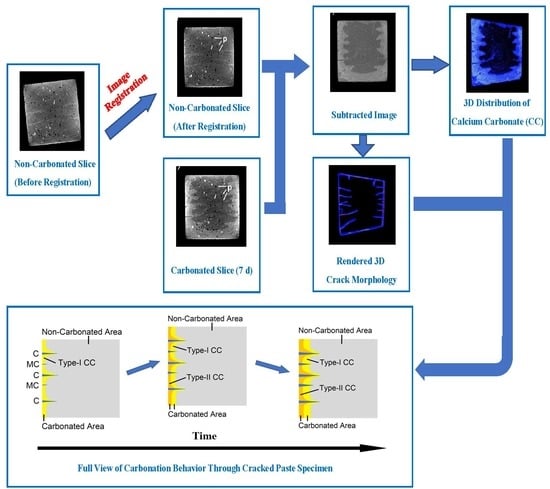
Tomography-Based Investigation on the Carbonation Behavior through the Surface-Opening Cracks of Sliced Paste Specimen
Abstract
1. Introduction
2. Materials and Methods
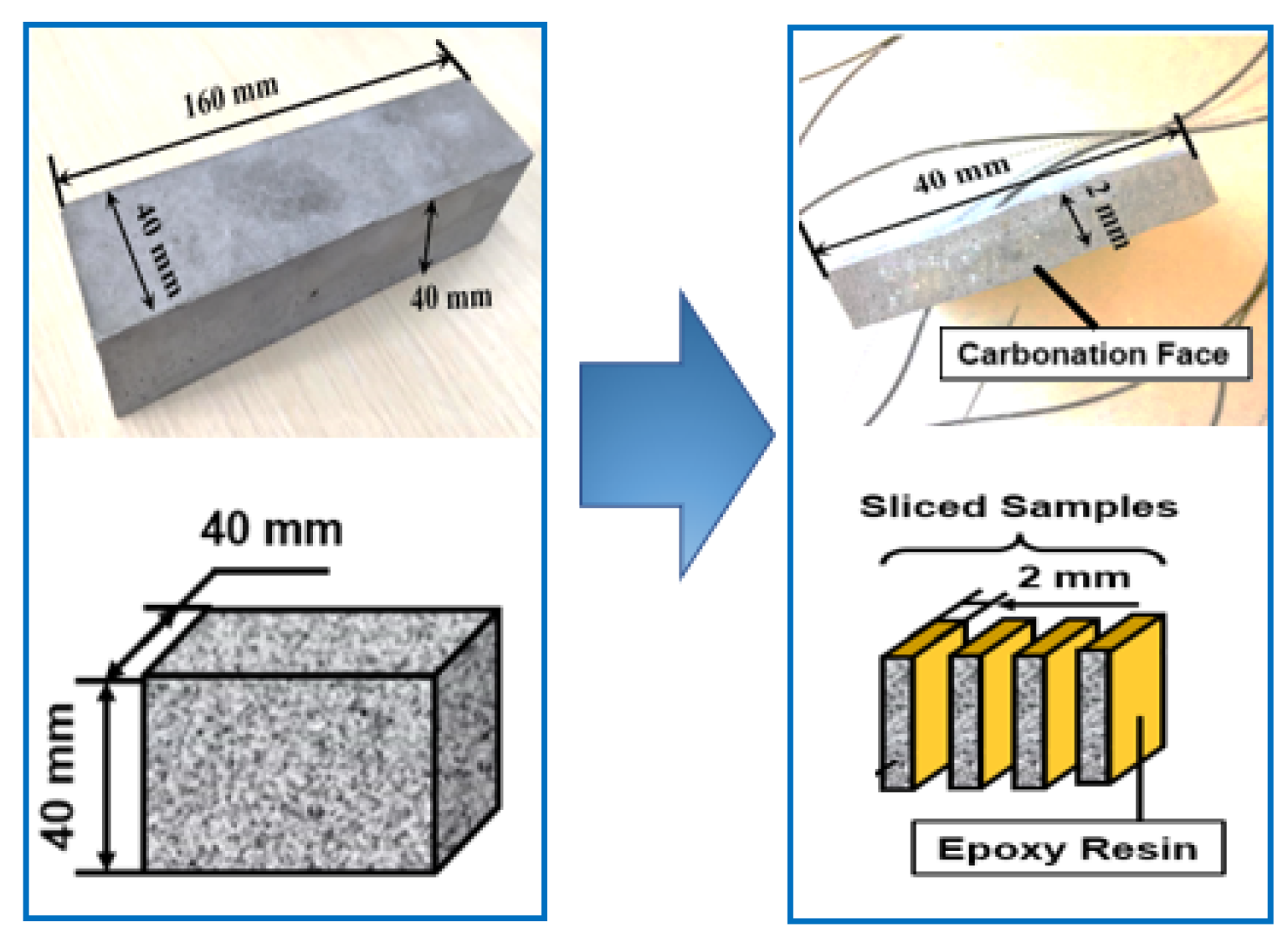
2.1. Sample Preparation
