Comparative Study on Flow-Accelerated Corrosion and Erosion–Corrosion at a 90° Carbon Steel Bend
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Media
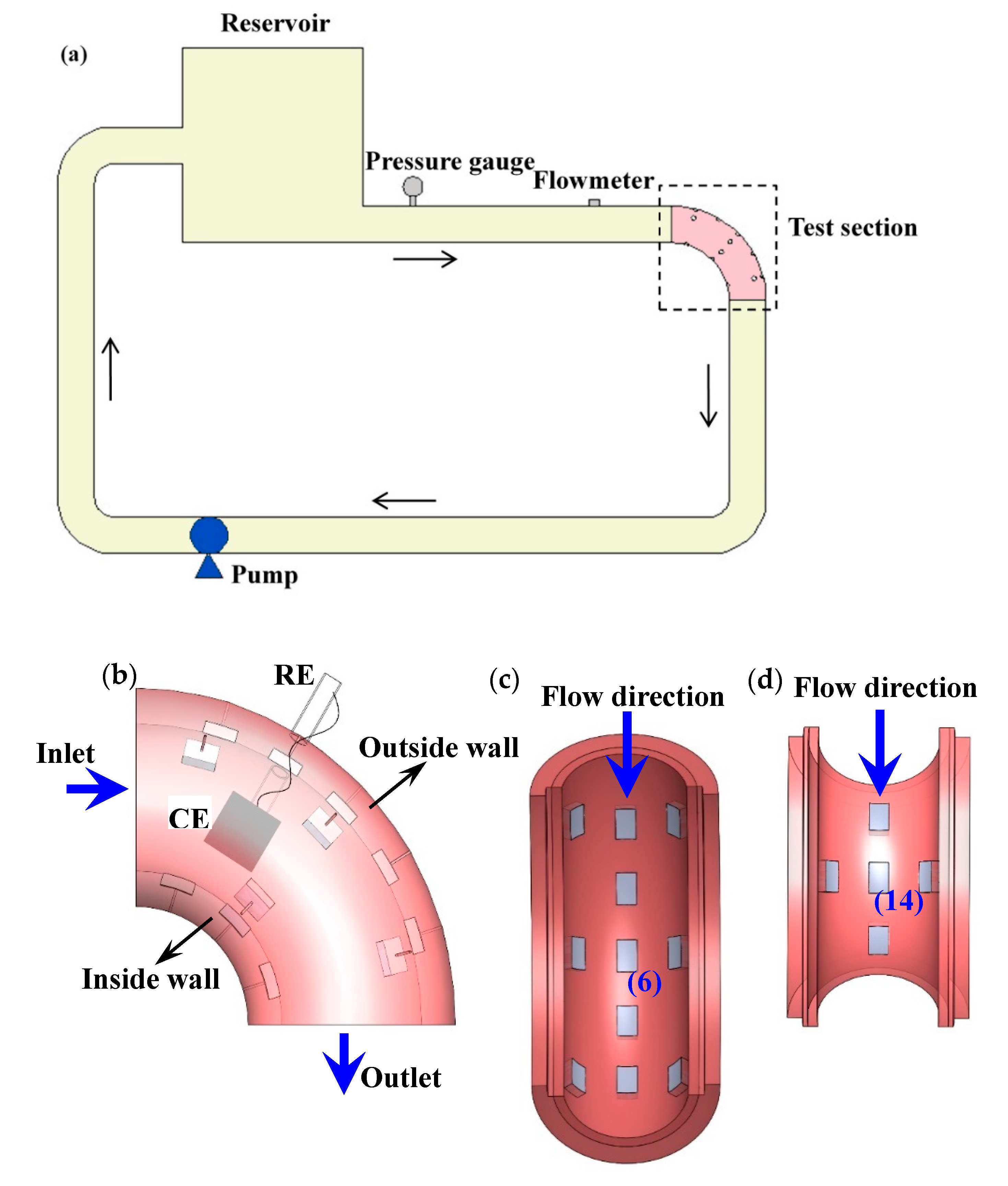
2.2. Experimental Apparatus
2.3. Flowing Tests
2.4. Surface Characterization
3. Results
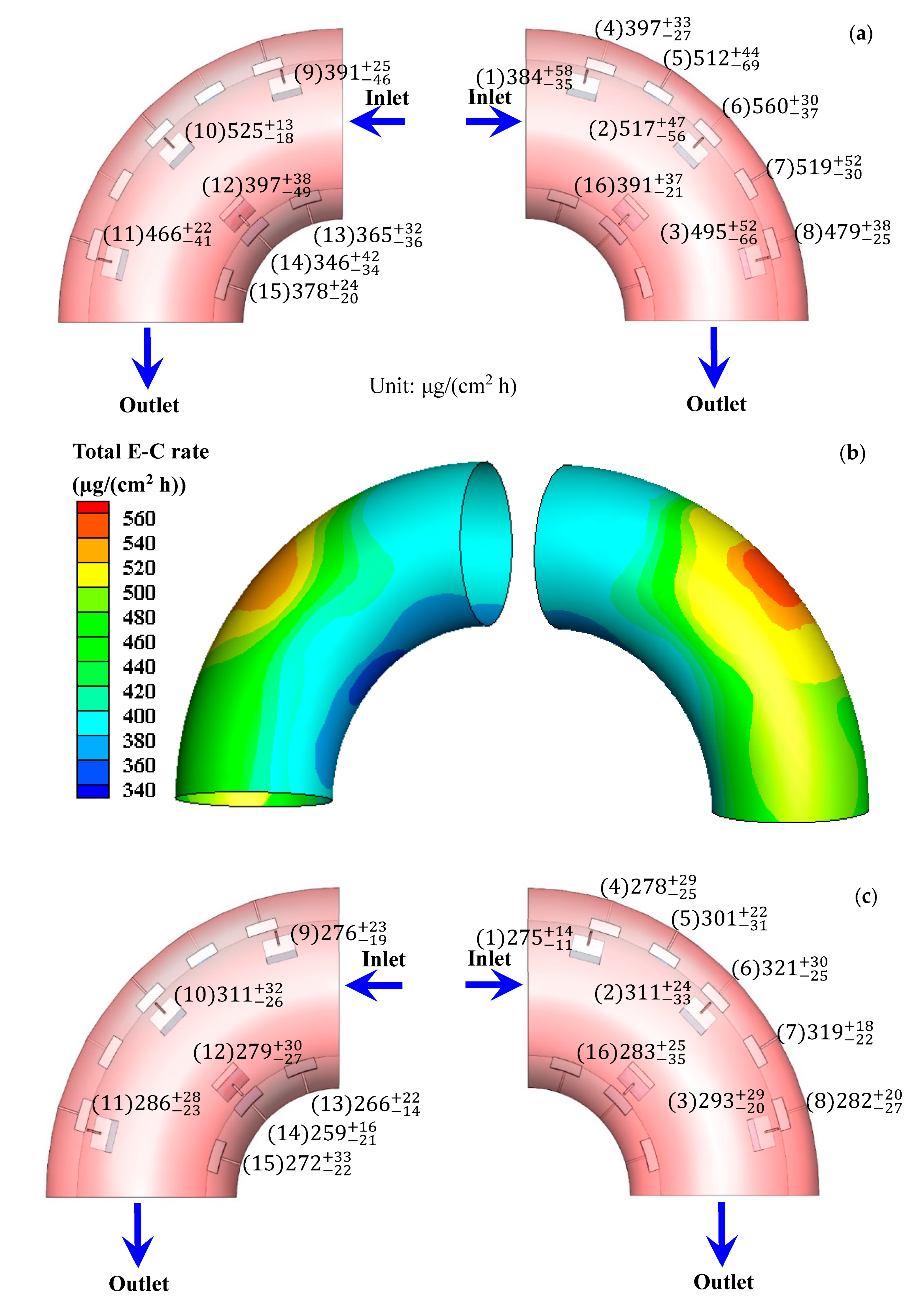
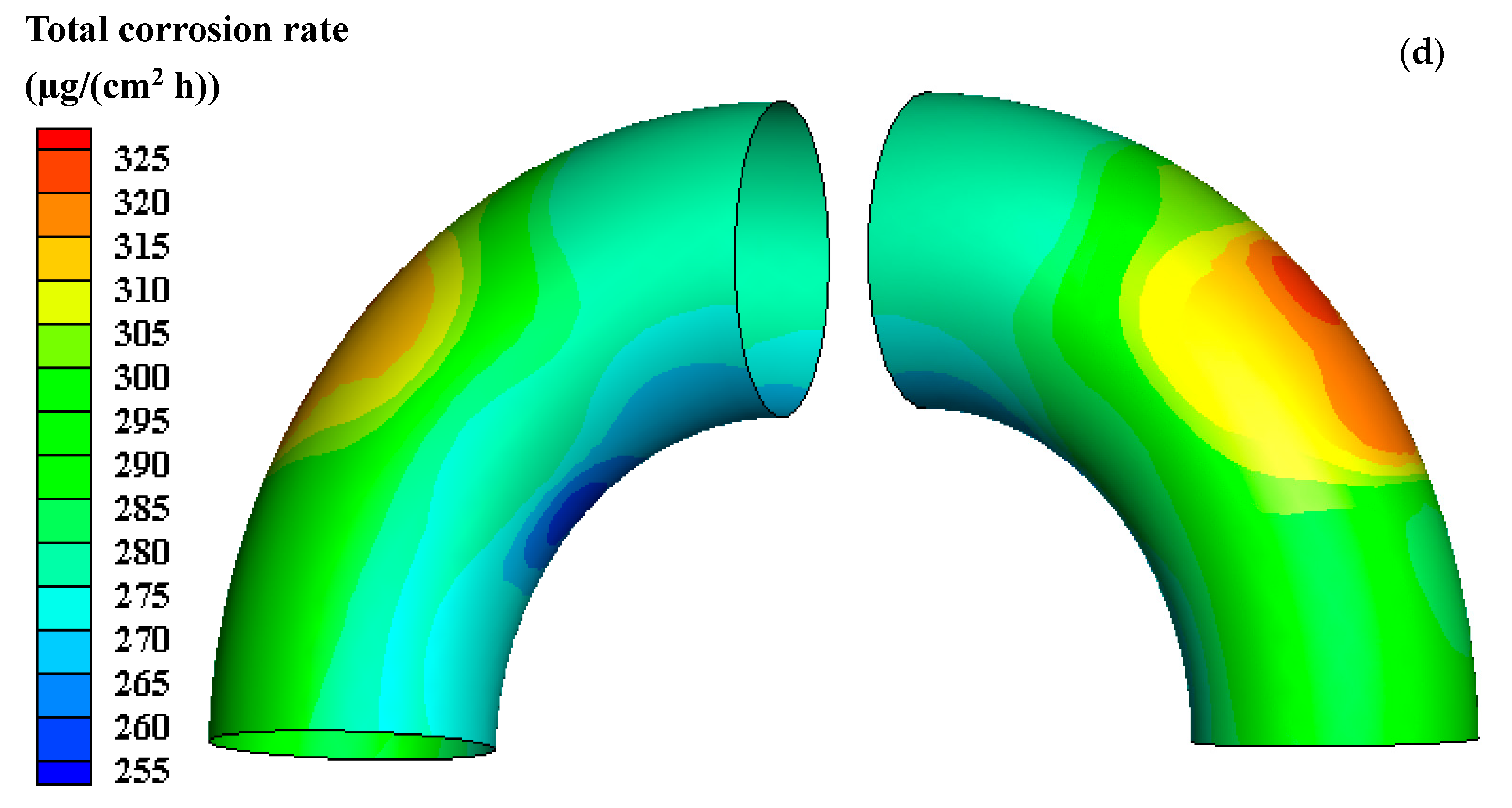
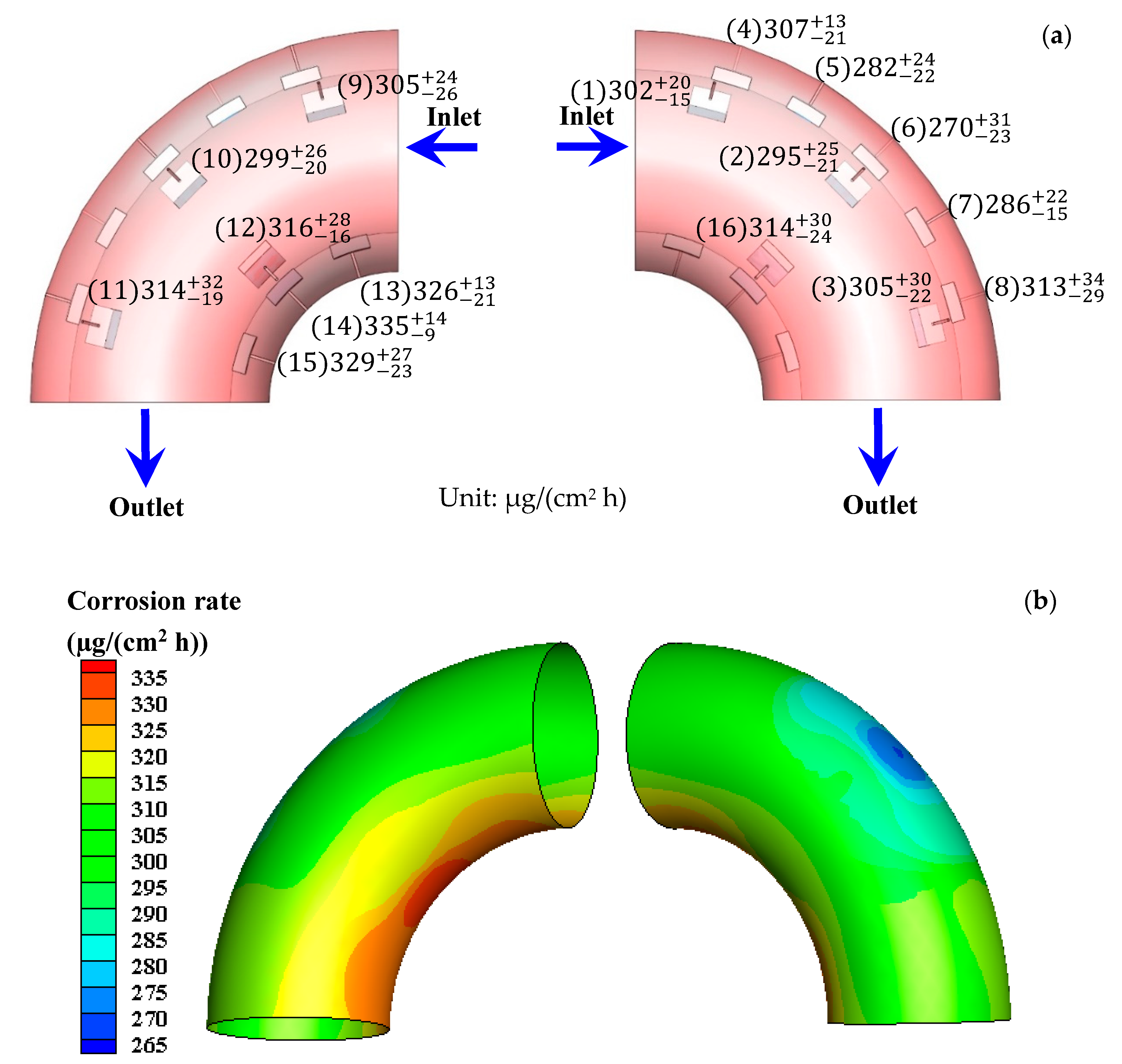
3.1. Total E-C Rate after E-C Test and Corrosion Rate after Flowing Test
3.2. Electrochemical Impedance Spectroscopy Measurements
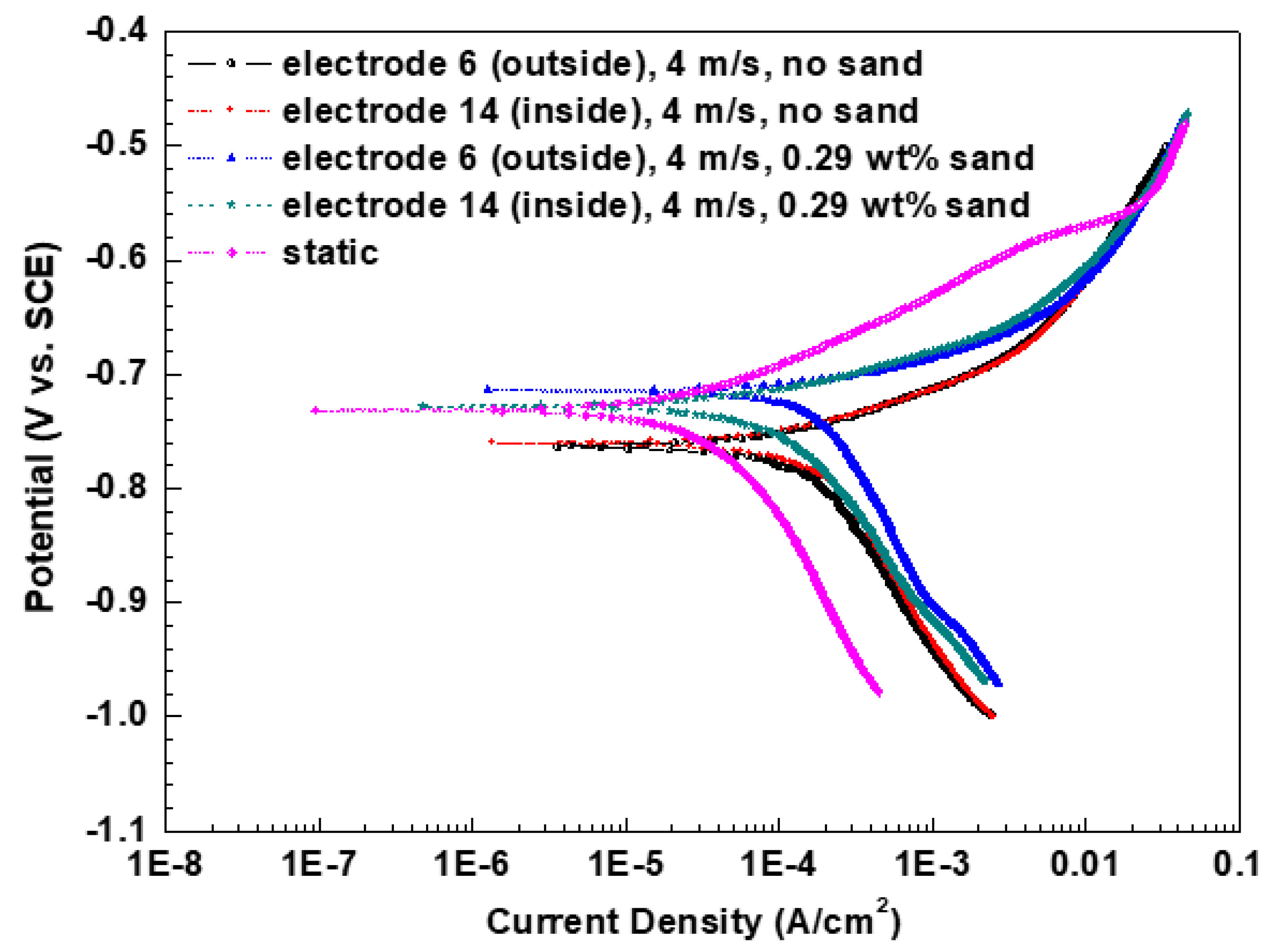
3.3. Polarization Curves Measurements
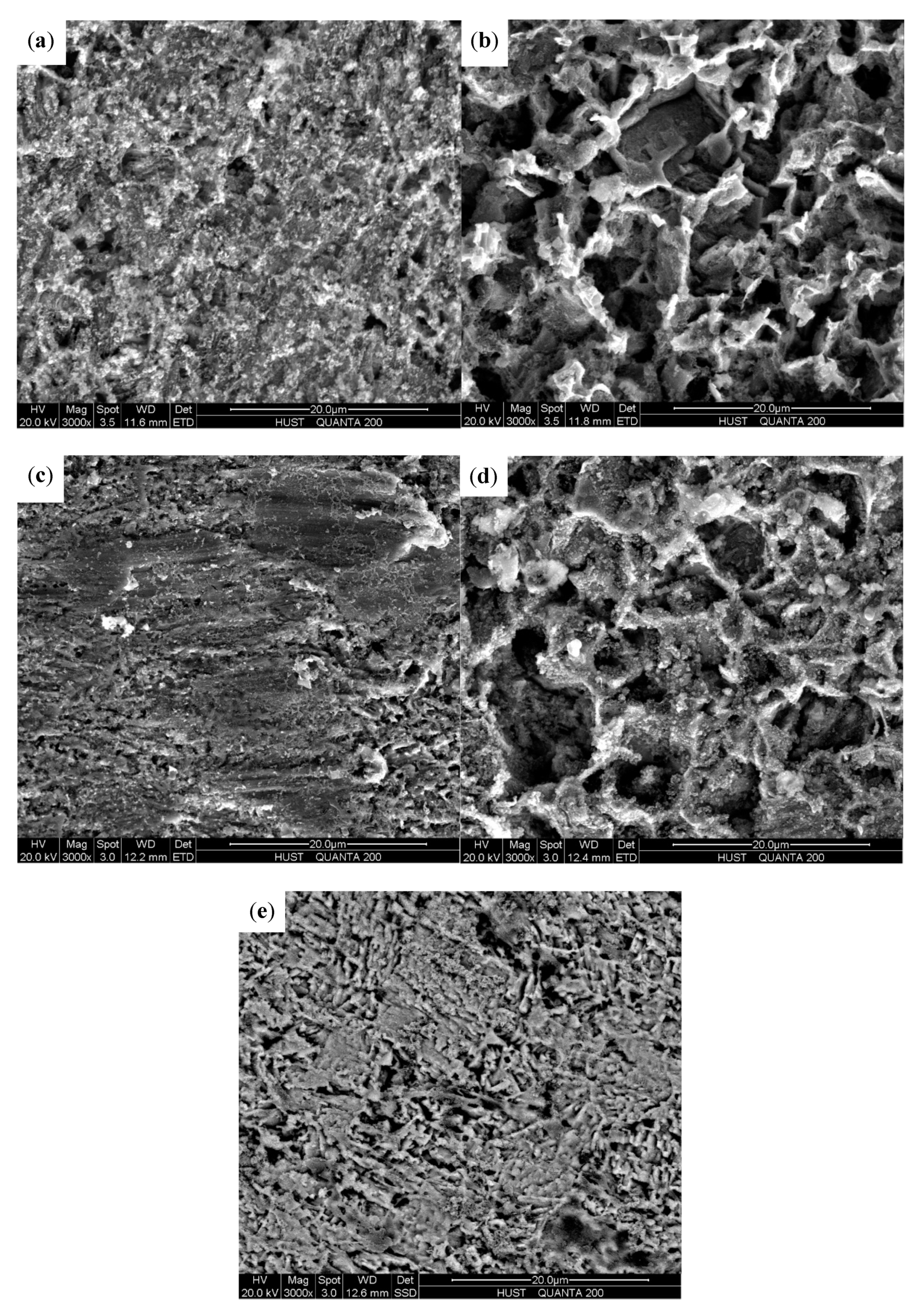
3.4. SEM Surface Morphology
4. Discussion
4.1. The Effect of Hydrodynamics on the Corrosion Behavior under Single-Phase and Two-Phase Flow
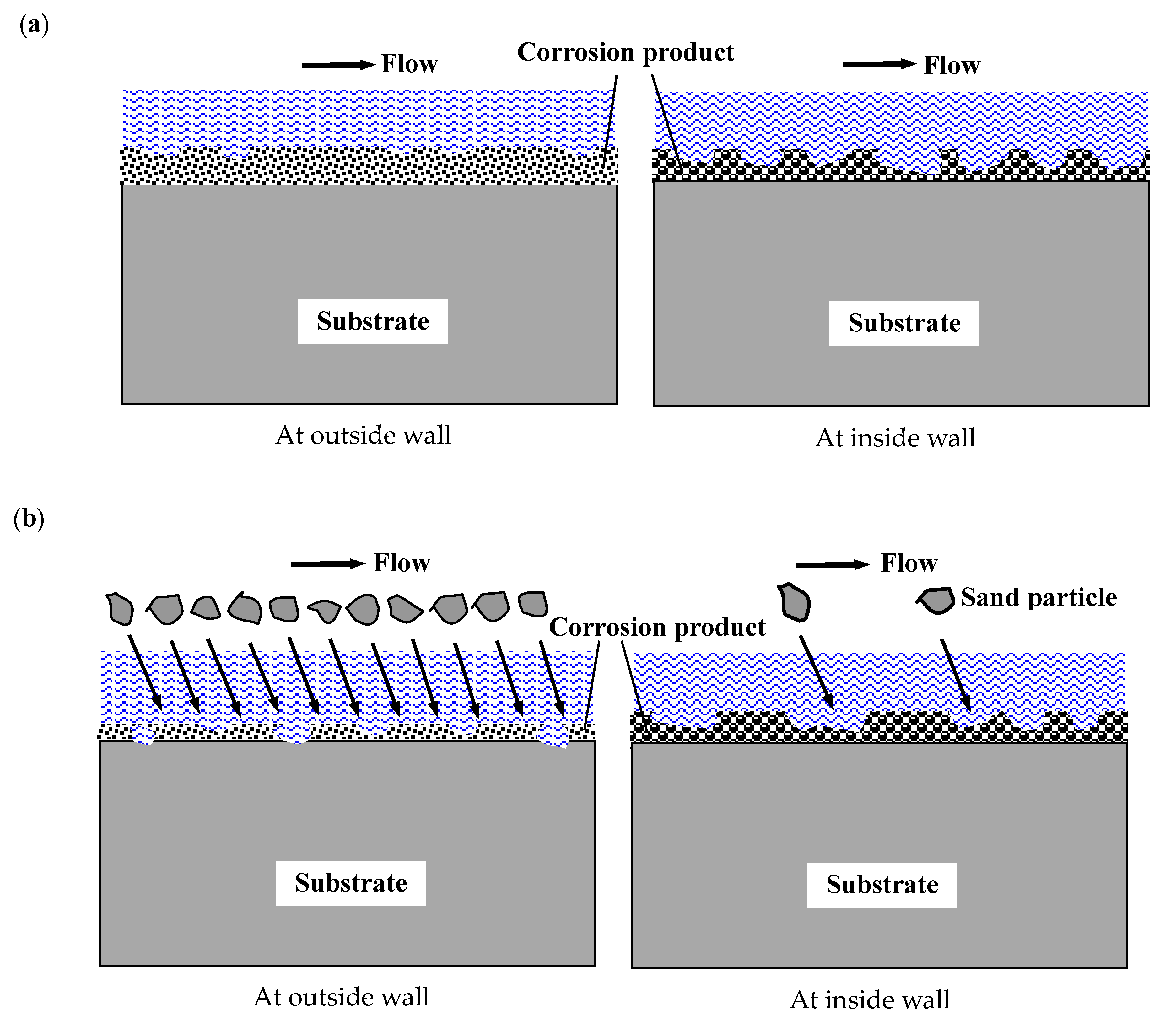
4.2. The Effect of Hydrodynamics on the FAC and E-C Behavior at the Bend
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Islam, M.A.; Farhat, Z. Erosion-corrosion mechanism and comparison of erosion-corrosion performance of API steels. Wear 2017, 376–377, 533–541. [Google Scholar] [CrossRef]
- Khayatan, N.; Ghasemi, H.M.; Abedini, M. Synergistic erosion-corrosion behavior of commercially pure titanium at various impingement angles. Wear 2017, 380–381, 154–162. [Google Scholar] [CrossRef]
