Effects of Different Divalent Cation Hydrothermal Treatments of Titanium Implant Surfaces for Epithelial Tissue Sealing
Abstract
1. Introduction
2. Materials and Methods
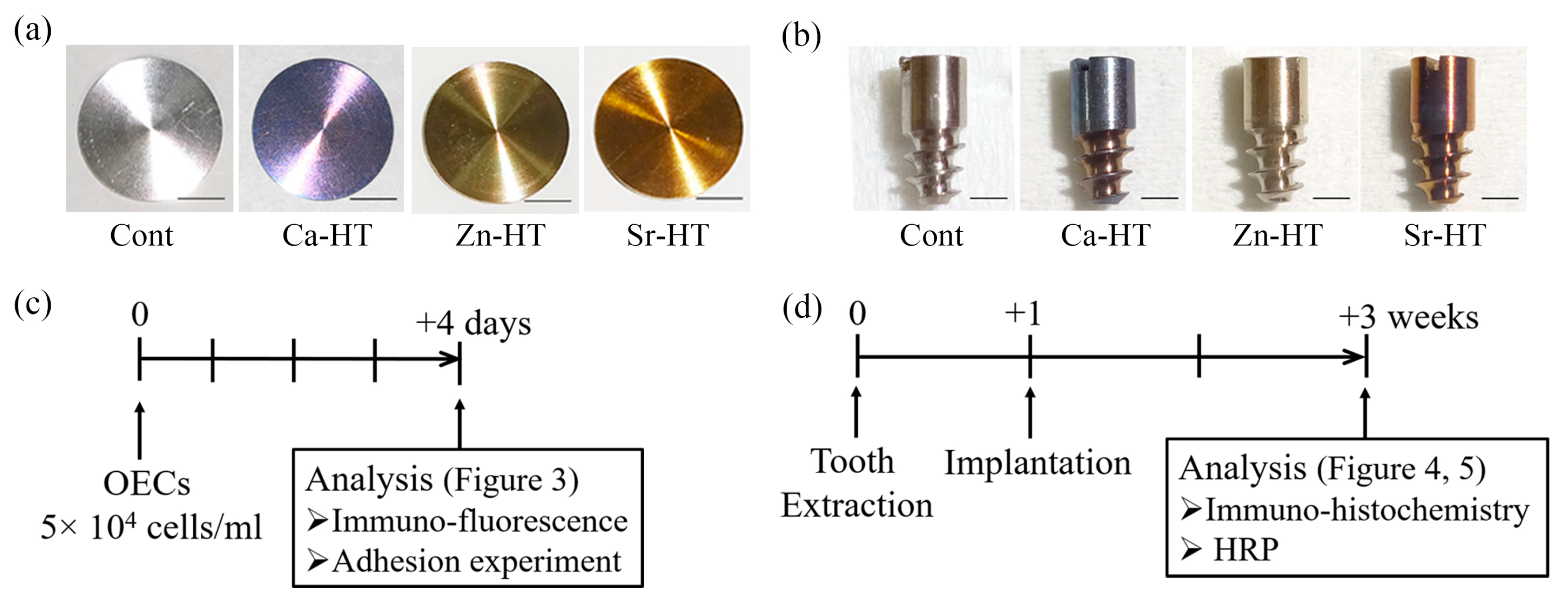
2.1. Hydrothermal Treatment of Ti Plates and Implants
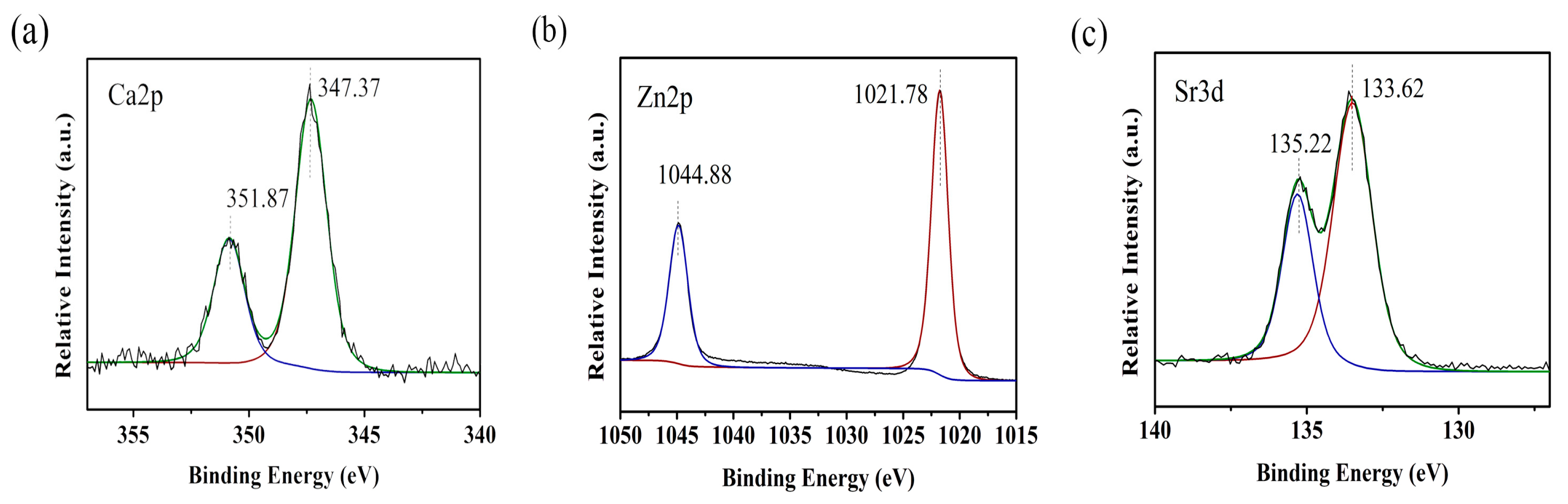
2.2. X-ray Photoelectron Spectroscopy Analysis of the Surface Characteristics
2.3. Epithelial Cell Culture
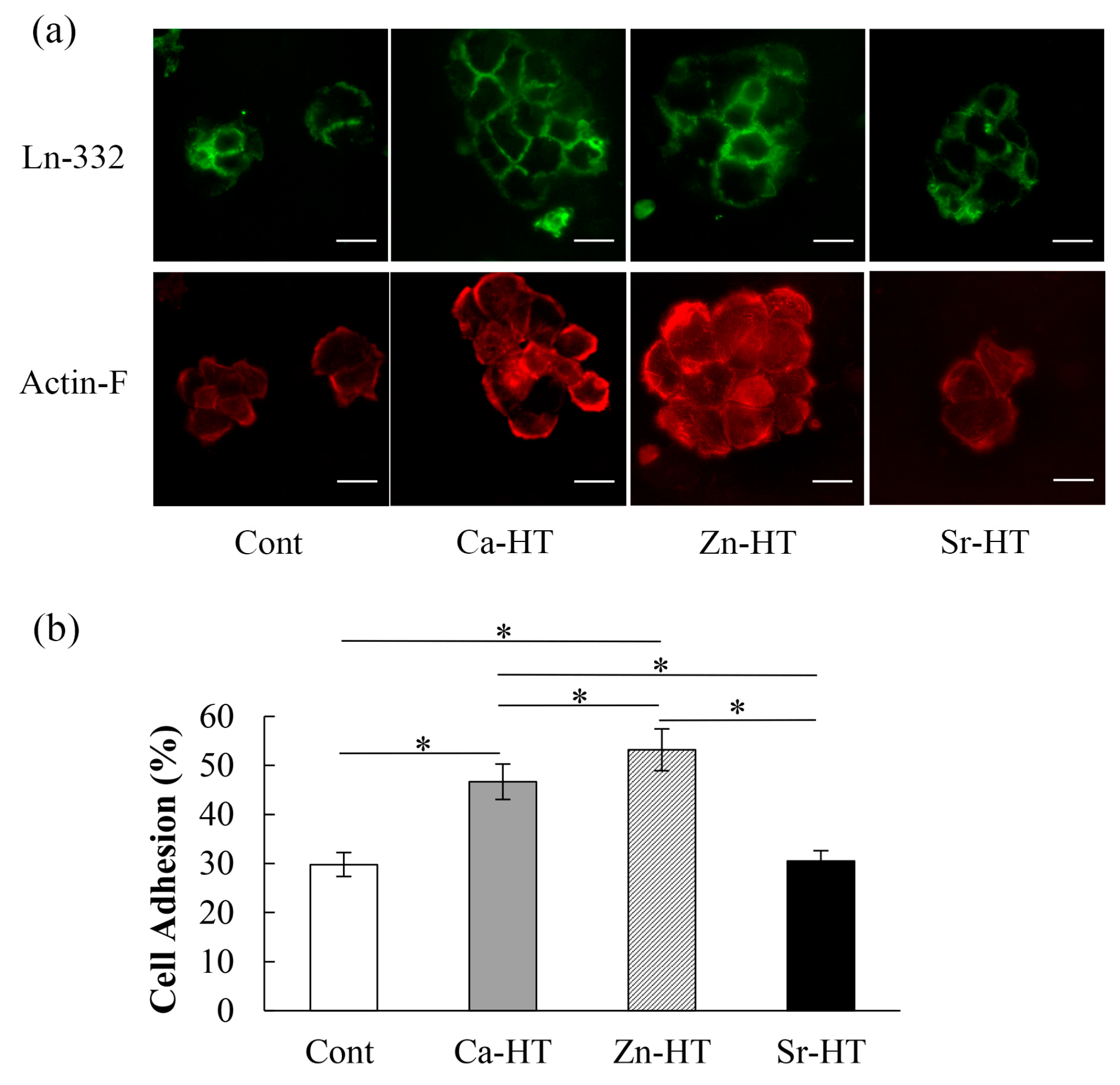
2.4. Immunofluorescence Staining for Adhesion Proteins
2.5. OEC Adhesion Evaluation
2.6. Animals and Implantation
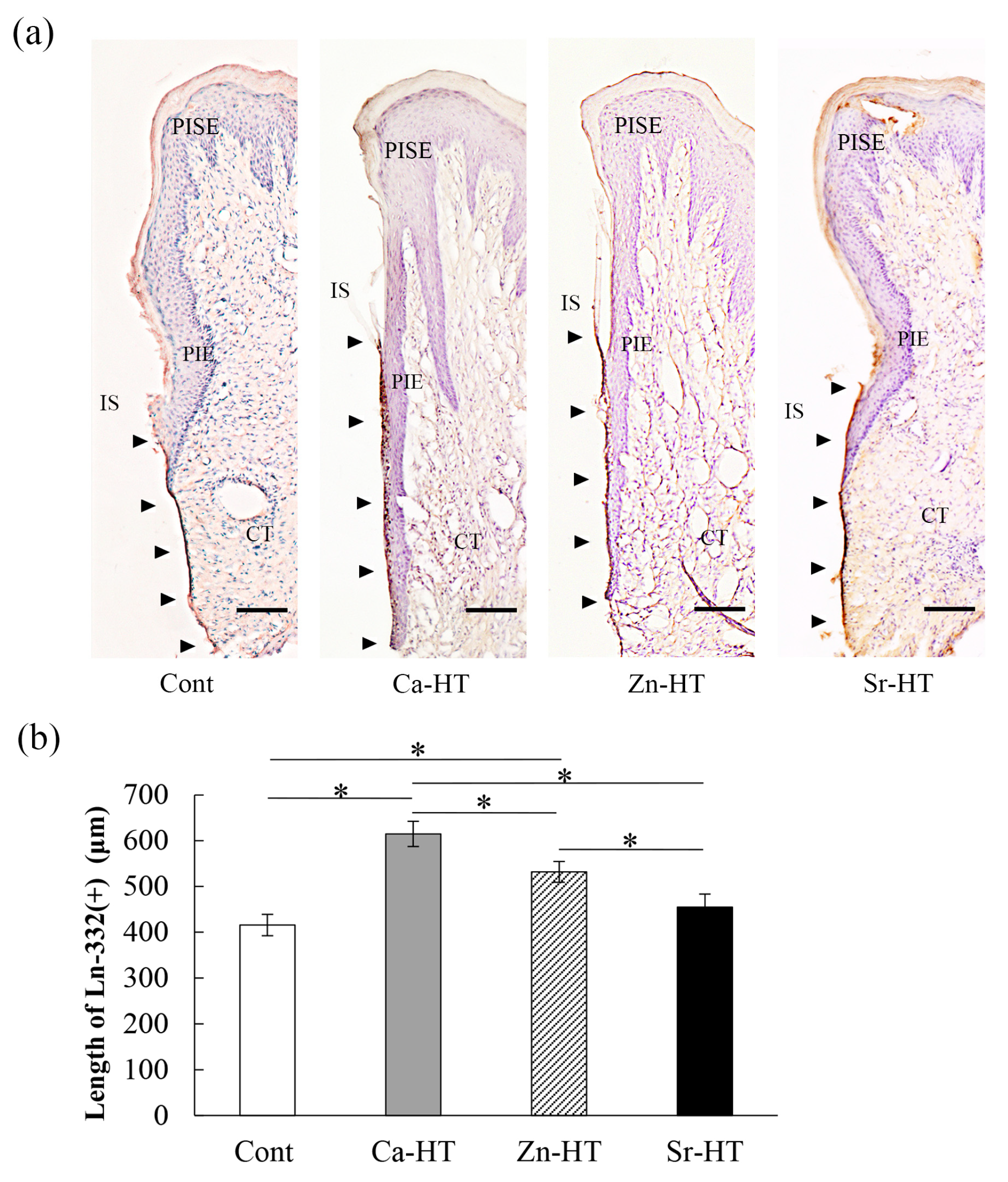
2.7. Tissue Preparation and Immunohistochemistry
2.8. Horseradish Peroxidase (HRP) Topical Application
2.9. Histochemistry of HRP
2.10. Statistical Analysis
3. Results
3.1. Surface Characteristics of the Specimens
3.2. Expression of Adhesion-Related Proteins in the Immunofluorescence Staining
3.3. OEC Adhesion Assay
3.4. Localization of Ln-332 in the Peri-Implant Epithelium (PIE)
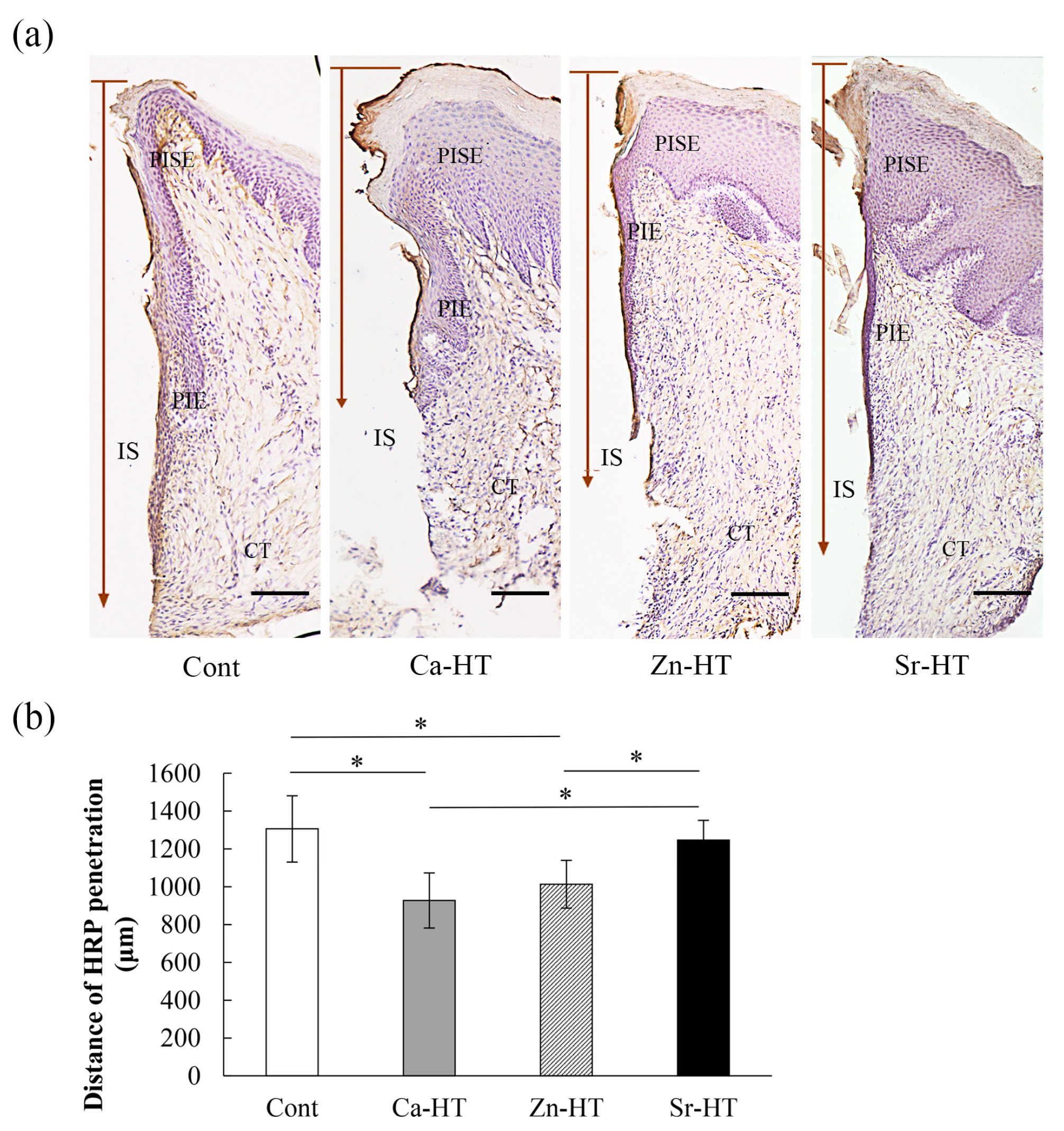
3.5. Evaluation of HRP Penetration
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berglundh, T.; Lindhe, J.; Marinello, C.; Ericsson, I.; Liljenberg, B. Soft tissue reaction to de novo plaque formation on implants and teeth. An experimental study in the dog. Clin. Oral Implants Res. 1992, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Lindhe, J.; Ericsson, I.; Marinello, C.P.; Liljenberg, B.; Thomsen, P. The soft tissue barrier at implants and teeth. Clin. Oral Implants Res. 1991, 2, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Diener, A.; Nebe, B.; Luthen, F.; Becker, P.; Beck, U.; Neumann, H.G.; Rychly, J. Control