Effects of Boric Acid Ester Modified Magnesium Borate Whisker on the Mechanical Properties and Crystallization Kinetics of Polypropylene Composites
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Composites Preparation
2.3. Mechanical Property Testing
2.4. DSC Tests
3. Results and Discussion
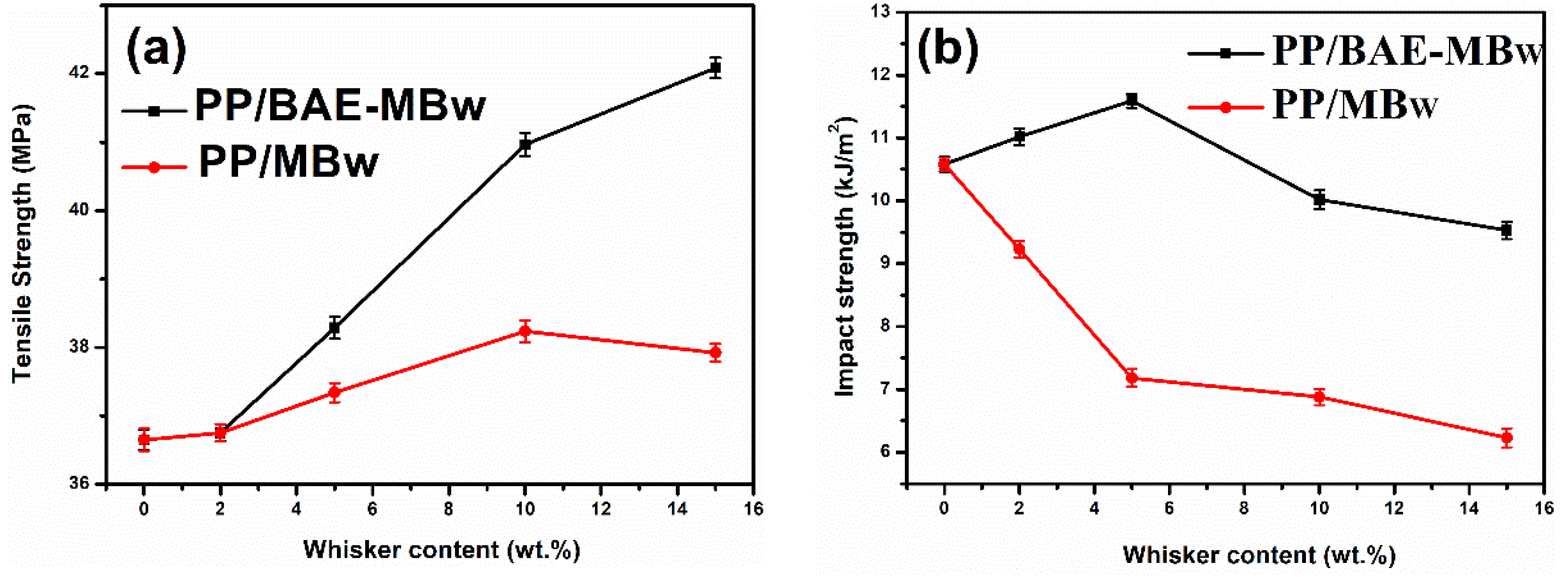
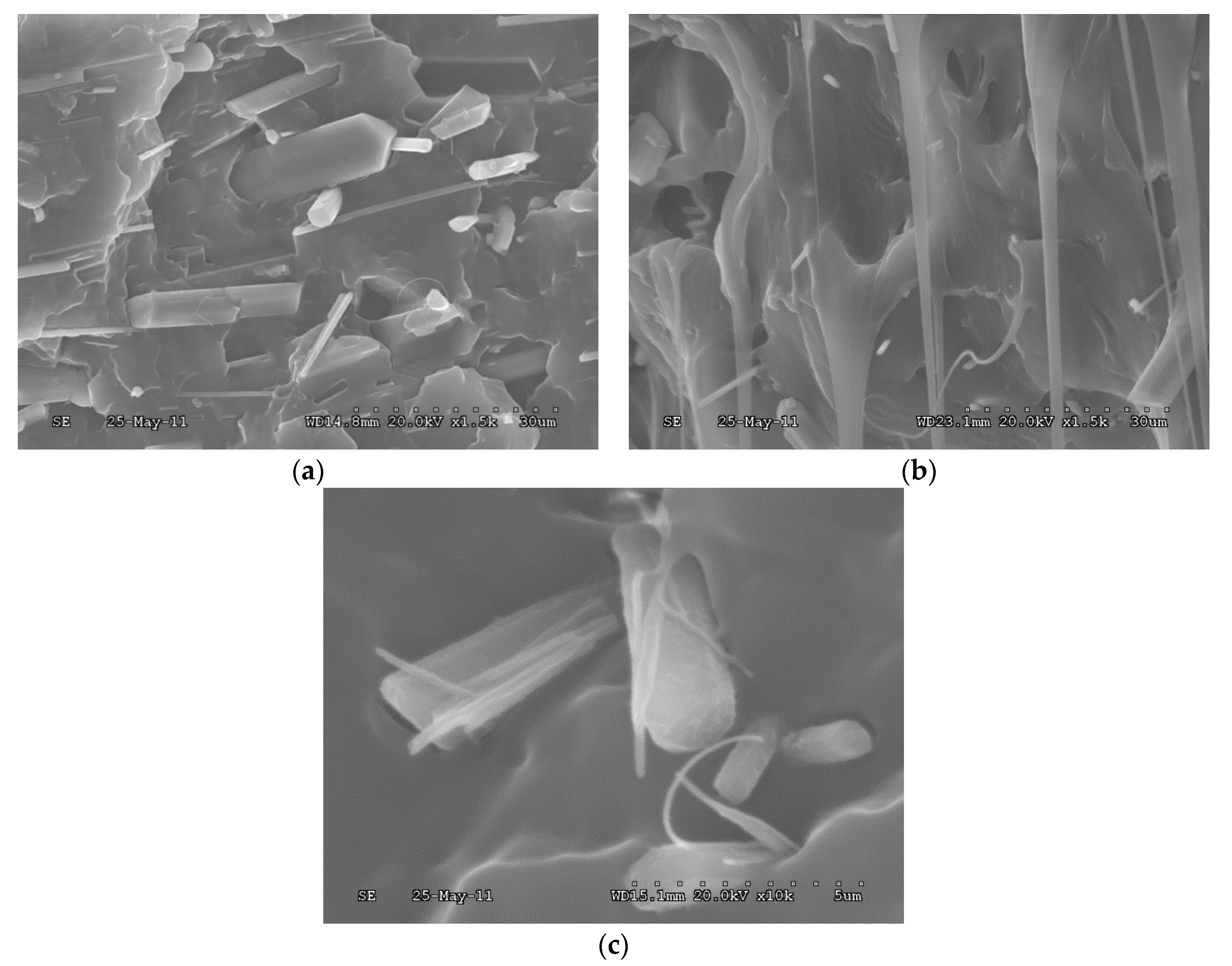
3.1. Mechanical Properties
3.2. Crystallization Behavior
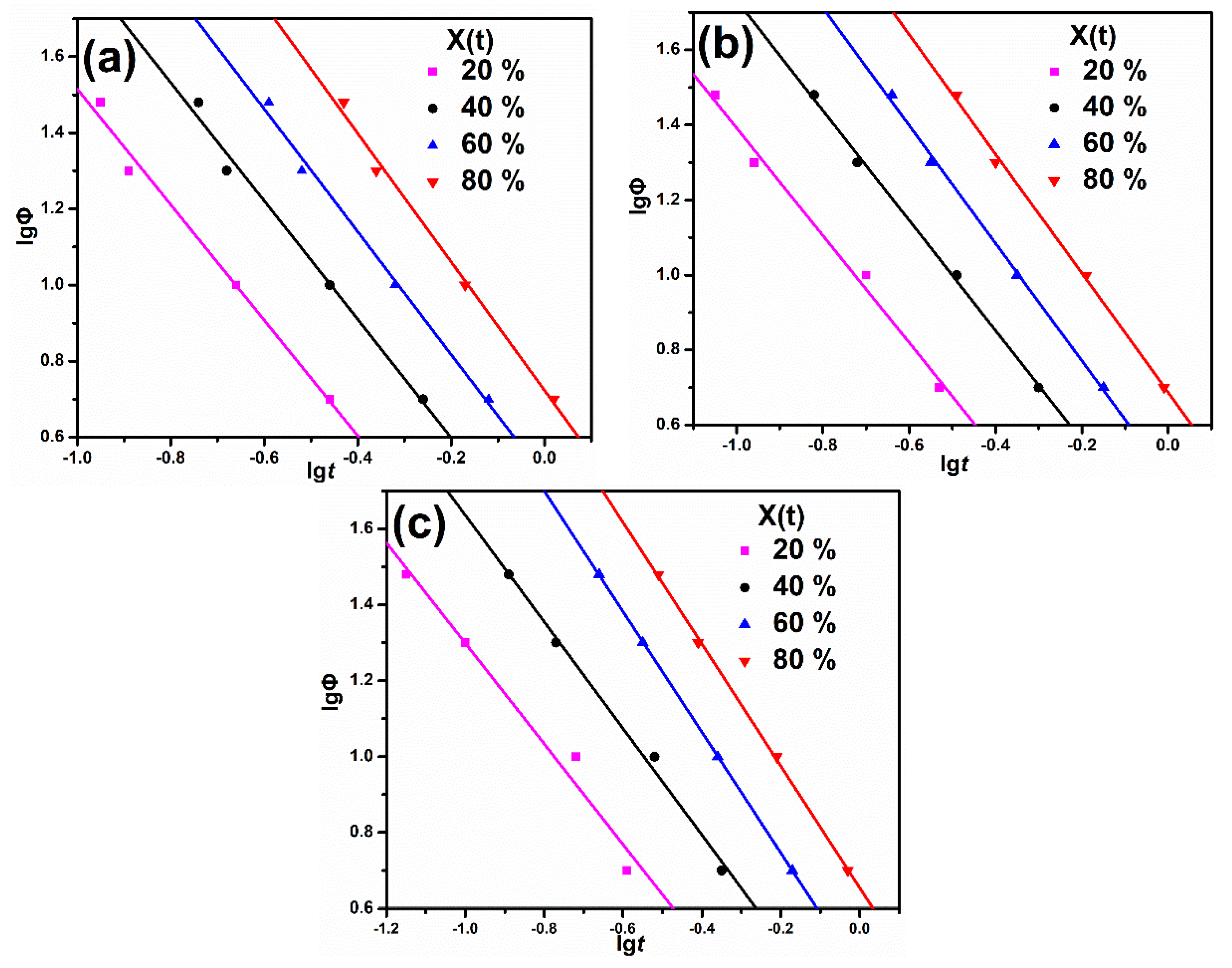
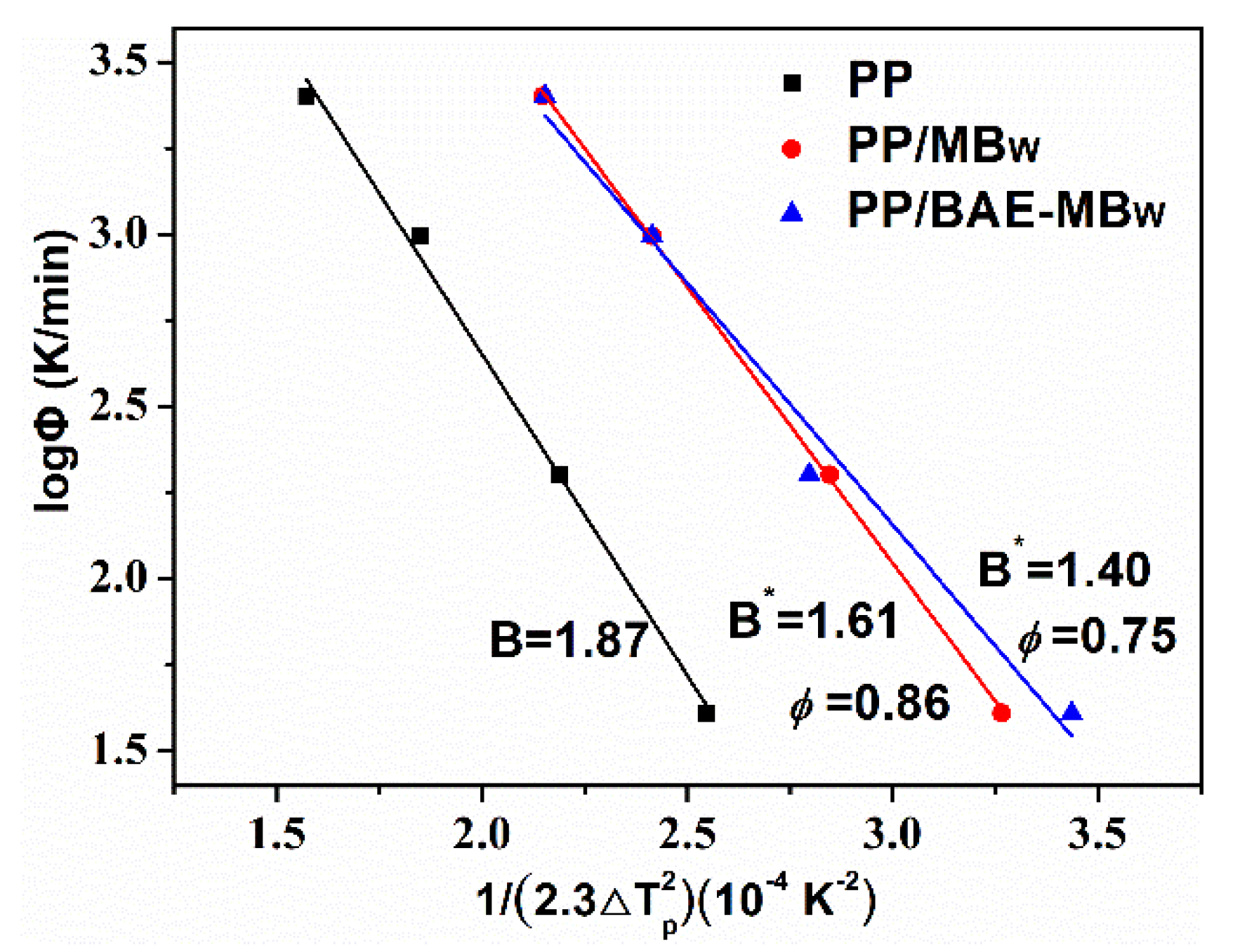
3.3. Non-Isothermal Crystallization Analysis
4. Conclusions
- The tensile strength and impact strength of PP/BAE-MBw are better than those of PP/MBw, which may be due to the more compatible interface between BAE-MBw and PP matrix.