- Cheng, J.R.; Li, Z.; Zhang, N.S.; Dou, Y.H.; Cui, L. Experimental study on erosion-corrosion of TP140 casing steel and 13Cr tubing steel in gas-solid and liquid-solid jet flows containing 2 wt % NaCl. Materials 2019, 12, 358. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.B.; Zheng, Y.G. Erosion-enhanced corrosion of stainless steel and carbon steel measured electrochemically under liquid and slurry impingement. Corros. Sci. 2016, 102, 259–268. [Google Scholar] [CrossRef]
- Zeng, L.; Guo, X.P.; Zhang, G.A. Inhibition of the erosion-corrosion of a 90° low alloy steel bend. J. Alloy. Compd. 2017, 724, 827–840. [Google Scholar] [CrossRef]
- Rajahram, S.S.; Harvey, T.J.; Wood, R.J.K. Electrochemical investigation of erosion-corrosion using a slurry pot erosion tester. Tribol. Int. 2011, 44, 232–240. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, J.D.; Chen, D.R.; Hao, X.P.; Wang, B.Y. The difference between synergistic erosion-corrosion and corrosion of mild steel in SiC suspension. J. Alloy. Compd. 2008, 466, 421–428. [Google Scholar] [CrossRef]
- Islam, M.A.; Jiang, J.R.; Xie, Y.S.; Fiala, P. Investigation of erosion-corrosion behavior of (WTi)C based weld overlays. Wear 2017, 390–391, 155–165. [Google Scholar] [CrossRef]
- Senatore, E.V.; Taleb, W.; Owen, J.; Hua, Y.; Gomes, J.A.C.P.; Barker, R.; Neville, A. Evaluation of high shear inhibitor performance in CO2-containing flow-induced corrosion and erosion-corrosion environments in the presence and absence of iron carbonate films. Wear 2018, 404–405, 143–152. [Google Scholar] [CrossRef]