of focal adhesion dynamics by material surface characteristics. Biomaterials 2005, 26, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Borradori, L.; Sonnenberg, A. Structure and function of hemidesmosomes: More than simple adhesion complexes. J. Investig. Dermatol. 1999, 112, 411–418. [Google Scholar] [CrossRef]
- Litjens, S.H.; de Pereda, J.M.; Sonnenberg, A. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 2006, 16, 376–383. [Google Scholar] [CrossRef]
- Goldfinger, L.E.; Hopkinson, S.B.; deHart, G.W.; Collawn, S.; Couchman, J.R.; Jones, J.C. The alpha3 laminin subunit, alpha6beta4 and alpha3beta1 integrin coordinately regulate wound healing in cultured epithelial cells and in the skin. J. Cell. Sci. 1999, 112, 2615–2629. [Google Scholar]
- Stern, I.B. Current concepts of the dentogingival junction: The epithelial and connective tissue attachments to the tooth. J. Periodontol. 1981, 52, 465–476. [Google Scholar] [CrossRef]
- Rousselle, P.; Lunstrum, G.P.; Keene, D.R.; Burgeson, R.E. Kalinin: An epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J. Cell Biol. 1991, 114, 567–576. [Google Scholar] [CrossRef]
- Brånemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindström, J.; Ohlsson, Å. Intra-osseous anchorage of dental prostheses: I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef]
- Atsuta, I.; Ayukawa, Y.; Furuhashi, A.; Ogino, Y.; Moriyama, Y.; Tsukiyama, Y.; Koyano, K. In vivo and in vitro studies of epithelial cell behavior around titanium implants with machined and rough surfaces. Clin. Implant Dent. Relat. Res. 2014, 16, 772–781. [Google Scholar] [CrossRef]
