Effects of Cryopreservation on Cell Metabolic Activity and Function of Biofabricated Structures Laden with Osteoblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Bioprinting and Culture of Osteoblast Constructs
2.3. Additive Manufacturing of Beta Tricalcium Phosphate Scaffolds
2.4. Cell Culture in Beta Tricalcium Phosphate Scaffolds
2.5. Cryopreservation of the Osteoblast Constructs
2.6. Cell Metabolic Activity
2.7. Alkaline Phosphatase Activity
2.8. Alizarin red S Staining
2.9. Cell Morphology in Tricalcium Phosphate Scaffolds
2.10. Statistical Analysis
3. Results
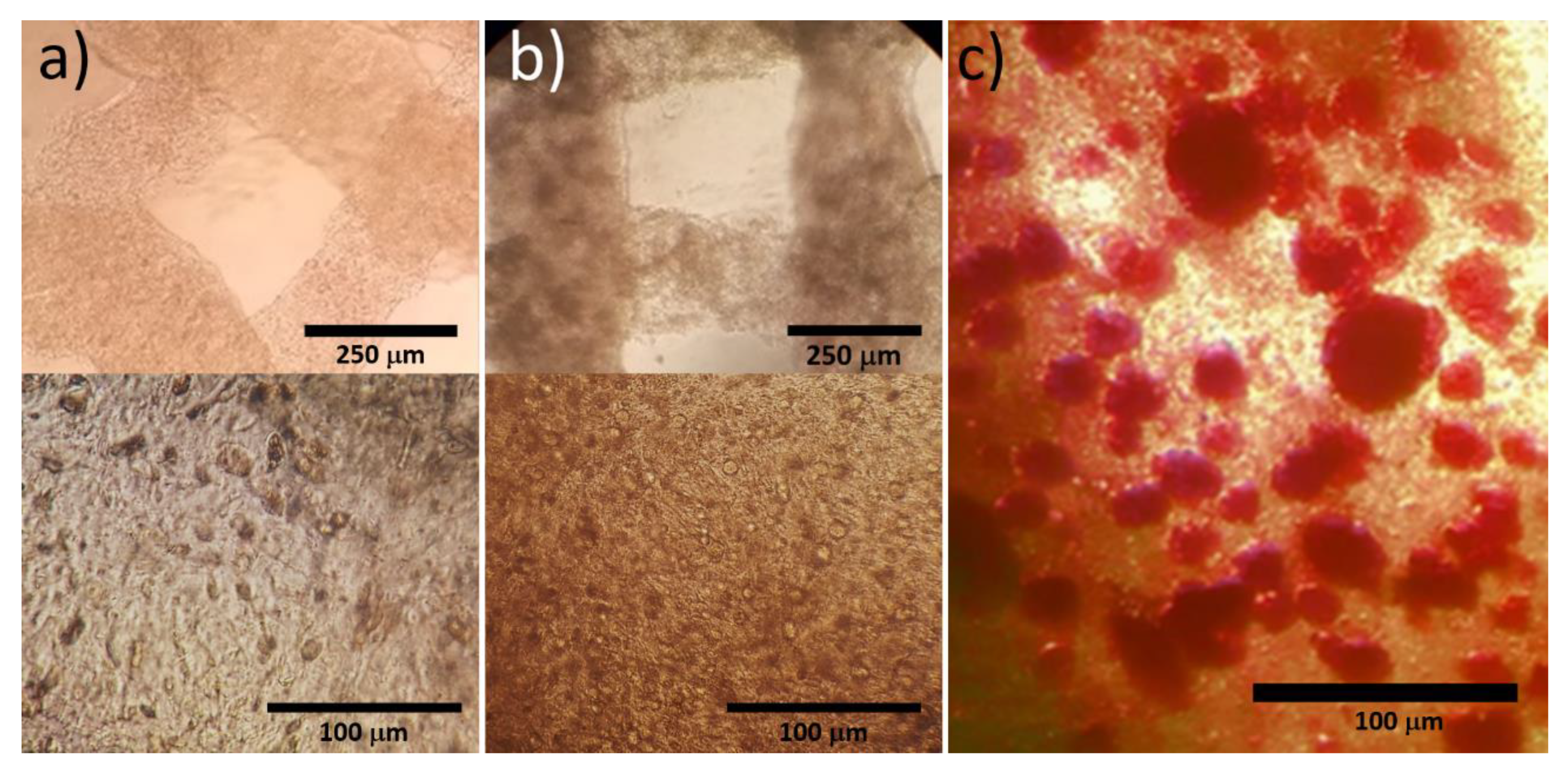
3.1. Bioprinted Osteoblast Constructs
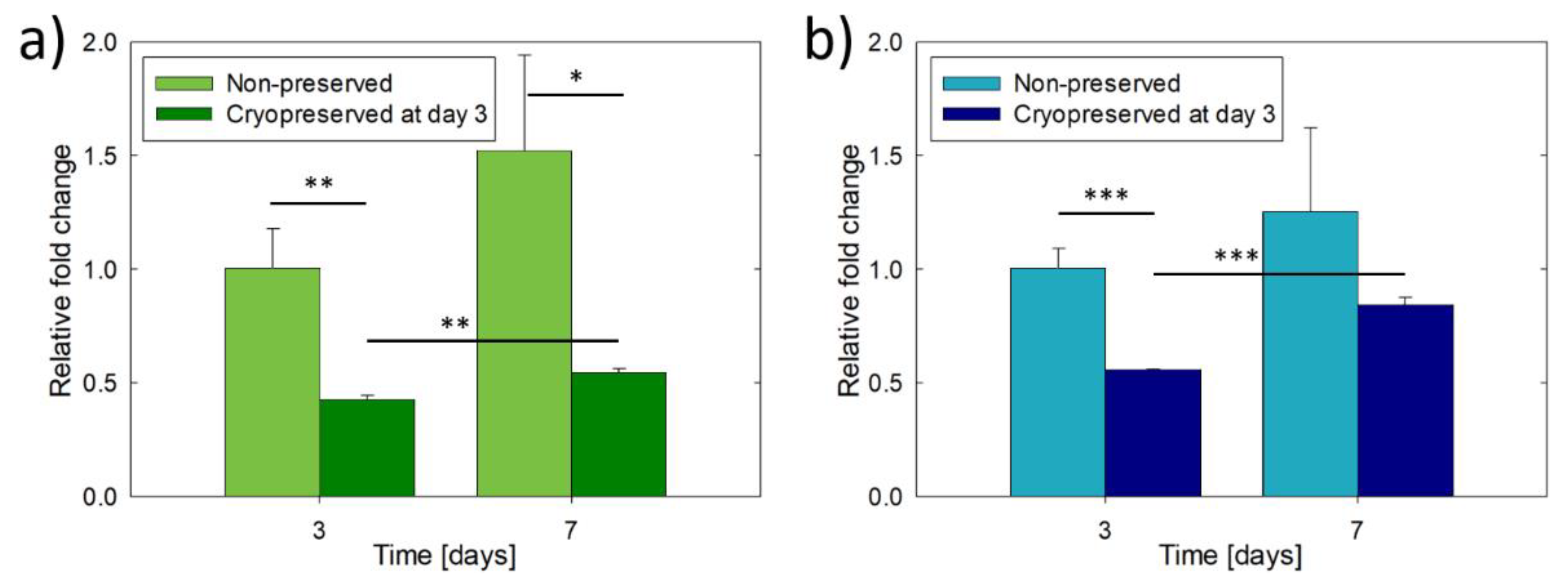
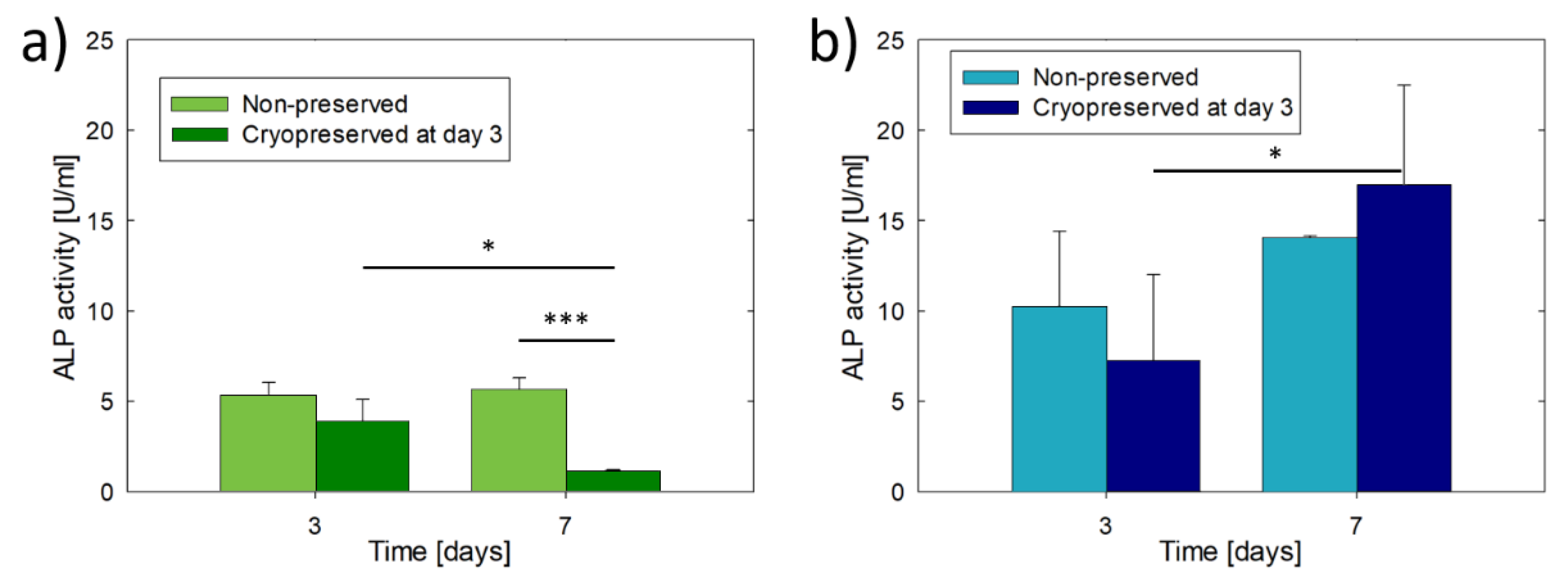
3.2. Culture and Cryopreservation of Bioprinted Osteoblast Constructs
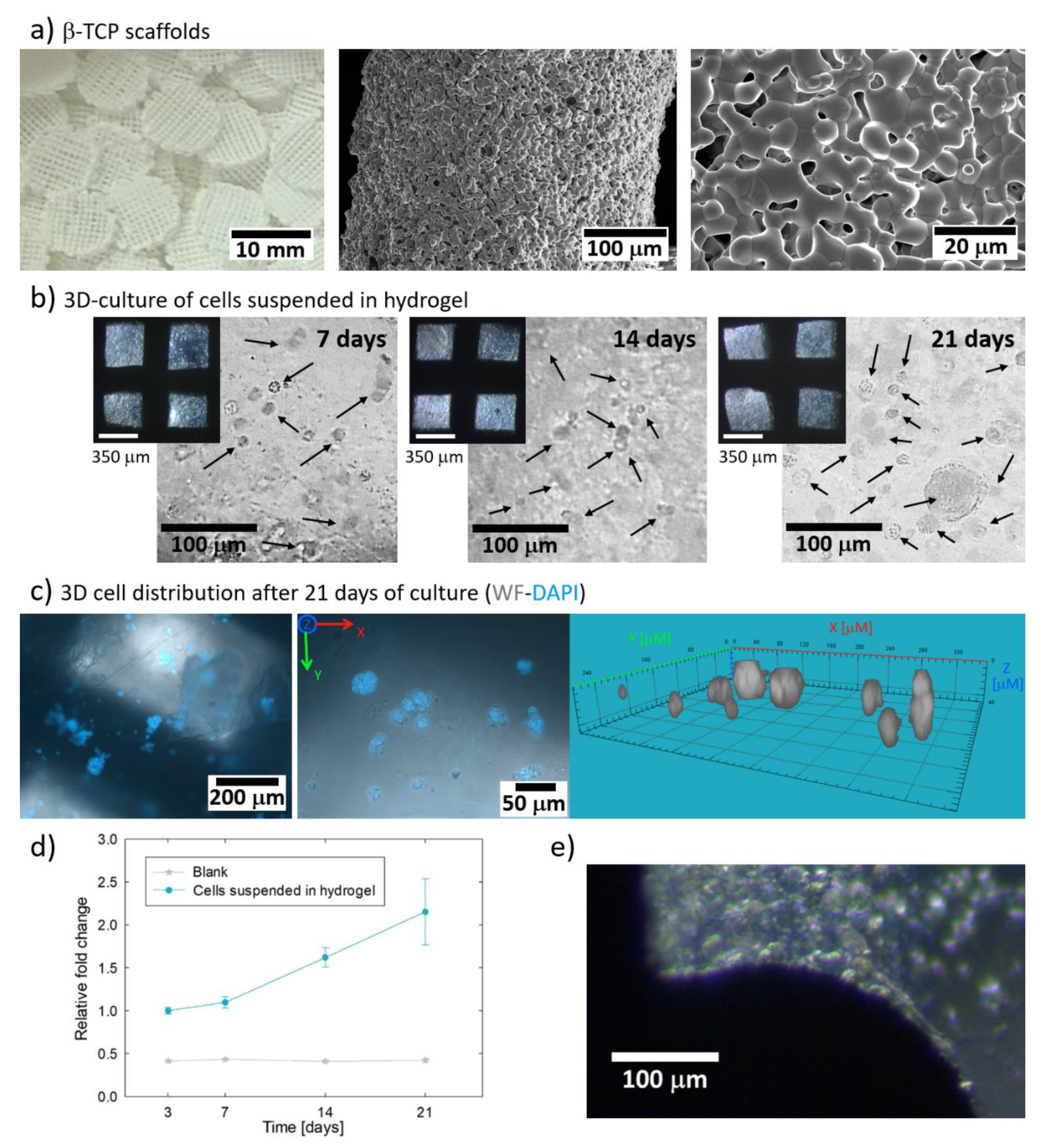
3.3. Culture of Hydrogel Encapsulated Saos-2 Cells in β-TCP Scaffolds
3.4. Cryopreservation of Hydrogel Encapsulated Saos-2 Cells Cultured in β-TCP Scaffolds
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A.; et al. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Marga, F.; Neagu, A.; Kosztin, I.; Forgacs, G. Developmental biology and tissue engineering. Birth Defects Res. Part C Embryo Today Rev. 2007, 81, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Tomaskovic-Crook, E.; Lozano, R.; Chen, Y.; Kapsa, R.M.; Zhou, Q.; Wallace, G.G.; Crook, J.M. Functional 3D Neural mini-tissues from printed gel-based bioink and human neural stem cells. Adv. Healthc. Mater. 2016, 5, 1429–1438. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Tasoglu, S.; Demirci, U. Bioprinting for stem cell research. Trends Biotechnol. 2013, 31, 10–19. [Google Scholar] [CrossRef]
