A Comparative Study on the Thermal Energy Storage Performance of Bio-Based and Paraffin-Based PCMs Using DSC Procedures
Abstract
1. Introduction
2. Materials and Methods
2.1. Paraffin Wax
2.2. Bio-based PCM
2.3. Methods
2.3.1. Dynamic DSC Method and Evaluation of TES Parameters
2.3.2. Stepwise DSC Method and Evaluation of TES Parameters
2.4. Experimental Program
3. Dynamic DSC Results
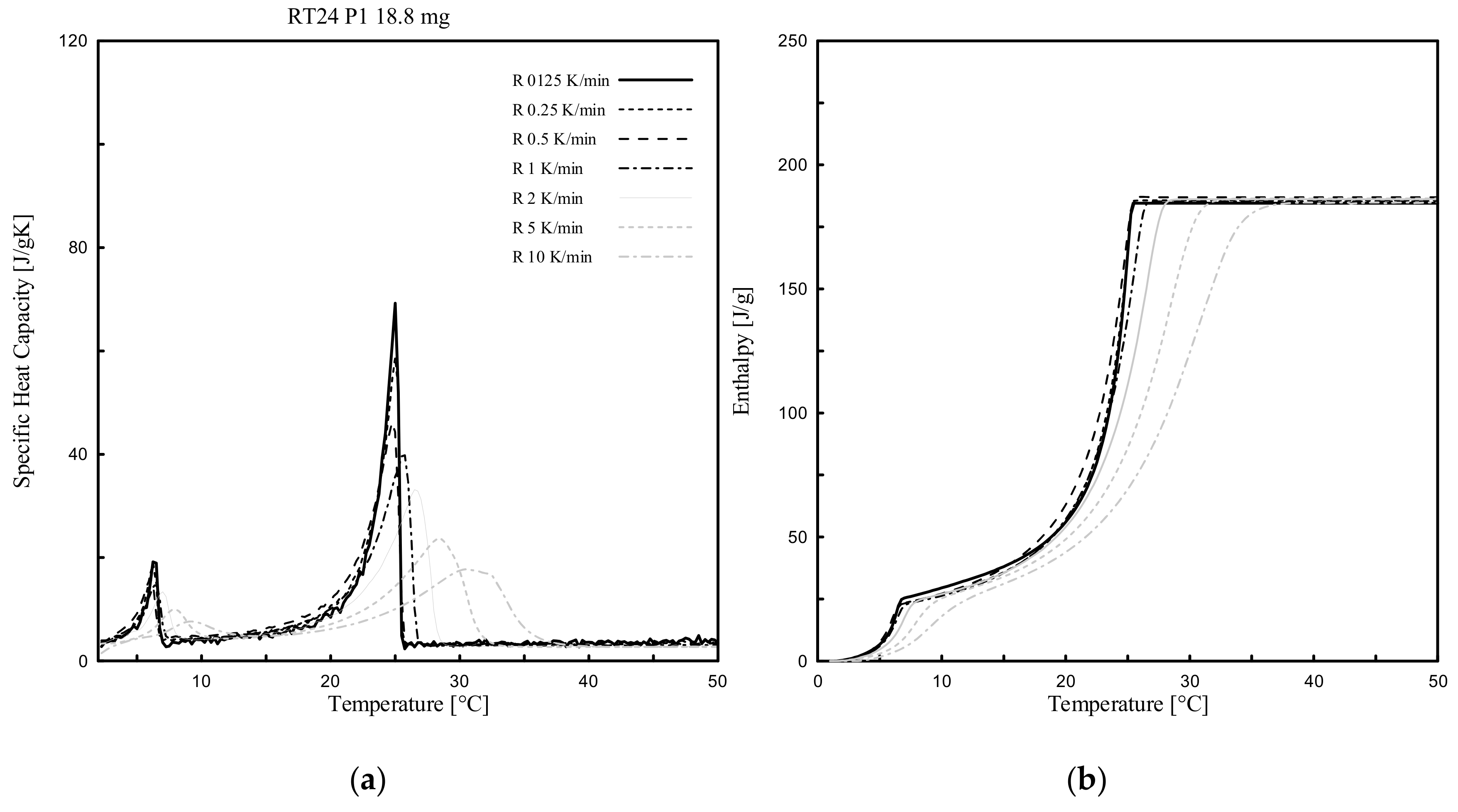
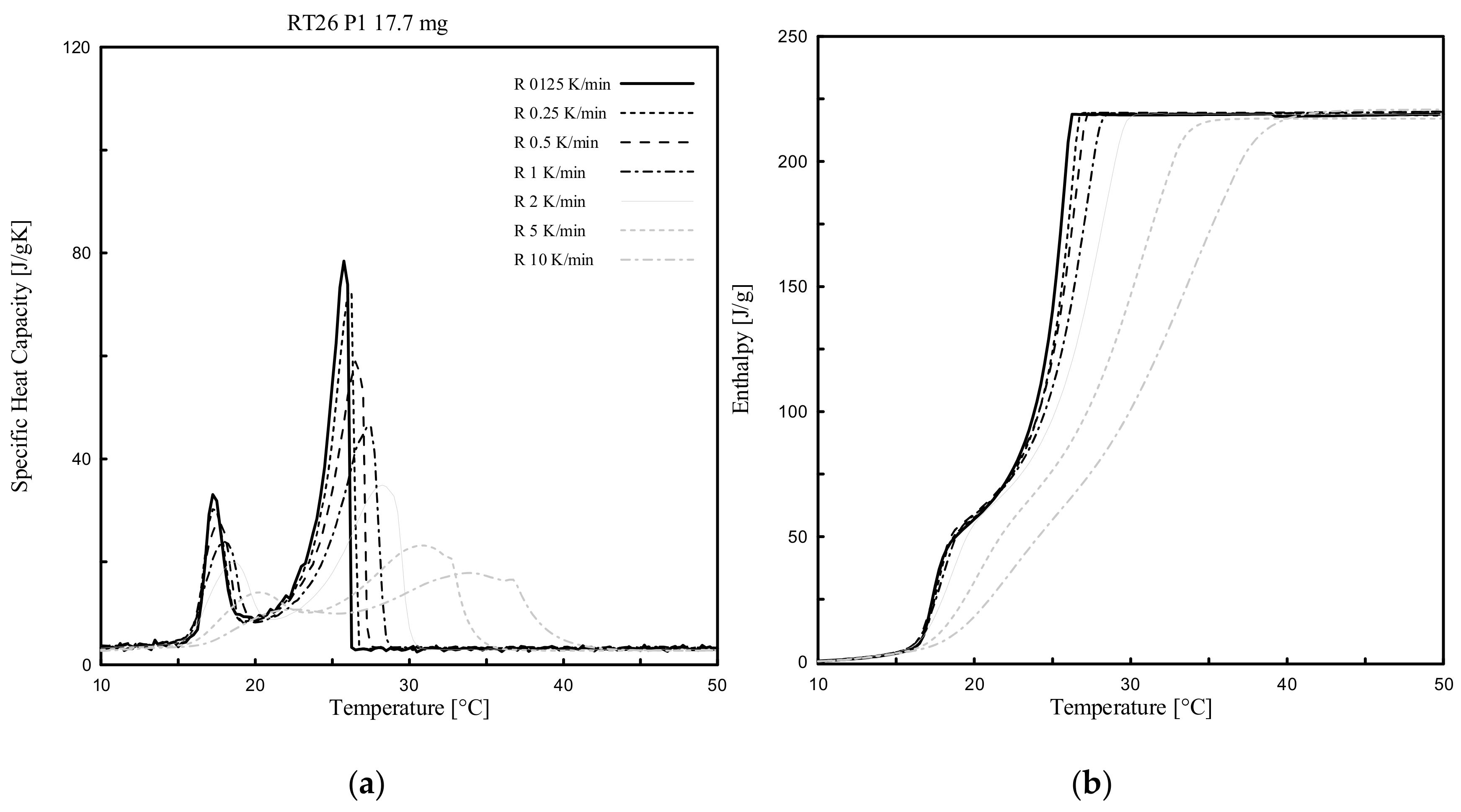
3.1. Heating Rate Effect
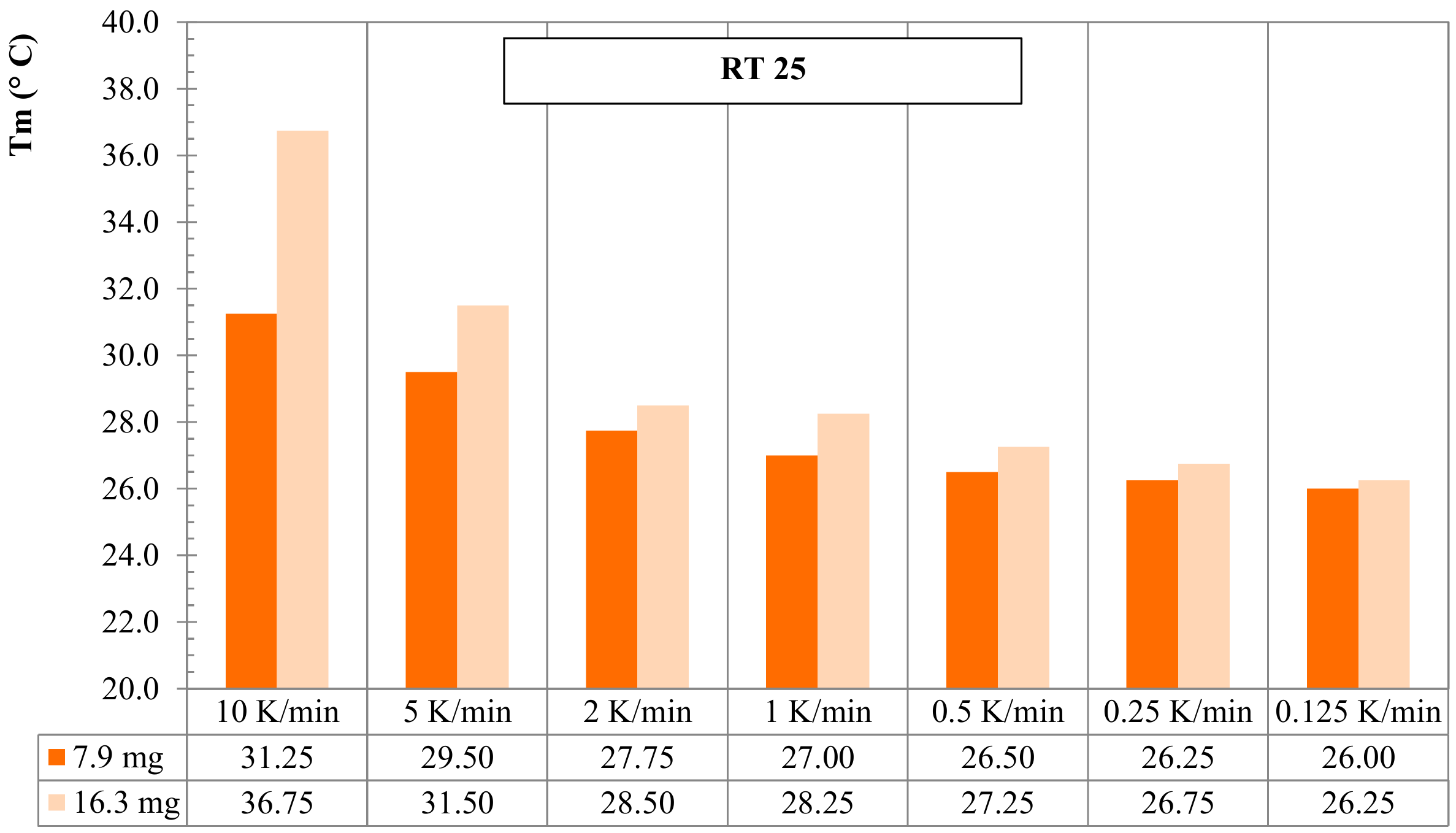
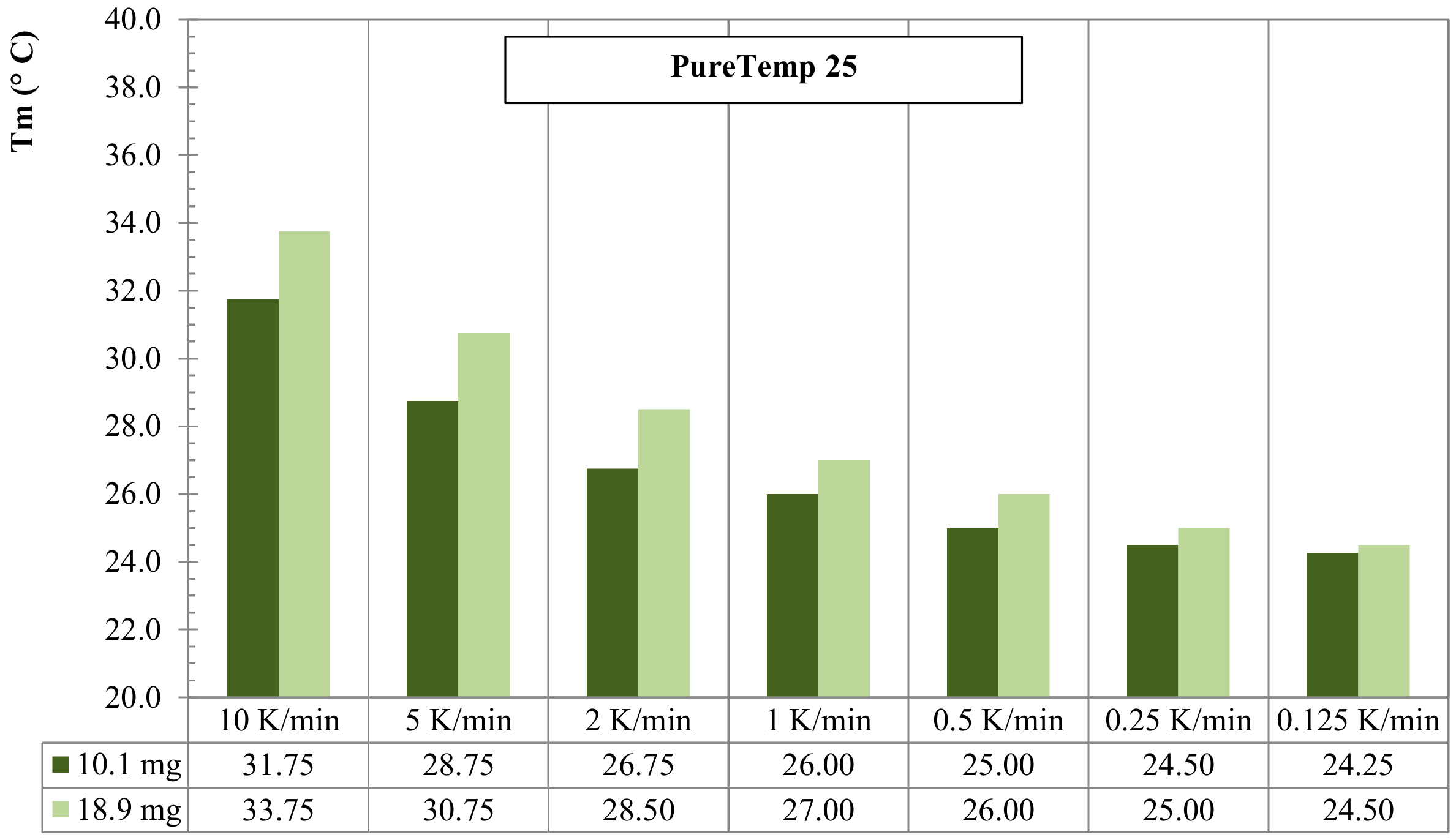
3.2. Mass of Sample Effect for RT25 and PureTemp 25
