Are Zirconia Bioceramics and Ceramics Intended to Come in Contact with Skin Inert?
Abstract
1. Introduction
- -
- oxides: aluminum oxide, zirconium oxide;
- -
- non-oxides: carbides, borides, nitrides, ceramics composed of silicate and atoms such as tungsten, magnesium, platinum, or even titanium;
- -
- composite ceramics: combination of oxides and non-oxides.
1.1. Dental Ceramics
1.2. CeramicsIntended to Come in Contact with Skin
2. Materials and Methods
2.1. Zirconia for Dental Applications
- ICP-OES: Al, Cr, Cu, Fe, Ni, P, S, Ti, V, Zn, As, Hg, Sb, Se.
- ICP-MS: Ba, Cd, Co, Hg, Li, Mo, Nb, Pb, Sb, Sn, Sr, Zn, Hf, Y, Zr.
- Fe (±22%), Cr, Cu, Mo, Ni, and Co (±17%) in 0.07 M HCl.
- Fe (±27%), Cr, Cu, Mo, Ni, and Co (±22%) in 0.1%NaF+0.1%KF.
2.2. Ceramics Intended to Come inContact with Skin
- -
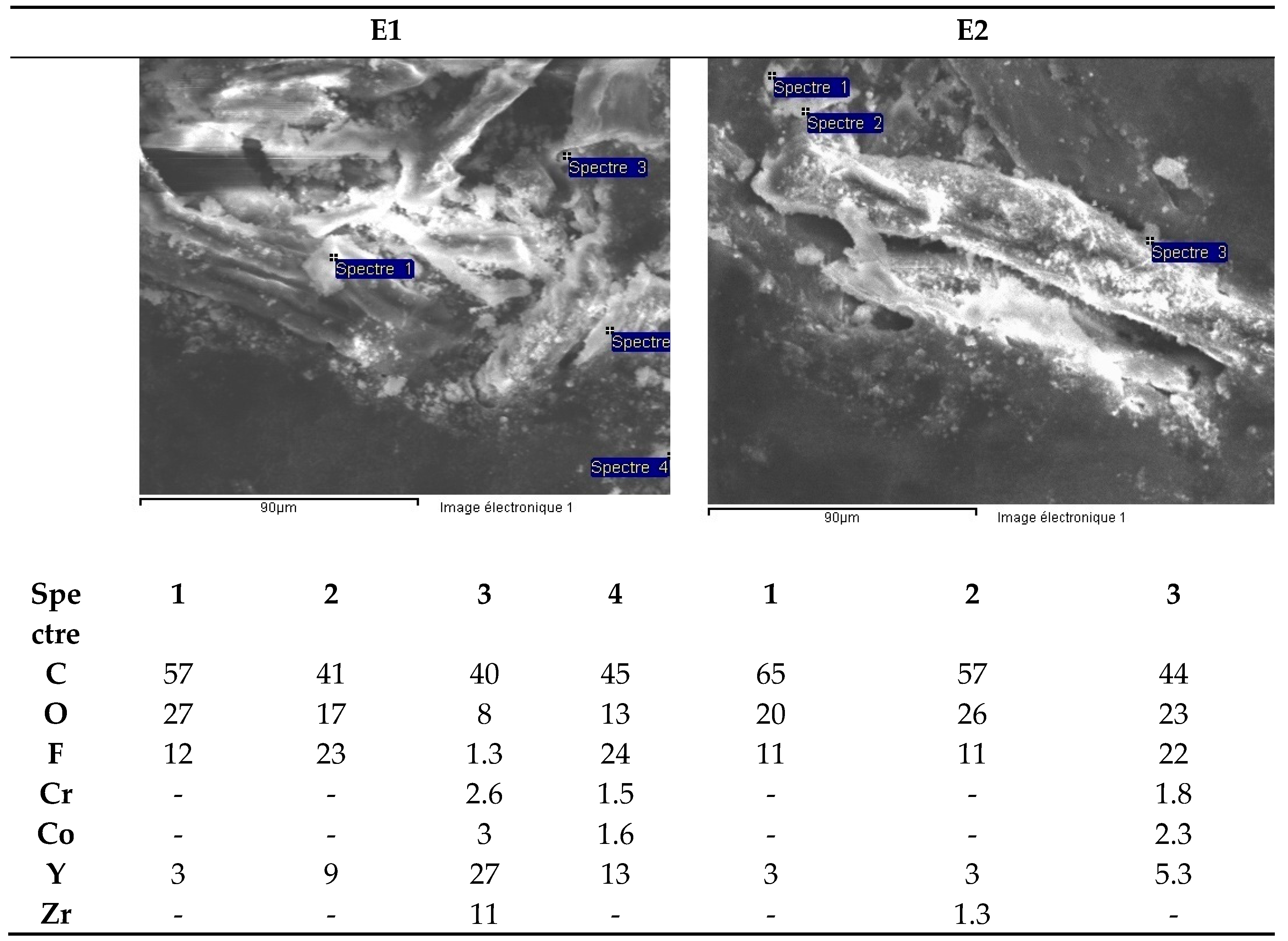
- dissolving ceramics in a mixture of HNO3 60% and HF 40% ultrapure (Figure 4).
- -
- extraction in appropriate media: HCl 0.07 M solution [31] and artificial sweat, followed by the analysis of the cations release by ICP-OES Optima 8300 (Perkin Elmer, Waltham, MA, USA) and ICP-MS Elan DRC (Perkin Elmer, Waltham, MA, USA).