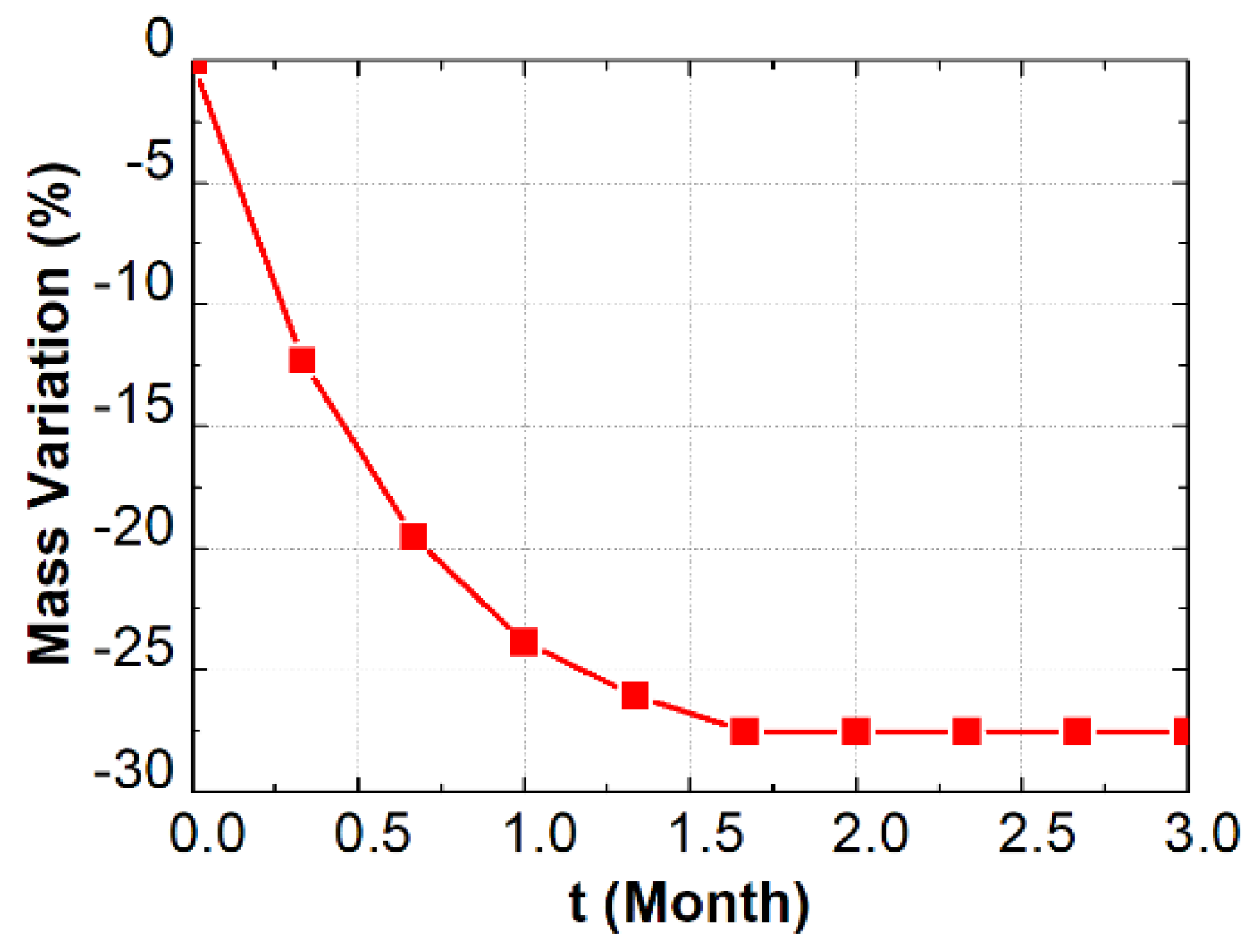
2.2. Preconditioning and Accelerated Carbonation Test
2.3. Testing Methods
2.3.1. Computed Tomography (CT)
2.3.2. Extension on Attenuation Method
2.3.3. Thermo-Gravimetric Analysis (TGA)
3. Results
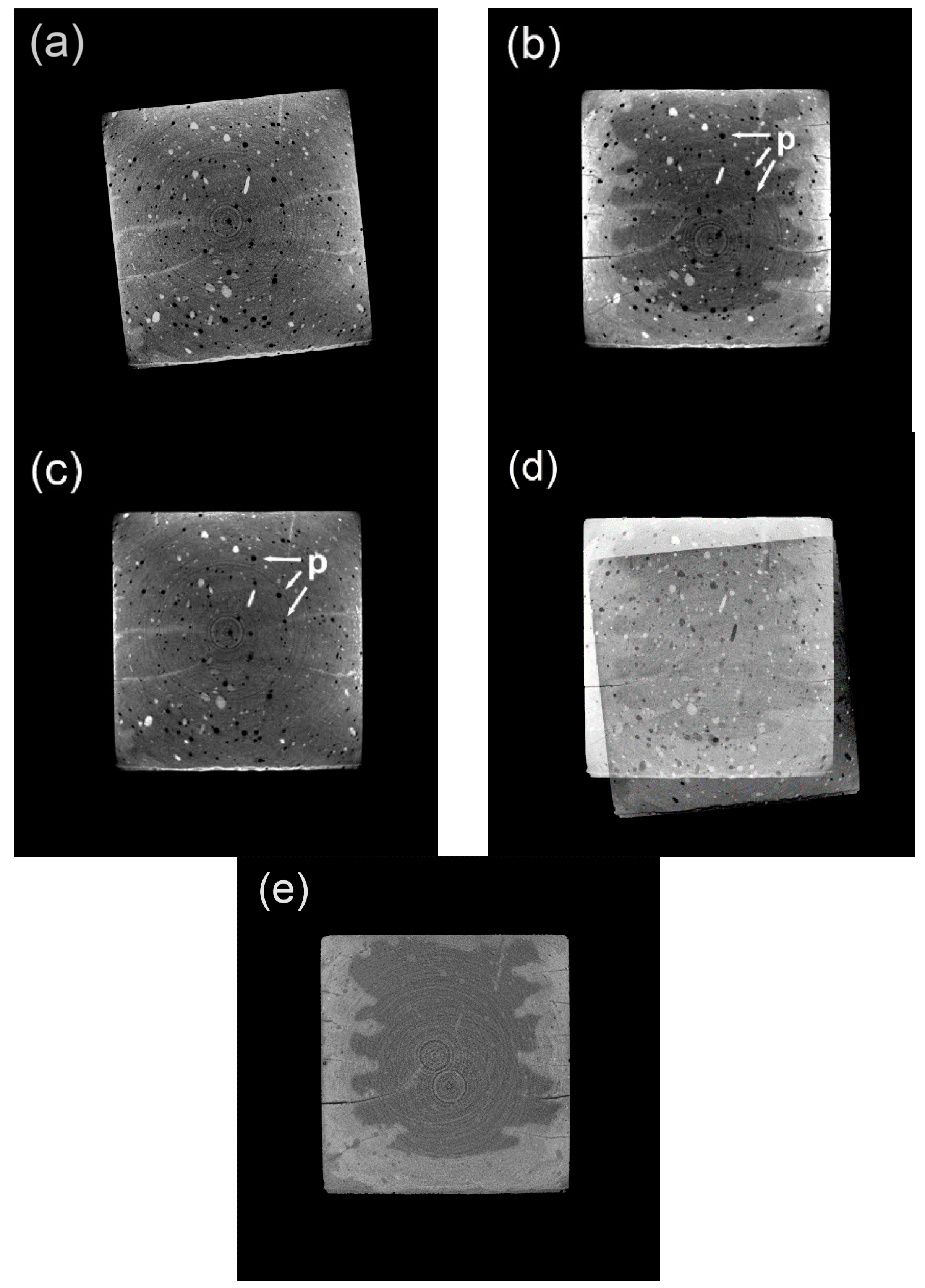
