Hydrogen Embrittlement and Oxide Layer Effect in the Cathodically Charged Zircaloy-2
Abstract
1. Introduction
2. Materials and Methods
3. Results
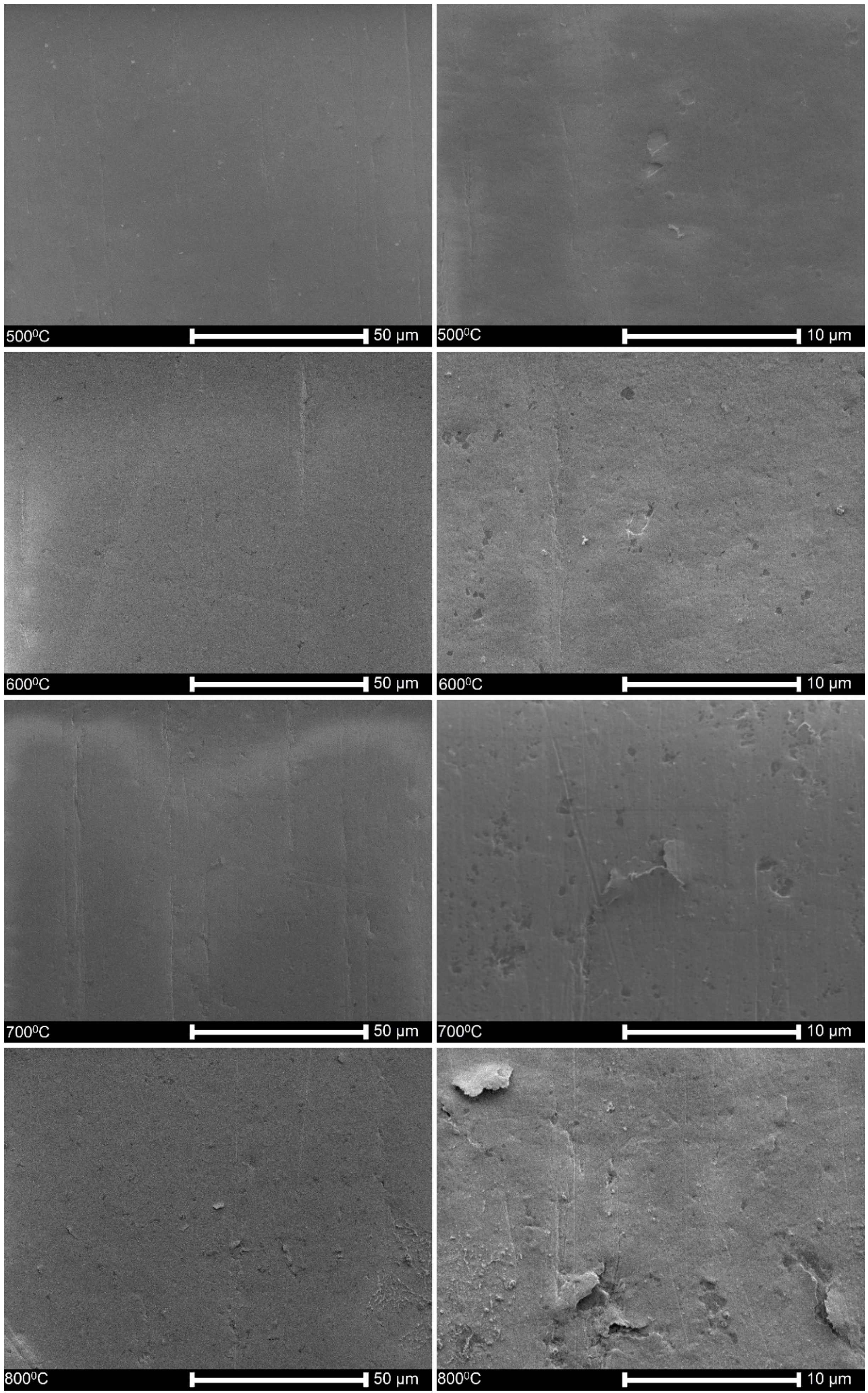
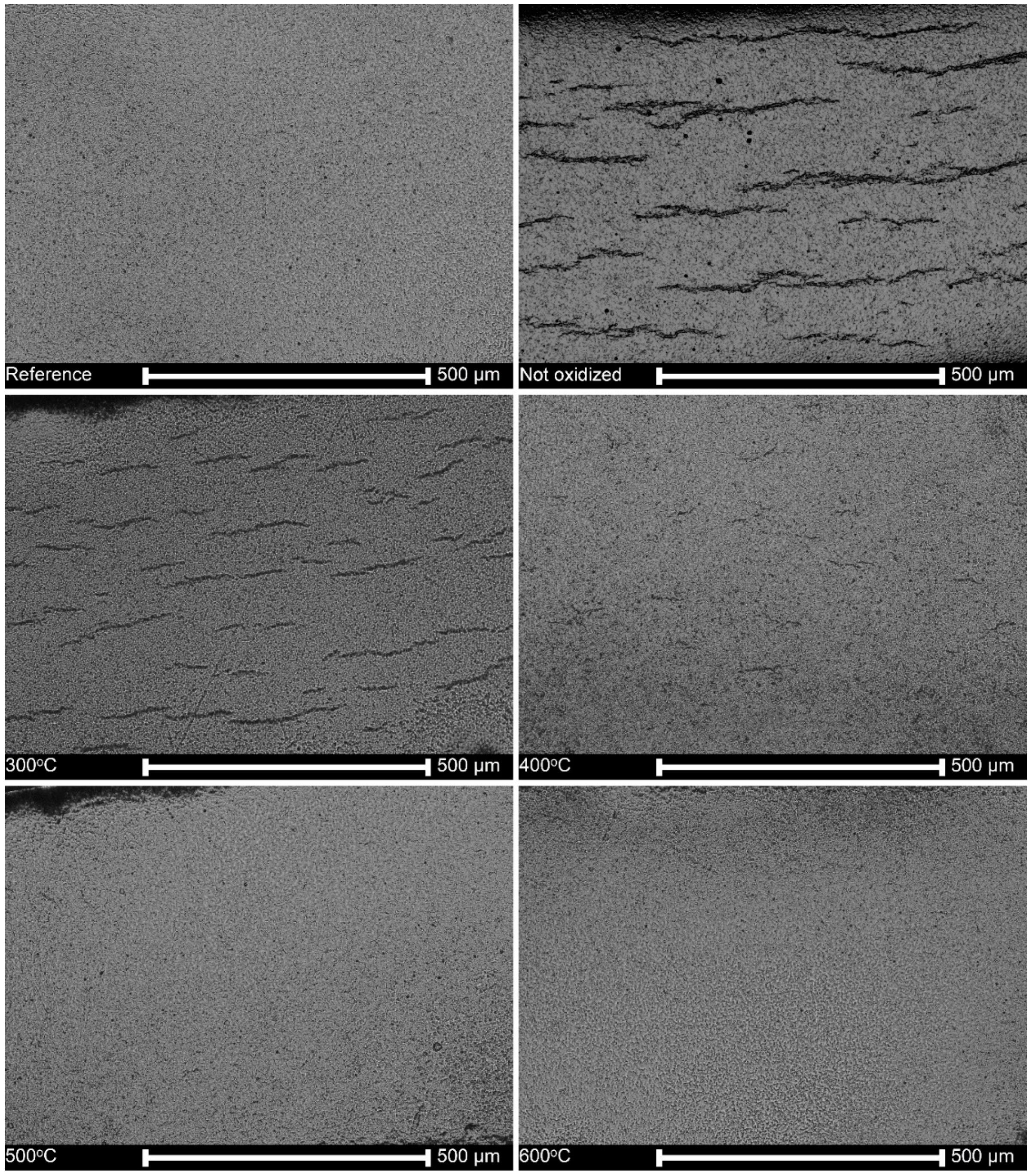
3.1. Surface Topography
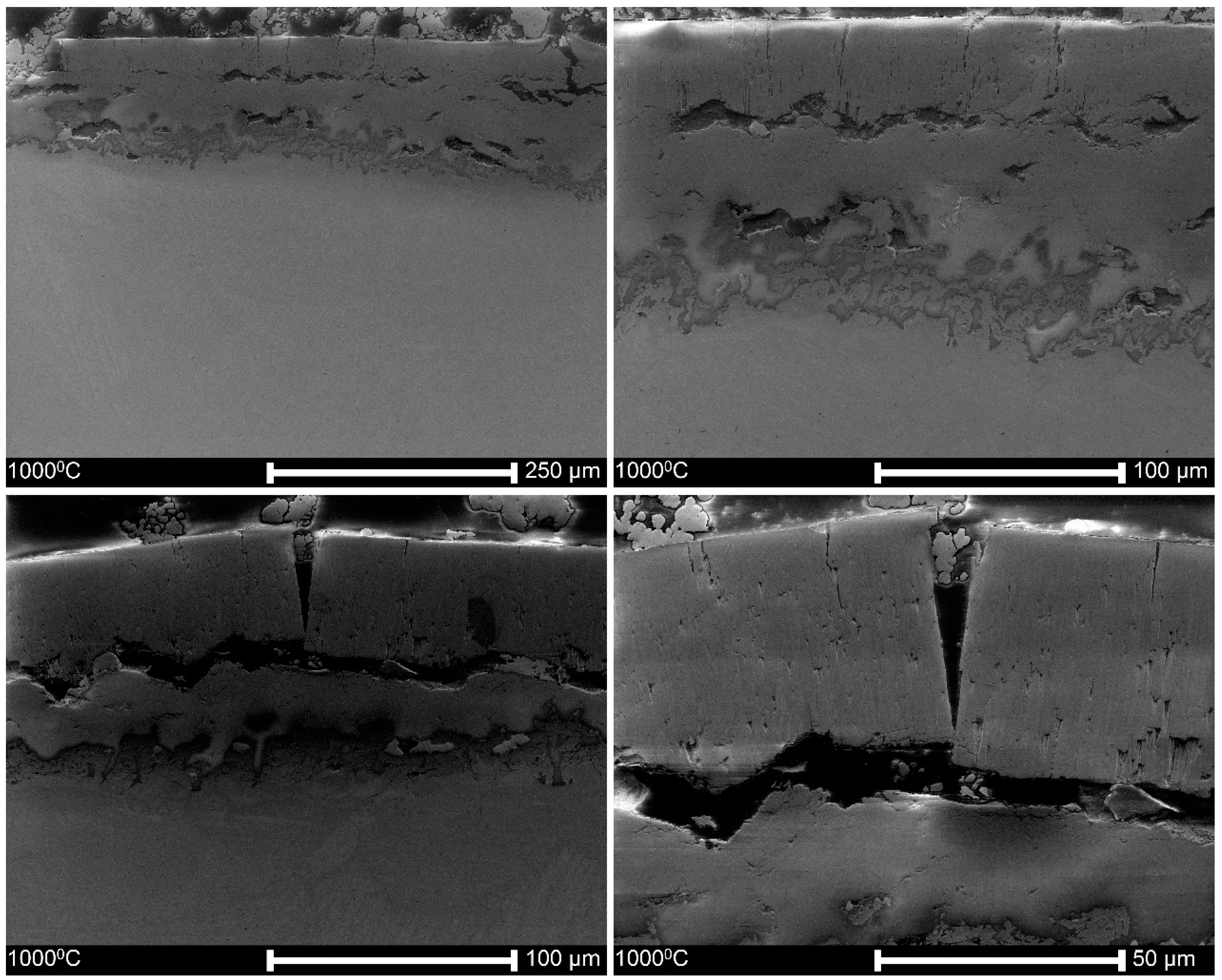
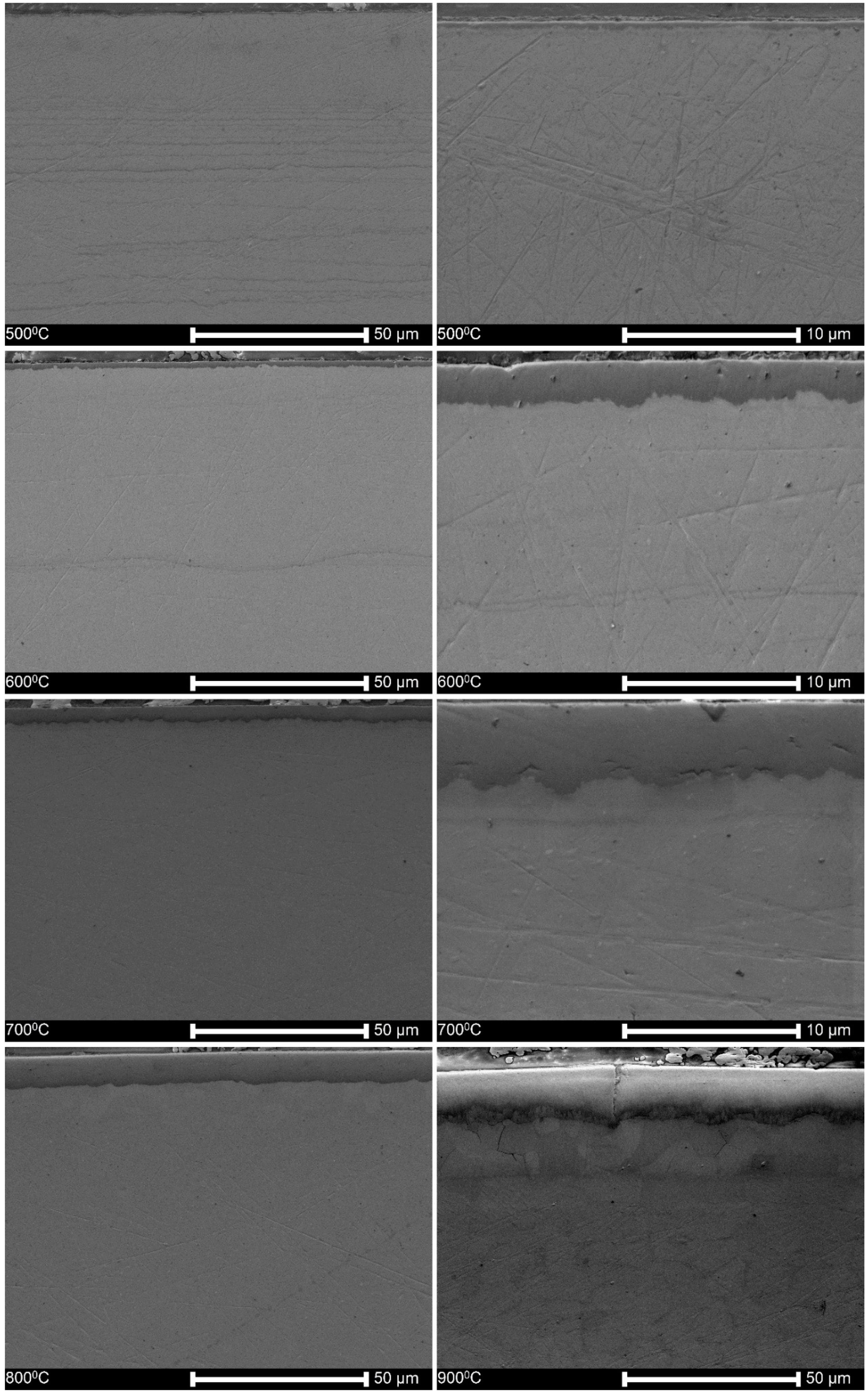
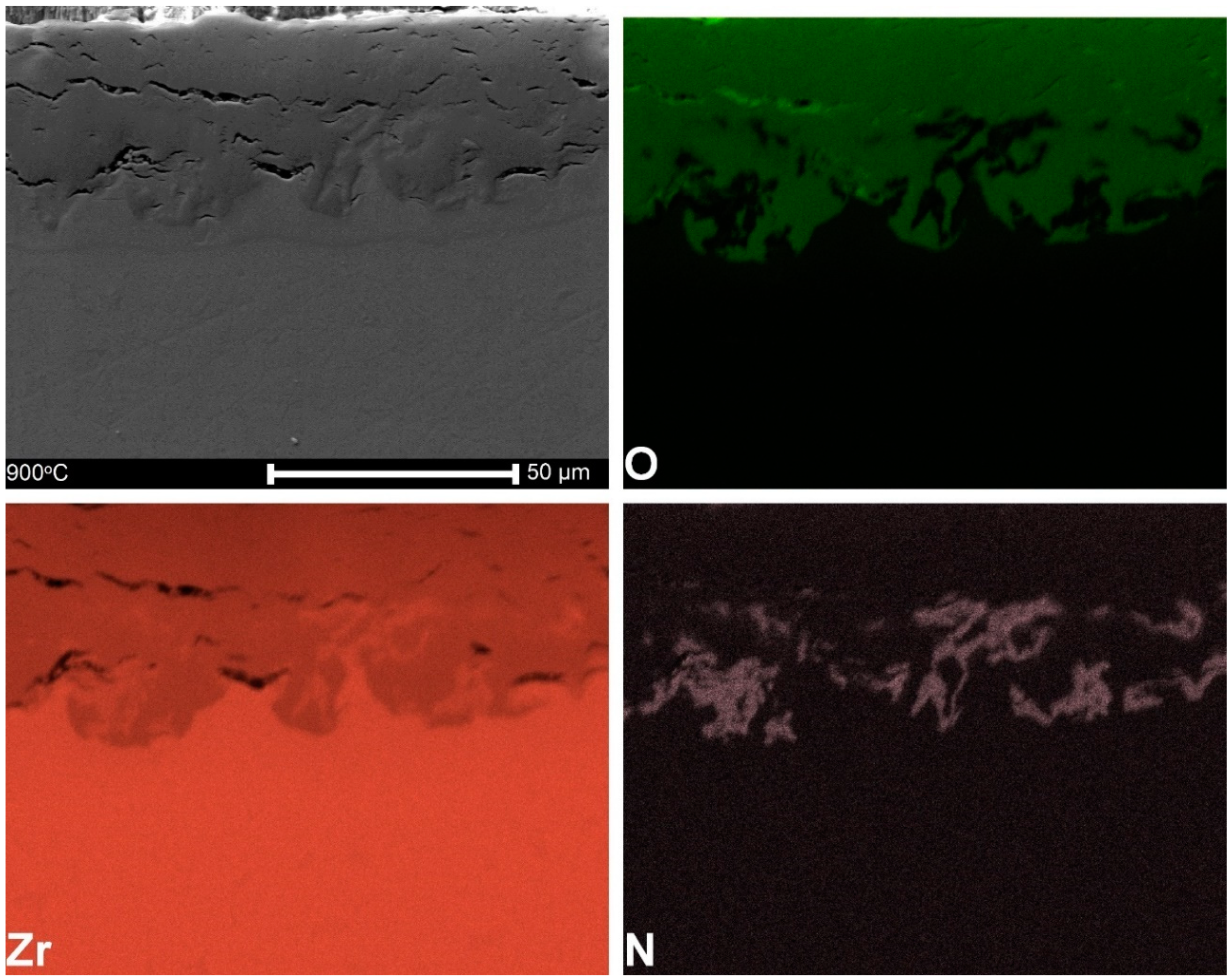
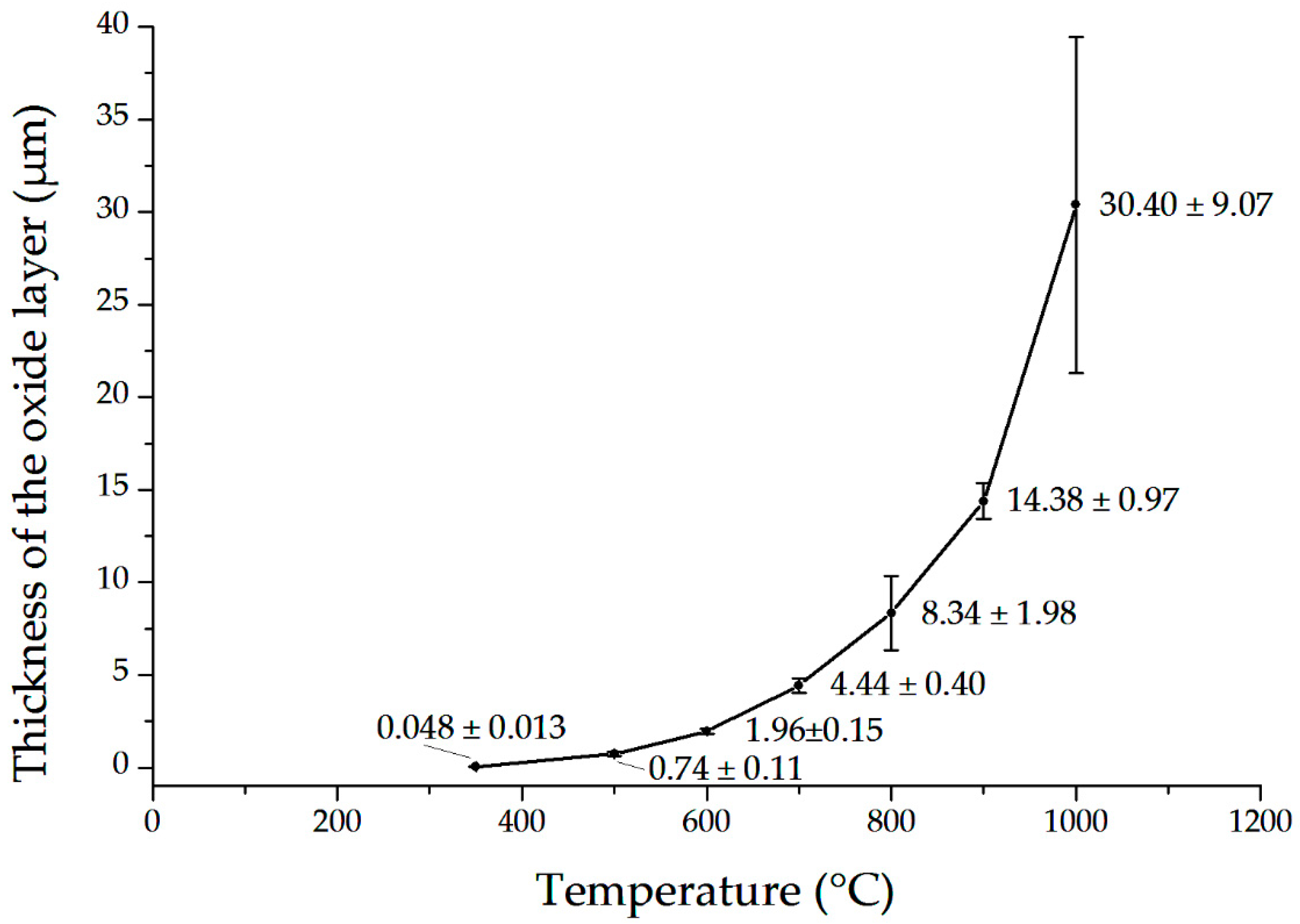
3.2. Microstructure and Thickness of Oxide Layers
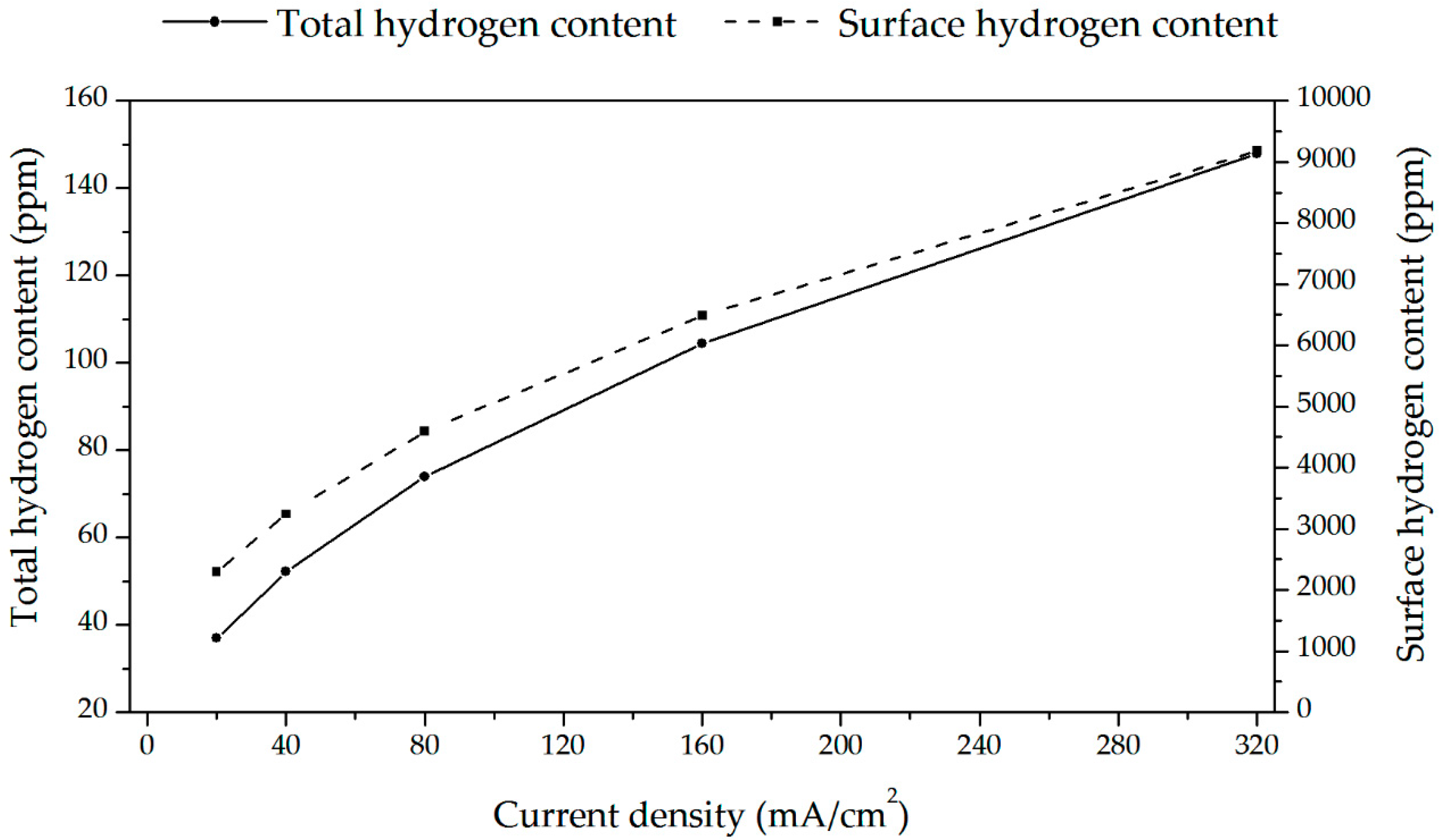
3.3. Hydrogen Permeation
3.4. Hydrogen Uptake
3.5. Microstructure of Hydrogen-Charged Specimens as Related to Oxidation Temperature
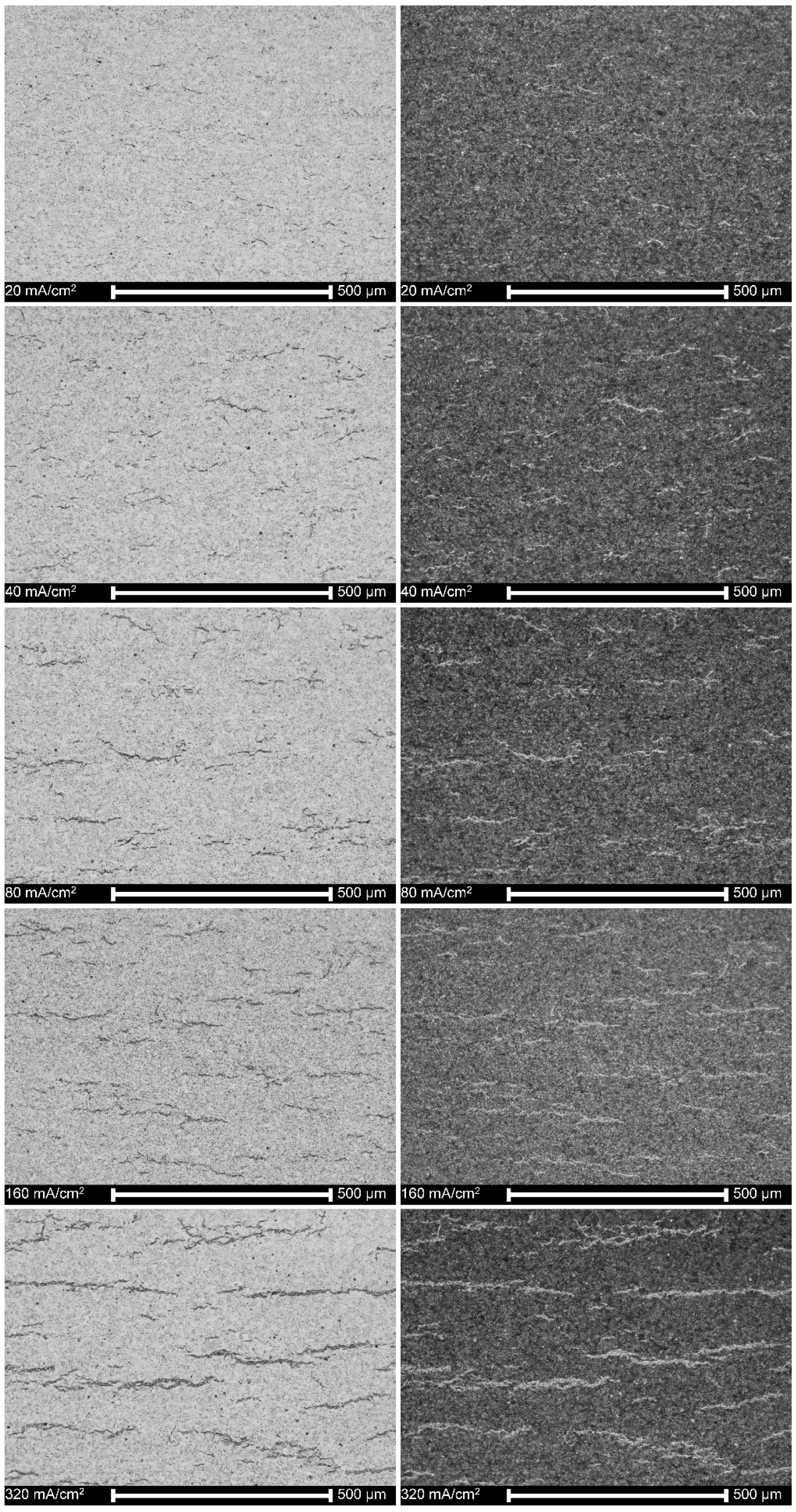
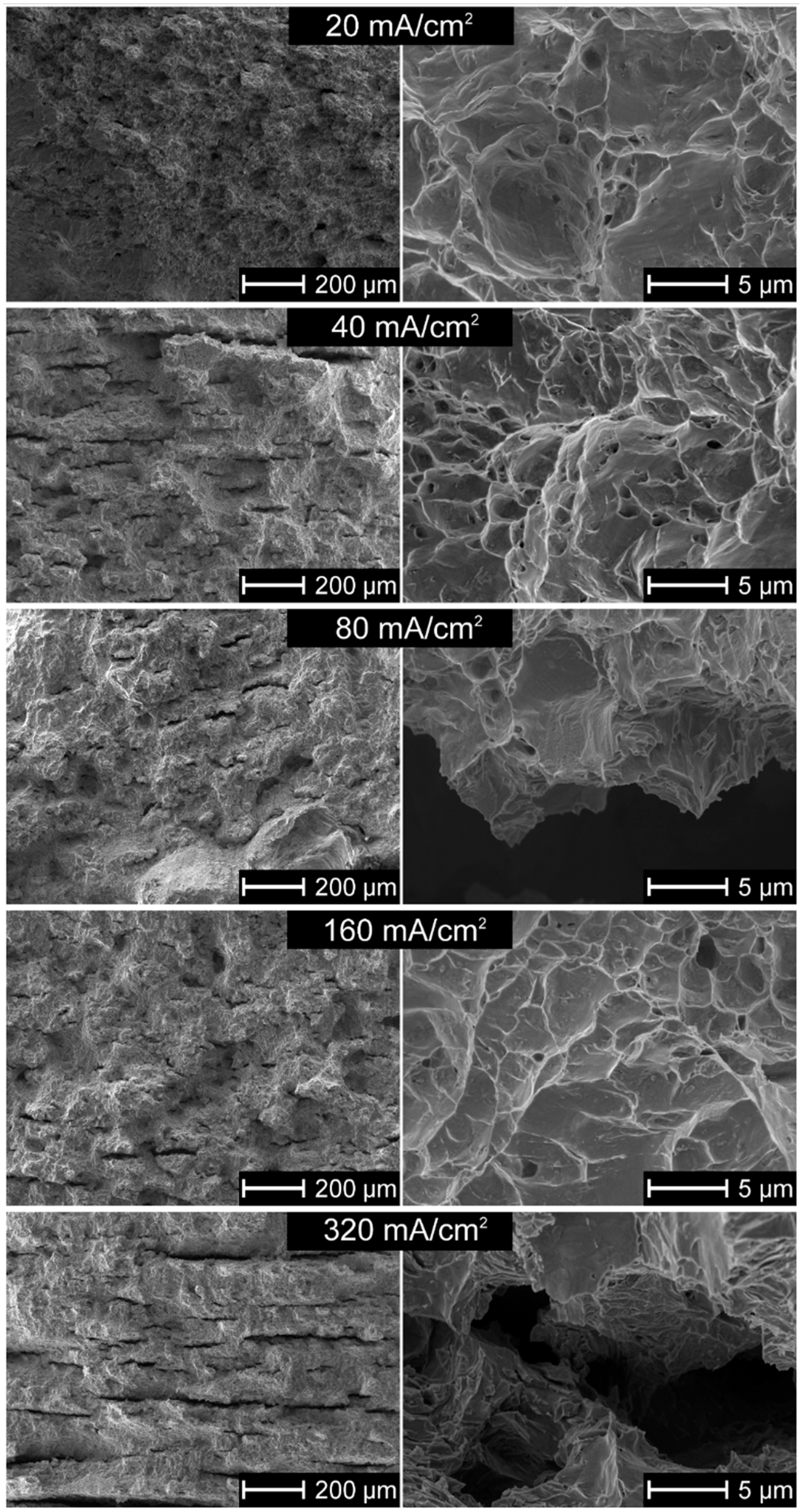
3.6. Microstructure of Hydrogen-Charged Alloy as Related to Cathodic Charging Density
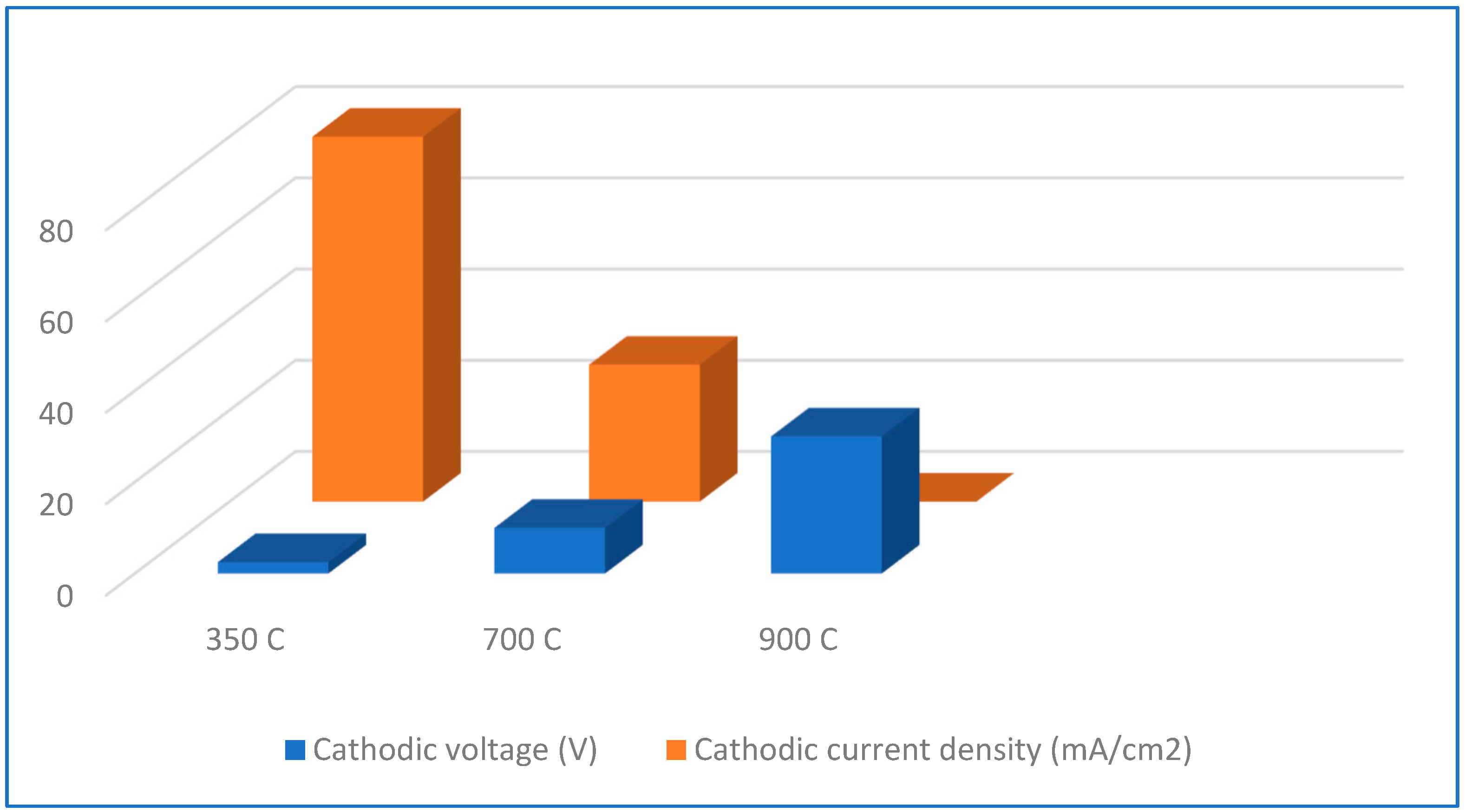
3.7. Mechanical Properties in Tensile Tests
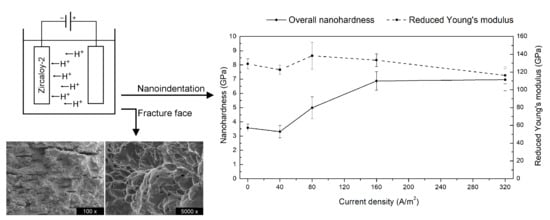
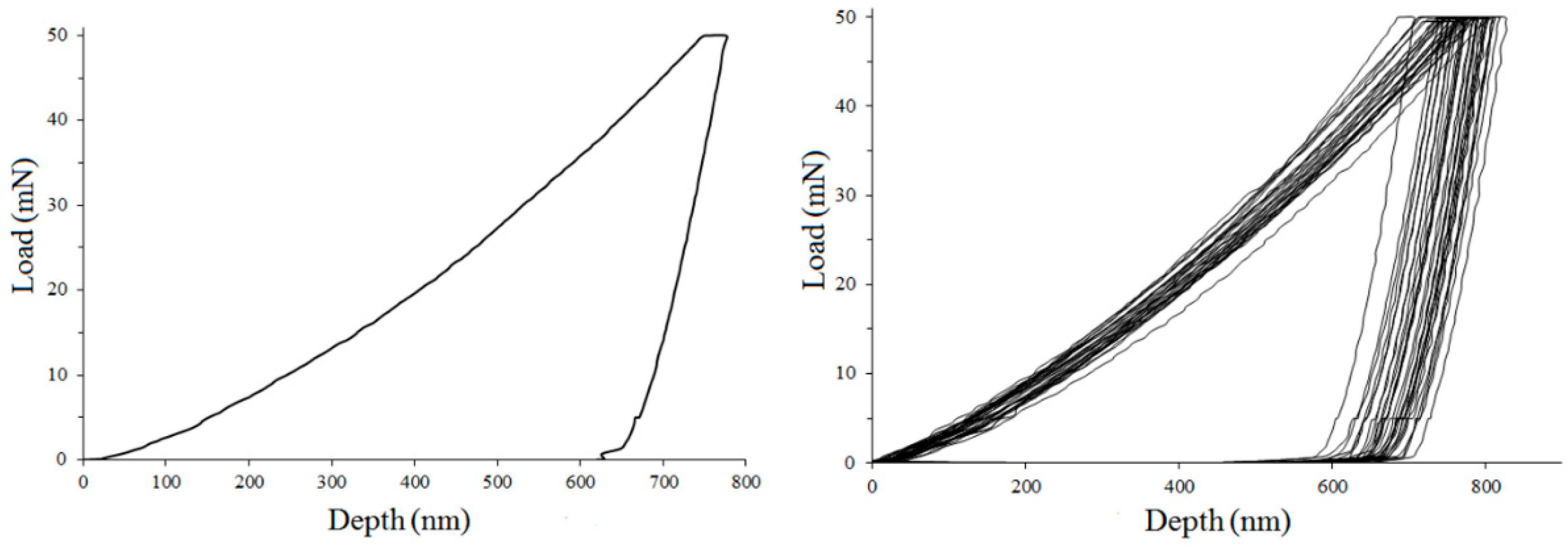
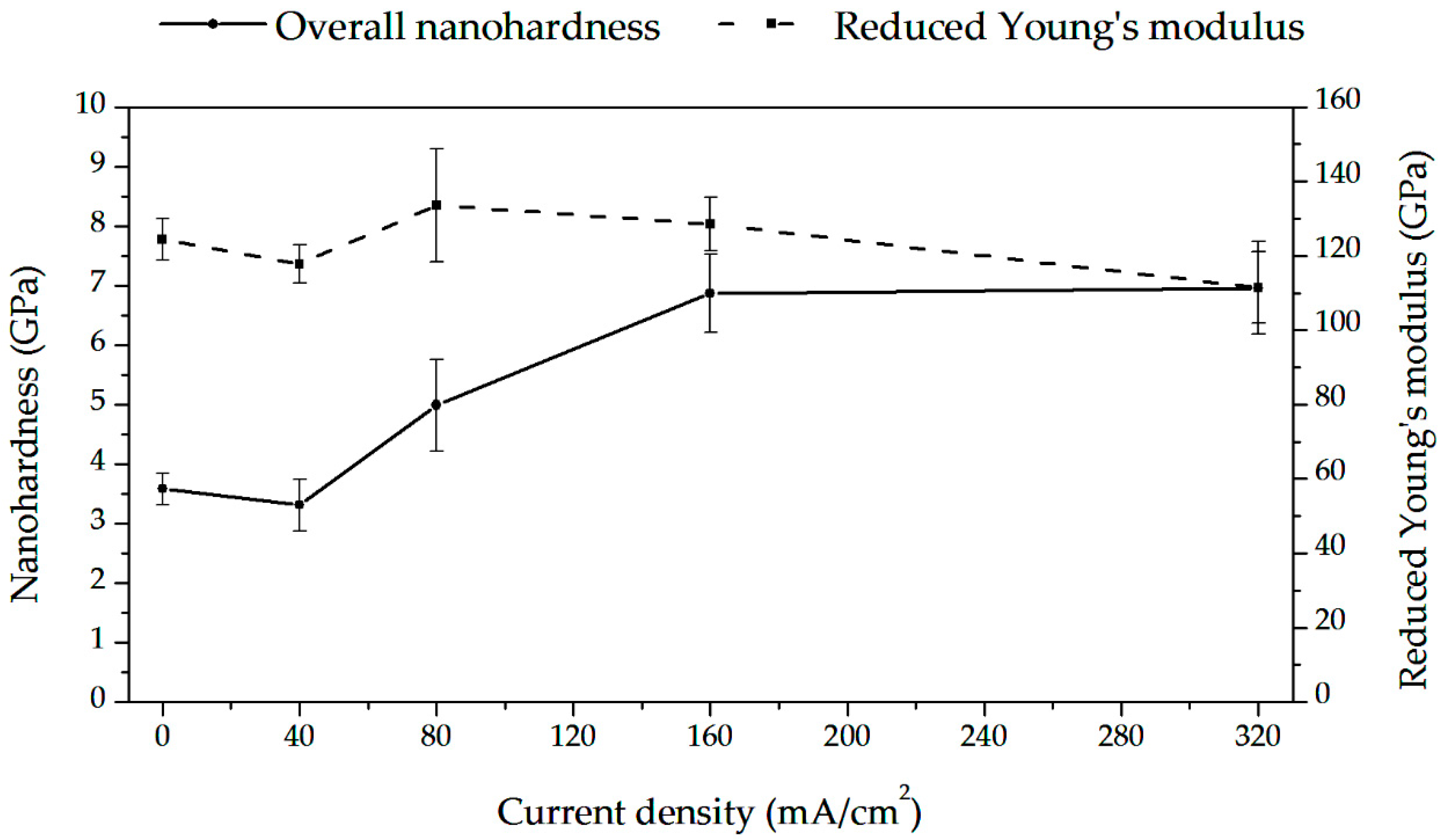
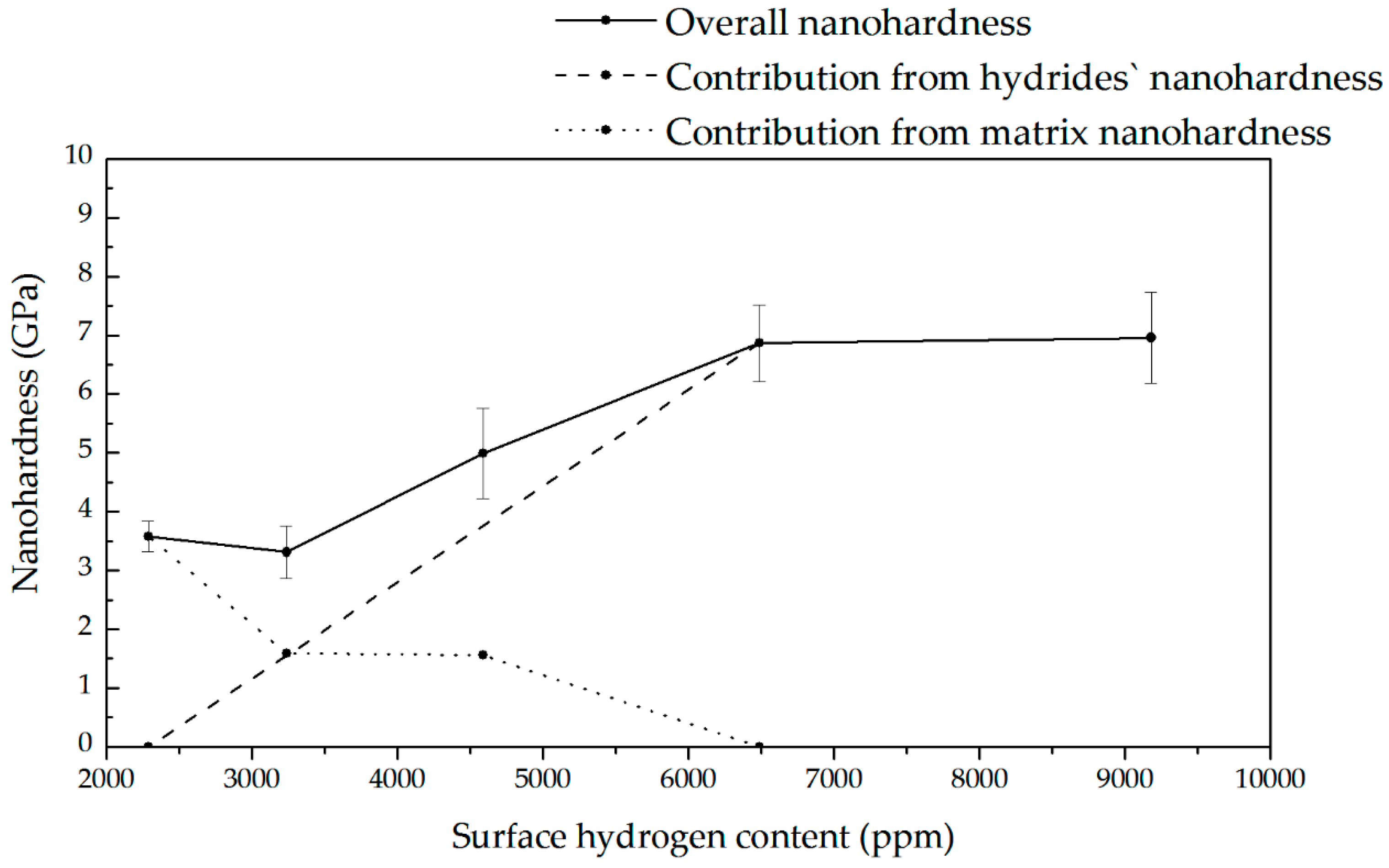
3.8. Nanoindentation Tests During Hydrogen Charging (Hydrogen Uptake)
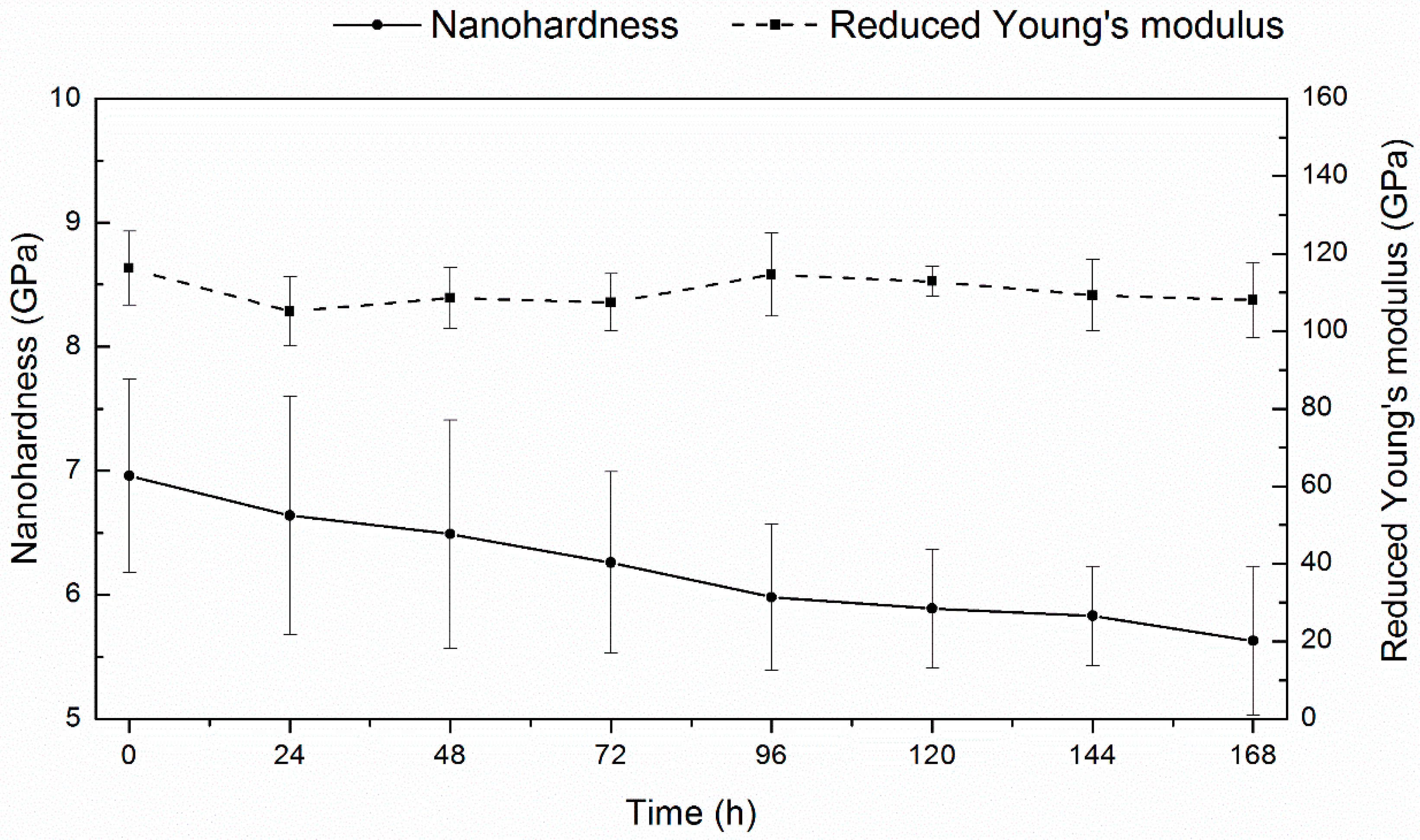
3.9. Nanoindentation Tests During Hydrogen Desorption
4. Discussion
4.1. Characteristics of Oxide Layers
4.2. Barrier Effect of the Oxide Layer
4.3. Effect of Hydrogen on Microstructure
4.4. Effect of Hydrogen on Fracture Mechanism and Path
4.5. Effect of Hydrogen Influence on Mechanical Properties
4.6. Hydrogen Influence on Nanomechanical Properties
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zieliński, A.; Sobieszczyk, S. Hydrogen-enhanced degradation and oxide effects in zirconium alloys for nuclear applications. Int. J. Hydrogen Energy 2011, 36, 8619–8629. [Google Scholar] [CrossRef]
- Allen, T.R.; Konings, R.J.M.; Motta, A.T. Corrosion of Zirconium Alloys. In Comprehensive Nuclear Materials; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 5, pp. 49–68. ISBN 9780080560335. [Google Scholar]
- Zinkle, S.J.; Was, G.S. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Mohamed, N.M.A. Study of Using Zirconium as a Reflector for Light Water Reactors Study of Using Zirconium as a Reflector for Light Water Reactors. Nucl. Sci. Eng. 2017, 173, 172–181. [Google Scholar] [CrossRef]
- Kamachi Mudali, U.; Ravi Shankar, A.; Natarajan, R.; Saibaba, N.; Raj, B. Application of zirconium alloys for reprocessing plant components. Nucl. Technol. 2013, 182, 349–357. [Google Scholar] [CrossRef]
- Jayaraj, J.; Thyagarajan, K.; Mallika, C.; Kamachi Mudali, U. Corrosion behavior of zirconium, titanium, and their alloys in simulated dissolver solution of fast breeder reactor spent nuclear fuel using Zircaloy-4 mock-up dissolver vessel. Nucl. Technol. 2015, 191, 58–70. [Google Scholar] [CrossRef]
- Duan, Z.; Yang, H.; Satoh, Y.; Murakami, K.; Kano, S.; Zhao, Z.; Shen, J.; Abe, H. Current status of materials development of nuclear fuel cladding tubes for light water reactors. Nucl. Eng. Des. 2017, 316, 131–150. [Google Scholar] [CrossRef]
- Krishnan, R.; Asundi, M.K. Zirconium alloys in nuclear power. Proc. Indian Aead. Sci. 1981, 4, 41–56. [Google Scholar]
- Olander, D.; Motta, A. Zirconium Alloys. In Light Water Reactor Materials; Chapter 17; American Nuclear Society: La Grange Park, IL, USA, 2011; Volume 7, pp. 1–40. [Google Scholar]
- Charit, I. Accident Tolerant Nuclear Fuels and Cladding Materials. JOM 2018, 70, 173–175. [Google Scholar] [CrossRef]
- Motta, A.T.; Couet, A.; Comstock, R.J. Corrosion of Zirconium Alloys Used for Nuclear Fuel Cladding. Annu. Rev. Mater. Res. 2015, 45, 311–343. [Google Scholar] [CrossRef]