- -
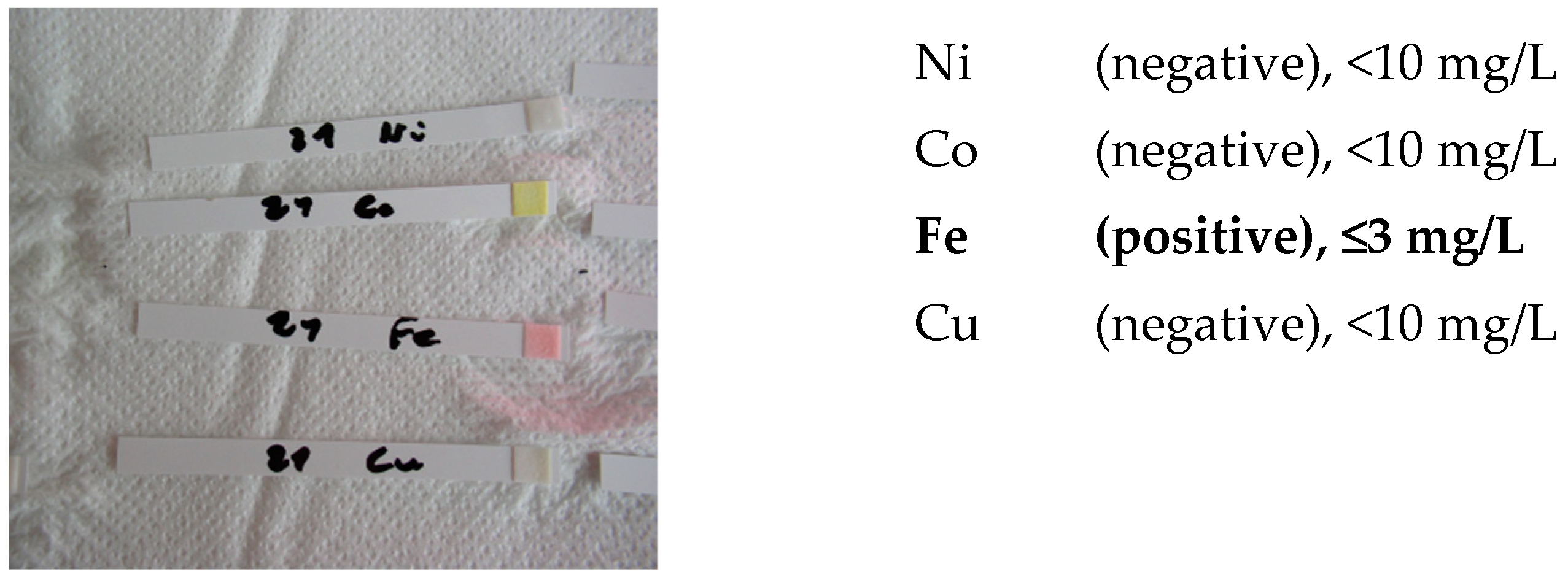
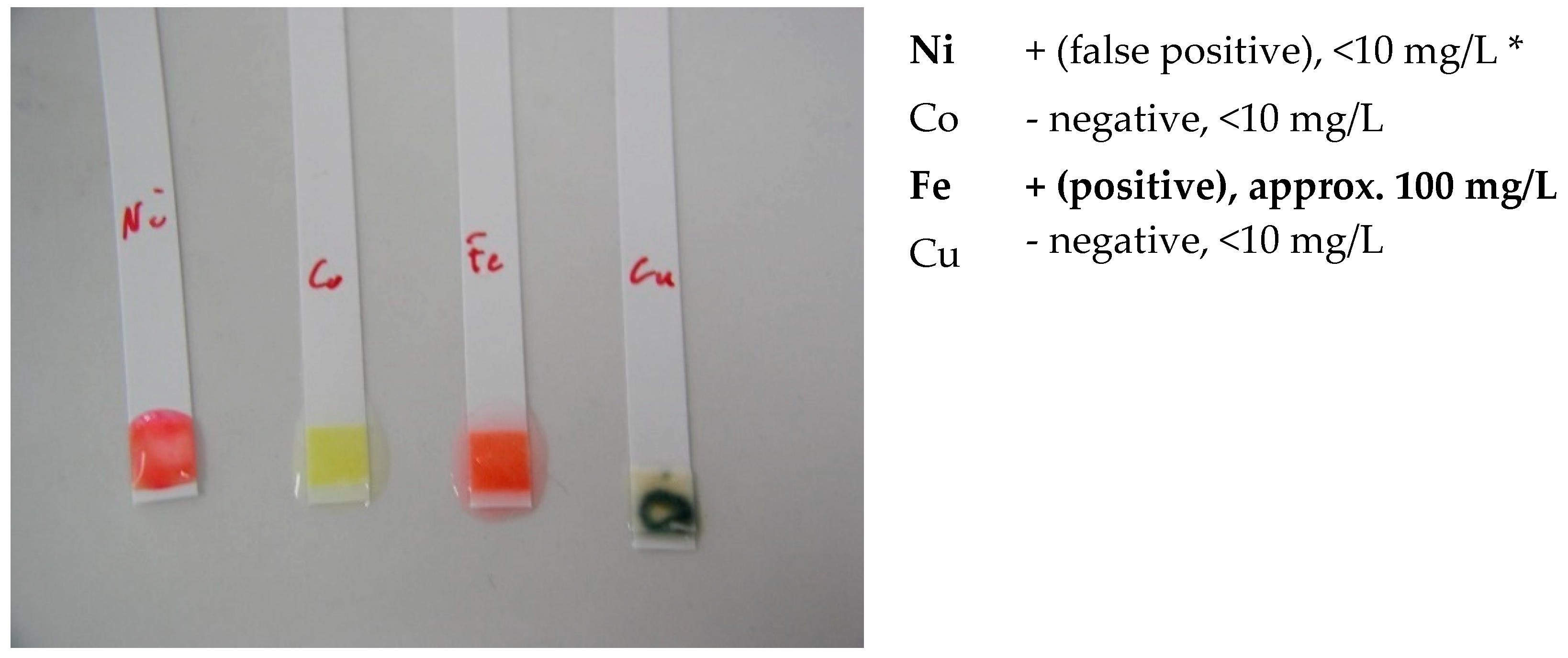
- screening identification test for Ni, Co, Cu and Fe with the strips type Merckoquant® (Merck, Kenilworth, NJ, USA).
- Ni (II) ions form a red complex with dimethylglyoxime. Measuring range: 10–500 mg/L.
- Co (II) ions form with the thiocyanate ions a blue complex. Measuring range: 10–1000 mg/L.
- Cu (II) ions are reduced by a mixture of reducers in copper (I) ions. These form a purple complex with 2,2′-biquinoline (cuproin). Measuring range: 10–300 mg/L.
- Fe (II) ions together with 2,2′-bipyridine form a red complex. Measuring range: 3–500 mg/L.
3. Results and Discussion
3.1. Zirconia for DentalApplications
3.2. Ceramics Intended to Come inContact with Skin
3.2.1. Cermet Ceramic
- -
- major elements: Ni, Cr and a lower concentration of Co and Fe (minor elements).
- -
- traces: some of which exceed 5 ppm (Hg, Te and Cu). The Hg concentration reaches 100 ppm and Cu 13 ppm.
3.2.2. Zirconia Ceramics for Watch making Applications: White Ceramic Watch Link and Black Ceramic Watch Band
- -
- elements: Hf (1.5%), Y (0.2% + precipitation as fluoride), Ta (about 50 mg/kg) and Nb (5 mg/kg).
- -
- toxic elements such as Te (0.02% = 200 mg/kg) and Ba (30 mg/kg) as well asAs, Pb and Cd with a concentration between 5 and 30 mg/kg.
- -
- among the rare earths, only Sm was measured in a concentration of about 7 mg/kg.
- -
- elements: Cr (0.1%), Ni (0.02%) and Co (0.02%) are present in the composition of the black ceramic watch band.
3.2.3. Black Zirconia Ceramic Bracelet Component
- -
- elements: Hf (3wt.%), Y (0.05wt.%+fluoride precipitation), Ta (aprox. 10 mg/kg) and Nb (4 mg/kg).During dissolution in the HF/HNO3 mixture, yttrium fluoride precipitates.
- -
- toxic elements such as Te (50 mg/kg) and Ba (10 mg/kg), as well asSm and Pb with a concentration of approx. 10 mg/kg.
- -
- elements: Cr (0.1%) and Co (0.5%) are present in the composition of the black zirconia ceramic bracelet component.