3.3. Specific Heat Capacities, Enthalpy and Melting Points for Heating and Cooling
4. Stepwise DSC Results
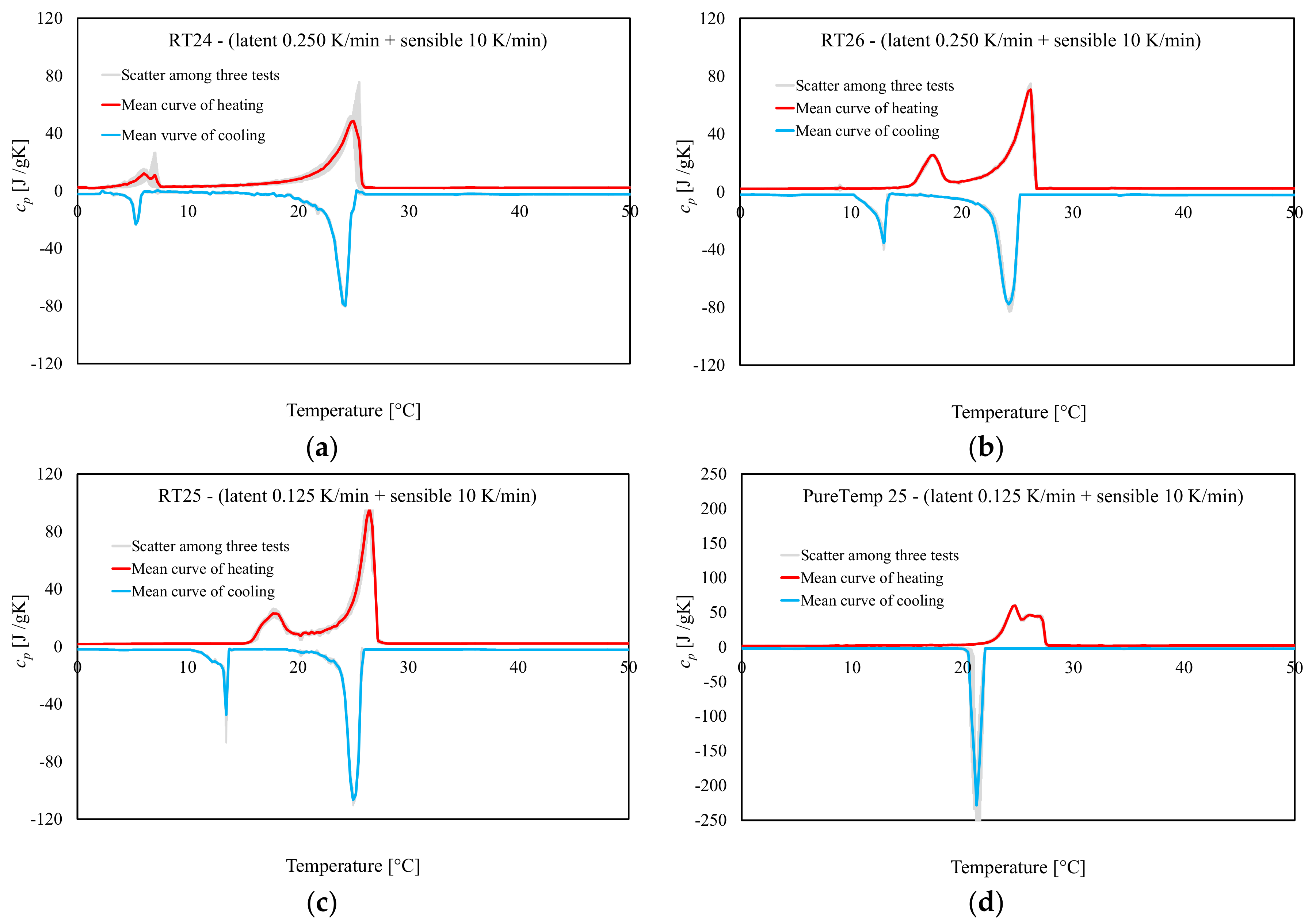
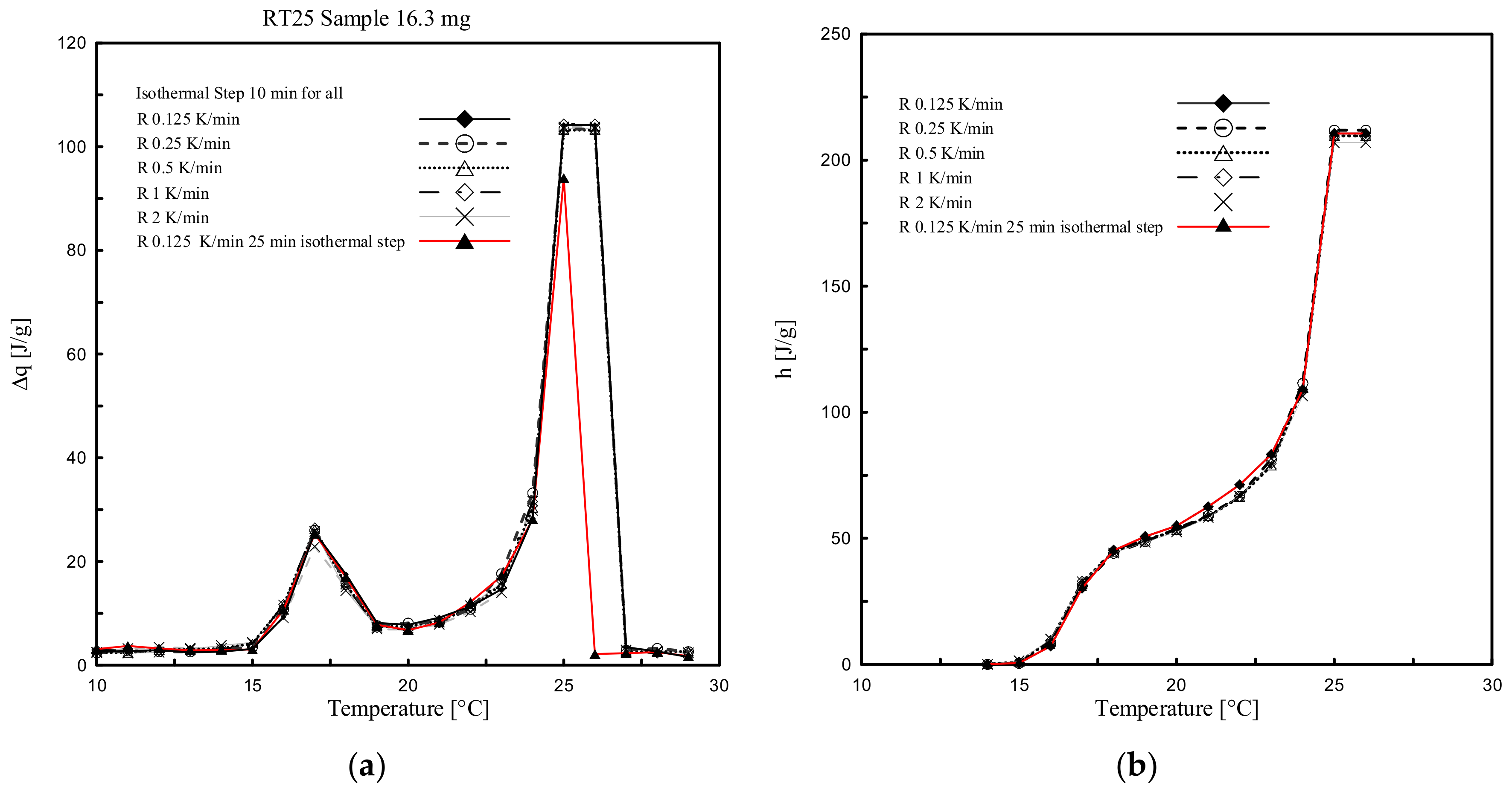
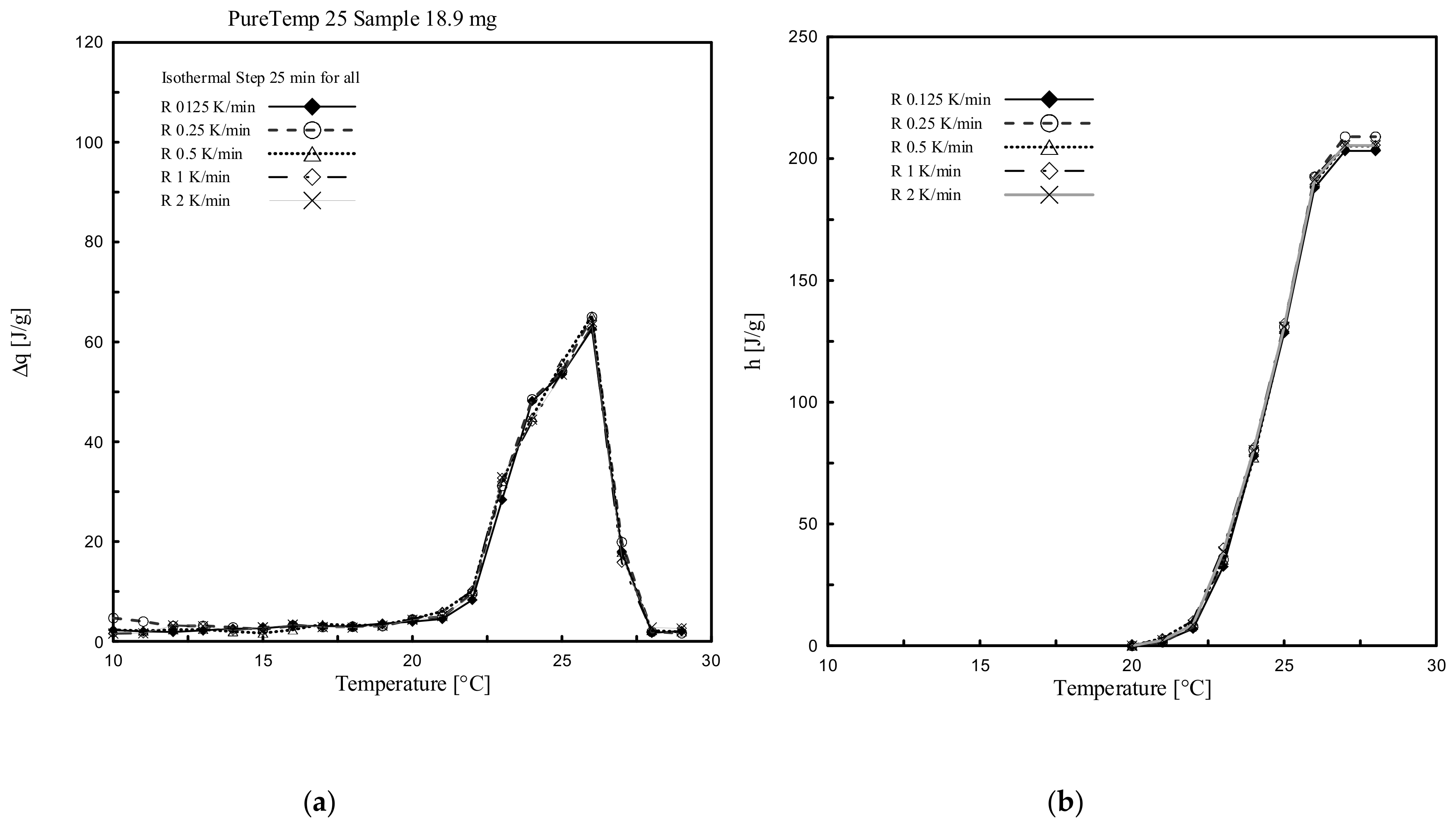
4.1. Heating Rate Effect
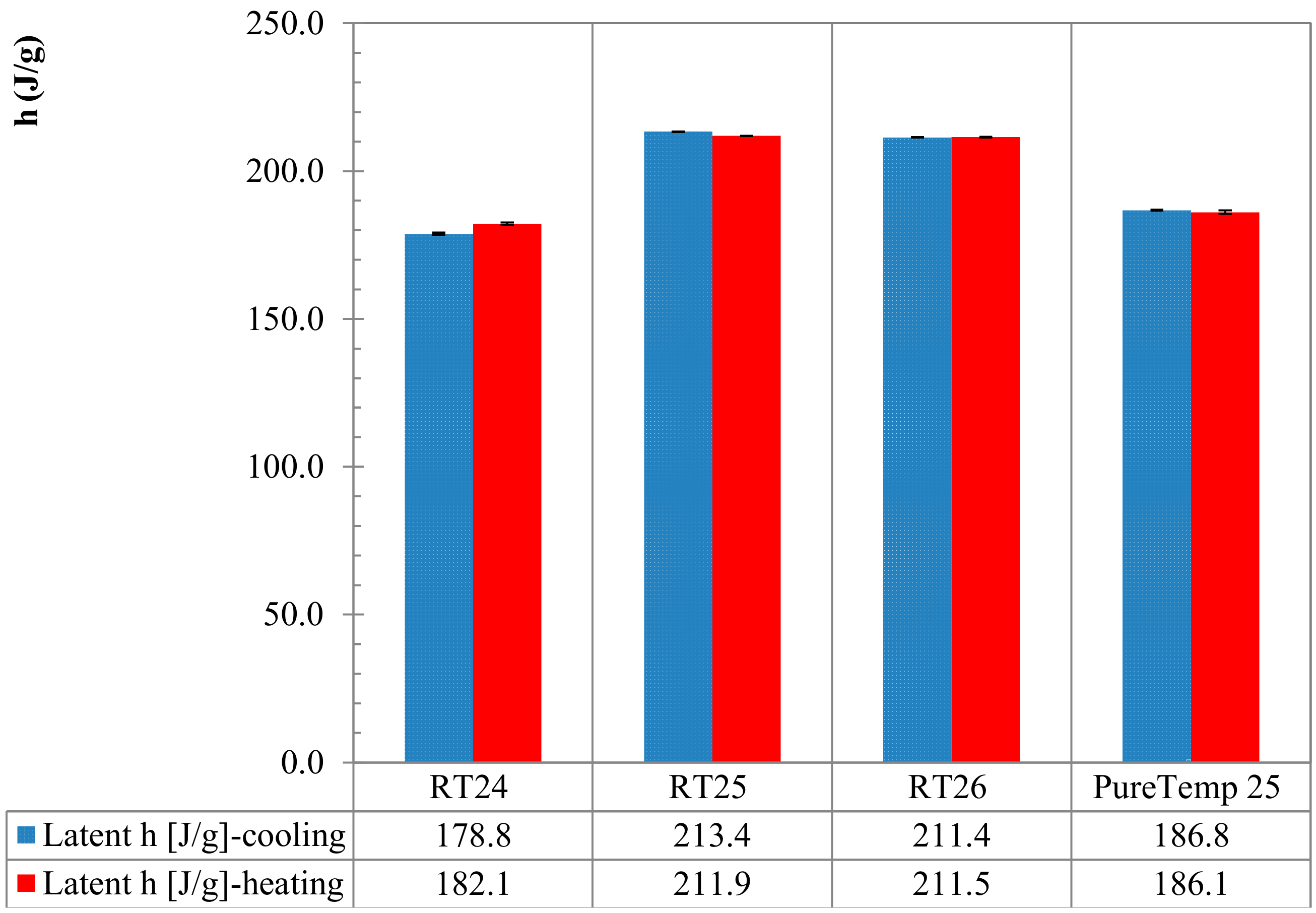
4.2. Enthalpy and Phase Change Temperature
5. Conclusions
- Dynamic DSC tests, performed for all 4 different PCMs and considering several heating rates (ranging from 10 K/min to 0.125 K/min), showed that the measurable melting peak temperatures Tm (indicated as that point at which the maximum peak of cp(T), or alternatively the inflection point of h(T), can be registered), mainly depends on the heating rate. Tm can be shifted progressively to lower values as the heating rate decreases and vice versa;
- The cp(T) and h(T) curves under dynamic DSC strongly depend on the selected heating rates. Only the related measure of the accumulated (total) specific (unitary) latent heat is independent to the heating rate. In general terms, the total achievable h is the same when different heating rates are assumed, even though the h(T) curves and their slopes, cp(T), are heating rate dependent;
- Dynamic DSC tests, performed by considering 2 different (low and high) sample masses, for RT25 and PureTemp 25, highlighted that the specimens with a lower mass are less affected by the heating rate. However, they suffer higher measurement noise and less representativity of the material under investigation;
- Dynamic DSC results of higher mass samples showed more stable measurements, especially in the sensible parts, and seemed to be more representative for the behavior of the investigated PCMs. However, they turned out to be more sensible to heating rate effects under latent heat storage.