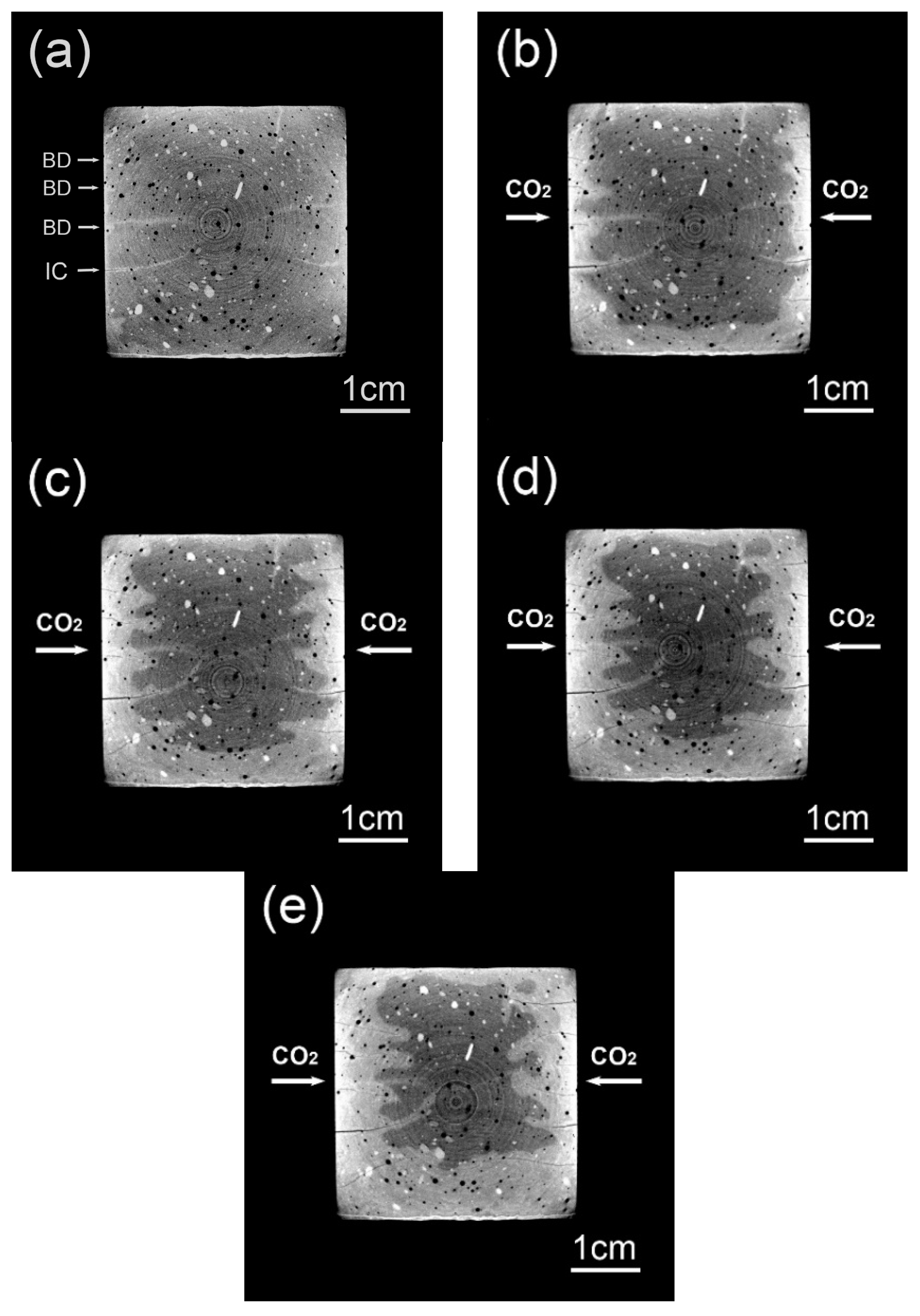
3.1. Original CT Data before and after Image Registration
3.2. Evolved Cross-sectional Areas Based on CT Renderings
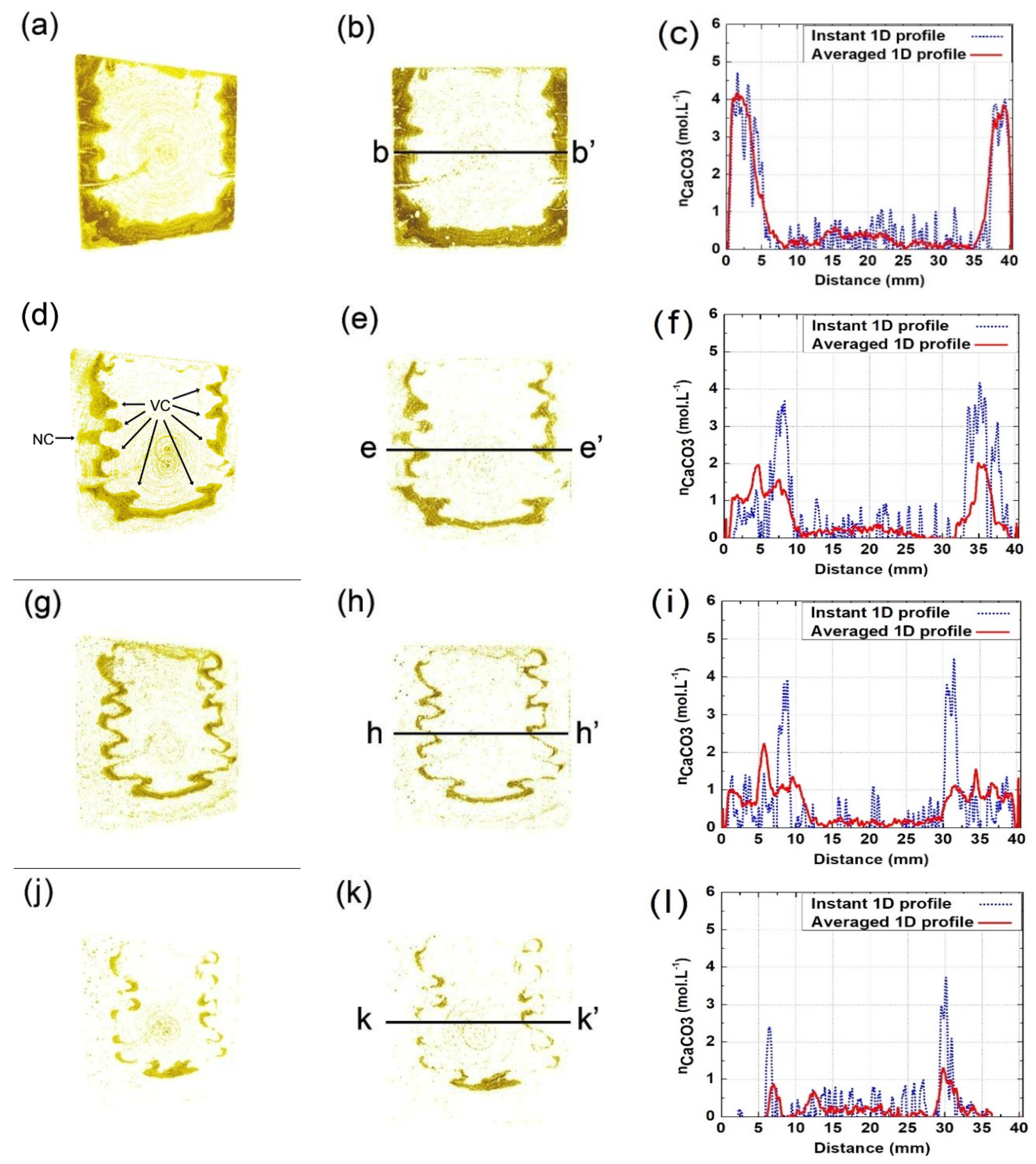
3.3. Spatial Distribution of
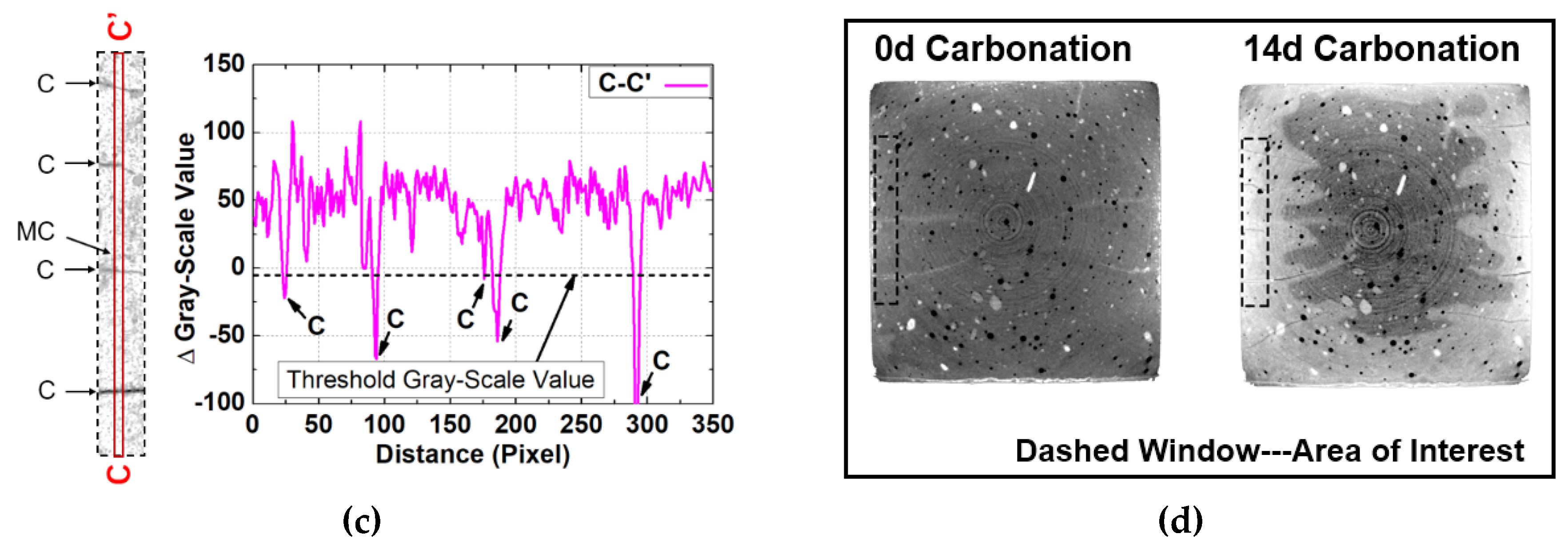