- Nakagawa, M.; Zhang, L.; Udoh, K.; Matsuya, S.; Ishikawa, K. Effects of hydrothermal treatment with CaCl2 solution on surface property and cell response of titanium implants. J. Mater. Sci. Mater. Med. 2005, 16, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Gibbs, D.F.; Inman, D.R.; Shah, B.; Fligiel, S.E.; Voorhees, J.J. Inhibition of epithelial cell adhesion by retinoic acid. Relationship to reduced extracellular matrix production and alterations in Ca2+ levels. Am. J. Pathol. 1991, 138, 887–895. [Google Scholar] [PubMed]
- Oshiro, W.; Ayukawa, Y.; Atsuta, I.; Furuhashi, A.; Yamazoe, J.; Kondo, R.; Sakaguchi, M.; Matsuura, Y.; Tsukiyama, Y.; Koyano, K. Effects of CaCl2 hydrothermal treatment of titanium implant surfaces on early epithelial sealing. Colloids Surf. B Biointerfaces 2015, 131, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ito, A.; Sogo, Y.; Li, X.; Oyane, A. Zinc-containing apatite layers on external fixation rods promoting cell activity. Acta Biomater. 2010, 6, 962–968. [Google Scholar] [CrossRef]
- Hie, M.; Tsukamoto, I. Administration of zinc inhibits osteoclastogenesis through the suppression of RANK expression in bone. Eur. J. Pharmacol. 2011, 668, 140–146. [Google Scholar] [CrossRef]
- Ancsin, J.B.; Kisilevsky, R. Laminin interactions important for basement membrane assembly are promoted by zinc and implicate laminin zinc finger-like sequences. J. Biol. Chem. 1996, 271, 6845–6851. [Google Scholar] [CrossRef]