- It can be concluded that the selection of the proper “heating rate” and “sample mass” (aimed at fulfilling the IEA requirements) arises from a compromise between accuracy of the measurements (i.e., enthalpy, specific heat capacities and melting point) and the mitigation of the heating/cooling rate effects.
- It can be stated that reliable and reproducible results can be achieved for characterizing the aforementioned (paraffin- and bio-based) PCMs by following the IEA standard procedure and adopting the dynamic DSC method.
- Results following the stepwise method demonstrated that both ∆q(T) and h(T) results, obtained by considering several heating rates (2, 1, 0.5, 0.25 and 0.125 K/min), presented almost the same trend and similar values (with differences significantly less than 0.2 °C and/or in terms of enthalpy of max. 5 J/g between them). This means that with the stepwise method the heating rate influence, which classically affects the dynamic DSC, can be fully controlled.
- The main drawbacks of the stepwise method, however, are related to its enormously time-consuming character, imposed by the test procedure. The required length of the relaxation time (namely, isothermal step) is sometimes too high to achieve proper results, especially in the melting region.
- The temperature resolution of the stored heat is considerably higher, using the stepwise method, in comparison to the dynamic one. However, a maximum resolution of 1 K, as those employed in the stepwise method of this study, is not high enough to obtain the cp(T) curve of the material, which would require smaller discretized temperature steps, and also does not allow describing the melting temperature of the material in a concise way.
- The results obtained following both dynamic and stepwise methods are dependent on the baseline construction, which allows separating the sensible from the latent heat part. In this regard, the baseline-construction can be more precisely built in the dynamic method than the stepwise one, because more valuable measurement data can be evaluated within very small temperature steps. For this reason, the stepwise method is more appropriate to measure the total enthalpy (both sensible and latent heat) in defined temperature steps and also validating the melting peak reached from the dynamic procedure, while the dynamic measurements can ascertain the stored latent heat much more quickly and precisely. Then using the IEA Standard, dynamic measurements are less time-consuming and also more precise to characterize the melting behavior of the material.
Author Contributions
Funding
Conflicts of Interest
References
- Rashid, K.; Ellingwood, K.; Safdarnejad, S.M.; Powell, K.M. Designing Flexibility into a Hybrid Solar Thermal Power Plant by Real-Time, Adaptive Heat Integration. Comput. Aided Chem. Eng. 2019, 47, 457–462. [Google Scholar]
- Ale, S.; Femeena, P.V.; Mehan, S.; Cibin, R. Environmental impacts of bioenergy crop production and benefits of multifunctional bioenergy systems. In Bioenergy with Carbon Capture and Storage; Academic Press: Cambridge, MA, USA, 2019; pp. 195–217. [Google Scholar]
- European Commission. Available online: https://ec.europa.eu/energy/en/topics/energy-efficiency/heating-and-cooling (accessed on 4 April 2020).
- EU-EC. Available online: https://ec.europa.eu/energy/sites/ener/files/documents/germany_de_version.pdf (accessed on 4 April 2020).
- Da Cunha, S.R.L.; de Aguiar, J.L.B. Phase change materials and energy efficiency of buildings: A review of knowledge. J. Energy Storage 2020, 27, 101083. [Google Scholar] [CrossRef]
- Casini, M. Smart Buildings: Advanced Materials and Nanotechnology to Improve Energy-Efficiency and Environmental Performance; Woodhead Publishing: Cambridge, MA, USA, 2016. [Google Scholar]
- Cárdenas-Ramírez, C.; Gómez, M.; Jaramillo, F. Characterization of a porous mineral as a promising support for shape-stabilized phase change materials. J. Energy Storage 2019, 26, 101041. [Google Scholar] [CrossRef]
- He, M.; Yang, L.; Lin, W.; Chen, J.; Mao, X.; Ma, Z. Preparation, thermal characterization and examination of phase change materials (PCMs) enhanced by carbon-based nanoparticles for solar thermal energy storage. J. Energy Storage 2019, 25, 100874. [Google Scholar] [CrossRef]
- Goia, F.; Perino, M.; Serra, V. Improving thermal comfort conditions by means of PCM glazing systems. Energy Build. 2013, 60, 442–452. [Google Scholar] [CrossRef]
- Stritih, U.; Tyagi, V.V.; Stropnik, R.; Paksoy, H.; Haghighat, F.; Joybari, M.M. Integration of passive PCM technologies for net-zero energy buildings. Sustain. Cities Soc. 2018, 41, 286–295. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.D.; De Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Dardir, M.; Panchabikesan, K.; Haghighat, F.; El Mankibi, M.; Yuan, Y. Opportunities and challenges of PCM-to-air heat exchangers (PAHXs) for building free cooling applications—A comprehensive review. J. Energy Storage 2019, 22, 157–175. [Google Scholar] [CrossRef]
- Sardari, P.T.; Grant, D.; Giddings, D.; Walker, G.S.; Gillott, M. Composite metal foam/PCM energy store design for dwelling space air heating. Energy Convers. Manag. 2019, 201, 112151. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Li, L.; Zhao, M. Experimental assessment on the use of phase change materials (PCMs)-bricks in the exterior wall of a full-scale room. Energy Convers. Manag. 2016, 120, 81–89. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Sanjayan, J.; Wang, X.; Alam, M.; Wilson, J. A novel paraffin/expanded perlite composite phase change material for prevention of PCM leakage in cementitious composites. Appl. Energy 2015, 157, 85–94. [Google Scholar] [CrossRef]
