3-D Printed Protective Equipment during COVID-19 Pandemic
Abstract
1. Introduction
2. Materials and Methods
2.1. Face Shield Characteristics
2.2. Manufacturing
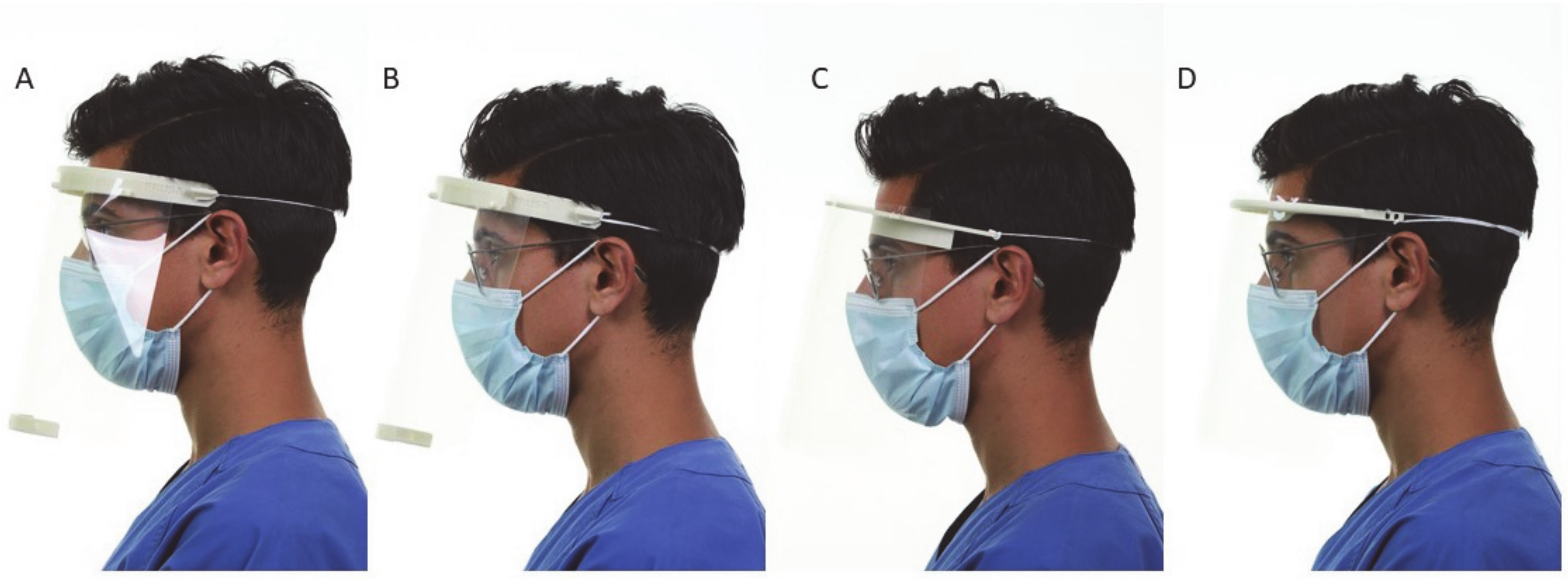
2.3. Evaluation
2.4. Statistical Analysis
3. Results
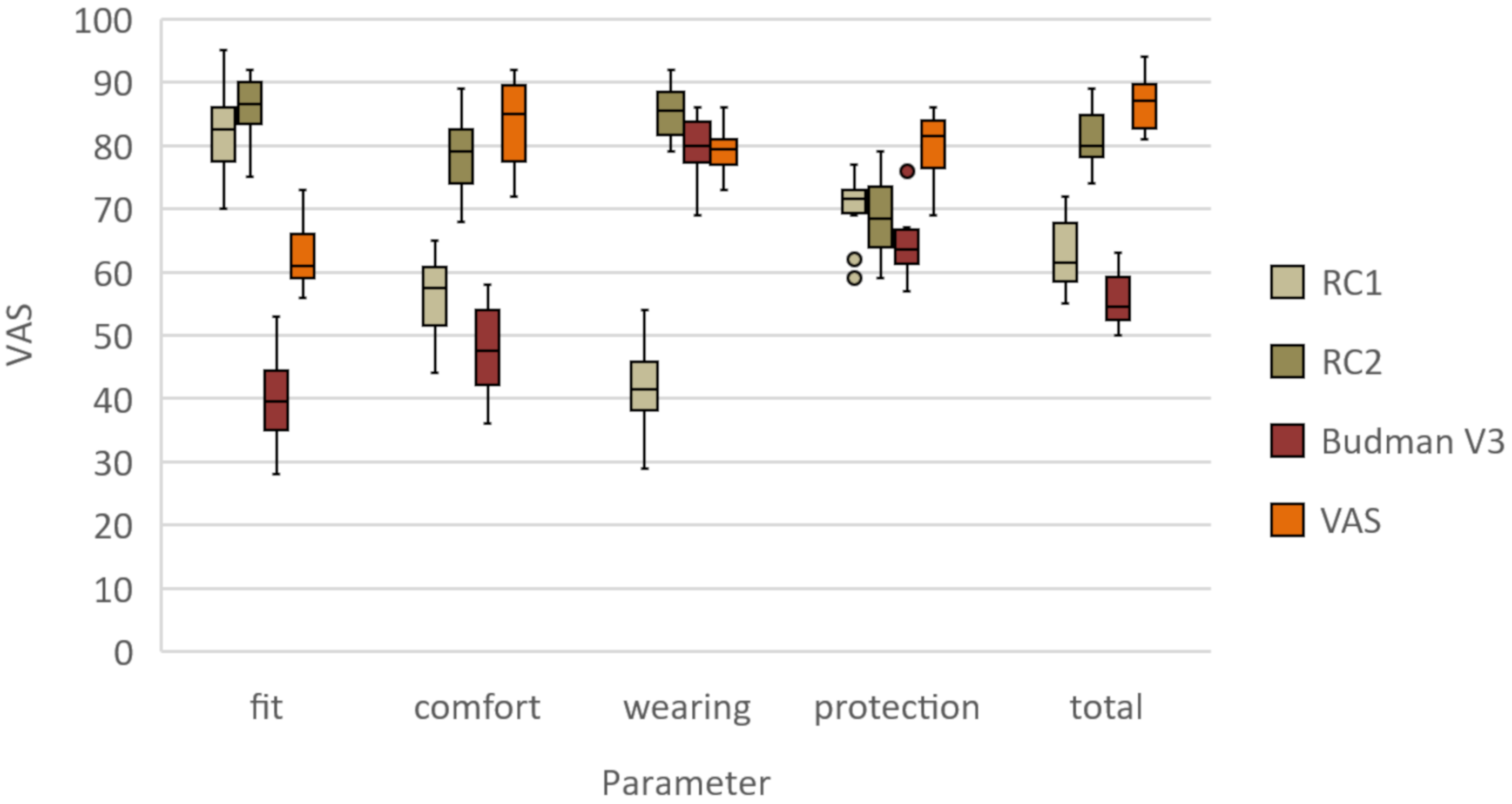
3.1. Clinical Assessment
3.2. RCI
3.3. RC2
3.4. Budmen V3
3.5. Easy 3D
4. Discussion
4.1. Rationale
4.2. Printing Technique
4.3. Printer Requirements
4.4. Material Selection
4.5. Assembly and Protection
4.6. Scalability
4.7. Future Developments
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, X.; Wang, W.; Zhao, X.; Zai, J.; Zhao, Q.; Li, Y.; Chaillon, A. Transmission dynamics and evolutionary history of 2019-nCoV. J. Med. Virol. 2020, 92, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Ranney, M.L.; Griffeth, V.; Jha, A.K. Critical Supply Shortages—The Need for Ventilators and Personal Protective Equipment during the Covid-19 Pandemic. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Strategies for Optimizing the Supply of Facemasks. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/face-masks.html (accessed on 6 April 2020).
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of sars-cov-2 as compared with sars-cov-1. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Yao, L.; Wei, T.; Tian, F.; Jin, D.-Y.; Chen, L.; Wang, M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA 2020, 323, 1406. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. Sars-cov-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Palacios Cruz, M.; Santos, E.; Velazquez Cervantes, M.A.; Leon Juarez, M. Covid-19, a worldwide public health emergency. Rev. Clin. Esp. 2020. [Google Scholar] [CrossRef]
- WHO. Rational Use of Personal Protective Equipment for Coronavirus Disease ( Covid-19): Interim Guidance, 27 february 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Roberge, R.J. Face shields for infection control: A review. J. Occup. Environ. Hyg. 2016, 13, 235–242. [Google Scholar] [CrossRef] [PubMed]
- ANSI/ISEA Z87.1-2015. American National Standard for Occupational and Educational Personal Eye and Face Protection Devices. Available online: https://safetyequipment.org/product/ansiisea-z87-1-2015/ (accessed on 3 April 2020).