- Féron, D. Overview of nuclear materials and nuclear corrosion science and engineering. In Nuclear Corrosion Science and Engineering; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 31–56. ISBN 9781845697655. [Google Scholar]
- McHugh, K.M.; Garnier, J.E.; Rashkeev, S.; Glazoff, M.V.; Griffith, G.W.; Bragg-Sitton, S.M. High Temperature Steam Corrosion of Cladding for Nuclear Applications: Experimental. In Ceramic Materials for Energy Applications III; Wiley: Hoboken, NJ, USA, 2013; pp. 149–160. ISBN 9781118807583. [Google Scholar]
- Singh, R.N.; Roychowdhury, S.; Sinha, V.P.; Sinha, T.K.; De, P.K.; Banerjee, S. Delayed hydride cracking in Zr-2.5Nb pressure tube material: Influence of fabrication routes. Mater. Sci. Eng. A 2004, 374, 342–350. [Google Scholar] [CrossRef]
- Singh, R.N.; Kishore, R.; Sinha, T.K.; Kashyap, B.P. Hydride blister formation in Zr-2.5wt%Nb pressure tube alloy. J. Nucl. Mater. 2002, 301, 153–164. [Google Scholar] [CrossRef]
- Ivanova, S.V. Effect of hydrogen on serviceability of zirconium items in VVER and RBMK-type reactors fuel assemblies. Int. J. Hydrogen Energy 2002, 27, 819–824. [Google Scholar] [CrossRef]
- Pierron, O.N.; Koss, D.A.; Motta, A.T.; Chan, K.S. The influence of hydride blisters on the fracture of Zircaloy-4. J. Nucl. Mater. 2003, 322, 21–35. [Google Scholar] [CrossRef]
- Steinbrück, M. Hydrogen absorption by zirconium alloys at high temperatures. J. Nucl. Mater. 2004, 334, 58–64. [Google Scholar] [CrossRef]
- Une, K.; Ishimoto, S.; Etoh, Y.; Ito, K.; Ogata, K.; Baba, T.; Kamimura, K.; Kobayashi, Y. The terminal solid solubility of hydrogen in irradiated Zircaloy-2 and microscopic modeling of hydride behavior. J. Nucl. Mater. 2009, 389, 127–136. [Google Scholar] [CrossRef]
- Bertolino, G.; Meyer, G.; Perez Ipiña, J. In situ crack growth observation and fracture toughness measurement of hydrogen charged Zircaloy-4. J. Nucl. Mater. 2003, 322, 57–65. [Google Scholar] [CrossRef]
- Grosse, M.; Steinbrueck, M.; Lehmann, E.; Vontobel, P. Kinetics of hydrogen absorption and release in zirconium alloys during steam oxidation. Oxid. Met. 2008, 70, 149–162. [Google Scholar] [CrossRef]
- Selmi, N.; Sari, A. Study of Oxidation Kinetics in Air of Zircaloy-4 by in Situ X-Ray Diffraction. Adv. Mater. Phys. Chem. 2013, 03, 168–173. [Google Scholar] [CrossRef]
- Qin, W.; Nam, C.; Li, H.L.; Szpunar, J.A. Tetragonal phase stability in ZrO2 film formed on zirconium alloys and its effects on corrosion resistance. Acta Mater. 2007, 55, 1695–1701. [Google Scholar] [CrossRef]
- Baek, J.H.; Jeong, Y.H. Breakaway phenomenon of Zr-based alloys during a high-temperature oxidation. J. Nucl. Mater. 2008, 372, 152–159. [Google Scholar] [CrossRef]
- Birchley, J.; Fernandez-Moguel, L. Simulation of air oxidation during a reactor accident sequence: Part 1—Phenomenology and model development. Ann. Nucl. Energy 2012, 40, 163–170. [Google Scholar] [CrossRef]
- Suman, S.; Khan, M.K.; Pathak, M.; Singh, R.N.; Chakravartty, J.K. Hydrogen in Zircaloy: Mechanism and its impacts. Int. J. Hydrogen Energy 2015, 40, 5976–5994. [Google Scholar] [CrossRef]
- Bair, J.; Asle Zaeem, M.; Tonks, M. A review on hydride precipitation in zirconium alloys. J. Nucl. Mater. 2015, 466, 12–20. [Google Scholar] [CrossRef]
- Shrivastava, K.C.; Kulkarni, A.S.; Ramanjaneyulu, P.S.; Sunil, S.; Saxena, M.K.; Singh, R.N.; Tomar, B.S.; Ramakumar, K.L. Determination of diffusion coefficients of hydrogen and deuterium in Zr-2.5%Nb pressure tube material using hot vacuum extraction-quadrupole mass spectrometry. J. Nucl. Mater. 2015, 461, 151–156. [Google Scholar] [CrossRef]