- The Mo and Kissinger models were found to describe the non-isothermal crystallization kinetics of PP, PP/MBw, and PP/BAE-MBw fairly well. The range of α values for virgin PP, PP/MBw, and PP/BAE-MBw are 1.52−1.69, 1.53−1.59, and 1.32−1.61, respectively. The crystallization rate of PP/BAE-MBw is the fastest among virgin PP, PP/MBw, and PP/BAE-MBw, which is in line with the values of t1/2. The Kissinger activation energy of PP, PP/MBw, and PP/BAE-MBw was calculated as 190.20 kJ/mol, 216.59 kJ/mol, and 258.98 kJ/mol, respectively. Thus, the MBw, especially for BAE-MBw, is an effective nucleating agent for PP matrix, and exhibits high nucleation activity in the hybrid melts.
Author Contributions
Funding
Conflicts of Interest
References
- Manias, E.; Touny, A.; Wu, L.; Strawhecker, K.; Lu, B.; Chung, T.C. Polypropylene/montmorillonite nanocomposites. Review of the synthetic routes and materials properties. Chem. Mater. 2001, 13, 3516–3523. [Google Scholar] [CrossRef]
- Shubhra, Q.T.H.; Alam, A.K.M.M.; Quaiyyum, M.A. Mechanical properties of polypropylene composites: A review. J. Thermoplast. Compos. 2013, 26, 362–391. [Google Scholar] [CrossRef]
- Olewnik, E.; Garman, K.; Czerwinski, W. Thermal properties of new composites based on nanoclay, polyethylene and polypropylene. J. Therm. Anal. Calorim. 2010, 101, 323–329. [Google Scholar] [CrossRef]
- Liang, J.Z.; Li, R.K.Y. Rubber toughening in polypropylene: A review. J. Appl. Polym. Sci. 2000, 77, 409–417. [Google Scholar] [CrossRef]
- Zhang, S.; Horrocks, A.R. A review of flame retardant polypropylene fibres. Prog. Polym. Sci. 2003, 28, 1517–1538. [Google Scholar] [CrossRef]
- Teh, J.W.; Rudin, A.; Keung, J.C. A review of polyethylene-polypropylene blends and their compatibilization. Adv. Polym. Tech. 1994, 13, 1–23. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, W.; Chen, G.; Liang, J.; Yang, S.; Wang, P.; Liu, L. Crystallization and melting behavior of isotactic polypropylene nucleated with individual and compound nucleating agents. J. Therm. Anal. Calorim. 2010, 102, 775–783. [Google Scholar] [CrossRef]
- Avella, M.; Martuscelli, E.; Raimo, M.; Partch, R.; Gangolli, S.G.; Pascucci, B. Polypropylene reinforced with silicon carbide whiskers. J. Mater. Sci. 1997, 32, 2411–2416. [Google Scholar] [CrossRef]
- Chen, L.F.; Leonelli, C. Alignment of silicon carbide whiskers in polymer matrix. J. Mater. Sci. 1997, 32, 627–631. [Google Scholar] [CrossRef]