- Lindsley, W.G.; Noti, J.D.; Blachere, F.M.; Szalajda, J.V.; Beezhold, D. Efficacy of face shields against cough aerosol droplets from a cough simulator. J. Occup. Environ. Hyg. 2014, 11, 509–518. [Google Scholar] [CrossRef]
- Zhou, P.; Huang, Z.; Xiao, Y.; Huang, X.; Fan, X.-G. Protecting Chinese Healthcare Workers While Combating the 2019 Novel Coronavirus. Infect. Control. Hosp. Epidemiol. 2020, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Shen, C.; Xia, N.; Song, W.; Fan, M.; Cowling, B.J. Rational use of face masks in the COVID-19 pandemic. Lancet Respir. Med. 2020. [Google Scholar] [CrossRef]
- Li, J.-P.O.; Lam, D.S.C.; Chen, Y.; Ting, D.S.W. Novel Coronavirus disease 2019 (COVID-19): The importance of recognising possible early ocular manifestation and using protective eyewear. Br. J. Ophthalmol. 2020, 104, 297–298. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.S.M. Precautions in ophthalmic practice in a hospital with a major acute SARS outbreak: An experience from Hong Kong. Eye 2006, 21, 304–305. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Xu, X.; Li, Y.; Cheng, L.; Zhou, X.; Ren, B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020, 12, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Bleasdale, S.C.; Maita, D.; Brosseau, L.M.; Program, C.P.E. A systematic risk-based strategy to select personal protective equipment for infectious diseases. Am. J. Infect. Control. 2019, 48, 46–51. [Google Scholar] [CrossRef]
- Szykiedans, K.; Credo, W. Mechanical Properties of FDM and SLA Low-cost 3-D Prints. Procedia Eng. 2016, 136, 257–262. [Google Scholar] [CrossRef]
- Ćwikła, G.; Grabowik, C.W.; Kalinowski, K.; Paprocka, I.; Ociepka, P. The influence of printing parameters on selected mechanical properties of FDM/FFF 3D-printed parts. IOP Conf. Ser. Mater. Sci. Eng. 2017, 227, 12033. [Google Scholar] [CrossRef]
- Andrzejewska, A. One Year Evaluation of Material Properties Changes of Polylactide Parts in Various Hydrolytic Degradation Conditions. Polymers 2019, 11, 1496. [Google Scholar] [CrossRef]
- Oth, O.; Dauchot, C.; Orellana, M.; Glineur, R. How to Sterilize 3D Printed Objects for Surgical Use? An Evaluation of the Volumetric Deformation of 3D-Printed Genioplasty Guide in PLA and PETG after Sterilization by Low-Temperature Hydrogen Peroxide Gas Plasma. Open Dent. J. 2019, 13, 410–417. [Google Scholar] [CrossRef]
- Hromadnik, V.; Pieralli, S.; Spies, B.; Beuer, F.; Wesemann, C. Accuracy of a workflow using sleeveless 3D printed surgical guides made from a cost-effective and biodegradable material: An in vitro study. Clin. Oral Implant. Res. 2019, 30, 519. [Google Scholar] [CrossRef]