- Lanzani, L.; Ruch, M. Comments on the stability of zirconium hydride phases in Zircaloy. J. Nucl. Mater. 2004, 324, 165–176. [Google Scholar] [CrossRef]
- Daum, R.S.; Chu, Y.S.; Motta, A.T. Identification and quantification of hydride phases in Zircaloy-4 cladding using synchrotron X-ray diffraction. J. Nucl. Mater. 2009, 392, 453–463. [Google Scholar] [CrossRef]
- Roustila, A.; Chêne, J.; Séverac, C. XPS study of hydrogen and oxygen interactions on the surface of the NiZr intermetallic compound. Int. J. Hydrogen Energy 2007, 32, 5026–5032. [Google Scholar] [CrossRef]
- Yamanaka, S.; Nishizaki, T.; Uno, M.; Katsura, M. Hydrogen dissolution into zirconium oxide. J. Alloy. Compd. 1999, 293, 38–41. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, H.; Wu, C.; Tian, H.; Wang, X. The role of alloying elements in the initiation of nanoscale porosity in oxide films formed on zirconium alloys. Corros. Sci. 2013, 77, 391–396. [Google Scholar] [CrossRef]
- Sundell, G.; Thuvander, M.; Andrén, H.O. Barrier oxide chemistry and hydrogen pick-up mechanisms in zirconium alloys. Corros. Sci. 2016, 102, 490–502. [Google Scholar] [CrossRef]
- Glazoff, M.V.; Tokuhiro, A.; Rashkeev, S.N.; Sabharwall, P. Oxidation and hydrogen uptake in zirconium, Zircaloy-2 and Zircaloy-4: Computational thermodynamics and ab initio calculations. J. Nucl. Mater. 2014, 444, 65–75. [Google Scholar] [CrossRef]
- Le Saux, M.; Besson, J.; Carassou, S.; Poussard, C.; Averty, X. Behavior and failure of uniformly hydrided Zircaloy-4 fuel claddings between 25 °C and 480 °C under various stress states, including RIA loading conditions. Eng. Fail. Anal. 2010, 17, 683–700. [Google Scholar] [CrossRef]
- Yamanaka, S.; Setoyama, D.; Muta, H.; Uno, M.; Kuroda, M.; Takeda, K.; Matsuda, T. Characteristics of zirconium hydrogen solid solution. J. Alloy. Compd. 2004, 372, 129–135. [Google Scholar] [CrossRef]
- Bertolino, G.; Meyer, G.; Perez Ipiña, J. Effects of hydrogen content and temperature on fracture toughness of Zircaloy-4. J. Nucl. Mater. 2003, 320, 272–279. [Google Scholar] [CrossRef]
- Blackmur, M.S.; Robson, J.D.; Preuss, M.; Zanellato, O.; Cernik, R.J.; Shi, S.Q.; Ribeiro, F.; Andrieux, J. Zirconium hydride precipitation kinetics in Zircaloy-4 observed with synchrotron X-ray diffraction. J. Nucl. Mater. 2015, 464, 160–169. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, M.H.; Choi, B.K.; Jeong, Y.H. Effect of the hydrogen contents on the circumferential mechanical properties of zirconium alloy claddings. J. Alloy. Compd. 2007, 431, 155–161. [Google Scholar] [CrossRef]
- Hong, S.I.; Lee, K.W.; Kim, K.T. Effect of the circumferential hydrides on the deformation and fracture of Zircaloy cladding tubes. J. Nucl. Mater. 2002, 303, 169–176. [Google Scholar] [CrossRef]
- Lee, K.W.; Hong, S.I. Zirconium hydrides and their effect on the circumferential mechanical properties of Zr-Sn-Fe-Nb tubes. J. Alloy. Compd. 2002, 346, 302–307. [Google Scholar] [CrossRef]
- Daunys, M.; Dundulis, R.; Grybenas, A.; Krasauskas, P. Hydrogen influence on mechanical and fracture mechanics characteristics of zirconium Zr-2.5Nb alloy at ambient and elevated temperatures. Nucl. Eng. Des. 2008, 238, 2536–2545. [Google Scholar] [CrossRef]
- Setoyama, D.; Yamanaka, S. Indentation creep study of zirconium hydrogen solid solution. J. Alloy. Compd. 2004, 379, 193–197. [Google Scholar] [CrossRef]
- Ito, M.; Muta, H.; Setoyama, D.; Uno, M.; Yamanaka, S. Nanoindentation studies of high-temperature oxidized Zircaloy-4 with and without hydrogen. J. Alloy. Compd. 2007, 446–447, 639–642. [Google Scholar] [CrossRef]
- Kuroda, M.; Setoyama, D.; Uno, M.; Yamanaka, S. Nanoindentation studies of zirconium hydride. J. Alloy. Compd. 2004, 368, 211–214. [Google Scholar] [CrossRef]