- Jubsilp, C.; Takeichi, T.; Hiziroglu, S.; Rimdusit, S. Effect of resin compositions on microwave processing and thermophysical properties of benzoxazine-epoxy-phenolic ternary systems filled with silicon carbide (SiC) whisker. Polym. Eng. Sci. 2009, 49, 1022–1029. [Google Scholar] [CrossRef]
- Tang, Y.S.; Liang, G.Z.; Zhang, Z.P.; Han, J. Performance of aluminum borate whisker reinforced cyanate ester resin. J. Appl. Polym. Sci. 2007, 106, 4131–4137. [Google Scholar] [CrossRef]
- Liang, G.Z.; Hu, X.L. Aluminum-borate-whiskers-reinforced bismaleimide composites. 1: Preparation and properties. Polym. Int. 2004, 53, 670–674. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Sun, Y.; Shang, Y.; Jiang, B.; Zhang, H.; Jiang, Z.H. Aluminium borate whiskers grafted with boric acid containing poly (ether ether ketone) as a reinforcing agent for the preparation of poly (ether ether ketone) composites. RSC Adv. 2015, 5, 100856–100864. [Google Scholar] [CrossRef]
- Tjong, S.C.; Meng, Y.Z. Mechanical and thermal properties of polycarbonate composites reinforced with potassium titanate whiskers. J. Appl. Polym. Sci. 1999, 72, 501–508. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Q.; Wang, T.; Pei, X. Preparation, damping and thermal properties of potassium titanate whiskers filled castor oil-based polyurethane/epoxy interpenetrating polymer network composites. Mater. Design 2011, 32, 803–807. [Google Scholar] [CrossRef]
- Tjong, S.C.; Meng, Y.Z. Properties and morphology of polyamide 6 hybrid composites containing potassium titanate whisker and liquid crystalline copolyester. Polymer 1999, 40, 1109–1117. [Google Scholar] [CrossRef]
- Kardos, J.L. The role of the interface in polymer composites-some myths, mechanisms, and modifications. In Molecular Characterization of Composite Interfaces; Springer: Berlin/Heidelberg, Germany, 1985; pp. 1–11. [Google Scholar]
- Tang, L.G.; Kardos, J.L. A review of methods for improving the interfacial adhesion between carbon fiber and polymer matrix. Polym. Compos. 1997, 18, 100–113. [Google Scholar] [CrossRef]
- Shokoohi, S.; Arefazar, A.; Khosrokhavar, R. Silane coupling agents in polymer-based Reinforced Composites: A review. J. Reinf. Plast. Comp. 2008, 27, 473–485. [Google Scholar] [CrossRef]