- Dang, Y.; Zhang, L.; Song, W.; Chang, B.; Han, T.; Zhang, Y.; Zhao, L. In vivo osseointegration of Ti implants with a strontium-containing nanotubular coating. Int. J. Nanomed. 2016, 11, 1003–1011. [Google Scholar] [CrossRef]
- Okawachi, H.; Ayukawa, Y.; Atsuta, I.; Furuhashi, A.; Sakaguchi, M.; Yamane, K.; Koyano, K. Effect of titanium surface calcium and magnesium on adhesive activity of epithelial-like cells and fibroblasts. Biointerphases 2012, 7, 27. [Google Scholar] [CrossRef]
- Zhang, L.; Ayukawa, Y.; Legeros, R.Z.; Matsuya, S.; Koyano, K.; Ishikawa, K. Tissue-response to calcium-bonded titanium surface. J. Biomed. Mater. Res. A 2010, 95, 33–39. [Google Scholar] [CrossRef]
- Shiraiwa, M.; Goto, T.; Yoshinari, M.; Koyano, K.; Tanaka, T. A study of the initial attachment and subsequent behavior of rat oral epithelial cells cultured on titanium. J. Periodontol. 2002, 73, 852–860. [Google Scholar] [CrossRef]
- Yasunami, N.; Ayukawa, Y.; Furuhashi, A.; Atsuta, I.; Rakhmatia, Y.D.; Moriyama, Y.; Masuzaki, T.; Koyano, K. Acceleration of hard and soft tissue healing in the oral cavity by a single transmucosal injection of fluvastatin-impregnated poly (lactic-co-glycolic acid) microspheres. An in vitro and rodent in vivo study. Biomed. Mater. 2015, 11, 015001. [Google Scholar] [CrossRef]
- Narimatsu, I.; Atsuta, I.; Ayukawa, Y.; Oshiro, W.; Yasunami, N.; Furuhashi, A.; Koyano, K. Epithelial and Connective Tissue Sealing around Titanium Implants with Various Typical Surface Finishes. ACS Biomater. Sci. Eng. 2019, 5, 4976–4984. [Google Scholar] [CrossRef]