- Rico, A.; Martin-Rengel, M.A.; Ruiz-Hervias, J.; Rodriguez, J.; Gomez-Sanchez, F.J. Nanoindentation measurements of the mechanical properties of zirconium matrix and hydrides in unirradiated pre-hydrided nuclear fuel cladding. J. Nucl. Mater. 2014, 452, 69–76. [Google Scholar] [CrossRef]
- Nedim Cinbiz, M.; Balooch, M.; Hu, X.; Amroussia, A.; Terrani, K. Nanoindentation study of bulk zirconium hydrides at elevated temperatures. J. Alloy. Compd. 2017, 726, 41–48. [Google Scholar] [CrossRef]
- Suman, S.; Khan, M.K.; Pathak, M.; Singh, R.N. Investigation of elevated-temperature mechanical properties of δ-hydride precipitate in Zircaloy-4 fuel cladding tubes using nanoindentation. J. Alloy. Compd. 2017, 726, 107–113. [Google Scholar] [CrossRef]
- Bertolino, G.; Meyer, G.; Perez Ipia, J. Degradation of the mechanical properties of Zircaloy-4 due to hydrogen embrittlement. Proc. J. Alloy. Compd. 2002, 330–332, 408–413. [Google Scholar] [CrossRef]
- Chakraborty, P.; Moitra, A.; Saha-Dasgupta, T. Effect of hydrogen on degradation mechanism of zirconium: A molecular dynamics study. J. Nucl. Mater. 2015, 466, 172–178. [Google Scholar] [CrossRef]
- Huang, J.H.; Yeh, M.S. Gaseous hydrogen embrittlement of a hydrided zirconium alloy. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 1998, 29, 1047–1056. [Google Scholar] [CrossRef]
- Oh, S.; Jang, C.; Kim, J.H.; Jeong, Y.H. Effect of Nb on hydride embrittlement of Zr-xNb alloys. Mater. Sci. Eng. A 2010, 527, 1306–1313. [Google Scholar] [CrossRef]
- Zhao, C.; Song, X.; Yang, Y.; Zhang, B. Hydrogen absorption cracking of zirconium alloy in the application of nuclear industry. Int. J. Hydrogen Energy 2013, 38, 10903–10911. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, B.K.; Baek, J.H.; Jeong, Y.H. Effects of oxide and hydrogen on the behavior of Zircaloy-4 cladding during the loss of the coolant accident (LOCA). Nucl. Eng. Des. 2006, 236, 2386–2393. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, Q.; Zhao, W.; Jiang, H. Hydride precipitation by cathodic hydrogen charging method in zirconium alloys. Mater. Chem. Phys. 2008, 110, 56–60. [Google Scholar] [CrossRef]
- Gou, Y.; Li, Y.; Liu, Y.; Chen, H.; Ying, S. Evaluation of a delayed hydride cracking in Zr-2.5Nb CANDU and RBMK pressure tubes. Mater. Des. 2009, 30, 1231–1235. [Google Scholar] [CrossRef]
- Shavkunov, S.P.; Tolkachev, A.B. Electrochemical hydrogen evolution at a single-crystal zirconium face in sulfuric acid solutions. Russ. J. Electrochem. 2002, 38, 714–719. [Google Scholar] [CrossRef]
- Bind, A.K.; Singh, R.N.; Khandelwal, H.K.; Sunil, S.; Avinash, G.; Chakravartty, J.K.; Ståhle, P. Influence of loading rate and hydrogen content on fracture toughness of Zr-2.5Nb pressure tube material. J. Nucl. Mater. 2015, 465, 177–188. [Google Scholar] [CrossRef]
- Zieliński, A.; Cymann, A.; Gumiński, A.; Hernik, A.; Gajowiec, G. Influence of High Temperature Oxidation on Hydrogen Absorption and Degradation of Zircaloy-2 and Zr 700 Alloys. High Temp. Mater. Process. 2019, 38, 8–15. [Google Scholar] [CrossRef]
- Arima, T.; Masuzumi, T.; Furuya, H.; Idemitsu, K.; Inagaki, Y. The oxidation kinetics and the structure of the oxide film on Zircaloy before and after the kinetic transition. J. Nucl. Mater. 2001, 294, 148–153. [Google Scholar] [CrossRef]
- Causey, R.A.; Cowgill, D.F.; Nilson, R.H. Review of the Oxidation Rate of Zirconium Alloys. 2005. Available online: https://pdfs.semanticscholar.org/71ac/99e8027773c2a04f2b9234ca85efb10dffb0.pdf (accessed on 18 April 2020). [CrossRef]