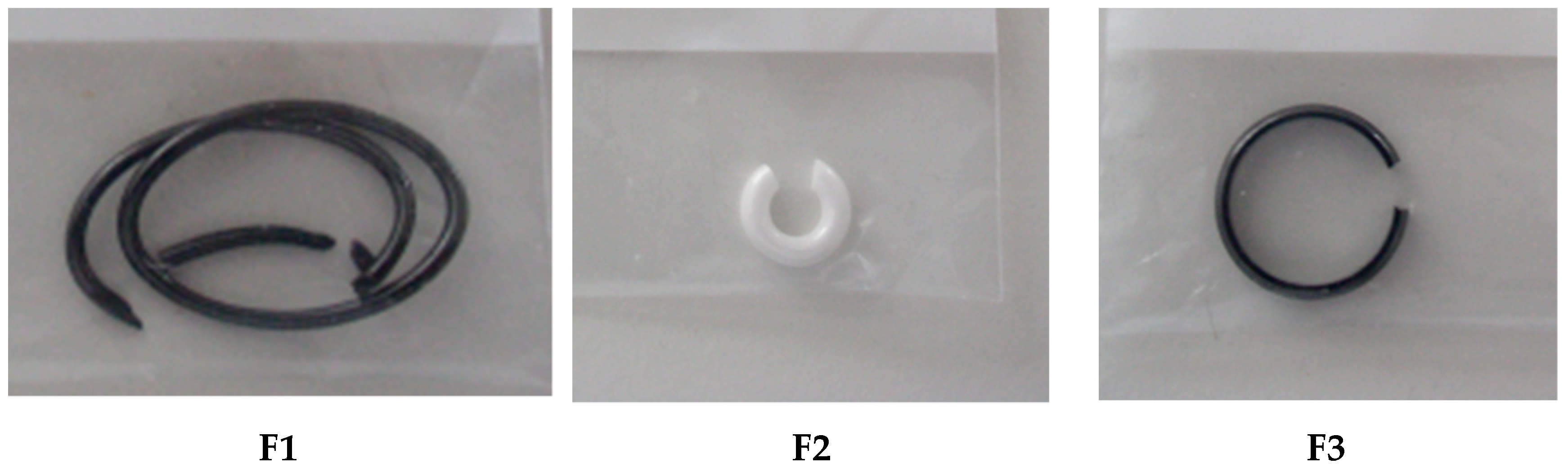
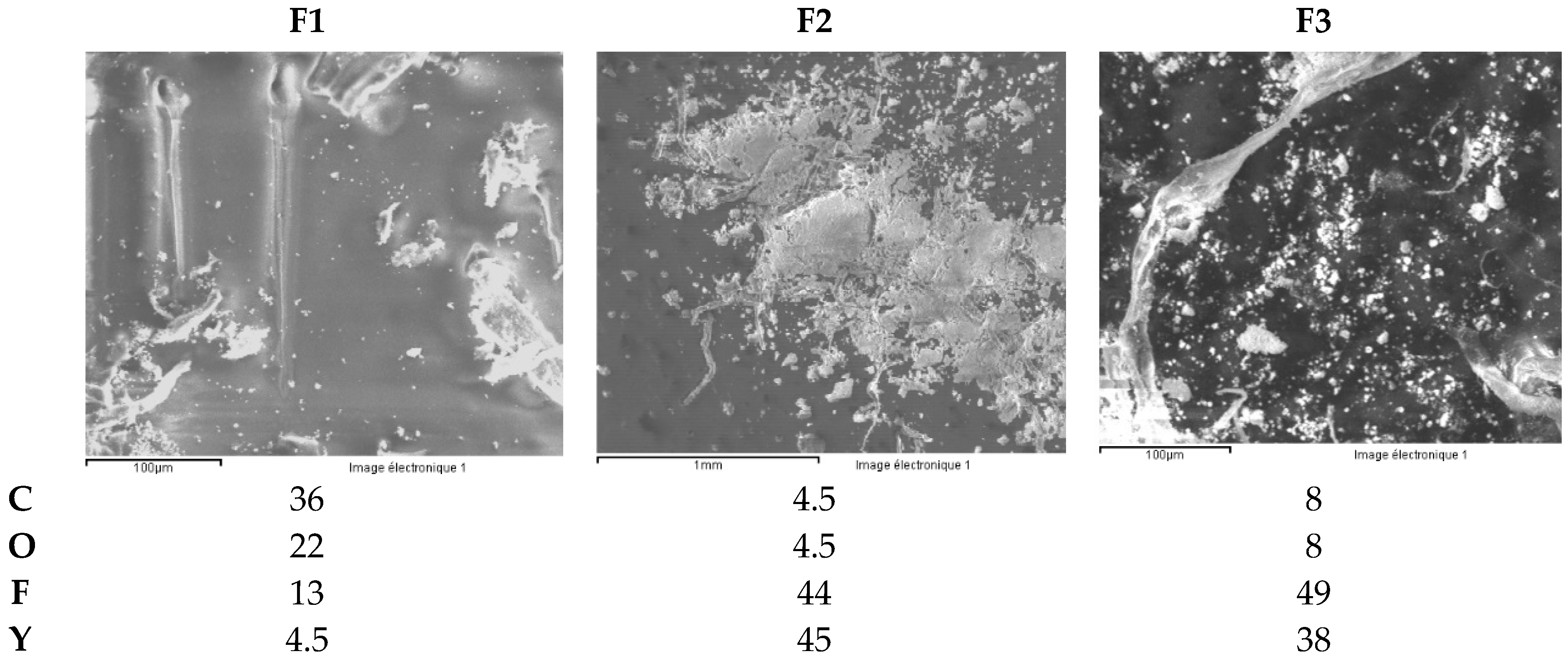
3.2.4. Zirconia Ceramic Rings, Jewelry Components, Two Black and One White (Figure 22)
- -
- elements: Hf, Y, Nb and Ta.
- -
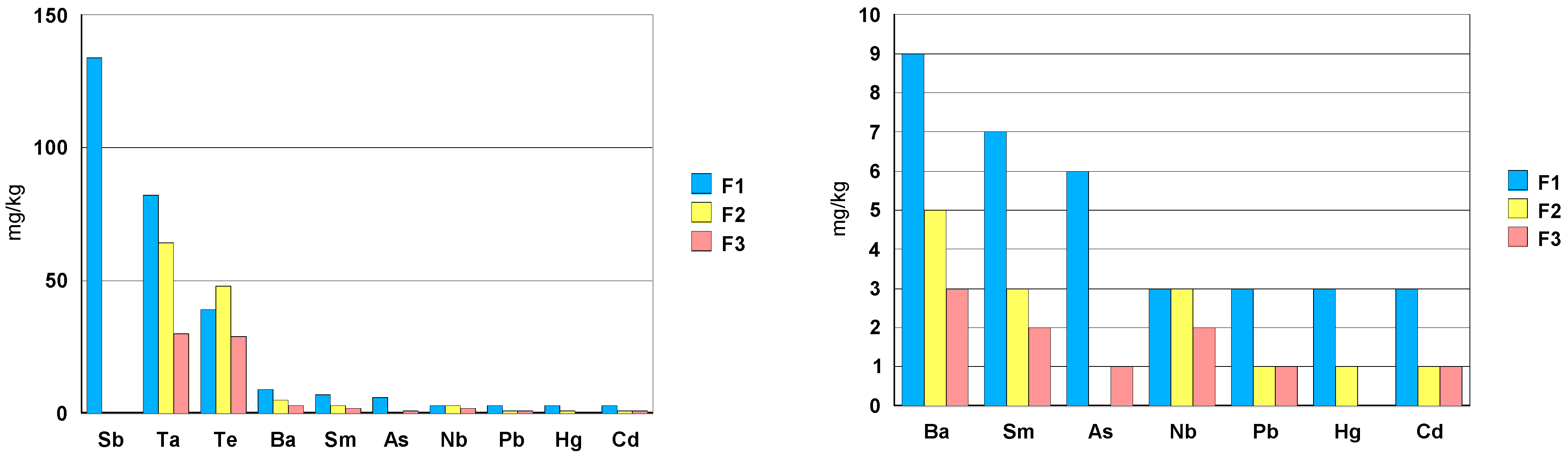
- toxic trace elements: Ba, As, Pb, Te, Cd and Hg in a concentration between 1mg/kg and 10 mg/kg.
- -
- ceramic F1 shows a Sb concentration of about 100 mg/kg.
- -
- among the rare earths, only Sm has been detected with a concentration of about 7 mg/kg.