- Liu, J.G.; BaKeDaShi, W.L.; Li, Z.L.; Xu, Y.Z.; Ji, W.R.; Zhang, C.; Cui, G.; Zhang, R.Y. Effect of flow velocity on erosion-corrosion of 90-degree horizontal elbow. Wear 2017, 376–377, 516–525. [Google Scholar] [CrossRef]
- Burstein, G.T.; Sasaki, K. Detecting electrochemical transients generated by erosion-corrosion. Electrochim. Acta 2001, 46, 3675–3683. [Google Scholar] [CrossRef]
- Jiang, X.; Zheng, Y.G.; Ke, W. Effect of flow velocity and entrained sand on inhibition performances of two inhibitors for CO2 corrosion of N80 steel in 3% NaCl solution. Corros. Sci. 2005, 47, 2636–2658. [Google Scholar] [CrossRef]
- Ma, F.L.; Li, J.L.; Zeng, Z.X.; Gao, Y.M. Tribocorrosion behavior in artificial seawater and anti-microbiologically influenced corrosion properties of TiSiN-Cu coating on F690 steel. J. Mater. Sci. Technol. 2019, 35, 448–459. [Google Scholar] [CrossRef]
- Li, W.; Pots, B.F.M.; Brown, B.; Kee, K.; Nesic, S. A direct measurement of wall shear stress in multiphase flow—Is it an important parameter in CO2 corrosion of carbon steel pipelines? Corros. Sci. 2016, 110, 35–45. [Google Scholar] [CrossRef]
- Gao, K.W.; Yu, F.; Pang, X.L.; Zhang, G.A.; Qiao, L.J.; Chu, W.Y.; Lu, M.X. Mechanical properties of CO2 corrosion product scales and their relationship to corrosion rates. Corros. Sci. 2008, 50, 2796–2803. [Google Scholar] [CrossRef]