- Coindreau, O.; Duriez, C.; Ederli, S. Air oxidation of Zircaloy-4 in the 600-1000 °C temperature range: Modeling for ASTEC code application. J. Nucl. Mater. 2010, 405, 207–215. [Google Scholar] [CrossRef]
- Park, K.; Yang, S.; Ho, K. The effect of high pressure steam on the oxidation of low-Sn Zircaloy-4 at temperatures between 700 and 900 °C. J. Nucl. Mater. 2012, 420, 39–48. [Google Scholar] [CrossRef]
- Steinbrück, M.; Böttcher, M. Air oxidation of Zircaloy-4, M5 Ò and ZIRLO TM cladding alloys at high temperatures. J. Nucl. Mater. 2011, 414, 276–285. [Google Scholar] [CrossRef]
- Yoo, H.I.; Koo, B.J.; Hong, J.O.; Hwang, I.S.; Jeong, Y.H. A working hypothesis on oxidation kinetics of Zircaloy. J. Nucl. Mater. 2001, 299, 235–241. [Google Scholar] [CrossRef]
- Duriez, C.; Dupont, T.; Schmet, B.; Enoch, F. Zircaloy-4 and M5® high temperature oxidation and nitriding in air. J. Nucl. Mater. 2008, 380, 30–45. [Google Scholar] [CrossRef]
- De Gabory, B.; Motta, A.T.; Wang, K. Transmission electron microscopy characterization of Zircaloy-4 and ZIRLOTM oxide layers. J. Nucl. Mater. 2015, 456, 272–280. [Google Scholar] [CrossRef]
- Kurpaska, L.; Jozwik, I.; Jagielski, J. Study of sub-oxide phases at the metal-oxide interface in oxidized pure zirconium and Zr-1.0% Nb alloy by using SEM/FIB/EBSD and EDS techniques. J. Nucl. Mater. 2016, 476, 56–62. [Google Scholar] [CrossRef]
- Sawabe, T.; Sonoda, T.; Furuya, M.; Kitajima, S.; Kinoshita, M.; Tokiwai, M. Microstructure of oxide layers formed on zirconium alloy by air oxidation, uniform corrosion and fresh-green surface modification. Proc. J. Nucl. Mater. 2011, 419, 310–319. [Google Scholar] [CrossRef]
- Tupin, M.; Martin, F.; Bisor, C.; Verlet, R.; Bossis, P.; Chene, J.; Jomard, F.; Berger, P.; Pascal, S.; Nuns, N. Hydrogen diffusion process in the oxides formed on zirconium alloys during corrosion in pressurized water reactor conditions. Corros. Sci. 2017, 116, 1–13. [Google Scholar] [CrossRef]
- Sawabe, T.; Sonoda, T.; Furuya, M.; Kitajima, S.; Takano, H. Residual stress distribution in oxide films formed on Zircaloy-2. J. Nucl. Mater. 2015, 466, 658–665. [Google Scholar] [CrossRef]
- Lindgren, M.; Geers, C.; Panas, I. Possible origin and roles of nano-porosity in ZrO2 scales for hydrogen pick-up in Zr alloys. J. Nucl. Mater. 2017, 492, 22–31. [Google Scholar] [CrossRef]
- Yardley, S.S.; Moore, K.L.; Ni, N.; Wei, J.F.; Lyon, S.; Preuss, M.; Lozano-Perez, S.; Grovenor, C.R.M. An investigation of the oxidation behaviour of zirconium alloys using isotopic tracers and high resolution SIMS. J. Nucl. Mater. 2013, 443, 436–443. [Google Scholar] [CrossRef]
- Cox, B. Hydrogen uptake during oxidation of zirconium alloys. J. Alloy. Compd. 1997, 256, 244–246. [Google Scholar] [CrossRef]
- Baris, S.; Abolhassani, Y.L.; Chiu, L.; Evans, H.E. Observation of crack microstructure in oxides and its correlation to oxidation and hydrogen-uptake by 3D FIB Tomography—Case of Zr-ZrO in reactor. Mater. High Temp. 2018, 35, 14–21. [Google Scholar] [CrossRef]
- Ni, N.; Lozano-Perez, S.; Sykes, J.M.; Smith, G.D.W.; Grovenor, C.R.M. Focused ion beam sectioning for the 3D characterisation of cracking in oxide scales formed on commercial ZIRLOTM alloys during corrosion in high temperature pressurised water. Corr. Sci. 2011, 53, 4073–4083. [Google Scholar] [CrossRef]
- Tejland, P.; Andrén, H.O. Origin and effect of lateral cracks in oxide scales formed on zirconium alloys. J. Nucl. Mater. 2012, 430, 64–71. [Google Scholar] [CrossRef]
- Dominguez, C. Effect of pre-oxide and hydrogen on creep of Zircaloy-4 at 1123 K. J. Nucl. Mater. 2018, 511, 446–458. [Google Scholar] [CrossRef]