- -
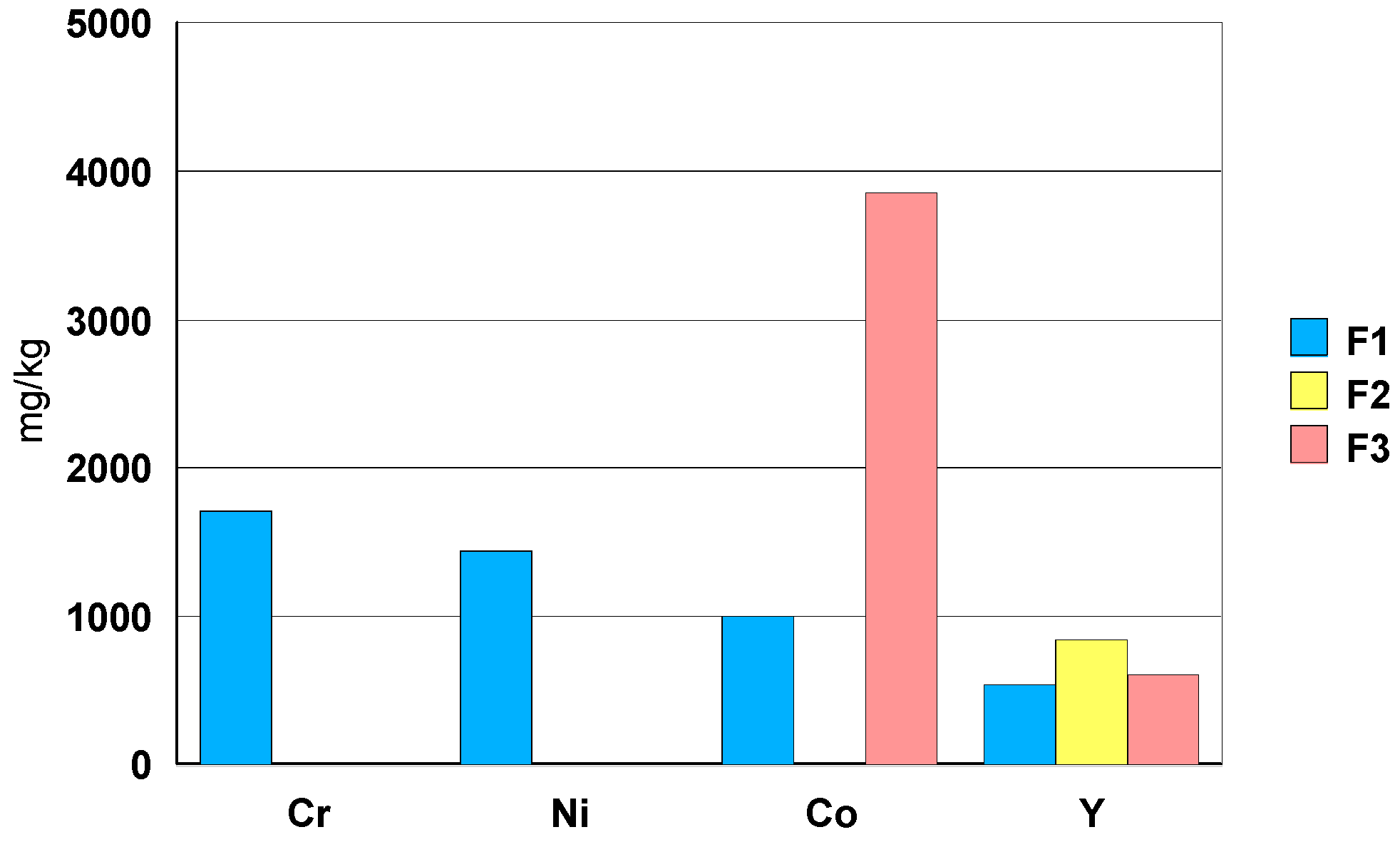
- in case of ceramic F1, the elements Cr, Ni and Co have a concentration order of 0.1wt.% (1000 mg/kg). Ceramic F3shows a Co concentration close to 0.4 wt.% (4000 mg/kg).
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- ASTM C242—19a Standard Terminology of Ceramic White Wares and Related Products. Available online: www.astm.org/Standards/C242.htm (accessed on 10 December 2019).
- CTIOA Tile Institute of America Ceramic, Tile and Stone Standards. Available online: www.ctioa.org (accessed on 10 December 2019).
- Buddy, D.; Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine, 3rd ed.; Academic Press, Elsevier: Amsterdam, The Netherlands, 2013; p. 144. [Google Scholar]
- Zhang, S. Biological and Biomedical Coatings Handbook: Processing and Characterization; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2011; p. 138. [Google Scholar]
- Haapasalo, M.; Parhar, M.; Huang, X.; Wei, X.; Lin, J.; Shen, Y. Clinical use of bioceramic materials. Endod. Top. 2015, 32, 97–117. [Google Scholar] [CrossRef]
- Kvam, K.; Karlsson, S. Solubilityand strength of zirconia-based dental materials after artificial aging. J. Prosthet. Dent. 2013, 110, 281–287. [Google Scholar] [CrossRef]
- Wen, J.; Chang, W.; Praisarnti, C.; Neelakantan, P. Increasing Use of Bioceramics in Endodontics: A Narrative Review. Oral Health 2018, 81, 1705–1728. [Google Scholar]
- Flinn, B.D.; de Groot, D.A.; Mancl, L.A.; Raigrodski, A.J. Accelerated aging characteristics of three yttria-stabilized tetragonal zirconia polycrystalline dental materials. J. Prosthet. Dent. 2012, 108, 223–230. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L.; Deville, S. Low temperature degradation of Zirconia and implications for biomedical implants. Annu. Rev. Mater. Res. 2007, 37, 1–32. [Google Scholar] [CrossRef]
- Dambreville, A.; Philippe, M.; Ray, A. Zirconia ceramics or by night, all cats are grey. Maitrise Orthop. 1999, 2, 56–63. [Google Scholar]
- Hohls, A.C. Investigation into Phase Transformation of Yttria Stabilized Zirconia Femoral Heads. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2011. [Google Scholar]
- CY3Z-R Grades. Available online: www.zirpro.com/sites/imdf.zirpro.com/files/documents/zirpro-tds-cy3z-r-zirconium_oxide-201904-e.pdf (accessed on 20 March 2020).
- 3 mol% Yttria-Stabilized Zirconia. Available online: www.zirpro.com/zirconia-beads-powders/3-mol-yttria-stabilized-zirconia (accessed on 10 December 2019).
- ISO 13356:2015 Implants for Surgery—Ceramic Materials Based on Yttria-Stabilized Tetragonal Zirconia (Y-TZP). Available online: www.iso.org/standard/62373.html (accessed on 10 December 2019).
- Zenostar, The Zirconia System. Available online: www.zenostar.de/en/dental-technician/zenostar-mt/ (accessed on 10 December 2019).
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/45/EC and Repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as Well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Available online: https://echa.europa.eu/regulations/reach/legislation (accessed on 10 December 2019).
- Graedel, T.E.; Harper, E.M.; Nassar, N.T.; Nuss, P.; Reck, B.K. Criticality of metals and metalloids. Proc. Natl. Acad. Sci. USA 2015, 14, 4257–4262. [Google Scholar] [CrossRef] [PubMed]
- Substances of Very High Concern Identification. Available online: https://echa.europa.eu/substances-of very-high-concern-identification (accessed on 10 January 2020).