- Mirzendehdel, A.M.; Suresh, K. Support structure constrained topology optimization for additive manufacturing. Comput. Des. 2016, 81, 1–13. [Google Scholar] [CrossRef]
- Rankin, T.M.; Giovinco, N.; Cucher, D.J.; Watts, G.; Hurwitz, B.; Armstrong, D.G. Three-dimensional printing surgical instruments: Are we there yet? J. Surg. Res. 2014, 189, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.; Kashlan, K.N.; Flanangan, C.L.; Wright, J.K.; Green, G.E.; Hollister, S.; Weatherwax, K.J. Regulatory Considerations in the Design and Manufacturing of Implantable 3D-Printed Medical Devices. Clin. Transl. Sci. 2015, 8, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Neches, R.Y.; Flynn, K.J.; Zaman, L.; Tung, E.; Pudlo, N. On the intrinsic sterility of 3D printing. PeerJ 2016, 4, e2661. [Google Scholar] [CrossRef] [PubMed]
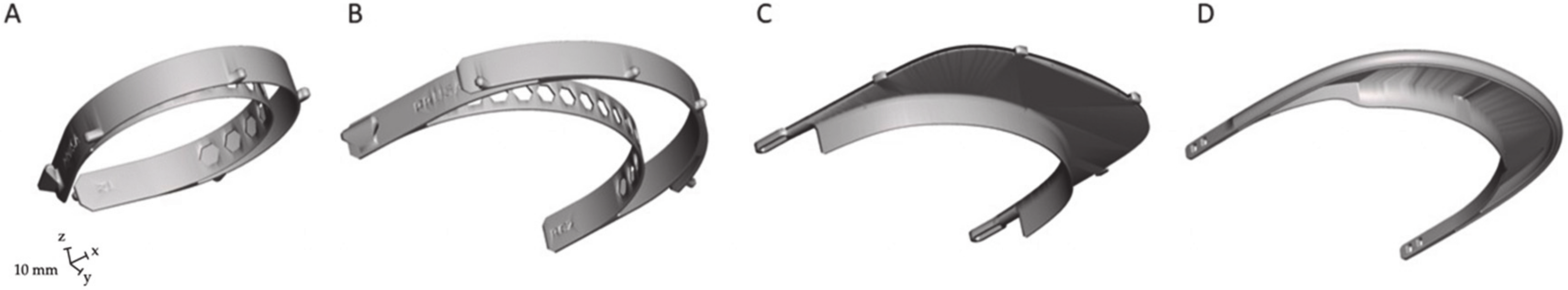
| Name | RC1 (Prusa) | RC2 (Prusa) | 3D Face Shield V3 (Budmen Industries) | Easy 3D Face Shield (Hanoch Hermann) |
|---|---|---|---|---|
| Print volume requirement | 135 × 120 × 20 mm | 144 × 191 × 20 mm | 141 × 187 × 20 mm | 146 × 165 × 15 mm |
| Filament weight | 30 g | 42 g | 33 g | 21 g |
| Total weight | 39 g | 51 g | 42 g | 30 g |
| Printing time | 2 h 30 min | 3 h 17 min | 2 h 06 min | 1 h 40 min |
| Tools for assembling | DIN A4 perforator | DIN A4 perforator | DIN A4 perforator | No |
| Fit | 82 ± 8 | 86 ± 5 | 40 ± 8 | 63 ± 6 |
| Comfort | 56 ± 7 | 78 ± 7 | 48 ± 8 | 83 ± 7 |
| Space for additional PPE | 42 ± 8 | 85 ± 5 | 80 ± 5 | 80 ± 4 |
| Protection | 70 ± 6 | 69 ± 7 | 65 ± 6 | 84 ± 4 |
| Overall | 63 ± 6 | 81 ± 5 | 56 ± 4 | 87 ± 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wesemann, C.; Pieralli, S.; Fretwurst, T.; Nold, J.; Nelson, K.; Schmelzeisen, R.; Hellwig, E.; Spies, B.C. 3-D Printed Protective Equipment during COVID-19 Pandemic. Materials 2020, 13, 1997. https://doi.org/10.3390/ma13081997
Wesemann C, Pieralli S, Fretwurst T, Nold J, Nelson K, Schmelzeisen R, Hellwig E, Spies BC. 3-D Printed Protective Equipment during COVID-19 Pandemic. Materials. 2020; 13(8):1997. https://doi.org/10.3390/ma13081997
Chicago/Turabian StyleWesemann, Christian, Stefano Pieralli, Tobias Fretwurst, Julian Nold, Katja Nelson, Rainer Schmelzeisen, Elmar Hellwig, and Benedikt Christopher Spies. 2020. "3-D Printed Protective Equipment during COVID-19 Pandemic" Materials 13, no. 8: 1997. https://doi.org/10.3390/ma13081997
APA StyleWesemann, C., Pieralli, S., Fretwurst, T., Nold, J., Nelson, K., Schmelzeisen, R., Hellwig, E., & Spies, B. C. (2020). 3-D Printed Protective Equipment during COVID-19 Pandemic. Materials, 13(8), 1997. https://doi.org/10.3390/ma13081997