- Szoka, A.; Gajowiec, G.; Serbinski, W.; Zielinski, A. Effect of surface state and stress on an oxidation of the Zircaloy-2 alloy. Best Int. J. Manag. Inf. Technol. Eng. 2016, 4, 55–64. [Google Scholar]
- Chen, W.; Wang, L.; Lu, S. Influence of oxide layer on hydrogen desorption from zirconium hydride. J. Alloy. Compd. 2009, 469, 142–145. [Google Scholar] [CrossRef]
- Große, M.; Lehmann, E.; Steinbrück, M.; Kühne, G.; Stuckert, J. Influence of oxide layer morphology on hydrogen concentration in tin and niobium containing zirconium alloys after high temperature steam oxidation. J. Nucl. Mater. 2009, 385, 339–345. [Google Scholar] [CrossRef]
- Couet, A.; Motta, A.T.; Comstock, R.J. Hydrogen pickup measurements in zirconium alloys: Relation to oxidation kinetics. J. Nucl. Mater. 2014, 451, 1–13. [Google Scholar] [CrossRef]
- Zienkiewicz, N.; Paradowska, J.; Serbiński, W.; Gajowiec, G.; Hernik, A.; Zieliński, A. Oxidation and Hydrogen Behaviur in Zr-2Mn Alloy. Adv. Mater. Sci. 2018, 18, 37–48. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, J.W.; Lee, Y.W.; Hong, S.I. Hydride formation by high temperature cathodic hydrogen charging method and its effect on the corrosion behavior of Zircaloy-4 tubes in acid solution. J. Nucl. Mater. 1998, 256, 124–130. [Google Scholar] [CrossRef]
- Singh, R.N.; Kumar, N.; Kishore, R.; Roychaudhury, S.; Sinha, T.K.; Kashyap, B.P. Delayed hydride cracking in Zr-2.5 Nb pressure tube material. J. Nucl. Mater. 2002, 304, 189–203. [Google Scholar] [CrossRef]
- Szoka, A.; Gajowiec, G.; Zielinski, A.; Serbinski, W.; Olive, J.-M.; Ossowska, A. Hydrogen degradation of pre-oxidized Zirconium alloy. Adv. Mater. Sci. 2017, 17, 1–21. [Google Scholar] [CrossRef]
- Takagi, I.; Une, K.; Miyamura, S.; Kobayashi, T. Deuterium diffusion in steam-corroded oxide layer of zirconium alloys. Proc. J. Nucl. Mater. 2011, 419, 339–346. [Google Scholar] [CrossRef]
- Une, K.; Sakamoto, K.; Takagi, I.; Sawada, K.; Watanabe, H.; Aomi, M. Deuterium diffusion in oxide layers of Zr-2.5Nb alloy. J. Nucl. Mater. 2013, 439, 84–92. [Google Scholar] [CrossRef]
- Kato, T.; Takagi, I.; Sakamoto, K.; Aomi, M. Hydrogen diffusivity in oxide layers formed in Zr alloy in air or steam. J. Nucl. Mater. 2017, 494, 79–86. [Google Scholar] [CrossRef]
- Elmoselhi, M.B. Hydrogen uptake by oxidized zirconium alloys. J. Alloy. Compd. 1995, 231, 716–721. [Google Scholar] [CrossRef]
- Sundell, G.; Thuvander, M.; Yatim, A.K.; Nordin, H.; Andrén, H.O. Direct observation of hydrogen and deuterium in oxide grain boundaries in corroded Zirconium alloys. Corros. Sci. 2015, 90, 1–4. [Google Scholar] [CrossRef]
- Shmakov, A.A.; Singh, R.N.; Yan, D.; Eadie, R.L.; Matvienko, Y.G. A combined SIF and temperature model of delayed hydride cracking in zirconium materials. Comput. Mater. Sci. 2007, 39, 237–241. [Google Scholar] [CrossRef]
- Qin, W.; Szpunar, J.A.; Kozinski, J. Hydride-induced degradation of zirconium alloys: A criterion for complete ductile-to-brittle transition and its dependence on microstructure. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015, 471. [Google Scholar] [CrossRef]
- Ponzoni, L.M.E.; Mieza, J.I.; De Las Heras, E.; Domizzi, G. Comparison of delayed hydride cracking behavior of two zirconium alloys. Proc. J. Nucl. Mater. 2013, 439, 238–242. [Google Scholar] [CrossRef]
- Shi, S.Q.; Shek, G.K.; Puls, M.P. Hydrogen concentration limit and critical temperatures for delayed hydride cracking in zirconium alloys. J. Nucl. Mater. 1995, 218, 189–201. [Google Scholar] [CrossRef]
- McRae, G.A.; Coleman, C.E.; Leitch, B.W. The first step for delayed hydride cracking in zirconium alloys. J. Nucl. Mater. 2010, 396, 130–143. [Google Scholar] [CrossRef]
