pure Ti layer at the bottom and the SiO: a pure Ti layer at the bottom of the sample resulted in a marginal reaction between Ti and Si already at 400 °C (see the O signal in the bottom Ti layer) and a strong reaction at 600 °C. In contrast to this, in the case of the Ti-Al alloy, such a reaction did not take place at these temperatures. There was also a stronger oxidation of the Al at the interface with the SiO cover layer in case of the pure Al layer, as can be seen from the thicker AlO interlayer.
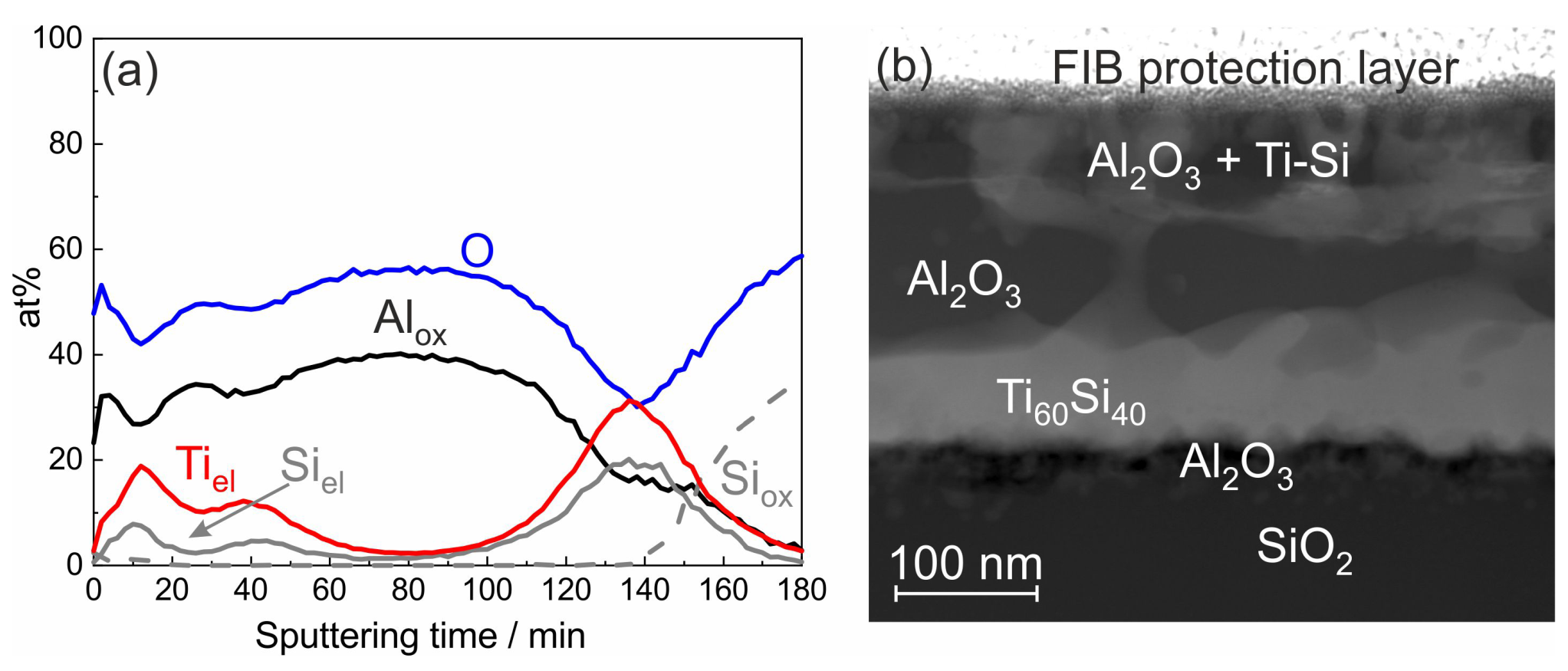
XPS was used to identify the chemical state of the elements in the degraded films. The result of an XPS depth profile analysis of the ML Ti/Al sample with 20 nm individual layer thickness and SiO
cover layer after annealing at 800 °C is shown in
Figure 4a. The measurements revealed that Al was only present in the oxidized state (marked by Al
), and the intensity curve follows that of O. Ti was only present in the elemental state (marked by Ti
) and was not oxidized at all. Unoxidized Si was found in the upper and lower region of the film, where its intensity curve was parallel to that of Ti. This behavior indicated the formation of a Ti-Si phase.
In contrast to the ML samples, in the co-sputtered films, the O signal was not parallel to that of Al (see
Figure 3d). Around the sputtering time of 30 min, the film without the cover layer showed a strong decrease in the Al, a slightly increasing O, and a maximum in the Ti signal. An analysis of the AES spectral shape revealed that in this region of the film a part of the Ti was oxidized. In the co-sputtered film with SiO
cover as well, at the sputtering time around 70 min, a stronger O signal as compared to Al was visible. Again, a part of the Ti was oxidized, however, to a much reduced amount as compared to the sample without cover layer.
The reaction between Ti and SiO
is widely described in the literature (see, e.g., [
27,
28]). There is a chemical reaction between Ti and SiO
at their interface, leading to the formation of a Ti silicide. The free O diffuses through the Ti layer, which is possible because of the high O solubility of 34 at% in this material [
29]. This solution of O in the bottom Ti layer was already visible for the ML systems after annealing at 400 °C (see
Figure 3b). During annealing at 600 °C, the interface reaction became stronger, and the O which diffused through the Ti layer then reacted with the Al on top of it to Al
O
. Ti not only reacted with the SiO
of the substrate, but also with the covering SiO
layer, so that at the film surface Ti-Si was also formed.