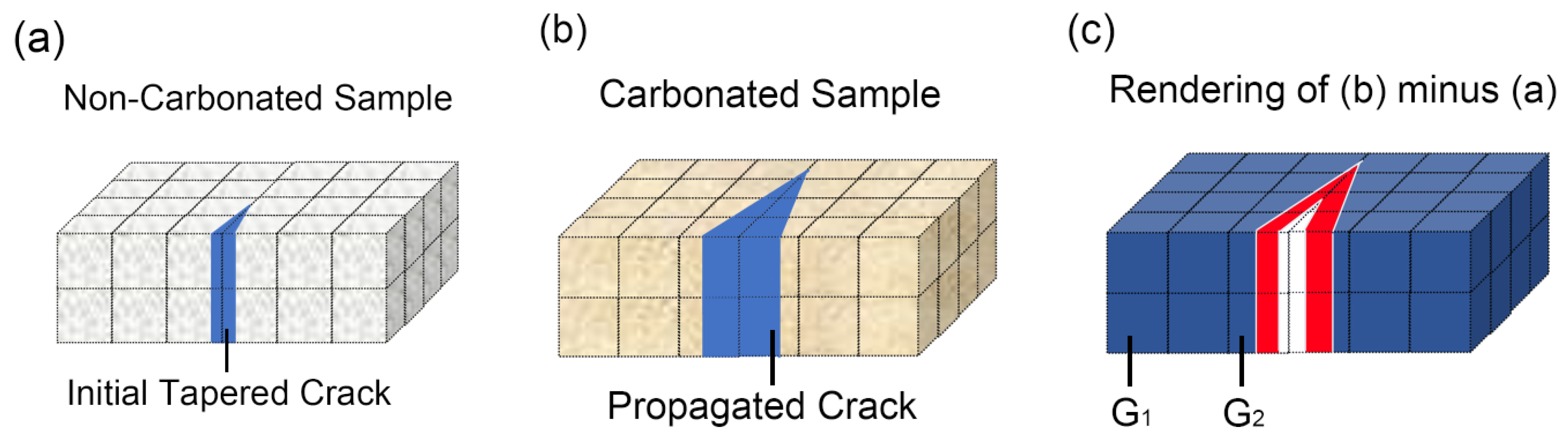
3.4. Method for Detection of Cracks for Partly Carbonated Specimen
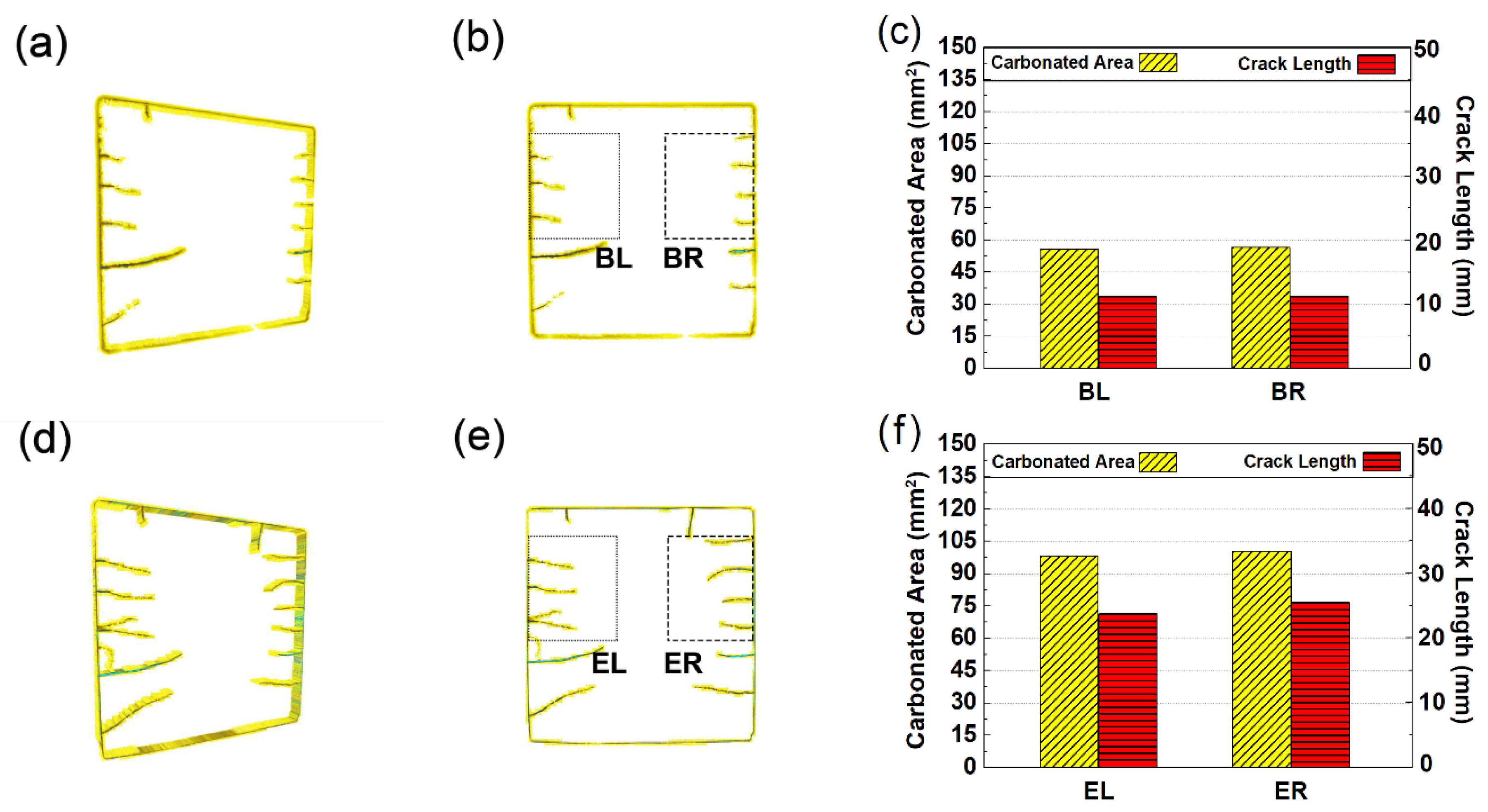
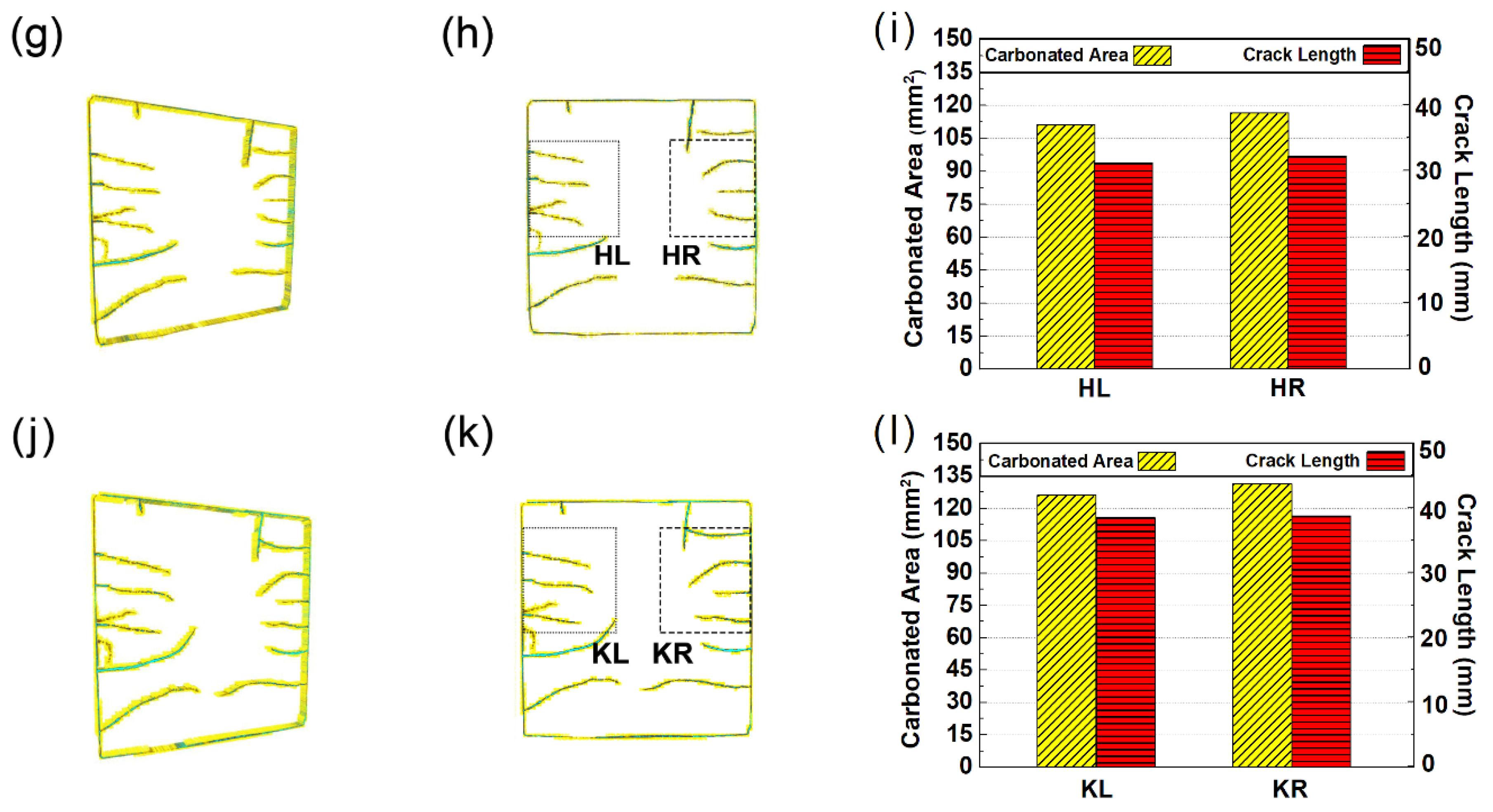
3.5. Rendered Cracking Behavior during Accelerated Carbonation
4. Verifications