- Xu, F.; Wu, J.; Wang, S.; Durmus, N.G.; Gurkan, U.A.; Demirci, U. Microengineering methods for cell-based microarrays and high-throughput drug-screening applications. Biofabrication 2011, 3, 1–22. [Google Scholar] [CrossRef]
- Samavedi, S.; Joy, N. 3D printing for the development of in vitro cancer models. Curr. Opin. Biomed. Eng. 2017, 2, 35–42. [Google Scholar] [CrossRef]
- Taubenberger, A.V. In vitro microenvironments to study breast cancer bone colonisation. Adv. Drug. Deliv. Rev. 2014, 79, 135–144. [Google Scholar] [CrossRef]
- Mir, T.A.; Nakamura, M. Three-Dimensional Bioprinting: Toward the era of manufacturing human organs as spare parts for healthcare and medicine. Tissue Eng. Part B Rev. 2017, 23, 245–256. [Google Scholar] [CrossRef]
- Raman, R.; Bashir, R. Biomimicry, biofabrication, and biohybrid systems: The emergence and evolution of biological desigin. Adv. Healthc. Mater. 2017, 6, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Shegarfi, H.; Reikeras, O. Review Article: Bone transplantation and immune response. J. Orthop. Surg. 2016, 17, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.J. Cryopreservation of human stem cells for clinical application: A review. Transfus. Med. Hemotherapy 2011, 38, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Pogozhykh, O.; Prokopyuk, V.; Prokopyuk, O.; Kuleshova, L.; Goltsev, A.; Figueiredo, C.; Pogozhykh, D. Towards biobanking technologies for natural and bioengineered multicellular placental constructs. Biomaterials 2018, 185, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kuleshova, L.L.; Gouk, S.S.; Hutmacher, D.W. Vitrification as a prospect for cryopreservation of tissue-engineered constructs. Biomaterials 2007, 28, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Dahl, S.L.; Chen, Z.; Solan, A.K.; Brockbank, K.G.; Niklason, L.E.; Song, Y.C. Feasibility of vitrification as a storage method for tissue-engineered blood vessels. Tissue Eng. 2006, 12, 291–300. [Google Scholar] [CrossRef]