- Shi, S.-Q.; Puls, M.P. Dependence of the threshold stress intensity factor on hydrogen concentration during delayed hydride cracking in zirconium alloys. J. Nucl. Mater. 1994, 218, 30–36. [Google Scholar] [CrossRef]
- Motta, A.T.; Capolungo, L.; Chen, L.Q.; Cinbiz, M.N.; Daymond, M.R.; Koss, D.A.; Lacroix, E.; Pastore, G.; Simon, P.C.A.; Tonks, M.R.; et al. Hydrogen in zirconium alloys: A review. J. Nucl. Mater. 2019, 518, 440–460. [Google Scholar] [CrossRef]
- Varias, A.G.; Massih, A.R. Simulation of hydrogen embrittlement in zirconium alloys under stress and temperature gradients. J. Nucl. Mater. 2000, 279, 273–285. [Google Scholar] [CrossRef]
- Xu, J.; Shi, S.Q. Investigation of mechanical properties of ε-zirconium hydride using micro- and nano-indentation techniques. J. Nucl. Mater. 2004, 327, 165–170. [Google Scholar] [CrossRef]
- Gao, X. Displacement burst and hydrogen effect during loading and holding in nanoindentation of an iron single crystal. Scr. Mater. 2005, 53, 1315–1320. [Google Scholar] [CrossRef]
- Zhao, Y.; Seok, M.Y.; Choi, I.C.; Lee, Y.H.; Park, S.J.; Ramamurty, U.; Suh, J.Y.; Jang, J. Il The role of hydrogen in hardening/softening steel: Influence of the charging process. Scr. Mater. 2015, 107, 46–49. [Google Scholar] [CrossRef]
- Wang, D.; Lu, X.; Deng, Y.; Guo, X.; Barnoush, A. Effect of hydrogen on nanomechanical properties in Fe-22Mn-0.6C TWIP steel revealed by in-situ electrochemical nanoindentation. Acta Mater. 2019, 166, 618–629. [Google Scholar] [CrossRef]
- Han, D.K.; Kim, Y.M.; Han, H.N.; Bhadeshia, H.K.D.H.; Suh, D.W. Hydrogen and aluminium in high-manganese twinning-induced plasticity steel. Scr. Mater. 2014, 80, 9–12. [Google Scholar] [CrossRef]
- Barnoush, A.; Asgari, M.; Johnsen, R. Resolving the hydrogen effect on dislocation nucleation and mobility by electrochemical nanoindentation. Scr. Mater. 2012, 66, 414–417. [Google Scholar] [CrossRef]
- Barnoush, A.; Zamanzade, M.; Vehoff, H. Direct observation of hydrogen-enhanced plasticity in super duplex stainless steel by means of in situ electrochemical methods. Scr. Mater. 2010, 62, 242–245. [Google Scholar] [CrossRef]
- Barnoush, A.; Asgari, M.; Johnsen, R.; Hoel, R. Hydrogen effect on nanomechanical properties of the nitrided steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2013, 44, 766–775. [Google Scholar] [CrossRef]
- Barnoush, A.; Vehoff, H. Hydrogen embrittlement of aluminum in aqueous environments examined by in situ electrochemical nanoindentation. Scr. Mater. 2008, 58, 747–750. [Google Scholar] [CrossRef]
- Tal-Gutelmacher, E.; Gemma, R.; Volkert, C.A.; Kirchheim, R. Hydrogen effect on dislocation nucleation in a vanadium (1 0 0) single crystal as observed during nanoindentation. Scr. Mater. 2010, 63, 1032–1035. [Google Scholar] [CrossRef]
- Barnoush, A.; Vehoff, H. Electrochemical nanoindentation: A new approach to probe hydrogen/deformation interaction. Scr. Mater. 2006, 55, 195–198. [Google Scholar] [CrossRef]
- Zhao, K.; He, J.; Mayer, A.E.; Zhang, Z. Effect of hydrogen on the collective behavior of dislocations in the case of nanoindentation. Acta Mater. 2018, 148, 18–27. [Google Scholar] [CrossRef]
- Zhou, X.; Ouyang, B.; Curtin, W.A.; Song, J. Atomistic investigation of the influence of hydrogen on dislocation nucleation during nanoindentation in Ni and Pd. Acta Mater. 2016, 116, 364–369. [Google Scholar] [CrossRef]
- Zhao, Y.; Park, J.M.; Lee, D.H.; Song, E.J.; Suh, J.Y.; Ramamurty, U.; Jang, J. Influences of hydrogen charging method on the hydrogen distribution and nanomechanical properties of face-centered cubic high-entropy alloy: A comparative study. Scr. Mater. 2019, 168, 76–80. [Google Scholar] [CrossRef]