- Ige, O.O.; Umoru, L.E. Effects of shear stress on the erosion-corrosion behaviour of X-65 carbon steel: A combined mass-loss and profilometry study. Tribol. Int. 2016, 94, 155–164. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, G.A.; Guo, X.P. Effect of hydrodynamics on the inhibition effect of thioureido imidazoline inhibitor for the flow accelerated corrosion of X65 pipeline steel. Corrosion-US 2016, 72, 598–614. [Google Scholar] [CrossRef]
- Gulbrandsen, E.; Grana, A. Testing of carbon dioxide corrosion inhibitor perfor-mance at high flow velocities in jet impingement geometry. Effects of mass transfer and flow forces. Corrosion-US 2007, 63, 1009–1020. [Google Scholar] [CrossRef]
- Hou, Y.; Aldrich, C.; Lepkova, K.; Kinsella, B. Detection of under deposit corrosion in a CO2 environment by using electrochemical noise and recurrence quantification analysis. Electrochim. Acta 2018, 274, 160–169. [Google Scholar] [CrossRef]
- Tan, Y.J.; Fwu, Y.; Bhardwaj, K. Electrochemical evaluation of under-deposit corrosion and its inhibition using the wire beam electrode method. Corros. Sci. 2011, 53, 1254–1261. [Google Scholar] [CrossRef]
- Barker, R.; Neville, A.; Hu, X.; Cushnaghan, S. Evaluating inhibitor performance in CO2-saturated erosion-corrosion environments. Corrosion-US 2015, 71, 14–29. [Google Scholar] [CrossRef]