- Agudelo, C.A.; Iwata, H. The development of alternative vitrification solutions for microencapsulated islets. Biomaterials 2008, 29, 1167–1176. [Google Scholar] [CrossRef]
- Malpique, R.; Osório, L.M.; Ferreira, D.S.; Ehrhart, F.; Brito, C.; Zimmermann, H.; Alves, P.M. Alginate encapsulation as a novel strategy for the cryopreservation of neurospheres. Tissue Eng. Part C Methods 2010, 16, 965–977. [Google Scholar] [CrossRef]
- Ahmad, H.F.; Sambanis, A. Cryopreservation effects on recombinant myoblasts encapsulated in adhesive alginate hydrogels. Acta Biomater. 2013, 9, 6814–6822. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, X.; Zhu, K.; He, X. Hydrogel encapsulation facilitates rapid-cooling cryopreservation of stem cell-laden core-shell microcapsules as cell-biomaterial constructs. Adv. Healthc. Mater. 2017, 6, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Koebe, H.G.; Dunn, J.C.; Toner, M.; Sterling, L.M.; Hubel, A.; Cravalho, E.G.; Yarmush, M.L.; Tompkins, R.G. A new approach to the cryopreservation of hepatocytes in a sandwich culture configuration. Cryobiology 1990, 27, 576–584. [Google Scholar] [CrossRef]
- Umemura, E.; Yamada, Y.; Nakamura, S.; Ito, K.; Hara, K.; Ueda, M. Viable cryopreserving tissue-engineered cell-biomaterial for cell banking therapy in an effective cryoprotectant. Tissue Eng. Part C Methods 2011, 17, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Teo, K.Y.; De Hoyos, T.O.; Dutton, J.C.; Grinnell, F.; Han, B. Effects of freezing-induced cell-fluid-matrix interactions on the cells and extracellular matrix of engineered tissues. Biomaterials 2011, 32, 5380–5390. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, W.; Wu, W.; Jin, Y.; Cen, L.; Kretlow, J.D.; Gao, W.; Dai, Z.; Wang, J.; Zhou, G.; et al. Cryopreservation of tissue-engineered epithelial sheets in trehalose. Biomaterials 2011, 32, 8426–8435. [Google Scholar] [CrossRef] [PubMed]