- Barnoush, A.; Kheradmand, N.; Hajilou, T. Correlation between the hydrogen chemical potential and pop-in load during in situ electrochemical nanoindentation. Scr. Mater. 2015, 108, 76–79. [Google Scholar] [CrossRef]
- Kirchheim, R. Solid solution softening and hardening by mobile solute atoms with special focus on hydrogen. Scr. Mater. 2012, 67, 767–770. [Google Scholar] [CrossRef]
- Kirchheim, R. Revisiting hydrogen embrittlement models and hydrogen-induced homogeneous nucleation of dislocations. Scr. Mater. 2010, 62, 67–70. [Google Scholar] [CrossRef]





| Stage Number | Test Kind | Determinants | Values | Measured Variables | Characterization Technique |
|---|---|---|---|---|---|
| 1 | Oxidation | Oxidation temperature | 300, 400, 500, 600, 700, 800, 900, 1000 °C | Surface state Oxide layer thickness | SEM |
| 2a | Oxidation followed by hydrogen charging | Oxidation temperature Cathodic current density | 350, 700, 1000 °C 80 and 300 mA/cm2 | Tensile properties | Slow Strain Rate Test |
| 2b | Oxidation temperature Cathodic current density | 300, 400, 500, 600 °C 80 mA/cm2 | Hydrogen content | Nondispersive Infrared Absorption | |
| 2c | Oxidation temperature Cathodic current density | 300, 400, 500, 600, 700, 800, 900, 1000 °C 80 mA/cm2 | Presence of hydrides | SEM | |
| 3 | Hydrogen charging | Cathodic current density Absorption time | 40, 80, 160, 320 mA/cm2 72 h | Nanoindentation mechanical properties | Nanoindentation |
| 4 | Hydrogen desorption | Cathodic current density Desorption time | 320 mA/cm2 168 h | Nanoindentation mechanical properties | Nanoindentation |
| Cathodic Current Density (mA/cm2) | Plastic Work (nJ) | Elastic Work (nJ) | Maximum Depth (nm) |
|---|---|---|---|
| 0 | 14.21 ± 0.67 | 2.59 ± 0.17 | 779.71 ± 27.46 |
| 40 | 15.08 ± 1.03 | 2.72 ± 0.25 | 816.56 ± 51.87 |
| 80 | 9.20 ± 1.03 | 3.24 ± 0.43 | 671.74 ± 48.09 |
| 160 | 9.54 ± 0.85 | 3.65 ± 0.24 | 585.95 ± 25.51 |
| 320 | 8.17 ± 0.44 | 4.00 ± 0.36 | 594.16 ± 35.82 |
| Desorption Time (h) | Plastic Work (nJ) | Elastic Work (nJ) | Maximum Depth (nm) |
|---|---|---|---|
| 0 | 8.17 ± 0.44 | 4.00 ± 0.36 | 594.16 ± 35.82 |
| 24 | 8.24 ± 0.45 | 4.52 ± 0.10 | 620.08 ± 41.95 |
| 48 | 8.60 ± 0.49 | 4.32 ± 0.17 | 621.48 ± 44.04 |
| 72 | 8.60 ± 0.50 | 4.31 ± 0.08 | 630.75 ± 36.92 |
| 96 | 8.40 ± 0.27 | 4.10 ± 0.21 | 634.44 ± 34.87 |
| 120 | 8.43 ± 0.42 | 4.14 ± 0.09 | 639.22 ± 21.09 |
| 144 | 8.69 ± 0.68 | 4.19 ± 0.16 | 645.28 ± 26.08 |
| 168 | 8.69 ± 0.72 | 4.19 ± 0.12 | 657.21 ± 38.38 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajowiec, G.; Bartmański, M.; Majkowska-Marzec, B.; Zieliński, A.; Chmiela, B.; Derezulko, M. Hydrogen Embrittlement and Oxide Layer Effect in the Cathodically Charged Zircaloy-2. Materials 2020, 13, 1913. https://doi.org/10.3390/ma13081913
Gajowiec G, Bartmański M, Majkowska-Marzec B, Zieliński A, Chmiela B, Derezulko M. Hydrogen Embrittlement and Oxide Layer Effect in the Cathodically Charged Zircaloy-2. Materials. 2020; 13(8):1913. https://doi.org/10.3390/ma13081913
Chicago/Turabian StyleGajowiec, Grzegorz, Michał Bartmański, Beata Majkowska-Marzec, Andrzej Zieliński, Bartosz Chmiela, and Marek Derezulko. 2020. "Hydrogen Embrittlement and Oxide Layer Effect in the Cathodically Charged Zircaloy-2" Materials 13, no. 8: 1913. https://doi.org/10.3390/ma13081913
APA StyleGajowiec, G., Bartmański, M., Majkowska-Marzec, B., Zieliński, A., Chmiela, B., & Derezulko, M. (2020). Hydrogen Embrittlement and Oxide Layer Effect in the Cathodically Charged Zircaloy-2. Materials, 13(8), 1913. https://doi.org/10.3390/ma13081913