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Blessy, B.M.; Beeregowda, K.M. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 2, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Hamann, D.; Hamann, C.R.; Thyssen, J.P. The impact of common metal allergens in daily devices. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Ziegenhagen, R.; Reclaru, L.; Ardelean, L.C.; Grecu, A.F. Corrosion Resistance of Stainless Steels Intended to Come into Direct or Prolonged Contact with the Skin. Materials 2019, 12, 987. [Google Scholar] [CrossRef] [PubMed]
- Godrey, A.; Abdel-Moneim, A.; Sepúlveda, M.S. Acute mixture toxicity of halogenated chemicals and their next generation counterparts on zebrafish embryos. Chemosphere 2017, 181, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Meyer, O. Testing and assessment strategies, including alternative and new approaches. Toxicol. Lett. 2003, 140–141, 21–30. [Google Scholar] [CrossRef]
- ECHA Substances Restricted under REACH. Available online: https://echa.europa.eu/web/guest/substances-restricted-under-reach (accessed on 10 January 2020).
- REACH Online. VII.html. Available online: https://reachonline.eu/REACH/EN/REACH_EN/article (accessed on 10 January 2020).
- REACH Online. VIII.html. Available online: https://reachonline.eu/REACH/EN/REACH_EN/article (accessed on 10 January 2020).
- Guidance on Information Requirements and Chemical Safety Assessment Chapter R.7a: Endpoint Specific Guidance. Available online: https://echa.europa.eu/documents/10162/13632/information_requirements_r7a_en.pdf (accessed on 10 January 2020).