- Kofron, M.D.; Opsitnick, N.C.; Attawia, M.A.; Laurencin, C.T. Cryopreservation of tissue engineered constructs for bone. J. Orthop. Res. 2003, 21, 1005–1010. [Google Scholar] [CrossRef]
- Liu, B.L.; McGrath, J. Vitrification solutions for the cryopreservation of tissue-engineered bone. Cell Preserv. Technol. 2004, 2, 133–143. [Google Scholar] [CrossRef]
- Luo, Y.; Lode, A.; Akkineni, A.R.; Gelinsky, M. Concentrated gelatin/alginate composites for fabrication of predesigned scaffolds with a favorable cell response by 3D plotting. RSC Adv. 2015, 5, 43480–43488. [Google Scholar] [CrossRef]
- Zehnder, T.; Sarker, B.; Boccaccini, A.R.; Detsch, R. Evaluation of an alginate–gelatine crosslinked hydrogel for bioplotting. Biofabrication 2015, 7, 025001. [Google Scholar] [CrossRef]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. Part A 2013, 101, 1255–1264. [Google Scholar] [CrossRef]
- Xia, Y.; Mei, F.; Duan, Y.; Gao, Y.; Xiong, Z.; Zhang, T. Bone tissue engineering using bone marrow stromal cells and an injectable sodium alginate/gelatin scaffold. J. Biomed. Mater. Res. Part A 2012, 100, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Stancu, I.C.; Dragusin, D.M.; Vasile, E.; Trusca, R.; Antoniac, I.; Vasilescu, D.S. Porous calcium alginate-gelatin interpenetrated matrix and its biomineralization potential. J. Mater. Sci.: Mater. Med. 2011, 22, 451–460. [Google Scholar] [CrossRef]
- Richards, R.; Czekanska, E.; Hayes, J.; Stoddart, M. In search of an osteoblast cell model for in vitro research. Eur. Cells Mater. 2016, 24, 1–17. [Google Scholar]