- Atsuta, I.; Ayukawa, Y.; Furuhashi, A.; Yamaza, T.; Tsukiyama, Y.; Koyano, K. Promotive effect of insulin-like growth factor-1 for epithelial sealing to titanium implants. J. Biomed. Mater. Res. A 2013, 101, 2896–2904. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Yamaza, T.; Yoshinari, M.; Ohsaki, Y.; Ayukawa, Y.; Kido, M.A.; Inoue, T.; Shimono, M.; Koyano, K.; Tanaka, T. Ultrastructural and immunoelectron microscopic studies of the peri-implant epithelium-implant (Ti-6Al-4V) interface of rat maxilla. J. Periodontol. 2000, 71, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Atsuta, I.; Yamaza, T.; Yoshinari, M.; Mino, S.; Goto, T.; Kido, M.A.; Terada, Y.; Tanaka, T. Changes in the distribution of laminin-5 during peri-implant epithelium formation after immediate titanium implantation in rats. Biomaterials 2005, 26, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Atsuta, I.; Yamaza, T.; Yoshinari, M.; Goto, T.; Kido, M.A.; Kagiya, T.; Mino, S.; Shimono, M.; Tanaka, T. Ultrastructural localization of laminin-5 (gamma2 chain) in the rat peri-implant oral mucosa around a titanium-dental implant by immuno-electron microscopy. Biomaterials 2005, 26, 6280–6287. [Google Scholar] [CrossRef]
- Yamaza, T.; Kido, M.A.; Kiyoshima, T.; Nishimura, Y.; Himeno, M.; Tanaka, T. A fluid-phase endocytotic capacity and intracellular degradation of a foreign protein (horseradish peroxidase) by lysosomal cysteine proteinases in the rat junctional epithelium. J. Periodont. Res. 1997, 32, 651–660. [Google Scholar] [CrossRef]
- Atsuta, I.; Ayukawa, Y.; Furuhashi, A.; Narimatsu, I.; Kondo, R.; Oshiro, W.; Koyano, K. Epithelial sealing effectiveness against titanium or zirconia implants surface. J. Biomed. Mater. Res. A 2019, 107, 1379–1385. [Google Scholar] [CrossRef]