- Worth, A.; Barroso, J.; Bremer, S.; Burton, J.; Casati, S.; Coecke, S.; Corvi, R.; Desprez, B.; Dumont, C.; Gouliarmou, V.; et al. Alternative Methods for Regulatory Toxicology–A State-of-the-Art Review. Available online: https://publications.jrc.ec.europa.eu/repository/bitstream/JRC9161/echa_jrc_sla_report_public_05-09-14_withcover%20ipo.pdf (accessed on 20 March 2020).
- EN 71-3:2019 Safety of Toys-Part 3: Migration of Certain Elements. Available online: https://www.eurofins.com/consumer-product-testing/media-centre/news/newsflash-europe-new-version-of-toy-safety-standard-en-71-32019-on-migration-of-certain-elements (accessed on 10 February 2020).
- Reclaru, L.; Mayer, J.M. Effects of fluoride on titanium and other dental alloys in dentistry. Biomaterials 1998, 19, 85–92. [Google Scholar] [CrossRef]
- ASTM F2923–14 Standard Specification for Consumer Product Safety for Children’s Jewelry. Available online: https://www.astm.org/Standards/F2923.htm (accessed on 10 February 2020).
- DIN EN 1811-2011 Reference Test Method for Release of Nickel from All Post Assemblies which are Inserted into Pierced Parts of the Human Body and Articles Intended to Come into Direct and Prolonged Contact with the Skin (includes Amendment A1:2015). Available online: https://www.en-standard.eu/din-en-1811 (accessed on 10 February 2020).
- Thoenen, T.; Hummel, W.; Berner, U.; Curti, E. Aqueous zirconium fluoride complexes. In The PSI/Nagra Chemical Thermodynamic Data Base 12/07 (Update of the Nagra/PSI TDB 01/01); Paul Scherer Institute: Villingen, Switzerland, 2014; pp. 362–365. [Google Scholar]