- Aditya, L.; Mahlia, T.M.I.; Rismanchi, B.; Ng, H.M.; Hasan, M.H.; Metselaar, H.S.C.; Aditiya, H.B. A review on insulation materials for energy conservation in buildings. Renew. Sustain. Energy Rev. 2017, 73, 1352–1365. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, Y.; Chen, S.; Sun, Y. Thermal performances study on a façade-built-in two-phase thermosyphon loop for passive thermo-activated building system. Energy Convers. Manag. 2019, 199, 112059. [Google Scholar] [CrossRef]
- Mehling, H.; Cabeza, L.F. Heat and Cold Storage with PCM; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Akeiber, H.J.; Hosseini, S.E.; Hussen, H.M.; Wahid, M.A.; Mohammad, A.T. Thermal performance and economic evaluation of a newly developed phase change material for effective building encapsulation. Energy Convers. Manag. 2017, 150, 48–61. [Google Scholar] [CrossRef]
- Castellón, C.; Günther, E.; Mehling, H.; Hiebler, S.; Cabeza, L.F. Determination of the enthalpy of PCM as a function of temperature using a heat-flux DSC—A study of different measurement procedures and their accuracy. Int. J. Energy Res. 2008, 32, 1258–1265. [Google Scholar] [CrossRef]
- Jin, X.; Xu, X.; Zhang, X.; Yin, Y. Determination of the PCM melting temperature range using DSC. Thermochim. Acta 2014, 595, 17–21. [Google Scholar] [CrossRef]
- Barreneche, C.; Solé, A.; Miró, L.; Martorell, I.; Fernández, A.I.; Cabeza, L.F. Study on differential scanning calorimetry analysis with two operation modes and organic and inorganic phase change material (PCM). Thermochim. Acta 2013, 553, 23–26. [Google Scholar] [CrossRef]
- Gschwander, S.; Haussmann, T.; Hagelstein, G.; Barreneche, C.; Ferrer, G.; Cabeza, L.; Hennemann, P. Standardization of PCM characterization via DSC. In Proceedings of the SHC 2015 International Conference on Solar Heating and Cooling for Buildings and Industry, Beijing, China, 19–21 May 2015; pp. 2–4. [Google Scholar]
- Feng, G.; Huang, K.; Xie, H.; Li, H.; Liu, X.; Liu, S.; Cao, C. DSC test error of phase change material (PCM) and its influence on the simulation of the PCM floor. Renew. Energy 2016, 87, 1148–1153. [Google Scholar] [CrossRef]
- Yinping, Z.; Yi, J. A simple method, the-history method, of determining the heat of fusion, specific heat and thermal conductivity of phase-change materials. Meas. Sci. Technol. 1999, 10, 201. [Google Scholar] [CrossRef]
- Badenhorst, H.; Cabeza, L.F. Critical analysis of the T-history method: A fundamental approach. Thermochim. Acta 2017, 650, 95–105. [Google Scholar] [CrossRef]
- Tan, P.; Brütting, M.; Vidi, S.; Ebert, H.P.; Johansson, P.; Kalagasidis, A.S. Characterizing phase change materials using the T-History method: On the factors influencing the accuracy and precision of the enthalpy-temperature curve. Thermochim. Acta 2018, 666, 212–228. [Google Scholar] [CrossRef]
- Buttitta, G.; Serale, G.; Cascone, Y. Enthalpy-temperature evaluation of slurry phase change materials with T-history method. Energy Procedia 2015, 78, 1877–1882. [Google Scholar] [CrossRef]
- Hong, H.; Kim, S.K.; Kim, Y.S. Accuracy improvement of T-history method for measuring heat of fusion of various materials. Int. J. Refrig. 2004, 27, 360–366. [Google Scholar] [CrossRef]
- Stanković, S.B.; Kyriacou, P.A. Improved measurement technique for the characterization of organic and inorganic phase change materials using the T-history method. Appl. Energy 2013, 109, 433–440. [Google Scholar] [CrossRef]
- Tan, P.; Brütting, M.; Vidi, S.; Ebert, H.P.; Johansson, P.; Jansson, H.; Kalagasidis, A.S. Correction of the enthalpy–temperature curve of phase change materials obtained from the T-History method based on a transient heat conduction model. Int. J. Heat Mass Transf. 2017, 105, 573–588. [Google Scholar] [CrossRef]
- Rathgeber, C.; Miró, L.; Cabeza, L.F.; Hiebler, S. Measurement of enthalpy curves of phase change materials via DSC and T-History: When are both methods needed to estimate the behaviour of the bulk material in applications? Thermochim. Acta 2014, 596, 79–88. [Google Scholar] [CrossRef]
- Solé, A.; Miró, L.; Barreneche, C.; Martorell, I.; Cabeza, L.F. Review of the T-history method to determine thermophysical properties of phase change materials (PCM). Renew. Sustain. Energy Rev. 2013, 26, 425–436. [Google Scholar] [CrossRef]
- Abhat, A. Low temperature latent heat thermal energy storage: Heat storage materials. Sol. Energy 1983, 30, 313–332. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, C.Y.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef]
- RUBITHERM-RT®, Technical Datasheets of RT24-RT25-RT26. Available online: https://www.rubitherm.eu/en/index.php/productcategory/organische-pcm-rt (accessed on 5 April 2020).
- PURETEMP 25, Technical Information, (2019 PURETEMP LLC). Available online: https://www.puretemp.com/stories/puretemp-technical-data-sheets (accessed on 5 April 2020).
- Standard DIN 51005. Thermal analysis (TA)—general Terms, German Institute for Standardisation. Available online: https://www.techstreet.com/standards/din-51005?product_id=2074265#jumps (accessed on 5 April 2020).
- Standard DIN 51007. Thermal analysis—Differential thermal analysis (DTA) and differential scanning calorimetry (DSC)—General Principles, German Institute for Standardisation. 2019. Available online: https://infostore.saiglobal.com/en-us/Standards/DIN-51007-2019-397024_SAIG_DIN_DIN_2718768/ (accessed on 5 April 2020).
- Gschwander, S.; Haussmann, T.; Hagelstein, G.; Sole, A.; Hohenauer, W.; Lager, D.; Mehling, H. Standard to determine the heat storage capacity of PCM using hf-DSC with constant heating/cooling rate (dynamic mode)— A technical report of subtask A2.1 of IEA-SHC 42 / ECES Annex 29; IEA SHC Task 42 / ECES Annex 29, DSC 4229 PCM Standard; IEA SHC: Paris, France, 2015. [Google Scholar]
- ISO 11357-3:2018. Plastics—Differential scanning calorimetry (DSC)—Part 3: Determination of temperature and enthalpy of melting and crystallization. March 2018. Available online: https://www.iso.org/standard/72460.html (accessed on 5 April 2020).