- Feng, B.; Chen, J.Y.; Qi, S.K.; He, L.; Zhao, J.Z.; Zhang, X.D. Characterization of surface oxide films on titanium and bioactivity. J. Mater. Sci. Mater. Med. 2002, 13, 457–464. [Google Scholar] [CrossRef]
- Zhu, L.; Ye, X.; Tang, G.; Zhao, N.; Gong, Y.; Zhao, Y.; Zhao, J.; Zhang, X. Biomimetic coating of compound titania and hydroxyapatite on titanium. J. Biomed. Mater. Res. A 2007, 83, 1165–1175. [Google Scholar] [CrossRef]
- Roach, P.; Farrar, D.; Perry, C.C. Interpretation of protein adsorption: Surface-induced conformational changes. J. Am. Chem. Soc. 2005, 127, 8168–8173. [Google Scholar] [CrossRef]
- Ayukawa, Y.; Oshiro, W.; Atsuta, I.; Furuhashi, A.; Kondo, R.; Jinno, Y.; Koyano, K. Long term retention of gingival sealing around titanium implants with CaCl2 hydrothermal treatment: A rodent study. J. Clin. Med. 2019, 8, 1560. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiong, W.; Zhang, C.; Gao, B.; Guan, H.; Cheng, H.; Fu, J.; Li, F. Enhanced osseointegration and antibacterial action of zinc-loaded titania-nanotube-coated titanium substrates: In vitro and in vivo studies. J. Biomed. Mater. Res. A 2014, 102, 3939–3950. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Y.; Shen, J.; Xu, J.; Zhu, L.; Gu, X.; He, F.; Wang, H. Positive modulation of osteogenesis on a titanium oxide surface incorporating strontium oxide: An in vitro and in vivo study. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Kinumatsu, T.; Hashimoto, S.; Muramatsu, T.; Sasaki, H.; Jung, H.S.; Yamada, S.; Shimono, M. Involvement of laminin and integrins in adhesion and migration of junctional epithelium cells. J. Periodont. Res. 2009, 44, 13–20. [Google Scholar] [CrossRef]