- Phuong, N.T.; Gilbert, V. Non-isothermal crystallization kinetics of short bamboo fiber-reinforced recycled polypropylene composites. J. Reinf. Plast. Comp. 2010, 29, 2576–2591. [Google Scholar] [CrossRef]
- Chen, S.H.; Han, S.H.; Chen, Y.H.; Xue, D.P.; Zhu, X.D. Surface modification of Mg2B2O5 whisker and its effect on the mechanical properties and thermostability of polycarbonate/MBw composites. Compos. Interface 2018, 25, 1019–1038. [Google Scholar] [CrossRef]
- Mo, Z.S. A method for the nonisothermal crystallization kinetics of polymers. Acta Polym. Sin. 2008, 8, 656–660. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. Ι general theory. J. Chem. Phy. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II transformation-time relations for random distribution of nuclei. J. Chem. Phy. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, phase change, and microstructure kinetics of phase change. III. J. Chem. Phy. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Ma, L.C.; Li, L.P.; Guo, C.G. Influence of m-isopropenyl-α, α-dimethylbenzyl isocyanate and styrene on non-isothermal crystallization behavior of polypropylene. J. Therm. Anal. Calorim. 2010, 101, 1101–1109. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Dobreva, A.; Gutzow, I. Activity of substrates in the catalyzed nucleation of glass-forming melts. I. Theory. J. Non-Cryst. Solids 1993, 162, 1–12. [Google Scholar] [CrossRef]
- Dobreva, A.; Gutzow, I. Activity of substrates in the catalyzed nucleation of glass-forming melts. II. experimental evidence. J. Non-Cryst. Solids 1993, 162, 13–25. [Google Scholar] [CrossRef]
- Ariffin, A.; Ariff, Z.M.; Jikan, E.E. Evaluation on nonisothermal crystallization kinetics of polypropylene/kaolin composites by employing Dobreva and Kissinger methods. J. Therm. Anal. Calorim. 2011, 103, 171–177. [Google Scholar] [CrossRef]
- Liu, J.Y.; Reni, L.; We, Q.; Wu, J.L.; Liu, S.; Wang, Y.J.; Li, G.Y. Fabrication and characterization of polycaprolactone/calcium sulfate whisker composites. Express Polym. Lett. 2011, 5, 742–752. [Google Scholar] [CrossRef]
- Tjong, S.C.; Jiang, W. Mechanical and thermal behavior of polycarbonate composites reinforced with aluminum borate whiskers. J. Appl. Polym. Sci. 1999, 73, 2247–2253. [Google Scholar] [CrossRef]
- Gu, H.; Gu, Y.; Wong, S.Y. Effect of interphase and strain-rate on the tensile properties of polyamide 6 reinforced with functionalized silica nanoparticles. Compos. Sci. Technol. 2013, 75, 62–69. [Google Scholar] [CrossRef]