- Czekanska, E.M.; Stoddart, M.J.; Ralphs, J.R.; Richards, R.G.; Hayes, J.S. A phenotypic comparison of osteoblast cell lines versus human primary osteoblasts for biomaterials testing. J. Biomed. Mater. Res. Part A 2014, 102, 2636–2643. [Google Scholar] [CrossRef] [PubMed]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef]
- Casas-Luna, M.; Tan, H.; Tkachenko, S.; Salamon, D.; Montufar, E.B. Enhancement of mechanical properties of 3D-plotted tricalcium phosphate scaffolds by rapid sintering. J. Eur. Cer. Soc. 2019, 39, 4366–4374. [Google Scholar] [CrossRef]
- Freshney, R.I. Cryopreservation. In Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications, 6th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2010; pp. 317–334. [Google Scholar]
- Takeo, T.; Kondo, T.; Haruguchi, Y.; Fukumoto, K.; Nakagawa, Y.; Takeshita, Y.; Nakamuta, Y.; Tsuchiyama, S.; Shimizu, N.; Hasegawa, T.; et al. Short-term storage and transport at cold temperatures of 2-cell mouse embryos produced by cryopreserved sperm. J Am. Assoc. Lab. Anim. Sci. 2010, 49, 415–419. [Google Scholar]
- Mazur, P.; Leibo, S.P.; Chu, E.H. A two-factor hypothesis of freezing injury. Evidence from Chinese hamster tissue-culture cells. Exp. Cell. Res. 1972, 71, 345–355. [Google Scholar] [CrossRef]
- Karlsson, J.O.; Toner, M. Long-term storage of tissues by cryopreservation: Critical issues. Biomaterials 1996, 17, 243–256. [Google Scholar] [CrossRef]
- Whittingham, D.G.; Leibo, S.P.; Mazur, P. Survival of Mouse Embryos Frozen to −196° and −269 °C. Science 1972, 178, 411–414. [Google Scholar] [CrossRef]