- Kainulainen, T.; Hakkinen, L.; Hamidi, S.; Larjava, K.; Kallioinen, M.; Peltonen, J.; Salo, T.; Larjava, H.; Oikarinen, A. Laminin-5 expression is independent of the injury and the microenvironment during reepithelialization of wounds. J. Histochem. Cytochem. 1998, 46, 353–360. [Google Scholar] [CrossRef]
- Ryan, M.C.; Tizard, R.; VanDevanter, D.R.; Carter, W.G. Cloning of the LamA3 gene encoding the alpha 3 chain of the adhesive ligand epiligrin. Expression in wound repair. J. Biol. Chem. 1994, 269, 22779–22787. [Google Scholar]
- Lansdown, A.B.; Mirastschijski, U.; Stubbs, N.; Scanlon, E.; Agren, M.S. Zinc in wound healing: Theoretical, experimental, and clinical aspects. Wound Repair Regen. 2007, 15, 2–16. [Google Scholar] [CrossRef]
- Zuk, A.; Matlin, K.S.; Hay, E.D. Type I collagen gel induces Madin-Darby canine kidney cells to become fusiform in shape and lose apical-basal polarity. J. Cell Biol. 1989, 108, 903–919. [Google Scholar] [CrossRef]
- Mills, J.W.; Zhou, J.H.; Cardoza, L.; Ferm, V.H. Zinc alters actin filaments in Madin-Darby canine kidney cells. Toxicol. Appl. Pharmacol. 1992, 116, 92–100. [Google Scholar] [CrossRef]
- Carulli, S.; Beck, K.; Dayan, G.; Boulesteix, S.; Lortat-Jacob, H.; Rousselle, P. Cell surface proteoglycans syndecan-1 and -4 bind overlapping but distinct sites in laminin alpha3 LG45 protein domain. J. Biol. Chem. 2012, 287, 12204–12216. [Google Scholar] [CrossRef]