- Mucha, M.; Królikowski, Z. Application of DSC to study crystallization kinetics of polypropylene containing fillers. J. Therm. Anal. Calorim. 2003, 74, 549–557. [Google Scholar] [CrossRef]
- Jiang, C.; Zhao, S.C.; Xin, Z. Influence of a novel beta-nucleating agent on the structure, mechanical properties, and crystallization behavior of isotactic polypropylene. J. Thermoplast. Compos. 2015, 28, 610–629. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, J.; Yang, H.; Lin, Z.; Tan, S. Mechanical properties, morphology, thermal performance, crystallization behavior, and kinetics of pp/microcrystal cellulose composites compatibilized by two different compatibilizers. J. Thermoplast. Compos. 2011, 24, 735–753. [Google Scholar]
- Zaman, H.U.; Hun, P.D.; Khan, R.A.; Yoon, K.B. Effect of surface-modified nanoparticles on the mechanical properties and crystallization behavior of PP/CaCO3 nanocomposites. J. Thermoplast. Compos. 2013, 26, 1057–1070. [Google Scholar] [CrossRef]
- Sahoo, R.K.; Mohanty, S.; Nayak, S.K. Effect of nanoclay on the nucleation, crystallization and melting behaviour of polypropylene: A study on non-isothermal crystallization kinetics. J. Thermoplast. Compos. 2015, 29, 1554–1572. [Google Scholar] [CrossRef]
- Nagarajan, K.; Levon, K.; Myerson, A.S. Nucleating agents in polypropylene. J. Therm. Anal. Calorim. 2000, 59, 497–508. [Google Scholar] [CrossRef]
- Borysiak, S. Determination of nucleating ability of wood for non-isothermal crystallisation of polypropylene. J. Therm. Anal. Calorim. 2007, 88, 455–462. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, Z.; Chen, C.; Li, M.; Tan, Y.; Mai, K. Non-isothermal crystallization kinetics of montmorillonite filled β-isotactic polypropylene nanocomposites. J. Therm. Anal. Calorim. 2015, 121, 829–838. [Google Scholar] [CrossRef]
- Vaaben, S.R.; Aguilar, A.; Avalos, F.; Valle, L.F.R. Carbon nanoparticles as effective nucleating agents for polypropylene. J. Therm. Anal. Calorim. 2008, 93, 947–952. [Google Scholar] [CrossRef]
- Li, J.; Zhou, C.; Gang, W. Study on nonisothermal crystallization of maleic anhydride grafted polypropylene/montmorillonite nanocomposite. Polym. Test. 2003, 22, 217–223. [Google Scholar] [CrossRef]
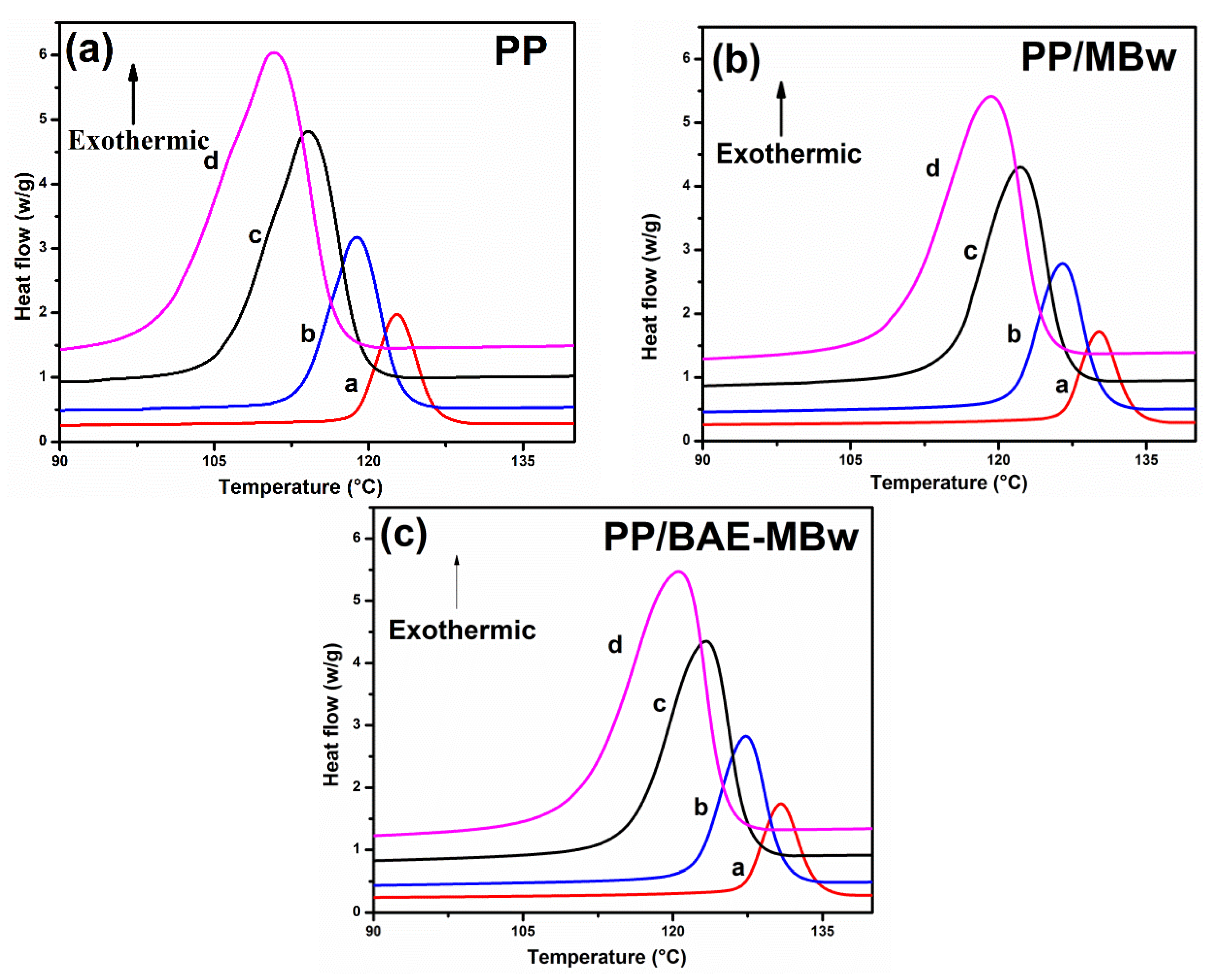
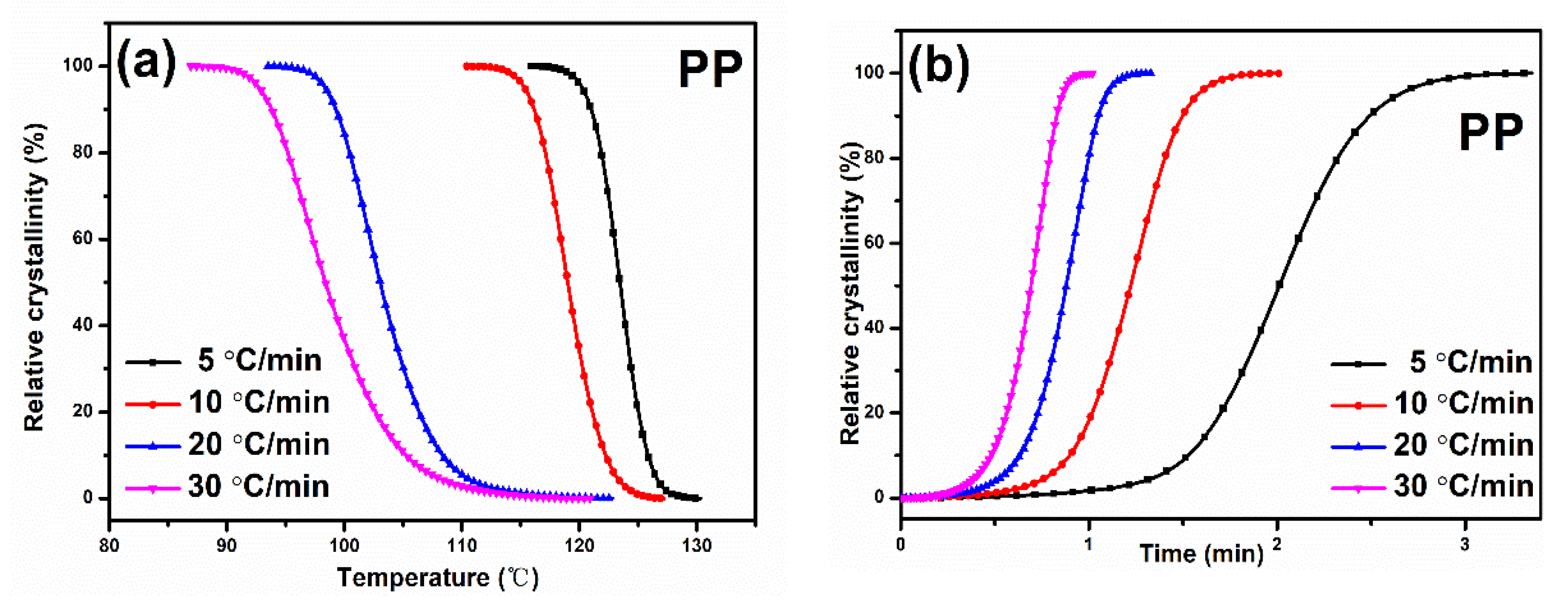