- Valéro, R.; Durand, B.; Guth, J.L.; Chopin, T. Influence des ions fluorure et de la silice amorphe sur la solubilité des gels de zircone et caractérisation des fluoro-complexes de zirconium en milieu moyennement acide. Rev. Can. Chim. 1999, 77, 2099–2104. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press: Boca Raton FL, USA, 2014; p. 499. [Google Scholar]
- Yttrium Chloride Hexahydrate. Available online: https://echa.europa.eu/fr/registration-dossier/-/registered-dossier/26898/4/9 (accessed on 10 February 2020).
- Spencer, J.F. The Metals of the Rare Earths; Longmans, Green and Company: London, UK, 1919; p. 135. [Google Scholar]
- Denry, I.; Holloway, J.A.; Gupta, P.K. Effect of crystallization heat treatment on the microstructure of niobium-doped fluorapatite glass-ceramics. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Denry, I.; Holloway, J.A. Low temperature sintering of fluorapatite glass-ceramics. Dent. Mater. 2014, 30, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.; Desel, H. Acute selenium poisoning by paradise nuts (Lecythisollaria). Hum. Exp. Toxicol. 2010, 29, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.W.; Clarkson, T.W.; Magos, L.; Suzuki, T. Toxicology of Metals; Lewis Publishers: Boca Raton, FL, USA, 1996; p. 427. [Google Scholar]
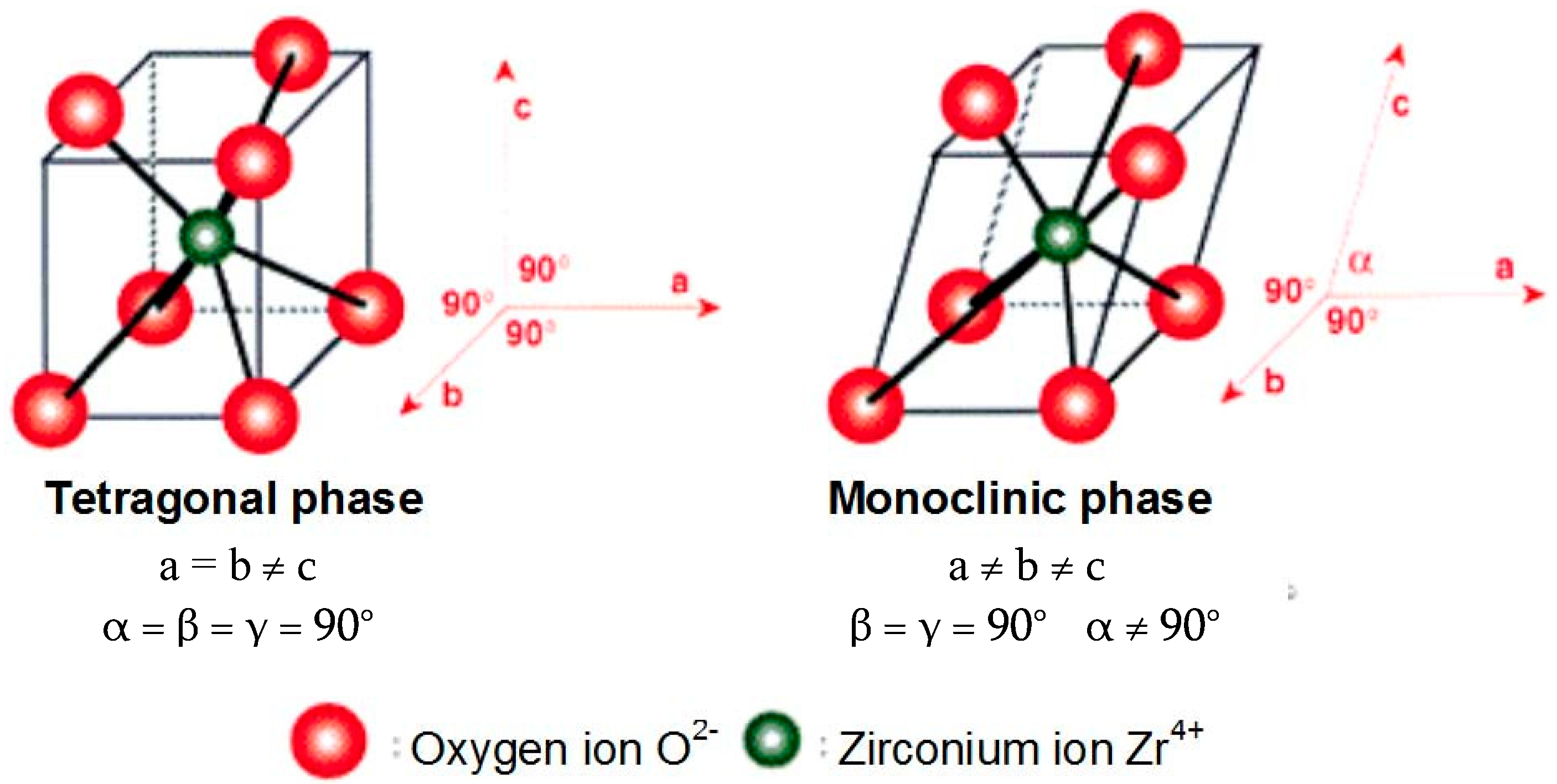
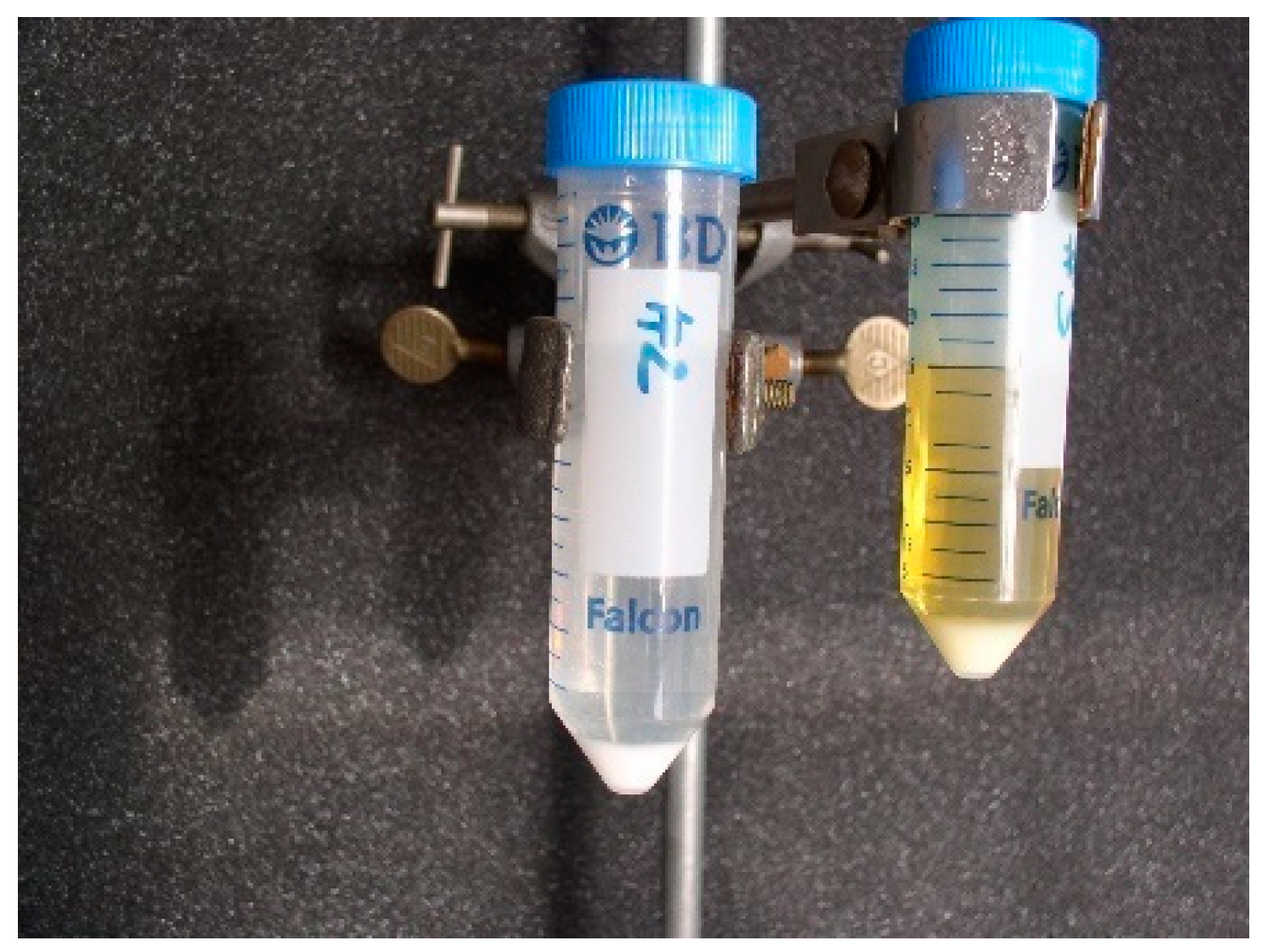

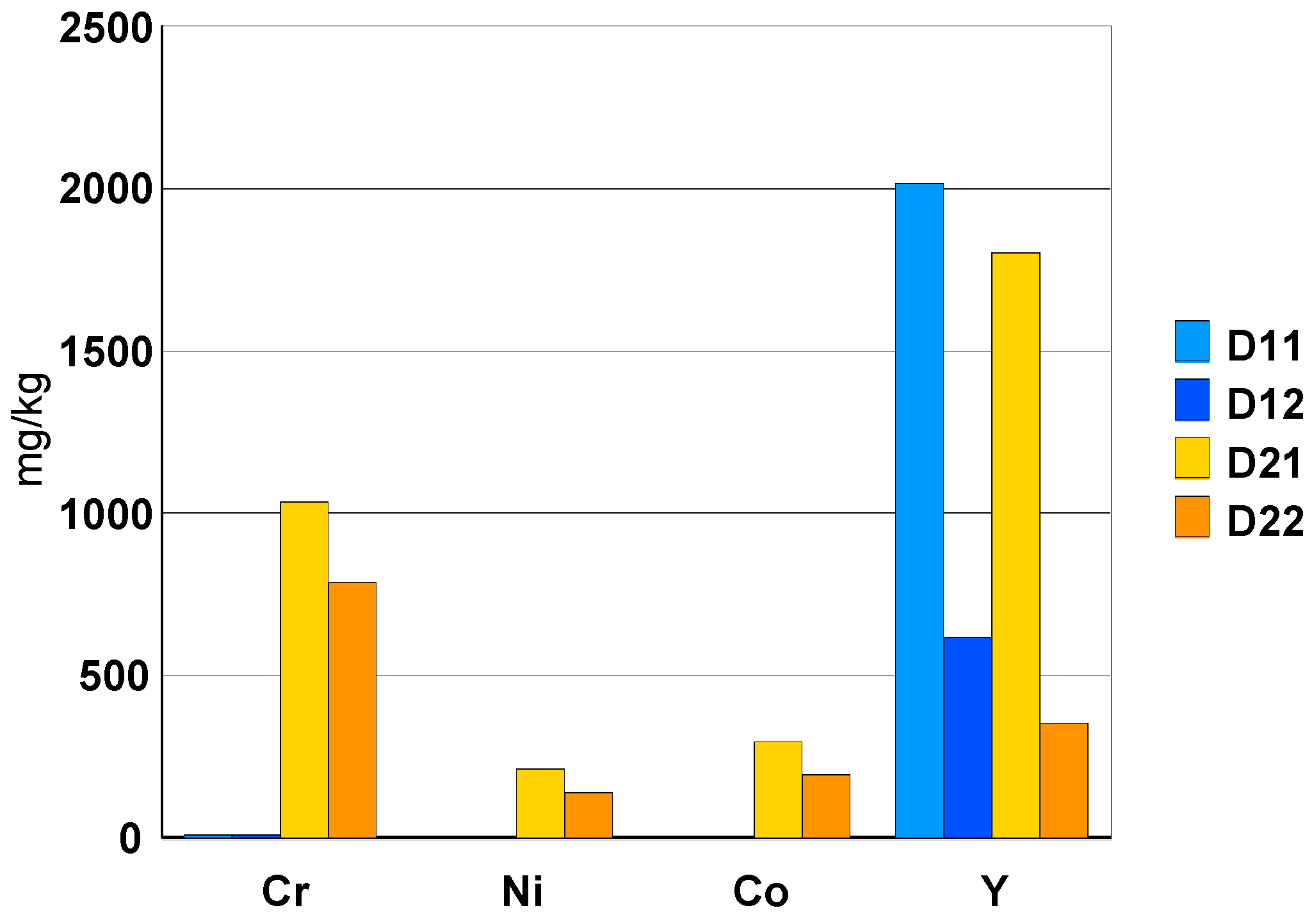



| Class | References | Form and Composition |
|---|---|---|
| Alumina-based ceramic | ISO 6574 | Dense sintered crystalline alumina A1203 |
| 99.9% porous alumina | ||
| 97% pure porous alumina | ||
| Zirconia reinforced alumina | ||
| Calcium-phosphate alumina | ||