- Zheng, Z.B.; Zheng, Y.G.; Zhou, X.; He, S.Y.; Sun, W.H.; Wang, J.Q. Determination of the critical flow velocities for erosion-corrosion of passive materials under impingement by NaCl solution containing sand. Corros. Sci. 2014, 88, 187–196. [Google Scholar] [CrossRef]
- El-Gammal, M.; Mazhar, H.; Cotton, J.S.; Shefski, C.; Pietralik, J.; Ching, C.Y. The hydrodynamic effects of single-phase flow on flow accelerated corrosion in a 90-degree elbow. Nucl. Eng. Des. 2010, 240, 1589–1598. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Q.; Zheng, J.Y.; Zhao, Y.Z. Numerical prediction of erosion in elbow based on CFD-DEM simulation. Powder Technol. 2016, 302, 236–246. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, G.A.; Guo, X.P.; Chai, C.W. Inhibition effect of thioureidoimidazoline inhibitor for the flow accelerated corrosion of an elbow. Corros. Sci. 2015, 90, 202–215. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, G.A.; Guo, X.P. Erosion-corrosion at different locations of X65 carbon steel elbow. Corros. Sci. 2014, 85, 318–330. [Google Scholar] [CrossRef]
- Zhang, G.A.; Cheng, Y.F. On the fundamentals of electrochemical corrosion of X65 steel in CO2-containing formation water in the presence of acetic acid in petroleum production. Corros. Sci. 2009, 51, 87–94. [Google Scholar] [CrossRef]
- Zhang, G.A.; Zeng, Y.; Guo, X.P.; Jiang, F.; Shi, D.Y.; Chen, Z.Y. Electrochemical corrosion behavior of carbon steel under dynamic high pressure H2S/CO2 environment. Corros. Sci. 2012, 65, 37–47. [Google Scholar] [CrossRef]