4.1. Verification Based on the Spatial Distribution of Formed during Each Reaction Interval
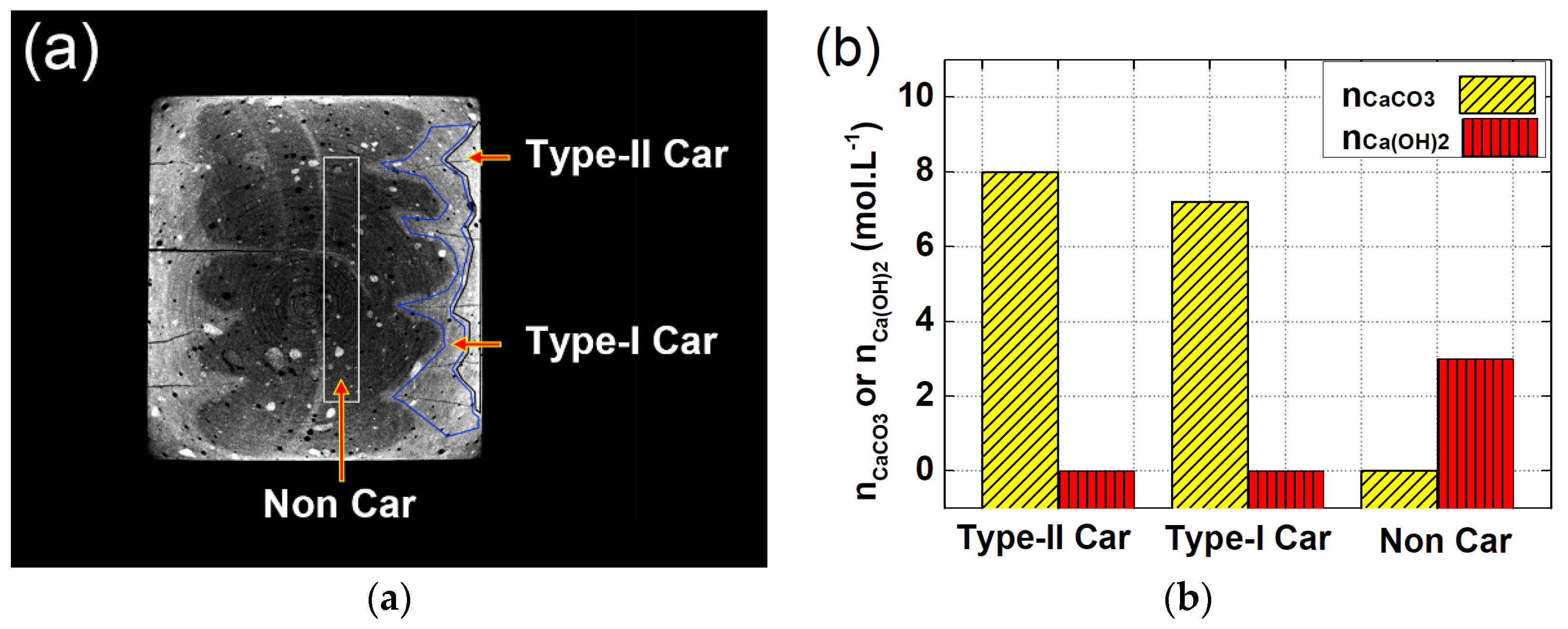
4.2. Verification Based on Thermo-Gravimetric Analysis (TGA)
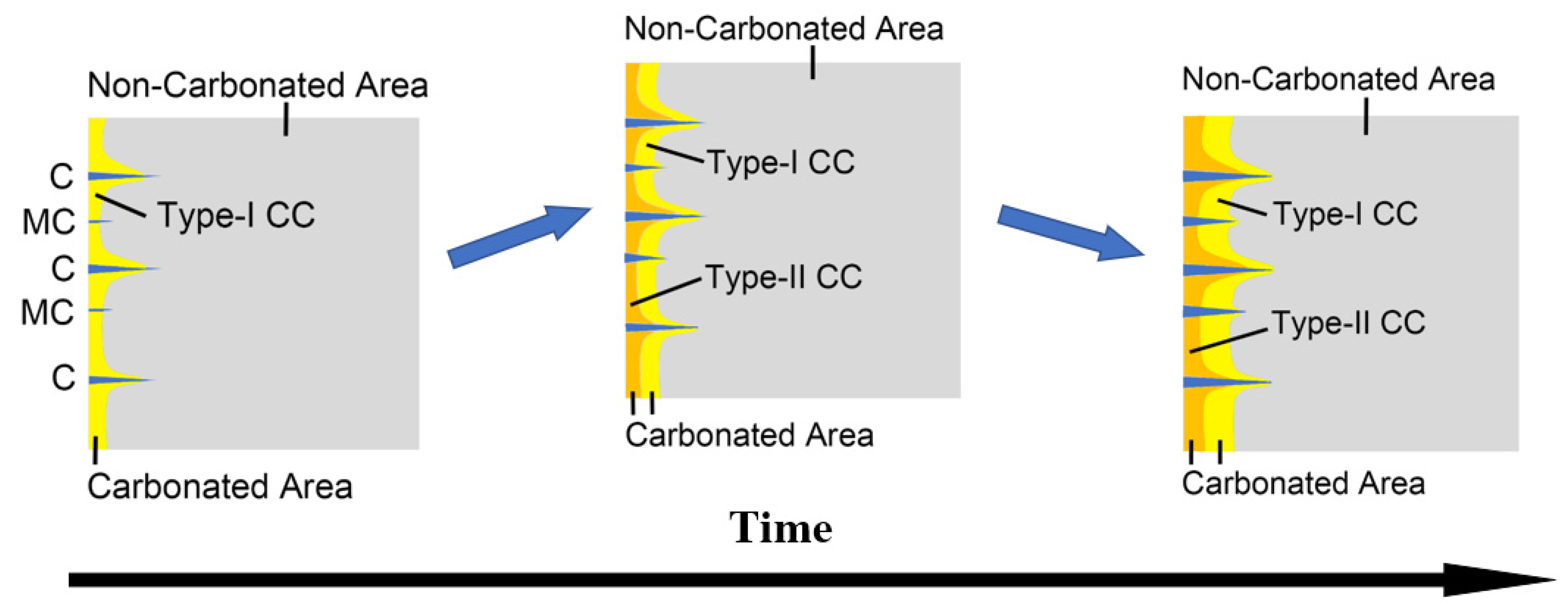
5. A Full View on the Carbonation-Cracking Behavior
6. Conclusions and Further Work
6.1. Conclusions
- (1)
- Compared with raw CT data, the identifiability of cracks was higher on the renderings obtained through XRAM, as the dark cordlike strips on the renderings were composed of both the crack itself and the naturally carbonated area flanking it.