- Mankel, C.; Caggiano, A.; Koenders, E. Thermal energy storage characterization of cementitious composites made with Recycled Brick Aggregates containing PCM. Energy Build. 2019, 202, 109395. [Google Scholar] [CrossRef]
- Caggiano, A.; Mankel, C.; Koenders, E. Reviewing theoretical and numerical models for PCM-embedded cementitious composites. Buildings 2019, 9, 3. [Google Scholar] [CrossRef]
- Hiebler, S. Kalorimetrische Methoden zur Bestimmung der Enthalpie von Latentwärmespeichermaterialien während des Phasenübergangs. Ph.D. Thesis, Technische Universität München, Munich, Germany, 2007. [Google Scholar]
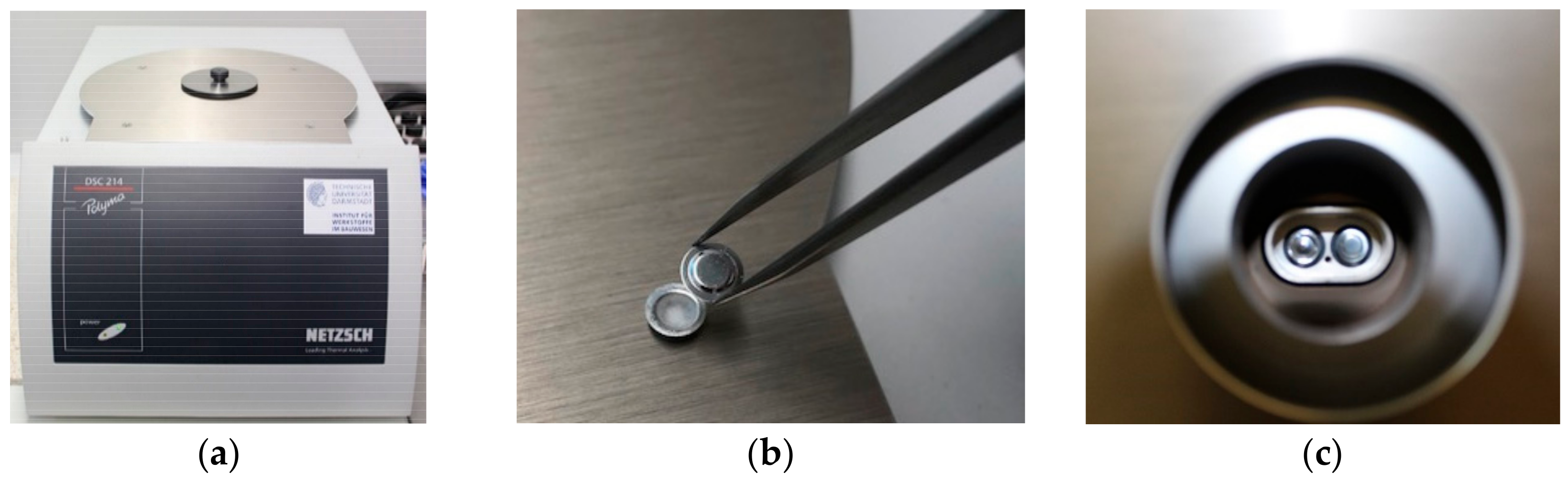
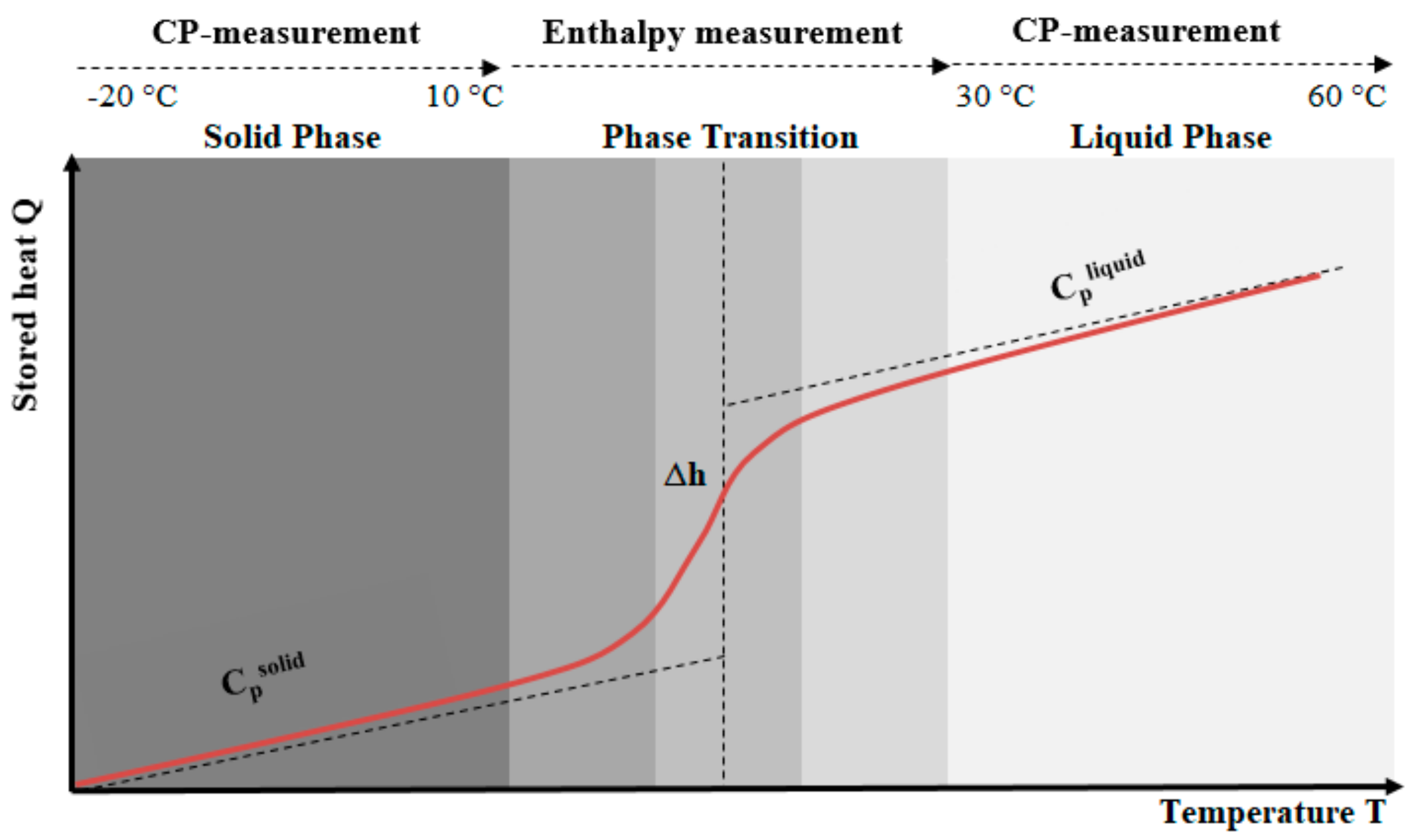

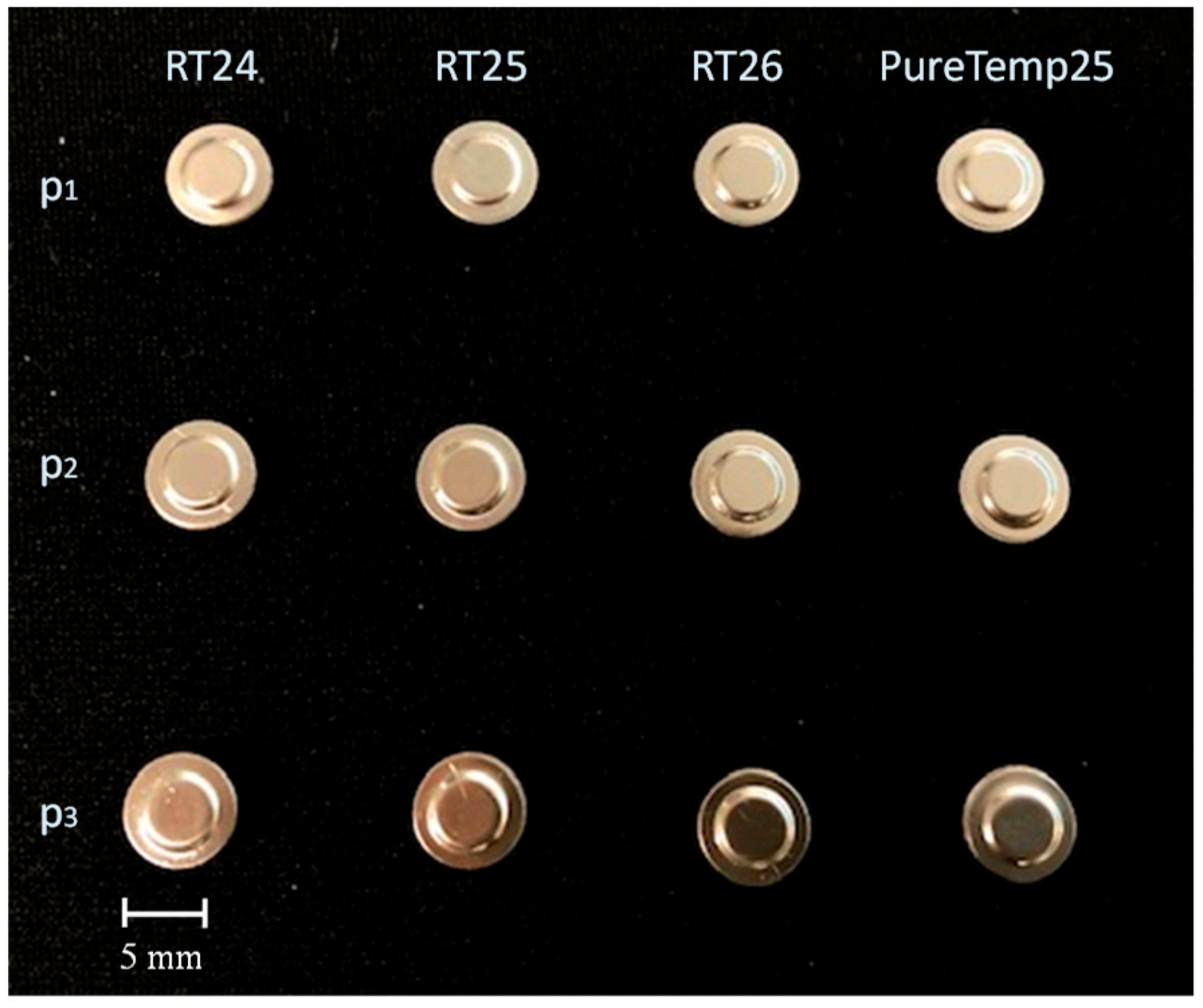






| Properties | RT24 | RT25 | RT26 | PureTemp25 |
|---|---|---|---|---|
| Tm (main peak) [°C] | 24 | 25 | 26 | 25 |
| Density liquid [kg/L] | 0.77 (40 °C) | 0.76 (at 40 °C) | 0.75 (at 30 °C) | 0.86 |
| Density solid [kg/L] | 0.88 (15 °C) | 0.88 (at 15 °C) | 0.90 (at 20 °C) | 0.95 |
| Specific Heat Capacity (liquid) [kJ/kg×K] | - | - | - | 2.29 |
| Specific Heat Capacity (solid) [kJ/kg×K] | - | - | - | 1.99 |
| Spec. Heat Capacity [kJ/kg×K] | 2 | 2 | 2 | - |
| Heat storage capacity [kJ/kg] | 160 (16–31 °C) | 170 (16–31 °C) | 180 (19–34 °C) | 187 * |
| Tests | Materials (Mass Values in mg) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT24 | RT25 | RT26 | PureTemp25 | |||||||||||||
| Dynamic Heat Rate (R), (R: 10, 5, 2, 1, 0.5, 0.25, 0.125 K/min) | P1 | P3 | P3 | P1 | P2 | P3 | P4 | P1 | P2 | P3 | P1 | P2 | P3 | P4 | ||
| ✔ | - | - | ✔ | - | - | - | ✔ | - | - | ✔ | - | - | - | |||
| 18.8 | 17.2 | 17.7 | 18.9 | |||||||||||||
| Dynamic Sample Mass (R: 0.125, 0.25 K/min) | - | - | - | ✔ | ✔ | ✔ | ✔ | - | - | - | ✔ | ✔ | ✔ | ✔ | ||
| 16.3 | 18.4 | 18 | 7.9 | 18.9 | 19 | 17.7 | 10.1 | |||||||||
| Dynamic - Enthalpy Temperature h (T) (R: 0.125 K/min) | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | - | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | - | ||
| 13.3 | 16.5 | 16.9 | 16.3 | 18.4 | 18 | 17.7 | 18.5 | 16.1 | 18.9 | 19 | 17.7 | |||||
| Dynamic - Heat Capacity cp (T) (R: 10 K/min) | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | - | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | - | ||
| 14.3 | 16 | 16.1 | 17.2 | 18.4 | 18 | 16.4 | 18.9 | 12.4 | 10.5 | 18.9 | 18 | |||||