- Hu, H.L.; Li, N. Electrochemical Measurements; National Defense Industry Press: Biejing, China, 2011; p. 235. [Google Scholar]
- Hassani, S.; Roberts, K.P.; Shirazi, S.A.; Shadley, J.R.; Rybicki, E.F.; Joia, C. Characterization and prediction of chemical inhibition performance for erosion-corrosion conditions in sweet oil and gas production. Corrosion 2012, 68, 885–896. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Wang, J.L.; Hou, B.S.; Xiang, J.; Chen, X.D.; Gu, T.Y.; Liu, H.F. The performance and mechanism of bifunctional biocide sodium pyrithione against sulfate reducing bacteria in X80 carbon steel corrosion. Corros. Sci. 2019, 150, 296–308. [Google Scholar] [CrossRef]
- Zhang, G.A.; Liu, D.; Li, Y.Z.; Guo, X.P. Corrosion behaviour of N80 carbon steel in formation water under dynamic supercritical CO2 condition. Corros. Sci. 2017, 120, 107–120. [Google Scholar] [CrossRef]
- Mora-Mendoza, J.L.; Turgoose, S. Fe3C influence on the corrosion rate of mild steel in aqueous CO2 systems under turbulent flow conditions. Corros. Sci. 2002, 44, 1223–1246. [Google Scholar] [CrossRef]
- Zhang, G.A.; Zeng, L.; Huang, H.L.; Guo, X.P. A study of flow accelerated corrosion at elbow of carbon steel pipeline by array electrode and computational fluid dynamics simulation. Corros. Sci. 2013, 77, 334–341. [Google Scholar] [CrossRef]