- (2)
- Carbonation incurs significant propagation of surface-opening cracks, due to which the grayscale value of local areas may decrease, and the decreased grayscale value can therefore be used as a clue for the identification of cracking.
- (3)
- Surface-opening cracks can serve as shortcuts for CO2 diffusion, leading to the formation of V-shaped carbonation cusps pervading the carbonation front and faster early age carbonation speed; in return, the shrinkage accumulated during carbonation can serve as the energy source, feeding further propagation of the surface-opening cracks.
- (4)
- Due to variant migrating rates of moisture and CO2, the carbonated area would divide into two independent zones with variant carbonation degrees. The carbonation degree for the sample surface was higher, while the carbonation degree for the inner carbonated zone flanking cracks was relatively lower.
- (5)
- Based on the carbonation-cracking behavior, the carbonation process can be divided mainly into three stages. At the first and second stages, cracks were propagating rapidly, accompanying drastic formation of V-shaped carbonation cusps, while at the third stage, due to finer microstructure at inner areas, both the carbonation and the cracking speed were slower.
6.2. Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Monteiro, I.; Branco, F.A.; De Brito, J.; Neves, R. Statistical analysis of the carbonation coefficient in open air concrete structures. Constr. Build. Mater. 2012, 29, 263–269. [Google Scholar] [CrossRef]
- Torgal, F.P.; Miraldo, S.; Labrincha, J.A.; De Brito, J. An overview on concrete carbonation in the context of eco-efficient construction: Evaluation, use of SCMs and/or RAC. Constr. Build. Mater. 2012, 36, 141–150. [Google Scholar] [CrossRef]
- Marques, P.F.; Chastre, C.; Nunes, Â. Carbonation service life modelling of RC structures for concrete with Portland and blended cements. Cem. Concr. Comp. 2013, 37, 171–184. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. Experimental investigation and mathematical modeling of the concrete carbonation problem. Chem. Eng. Sci. 1991, 46, 1333–1338. [Google Scholar] [CrossRef]
- Liu, R.; Jiang, L.; Xu, J.; Xiong, C.; Song, Z. Influence of carbonation on chloride-induced reinforcement corrosion in simulated concrete pore solutions. Constr. Build. Mater. 2014, 56, 16–20. [Google Scholar] [CrossRef]
- Talukdar, S.; Banthia, N.; Grace, J.R. Carbonation in concrete infrastructure in the context of global climate change—Part 1: Experimental results and model development. Cem. Concr. Comp. 2012, 34, 924–930. [Google Scholar] [CrossRef]
- Talukdar, S.; Banthia, N.; Grace, J.R.; Cohen, S. Carbonation in concrete infrastructure in the context of global climate change—Part 2: Canadian urban simulations. Cem. Concr. Comp. 2012, 34, 931–935. [Google Scholar] [CrossRef]
- Xuan, D.; Zhan, B.; Poon, C.S. Assessment of mechanical properties of concrete incorporating carbonated recycled concrete aggregates. Cem. Concr. Comp. 2016, 65, 67–74. [Google Scholar] [CrossRef]
- Matsushita, F.; Aono, Y.; Shibata, S. Calcium silicate structure and carbonation shrinkage of a tobermorite-based material. Cem. Concr. Res. 2004, 34, 1251–1257. [Google Scholar] [CrossRef]
- Chen, J.J.; Thomas, J.J.; Jennings, H.M. Decalcification shrinkage of cement paste. Cem. Concr. Res. 2006, 36, 801–809. [Google Scholar] [CrossRef]
- Metalssi, O.O.; Aït-Mokhtar, A.; Turcry, P.; Ruot, B. Consequences of carbonation on microstructure and drying shrinkage of a mortar with cellulose ether. Constr. Build. Mater. 2012, 34, 218–225. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A.; Neves, J. Drying and carbonation shrinkage of cement paste containing alkalis. Mater. Struct. 2017, 50, 132. [Google Scholar] [CrossRef]
- Rodriguez, O.G.; Hooton, R.D. Influence of cracks on chloride ingress into concrete. Mater. J. 2003, 100, 120–126. [Google Scholar]
- Sideris, K.K.; Savva, A.E.; Papayianni, J. Sulfate resistance and carbonation of plain and blended cements. Cem. Concr. Comp. 2006, 28, 47–56. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, Y.; Jiao, Y.; Yang, J. Quantitative analysis of concrete property under effects of crack, freeze-thaw and carbonation. Constr. Build. Mater. 2016, 129, 106–115. [Google Scholar] [CrossRef]
- Akçaoğlu, T.; Tokyay, M.; Çelik, T. Assessing the ITZ microcracking via scanning electron microscope and its effect on the failure behavior of concrete. Cem. Concr. Res. 2005, 35, 358–363. [Google Scholar] [CrossRef]
- Han, J.; Sun, W.; Pan, G.; Wang, C.; Rong, H. Application of X-ray computed tomography in characterization microstructure changes of cement pastes in carbonation process. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2012, 27, 358–363. [Google Scholar] [CrossRef]
- Suzuki, T.; Ogata, H.; Takada, R.; Aoki, M.; Ohtsu, M. Use of acoustic emission and X-ray computed tomography for damage evaluation of freeze-thawed concrete. Constr. Build. Mater. 2010, 24, 2347–2352. [Google Scholar] [CrossRef]
- Thiéry, M.; Faure, P.; Morandeau, A.; Platret, G.; Bouteloup, J.-F.; Dangla, P.; Baroghel-Bouny, V. Effect of carbonation on the microstructure and moisture properties of cement-based materials. In Proceedings of the XII DBMC 12th International Conference on Building Materials and Components, Porto, Portugal, 12–15 April 2011; Volume 3, pp. 1–8. [Google Scholar]