| Step-wise Heat Rate (R:2, 1, 0.5, 0.25, 0.125 K/min) | - | - | - | ✔ 16.3 | - | - | - | - | - | - | ✔ 18.9 | - | - | - | ||
| Step-wise - Enthalpy Temperature h (T) (R: VAR) | - | - | - | ✔ 16.3 | - | - | - | - | - | - | ✔ 18.9 | - | - | - | ||
| PCM Type | RT24 R 0.250 K/min | RT25 0.125 K/min | RT26 0.250 K/min | PureTemp 25 0.125 K/min | ||||
|---|---|---|---|---|---|---|---|---|
| Tonset, heating | Tonset, cooling | Tonset, heating | Tonset, heating | Tonset, heating | Tonset, heating | Tonset, heating | Tonset, heating | |
| P1,2,3 | 3.0 | 24.3 | 15.5 | 25.7 | 15.6 | 25.1 | 23.0 | 21.9 |
| Temp. Range [°C] | RT25 (Mass: 16.3 mg) | PureTemp25 (Mass: 18.9 mg) | ||
|---|---|---|---|---|
| ∆q (total: S + L) | ∆q (L) | ∆q (total: S + L) | ∆q (L) | |
| [J/g] | [J/g] | [J/g] | [J/g] | |
| 15 to 16 | 3.03 | 0.93 | - | - |
| 16 to 17 | 10.70 | 8.60 | - | - |
| 17 to 18 | 25.33 | 23.23 | - | - |
| 18 to 19 | 16.96 | 14.86 | - | - |
| 19 to 20 | 7.77 | 5.67 | - | - |
| 20 to 21 | 6.77 | 4.67 | - | - |
| 21 to 22 | 8.26 | 6.16 | 4.48 | 1.59 |
| 22 to 23 | 12.18 | 10.08 | 8.30 | 5.41 |
| 23 to 24 | 17.23 | 15.13 | 28.39 | 25.50 |
| 24 to 25 | 28.13 | 26.03 | 48.16 | 45.27 |
| 25 to 26 | 93.95 | 91.85 | 53.53 | 50.64 |
| 26 to 27 | - | - | 62.56 | 59.67 |
| 27 to 28 | - | - | 22.32 | 19.43 |
| - | 230.31 | 207.21 | 227.74 | 207.48 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazari Sam, M.; Caggiano, A.; Mankel, C.; Koenders, E. A Comparative Study on the Thermal Energy Storage Performance of Bio-Based and Paraffin-Based PCMs Using DSC Procedures. Materials 2020, 13, 1705. https://doi.org/10.3390/ma13071705
Nazari Sam M, Caggiano A, Mankel C, Koenders E. A Comparative Study on the Thermal Energy Storage Performance of Bio-Based and Paraffin-Based PCMs Using DSC Procedures. Materials. 2020; 13(7):1705. https://doi.org/10.3390/ma13071705
Chicago/Turabian StyleNazari Sam, Mona, Antonio Caggiano, Christoph Mankel, and Eddie Koenders. 2020. "A Comparative Study on the Thermal Energy Storage Performance of Bio-Based and Paraffin-Based PCMs Using DSC Procedures" Materials 13, no. 7: 1705. https://doi.org/10.3390/ma13071705
APA StyleNazari Sam, M., Caggiano, A., Mankel, C., & Koenders, E. (2020). A Comparative Study on the Thermal Energy Storage Performance of Bio-Based and Paraffin-Based PCMs Using DSC Procedures. Materials, 13(7), 1705. https://doi.org/10.3390/ma13071705