- Ji, L.; De Pablo, L.L.; Palecek, S.P. Cryopreservation of adherent human embryonic stem cells. Biotechnol. Bioeng. 2004, 88, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Goh, B.C.; Thirumala, S.; Kilroy, G.; Devireddy, R.V.; Gimble, J.M. Cryopreservation characteristics of adipose-derived stem cells: Maintenance of differentiation potential and viability. J. Tissue Eng. Regen. Med. 2012, 1, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.; Wood, M.J.; Whittingham, D.G. Normal fertilization and development of frozen-thawed Mouse Oocytes: Protective action of certain macromolecules. Biol. Reprod. 1993, 48, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Sambu, S.; Xu, X.; Schiffter, H.A.; Cui, Z.F.; Ye, H. RGDS-Fuctionalized alginates improve the survival rate of encapsulated embryonic stem cells during cryopreservation. Cryo-Letters 2011, 32, 389–401. [Google Scholar] [PubMed]
- Miranda, P.; Saiz, E.; Gryn, K.; Tomsia, A.P. Sintering and robocasting of beta-tricalcium phosphate scaffolds for orthopaedic applications. Acta Biomater. 2006, 2, 457–466. [Google Scholar] [CrossRef]
- Varghese, S.; Chien, S.; Gianneschi, N.C.; Kang, H.; Vecchio, K.S.; Caro, E.J.; Phadke, A.; Theodorakis, E.A.; Siu, M.; Hwang, Y.; et al. Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 990–995. [Google Scholar]
- Lee, M.N.; Hwang, H.S.; Oh, S.O.; Roshanzadeh, A.; Kim, J.W.; Song, J.H.; Kim, E.S.; Koh, J.T. Elevated extracellular calcium ions promote proliferation and migration of mesenchymal stem cells via increasing osteopontin expression. Exp. Mol. Med. 2018, 50, 1–16. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, L.; Liu, J.; Zhou, X.; Weir, M.D. Bone tissue engineering via nanostructured calcium phosphate biomaterials and stem cells. Bone Res. 2014, 2, 1–13. [Google Scholar] [CrossRef]
- Pautke, C.; Schieker, M.; Tischer, T.; Kolk, A.; Neth, P.; Mutscher, W.; Milz, S. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2OS in comparison to human osteoblasts. Anticancer Res. 2004, 24, 3743–3748. [Google Scholar]