- Mizushima, H.; Takamura, H.; Miyagi, Y.; Kikkawa, Y.; Yamanaka, N.; Yasumitsu, H.; Misugi, K.; Miyazaki, K. Identification of integrin-dependent and -independent cell adhesion domains in COOH-terminal globular region of laminin-5 alpha 3 chain. Cell Growth Differ. 1997, 8, 979–987. [Google Scholar] [PubMed]
- Takeuchi, Y.; Yanagishita, M.; Hascall, V.C. Effects of MgCl2 on the release and recycling of heparan sulfate proteoglycans in a rat parathyroid cell line. Arch. Biochem. Biophys. 1992, 298, 371–379. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Atsuta, I.; Ayukawa, Y.; Narimatsu, I.; Zhou, T.; Hu, J.; Koyano, K. Effects of Different Divalent Cation Hydrothermal Treatments of Titanium Implant Surfaces for Epithelial Tissue Sealing. Materials 2020, 13, 2038. https://doi.org/10.3390/ma13092038
Zhou X, Atsuta I, Ayukawa Y, Narimatsu I, Zhou T, Hu J, Koyano K. Effects of Different Divalent Cation Hydrothermal Treatments of Titanium Implant Surfaces for Epithelial Tissue Sealing. Materials. 2020; 13(9):2038. https://doi.org/10.3390/ma13092038
Chicago/Turabian StyleZhou, Xudiyang, Ikiru Atsuta, Yasunori Ayukawa, Ikue Narimatsu, Tianren Zhou, Jiangqi Hu, and Kiyoshi Koyano. 2020. "Effects of Different Divalent Cation Hydrothermal Treatments of Titanium Implant Surfaces for Epithelial Tissue Sealing" Materials 13, no. 9: 2038. https://doi.org/10.3390/ma13092038
APA StyleZhou, X., Atsuta, I., Ayukawa, Y., Narimatsu, I., Zhou, T., Hu, J., & Koyano, K. (2020). Effects of Different Divalent Cation Hydrothermal Treatments of Titanium Implant Surfaces for Epithelial Tissue Sealing. Materials, 13(9), 2038. https://doi.org/10.3390/ma13092038