- Morandeau, A.; Thiery, M.; Dangla, P. Investigation of the carbonation mechanism of CH and CSH in terms of kinetics, microstructure changes and moisture properties. Cem. Concr. Res. 2014, 56, 153–170. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiéry, M.; Dangla, P. Impact of accelerated carbonation on OPC cement paste blended with fly ash. Cem. Concr. Res. 2015, 67, 226–236. [Google Scholar] [CrossRef]
- Ketcham, R.A.; Iturrino, G.J. Nondestructive high-resolution visualization and measurement of anisotropic effective porosity in complex lithologies using high-resolution X-ray computed tomography. J. Hydrol. 2005, 302, 92–106. [Google Scholar] [CrossRef]
- Withjack, E.M. Method of Porosity Determination in Porous Media by X-ray Computed Tomography. U.S. Patent 4,982,086, 1 January 1991. [Google Scholar]
- Cui, D.; Sun, W.; Wan, K.; Banthia, N. Porosity characterization in interfacial transition zone using dual CT scans. J. Test. Eval. 2017, 45, 408–418. [Google Scholar] [CrossRef]
- Cui, D.; Sun, W.; Wang, Q.; Gu, C. Use of tomography to estimate the representative elementary volume in mortars stained with potassium iodide. Mater. Des. 2018, 147, 80–91. [Google Scholar] [CrossRef]
- Cui, D.; Sun, W.; Banthia, N. Use of tomography to understand the influence of preconditioning on carbonation tests in cement-based materials. Cem. Concr. Comp. 2018, 88, 52–63. [Google Scholar] [CrossRef]
- Wan, K.; Xu, Q.; Wang, Y.; Pan, G. 3D spatial distribution of the calcium carbonate caused by carbonation of cement paste. Cem. Concr. Comp. 2014, 45, 255–263. [Google Scholar] [CrossRef]
- Standard for Test Methods of Long-term Performance and Durability of Ordinary Concrete; China Architecture and Building Press: Beijing, China, 2009; G.B. T50082-2009; pp. 61–63.
- Turcry, P.; Oksri-Nelfia, L.; Younsi, A.; Aît-Mokhtar, A. Analysis of an accelerated carbonation test with severe preconditioning. Cem. Concr. Res. 2014, 57, 70–78. [Google Scholar] [CrossRef]
- Wu, Q.; Rougelot, T.; Burlion, N.; Bourbon, X. Representative volume element estimation for desorption isotherm of concrete with sliced samples. Cem. Concr. Res. 2015, 76, 1–9. [Google Scholar] [CrossRef]
- Iswar, S.; Griffa, M.; Kaufmann, R.; Beltran, M.; Huber, L.; Brunner, S.; Lattuada, M.; Koebel, M.M.; Malfait, W.J. Effect of aging on thermal conductivity of fiber-reinforced aerogel composites: An X-ray tomography study. Microporous Mesoporous Mater. 2019, 278, 289–296. [Google Scholar] [CrossRef]
- Latham, S.; Varslot, T.; Sheppard, A. Image registration: Enhancing and calibrating X-ray micro-CT imaging. In Proceedings of the Society of Core Analysts, Abu Dhabi, UAE, 29 October–2 November 2008. [Google Scholar]
- Cui, D.; Banthia, N.; Wang, Q.; Sun, W. Investigation on porosity of partly carbonated paste specimens blended with fly ash through dual CT scans. Constr. Build. Mater. 2019, 196, 692–702. [Google Scholar] [CrossRef]
- Leemann, A.; Moro, F. Carbonation of concrete: The role of CO2 concentration, relative humidity and CO2 buffer capacity. Mater. Struct. 2017, 50, 30. [Google Scholar] [CrossRef]
- Galan, I.; Andrade, C.; Castellote, M. Natural and accelerated CO2 binding kinetics in cement paste at different relative humidities. Cem. Concr. Res. 2013, 49, 21–28. [Google Scholar] [CrossRef]
- Jana, D.; Erlin, B. Carbonation as an indicator of crack age. Concr. Int. 2007, 29, 39–42. [Google Scholar]
- Sullivan-Green, L.; Hime, W.; Dowding, C. Accelerated protocol for measurement of carbonation through a crack surface. Cem. Concr. Res. 2007, 37, 916–923. [Google Scholar] [CrossRef]
- Vicente, M.A.; González, D.C.; Mínguez, J. Recent advances in the use of computed tomography in concrete technology and other engineering fields. Micron 2019, 118, 22–34. [Google Scholar] [CrossRef]
- Du Plessis, A.; Boshoff, W.P. A review of X-ray computed tomography of concrete and asphalt construction materials. Constr. Build. Mater. 2019, 199, 637–651. [Google Scholar] [CrossRef]



| Cement | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | Others | LOI | Total |
|---|---|---|---|---|---|---|---|---|---|
| Mass ratio | 62.60 | 21.35 | 4.67 | 3.31 | 3.08 | 2.25 | 1.29 | 1.45 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, D.; Zuo, X.; Zheng, K.; Talukdar, S. Tomography-Based Investigation on the Carbonation Behavior through the Surface-Opening Cracks of Sliced Paste Specimen. Materials 2020, 13, 1804. https://doi.org/10.3390/ma13081804
Cui D, Zuo X, Zheng K, Talukdar S. Tomography-Based Investigation on the Carbonation Behavior through the Surface-Opening Cracks of Sliced Paste Specimen. Materials. 2020; 13(8):1804. https://doi.org/10.3390/ma13081804
Chicago/Turabian StyleCui, Dong, Xiaobao Zuo, Keren Zheng, and Sudip Talukdar. 2020. "Tomography-Based Investigation on the Carbonation Behavior through the Surface-Opening Cracks of Sliced Paste Specimen" Materials 13, no. 8: 1804. https://doi.org/10.3390/ma13081804
APA StyleCui, D., Zuo, X., Zheng, K., & Talukdar, S. (2020). Tomography-Based Investigation on the Carbonation Behavior through the Surface-Opening Cracks of Sliced Paste Specimen. Materials, 13(8), 1804. https://doi.org/10.3390/ma13081804