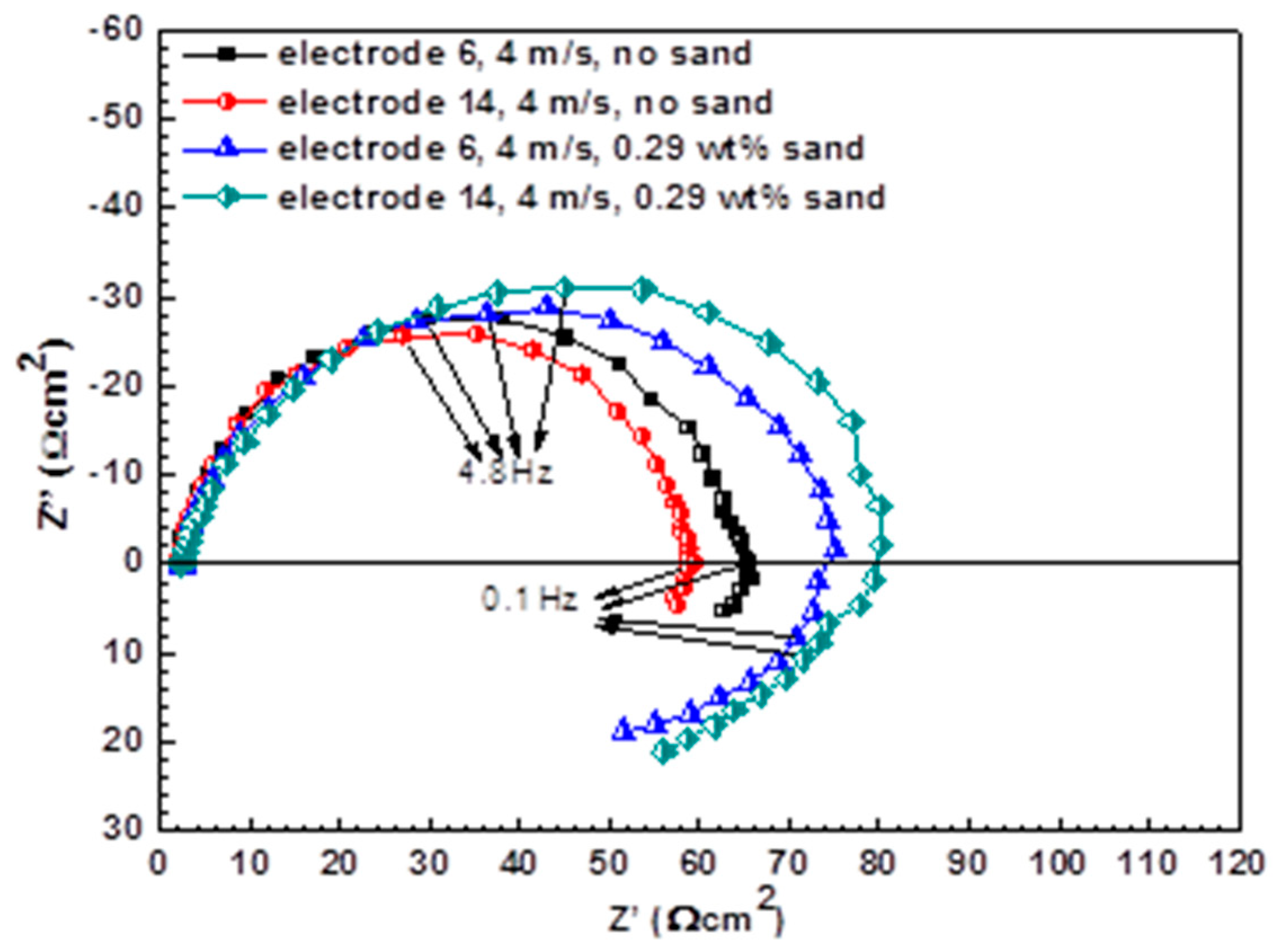
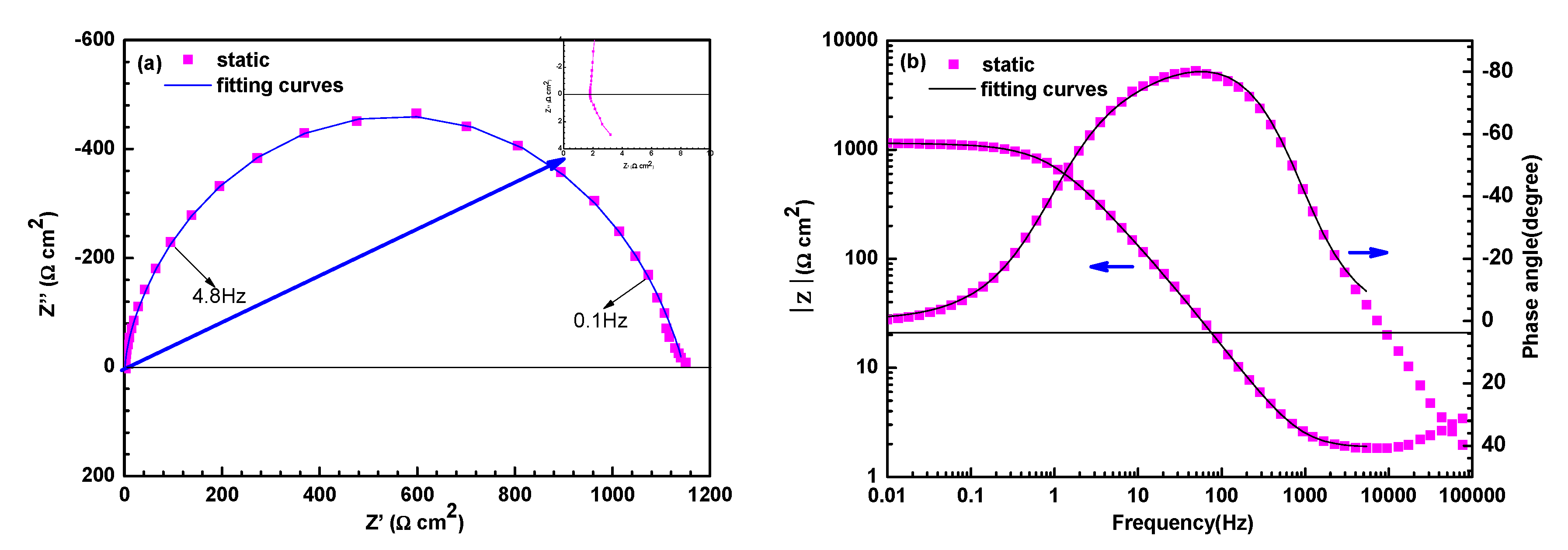
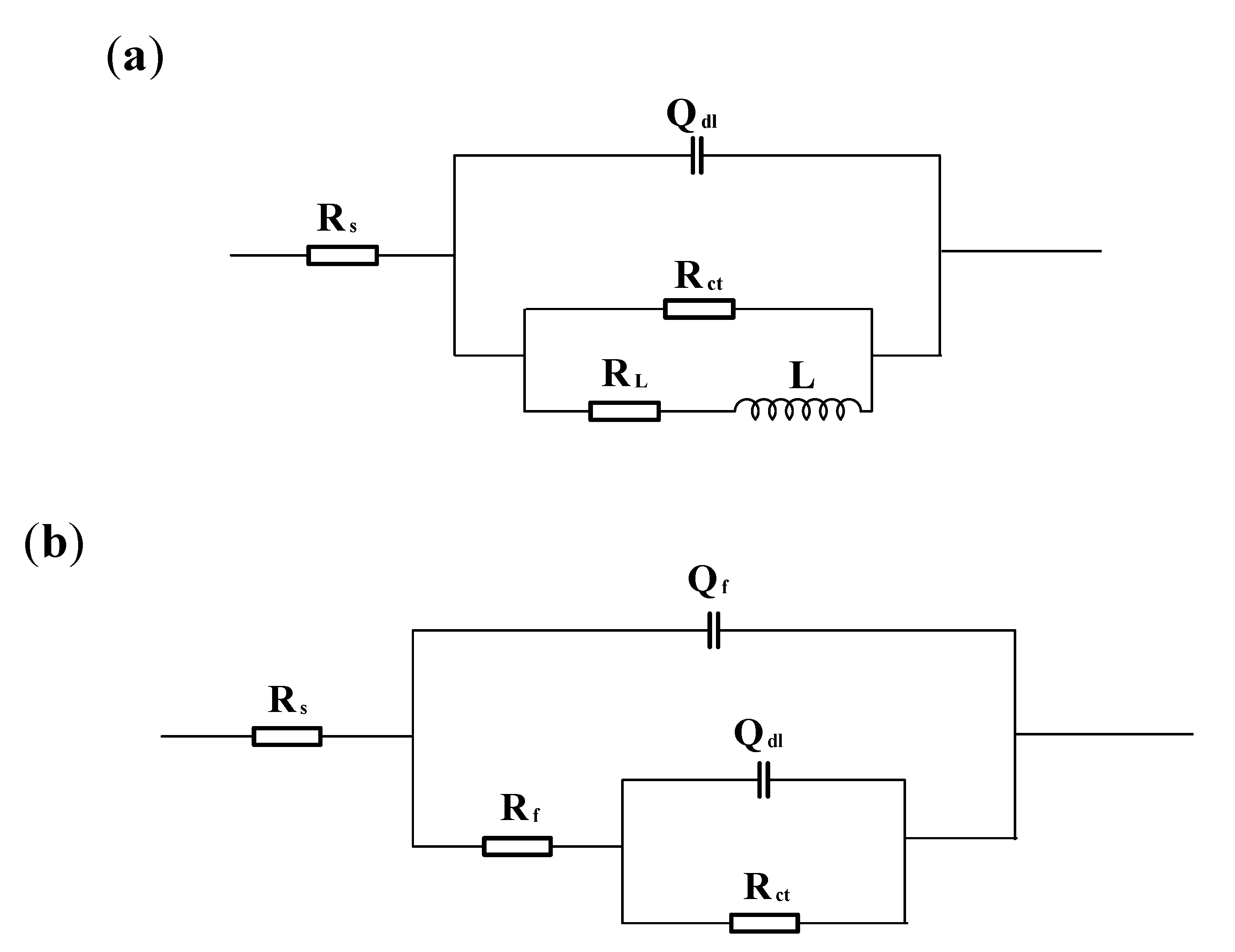
| Electrode | Sand Concentration (wt %) | Rs (Ω·cm2) | Qdl (×10−4Ω−1cm−2 sn) | n | Rct (Ω cm2) | RL (Ω cm2) | L (H/cm2) |
|---|---|---|---|---|---|---|---|
| Electrode 6 | 0 | 2.24 | 7.94 | 0.92 | 62.86 | 126.10 | 7171 |
| Statistical errors (%) | 3.87 | 5.16 | 1.82 | 7.33 | 17.94 | 6.03 | |
| Electrode 14 | 0 | 2.16 | 8.48 | 0.93 | 57.17 | 175.70 | 6558 |
| Statistical errors (%) | 5.18 | 3.61 | 1.42 | 6.29 | 18.56 | 7.02 | |
| Electrode 6 | 0.29 | 2.37 | 7.46 | 0.89 | 71.48 | 67.42 | 846 |
| Statistical errors (%) | 7.29 | 4.81 | 0.75 | 6.37 | 16.42 | 15.87 | |
| Electrode 14 | 0.29 | 2.34 | 6.08 | 0.85 | 78.59 | 81.64 | 768 |
| Statistical errors (%) | 5.86 | 6.67 | 1.95 | 7.92 | 13.09 | 18.85 | |
| Rs (Ω cm2) | Qf (×10−4Ω−1cm−2 sn) | n1 | Rf (Ω cm2) | Qdl (×10−4Ω−1cm−2 sn) | n2 | Rct (Ω cm2) | |
|---|---|---|---|---|---|---|---|
| Calculated values | 1.03 | 1.06 | 0.99 | 222.30 | 1.68 | 0.70 | 924.40 |
| Fitting errors (%) | 1.22 | 6.44 | 0.84 | 8.72 | 10.41 | 4.40 | 9.16 |
| Statistical errors (%) | 7.78 | 14.93 | 0.68 | 5.91 | 13.25 | 3.74 | 10.26 |
| Electrode | Velocity (m/s) | Sand Concentration (wt %) | Ecorr (V vs. SCE) | ba(V/dec) | bc(V/dec) | Icorr (A/cm2) |
|---|---|---|---|---|---|---|
| Electrode 6 | 4 | 0 | −0.763 | 0.079 | −0.200 | 1.37 × 10−4 |