- Park, S.; Nam, J.S.; Lee, D.R.; Kim, H.; Ahn, C.W. Fetal bovine serum-free cryopreservation methods for clinical banking of human adipose-derived stem cells. Cryobiology 2018, 81, 65–73. [Google Scholar] [CrossRef]
- Liang, X.; Hu, X.; Hu, Y.; Zeng, W.; Zeng, G.; Ren, Y.; Liu, Y.; Chen, K.; Peng, H.; Ding, H.; et al. Recovery and functionality of cryopreserved peripheral blood mononuclear cells using five different xeno-free cryoprotective solutions. Cryobiology 2019, 86, 25–32. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Tapia, L.G.; Fohlerová, Z.; Žídek, J.; Alvarez-Perez, M.A.; Čelko, L.; Kaiser, J.; Montufar, E.B. Effects of Cryopreservation on Cell Metabolic Activity and Function of Biofabricated Structures Laden with Osteoblasts. Materials 2020, 13, 1966. https://doi.org/10.3390/ma13081966
Hernández-Tapia LG, Fohlerová Z, Žídek J, Alvarez-Perez MA, Čelko L, Kaiser J, Montufar EB. Effects of Cryopreservation on Cell Metabolic Activity and Function of Biofabricated Structures Laden with Osteoblasts. Materials. 2020; 13(8):1966. https://doi.org/10.3390/ma13081966
Chicago/Turabian StyleHernández-Tapia, Laura G., Zdenka Fohlerová, Jan Žídek, Marco A. Alvarez-Perez, Ladislav Čelko, Jozef Kaiser, and Edgar B. Montufar. 2020. "Effects of Cryopreservation on Cell Metabolic Activity and Function of Biofabricated Structures Laden with Osteoblasts" Materials 13, no. 8: 1966. https://doi.org/10.3390/ma13081966
APA StyleHernández-Tapia, L. G., Fohlerová, Z., Žídek, J., Alvarez-Perez, M. A., Čelko, L., Kaiser, J., & Montufar, E. B. (2020). Effects of Cryopreservation on Cell Metabolic Activity and Function of Biofabricated Structures Laden with Osteoblasts. Materials, 13(8), 1966. https://doi.org/10.3390/ma13081966