| Sample | Φ/°C∙min−1 | ∆HC/J∙g−1 | T0/°C | TP/°C | (T0 − TP)/°C | t1/2/min | Tm/°C |
|---|---|---|---|---|---|---|---|
| PP | 5 | 102.17 | 126.43 | 122.80 | 3.63 | 2.01 | 164.12 |
| 10 | 99.51 | 123.07 | 118.90 | 4.17 | 1.22 | 163.46 | |
| 20 | 98.70 | 119.23 | 114.15 | 5.08 | 0.88 | 162.64 | |
| 30 | 95.48 | 116.64 | 110.79 | 5.85 | 0.69 | 163.37 | |
| PP/MBw | 5 | 85.98 | 133.73 | 130.21 | 3.52 | 1.52 | 165.78 |
| 10 | 82.91 | 130.39 | 126.49 | 3.90 | 1.16 | 165.92 | |
| 20 | 80.17 | 126.77 | 122.17 | 4.60 | 0.84 | 164.61 | |
| 30 | 77.46 | 124.45 | 119.24 | 5.21 | 0.71 | 164.18 | |
| PP/BAE-MBw | 5 | 87.42 | 134.30 | 130.83 | 3.47 | 1.38 | 167.32 |
| 10 | 86.45 | 131.05 | 127.34 | 3.71 | 0.99 | 166.42 | |
| 20 | 81.89 | 127.48 | 123.32 | 4.16 | 0.83 | 165.76 | |
| 30 | 78.71 | 125.18 | 120.49 | 4.69 | 0.61 | 165.49 |
| Sample | X(t)/% | α | F(T) | ΔE/kJ·mol−1 |
|---|---|---|---|---|
| PP | 20 | 1.52 | 0.99 | 190.20 |
| 40 | 1.56 | 1.95 | ||
| 60 | 1.61 | 3.16 | ||
| 80 | 1.69 | 5.28 | ||
| PP/MBw | 20 | 1.43 | 0.91 | 206.59 |
| 40 | 1.47 | 1.84 | ||
| 60 | 1.57 | 2.86 | ||
| 80 | 1.59 | 4.79 | ||
| PP/BAE-MBw | 20 | 1.32 | 0.92 | 218.98 |
| 40 | 1.40 | 1.70 | ||
| 60 | 1.59 | 2.68 | ||
| 80 | 1.61 | 4.47 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.-H.; Han, S.-H.; Wang, J.; Liu, H.; Zhu, X.-D.; Chen, S.-H. Effects of Boric Acid Ester Modified Magnesium Borate Whisker on the Mechanical Properties and Crystallization Kinetics of Polypropylene Composites. Materials 2020, 13, 1698. https://doi.org/10.3390/ma13071698
Luo J-H, Han S-H, Wang J, Liu H, Zhu X-D, Chen S-H. Effects of Boric Acid Ester Modified Magnesium Borate Whisker on the Mechanical Properties and Crystallization Kinetics of Polypropylene Composites. Materials. 2020; 13(7):1698. https://doi.org/10.3390/ma13071698
Chicago/Turabian StyleLuo, Jin-Hua, Shi-Hu Han, Juan Wang, Hui Liu, Xiao-Dong Zhu, and Shan-Hua Chen. 2020. "Effects of Boric Acid Ester Modified Magnesium Borate Whisker on the Mechanical Properties and Crystallization Kinetics of Polypropylene Composites" Materials 13, no. 7: 1698. https://doi.org/10.3390/ma13071698
APA StyleLuo, J.-H., Han, S.-H., Wang, J., Liu, H., Zhu, X.-D., & Chen, S.-H. (2020). Effects of Boric Acid Ester Modified Magnesium Borate Whisker on the Mechanical Properties and Crystallization Kinetics of Polypropylene Composites. Materials, 13(7), 1698. https://doi.org/10.3390/ma13071698