In the upper region of the film, a small carbon signal of less than 5 at% was measured by XPS, indicating C which was present in a carbide state. However, most likely the carbide was formed by the Ar sputtering and did not originate from the sample. (More details on the XPS measurement of the Ti-Al samples were published by Oswald et al. [
30].)
To get more information on the morphology of this sample, a STEM analysis was performed. A STEM image predominantly showing the chemical contrast is presented in
Figure 4b. The EDX analysis confirmed the formation of Al
O
and the presence of Ti-Si in the upper and lower region of the film. The chemical composition of the Ti-Si grains in the lower region of the film was about Ti
Al
. Close to this composition, there are the two Ti-Si phases: Ti
Si
and Ti
Si
[
31]. There are reports describing the formation of the Ti
Si
phase [
27,
28], and for our samples this phase was confirmed by the small Ti
Si
(002) peak in the XRD data at
in
Figure 1c,d.
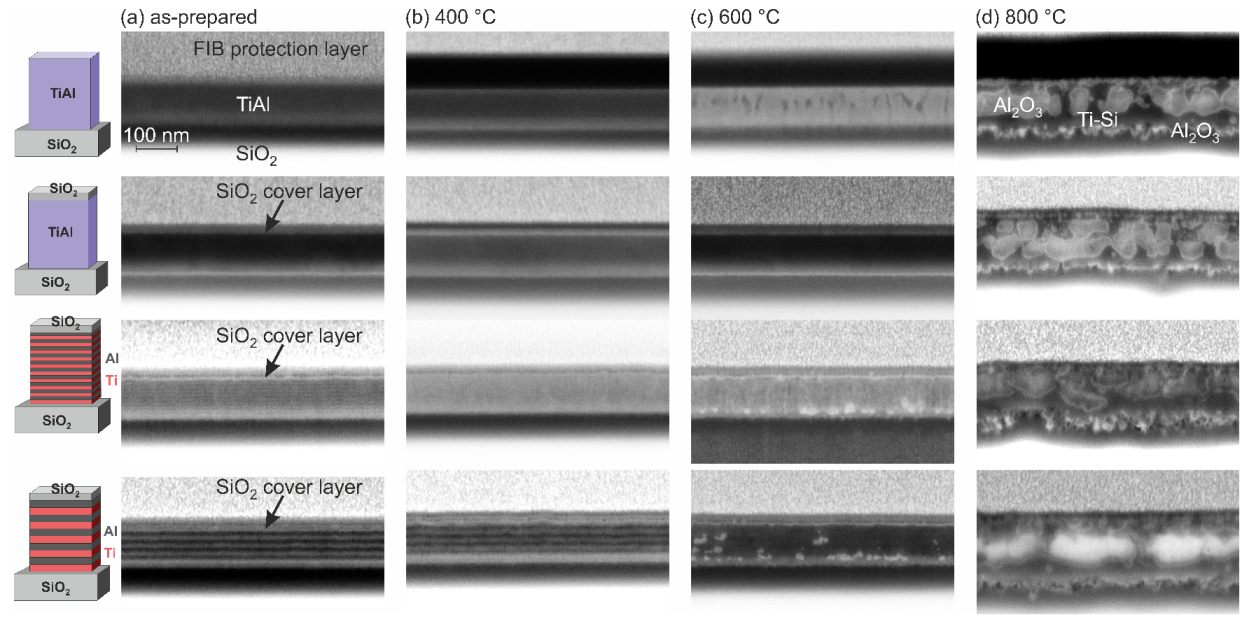
Figure 5 summarizes SEM images of the FIB cross sections of the different samples in the as-prepared state and after the annealing procedures. In the as-prepared state (
Figure 5a), the co-sputtered films showed a homogeneous structure. It was challenging to resolve the 10 nm multilayers; however, the 20-nm Ti and Al layers were clearly visible (dark layers: Ti; brighter layers: Al). After annealing at 400 °C, the co-sputtered samples as well as the 10-nm ML film appeared homogeneous. In the 20-nm ML sample, it was still possible to identify the layer structure (
Figure 5b).
Changes in the microstructure became visible in the co-sputtered film without cover layer after annealing at 600 °C. The SEM image showed that darker grains were present in the upper region of the film, and that there are no visible inhomogeneities in the lower region. The dark structures were most likely Ti rich grains. This is in agreement with the AES measurements (
Figure 3c), which showed besides Al and O a strong Ti signal in the upper region of the film. In contrast to the uncovered film, the co-sputtered film with SiO
layer did not possess such inhomogeneities as was expected from the AES measurements, which revealed a homogeneous composition. After annealing at 600 °C, in the images of both ML samples, small bright structures were present—in the case of the 10-nm ML in the lower region of the film and in the case of the 20-nm ML additionally in the center region of the TiAl layer. These bright structures were Al
O
grains on top of the Ti
Si
layer, which is in agreement with the AES results. In the 20-nm ML films, the AES showed a small O signal during the sputtering time between 40 and 55 min, which was not present in the 10-nm ML sample, caused by the Al
O
grains that were distributed in the center region of the TiAl layer.
For all samples, strong changes in the morphology appeared after annealing at 800 °C (
Figure 5d). Irregular formed structures were visible and the film thickness increased significantly. From the evaluation of the EDX data shown in
Figure 4 in combination with the AES measurements, it can be derived that the large bright structures were Al
O
grains. In the case of the uncovered film, these grains were embedded in a Ti and Ti-O matrix, and, in the case of the films with the SiO
cover layer, the matrix consisted of Ti-Si. In all samples, the interface between the film and substrate was no longer clearly defined and showed a large roughness due to the reaction between Ti and SiO
.