| Statistical errors (%) | 0.74 | 4.18 | 1.99 | 6.85 | ||
| Electrode 14 | 4 | 0 | −0.760 | 0.080 | −0.227 | 1.72 × 10−4 |
| Statistical errors (%) | 1.05 | 4.60 | 2.51 | 7.31 | ||
| Electrode 6 | 4 | 0.29 | −0.714 | 0.096 | −0.222 | 1.58 × 10−4 |
| Statistical errors (%) | 1.59 | 5.92 | 2.10 | 8.14 | ||
| Electrode 14 | 4 | 0.29 | −0.728 | 0.073 | −0.165 | 8.86 × 10−5 |
| Statistical errors (%) | 1.33 | 5.88 | 3.44 | 1.51 | ||
| 0 | 0 | −0.731 | 0.063 | −0.202 | 3.34 × 10−5 | |
| Statistical errors (%) | 0.82 | 3.07 | 1.81 | 2.00 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, L.; Chen, G.; Chen, H. Comparative Study on Flow-Accelerated Corrosion and Erosion–Corrosion at a 90° Carbon Steel Bend. Materials 2020, 13, 1780. https://doi.org/10.3390/ma13071780
Zeng L, Chen G, Chen H. Comparative Study on Flow-Accelerated Corrosion and Erosion–Corrosion at a 90° Carbon Steel Bend. Materials. 2020; 13(7):1780. https://doi.org/10.3390/ma13071780
Chicago/Turabian StyleZeng, Li, Geng Chen, and Hanxin Chen. 2020. "Comparative Study on Flow-Accelerated Corrosion and Erosion–Corrosion at a 90° Carbon Steel Bend" Materials 13, no. 7: 1780. https://doi.org/10.3390/ma13071780
APA StyleZeng, L., Chen, G., & Chen, H. (2020). Comparative Study on Flow-Accelerated Corrosion and Erosion–Corrosion at a 90° Carbon Steel Bend. Materials, 13(7), 1780. https://doi.org/10.3390/ma13071780