| Single crystal alumina | ||
| Silicate-based ceramic | Porous vitroceramic | |
| Dense vitroceramic | ||
| Calcium-phosphate-based ceramic | Dense sintered hydroxy-apatite | |
| Porous sintered hydroxy-apatite | ||
| Dense sintered beta tricalcium phosphate | ||
| Porous sintered beta tricalcium phosphate | ||
| Other types | ||
| Calcium carbonate-based ceramic | Porous sintered calcium carbonate | |
| Natural porous calcium carbonate | ||
| Zirconia-based ceramic | Alumina stabilized zirconia | |
| Yttria partially stabilized zirconia | ||
| Magnezium stabilized zirconia | ||
| Ceria partially stabilized zirconia | ||
| Felds pat-based ceramic | ISO 6872 ISO 9693 | Reinforced with leucit |
| Reinforced with alumina | ||
| Reinforced with mica |
| Property | Y-TZP [12,13] | ISO 13356 [14] | Zenostar [15] |
|---|---|---|---|
| Chemical composition | |||
| ZrO2+HfO2+Y2O3 | >99.0 | >99.0 | >99.0 |
| Y2O3 | 4.5–5.4 | 4.5–5.4 | >4.5 |
| Al2O3 | <0.5 | <0.5 | <0.5 |
| Other total oxides | <0.5 | <0.5 | <1.5 |
| Physical properties | |||
| Bulk density (g/cm3) | 6.05 | >6.00 | >6.00 |
| Grainsize (μm) | 0.2 | <0.6 | <0.6 |
| Monoclinic phase (%) | 1 | - | <5 |
| Mechanical properties | |||
| Flexural strength-4 point bend (MPa) | 1666 | >800 | 1200±200 |
| Elastic modulus (GPa) | 201 | - | 210 |
| Vickers hardness (HV) | 1270 | - | >1300 |
| Fracture toughness (Kgf/mm2/3) | 16.8 | - | - |
| Compressive strength (MPa) | 4900 | - | - |
| Impact strength (MPa) | 137 | - | - |
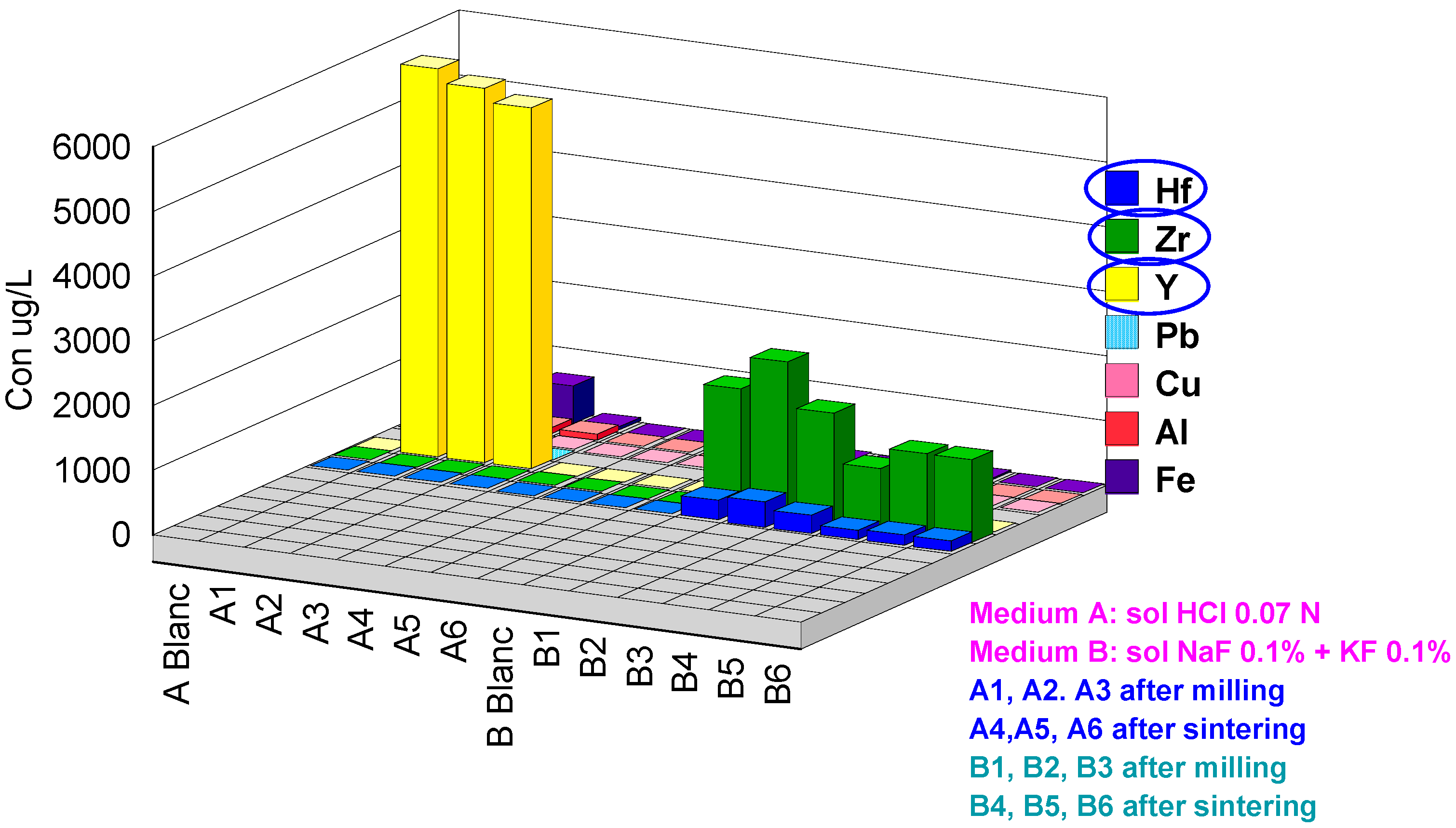
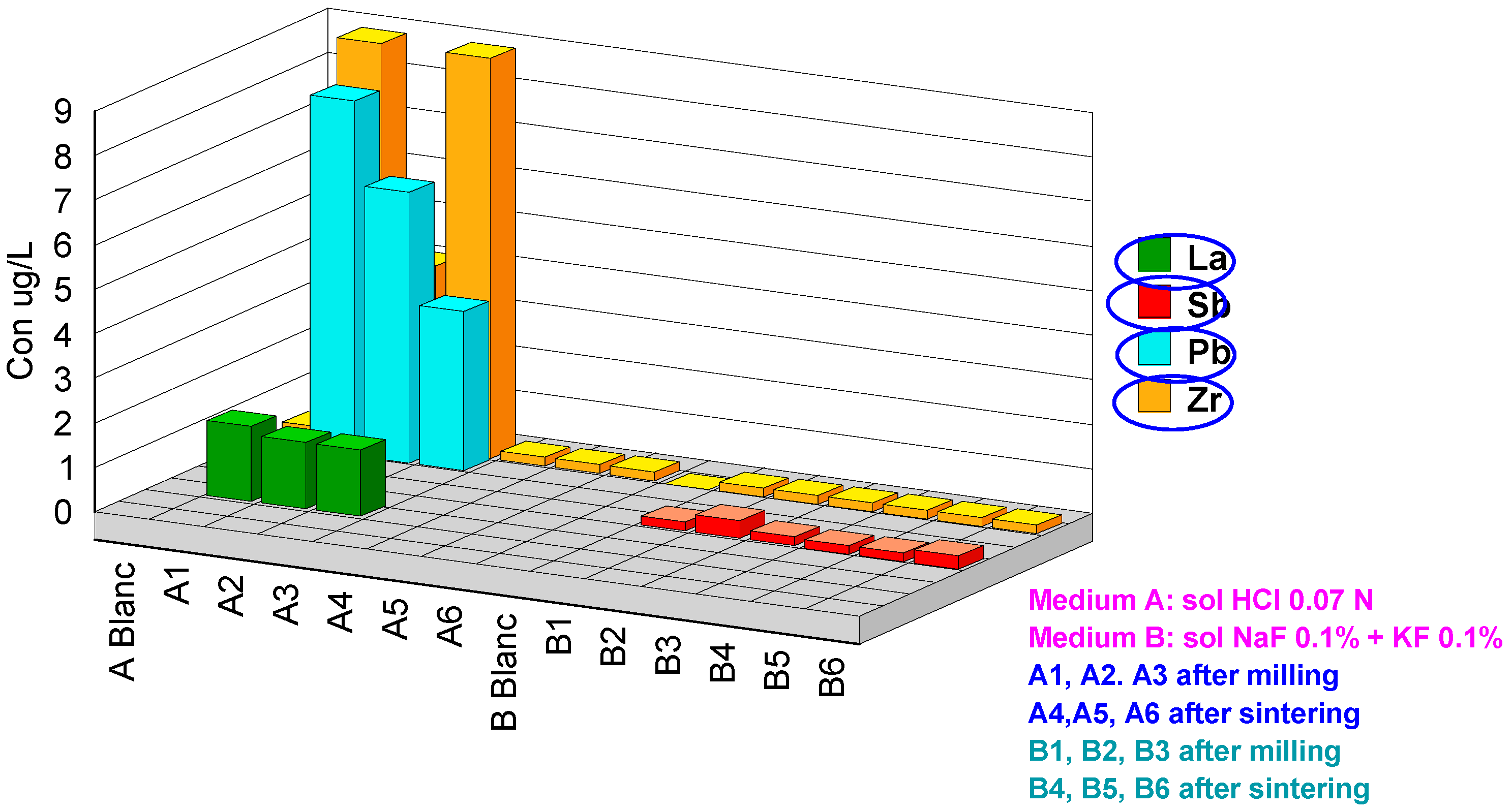
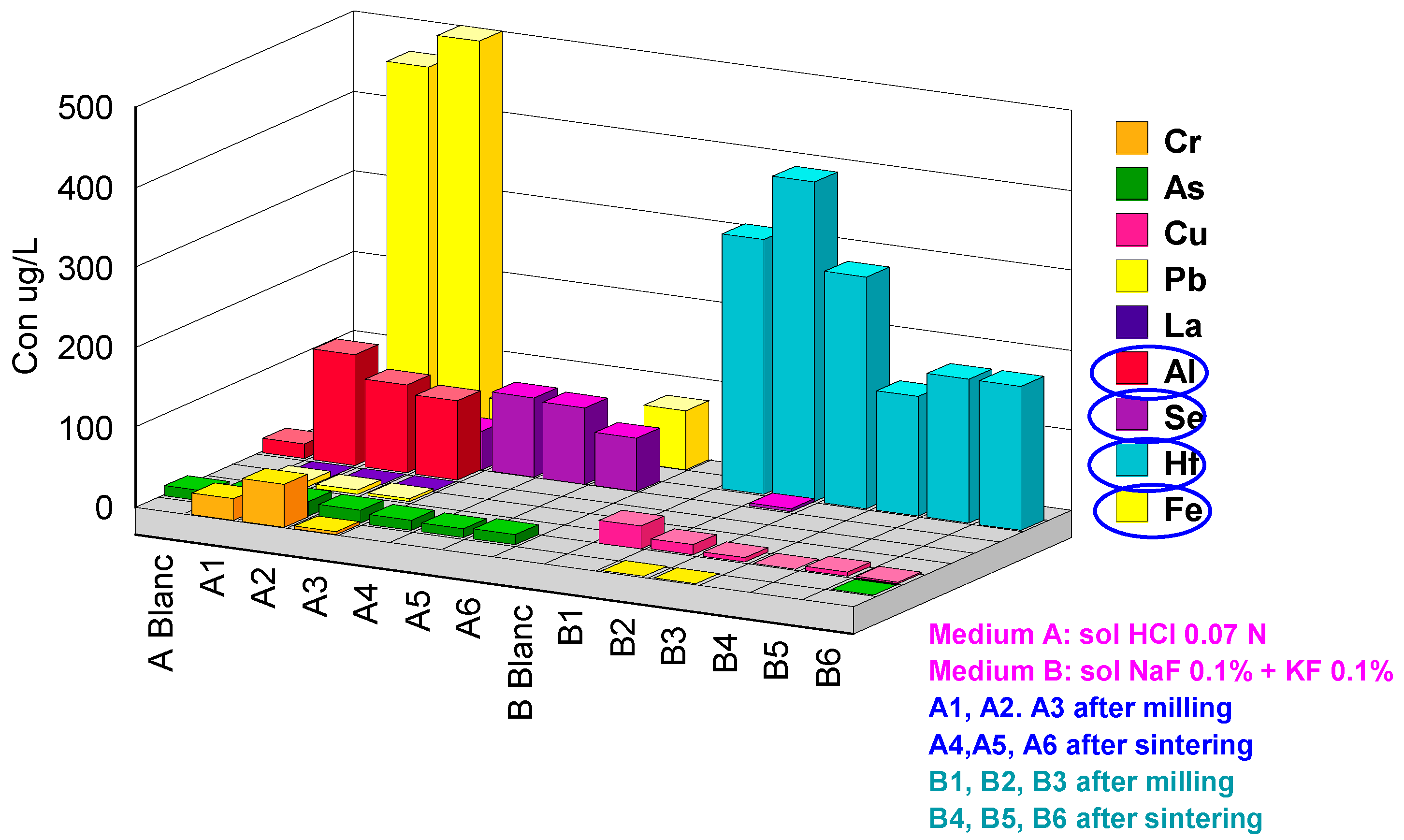
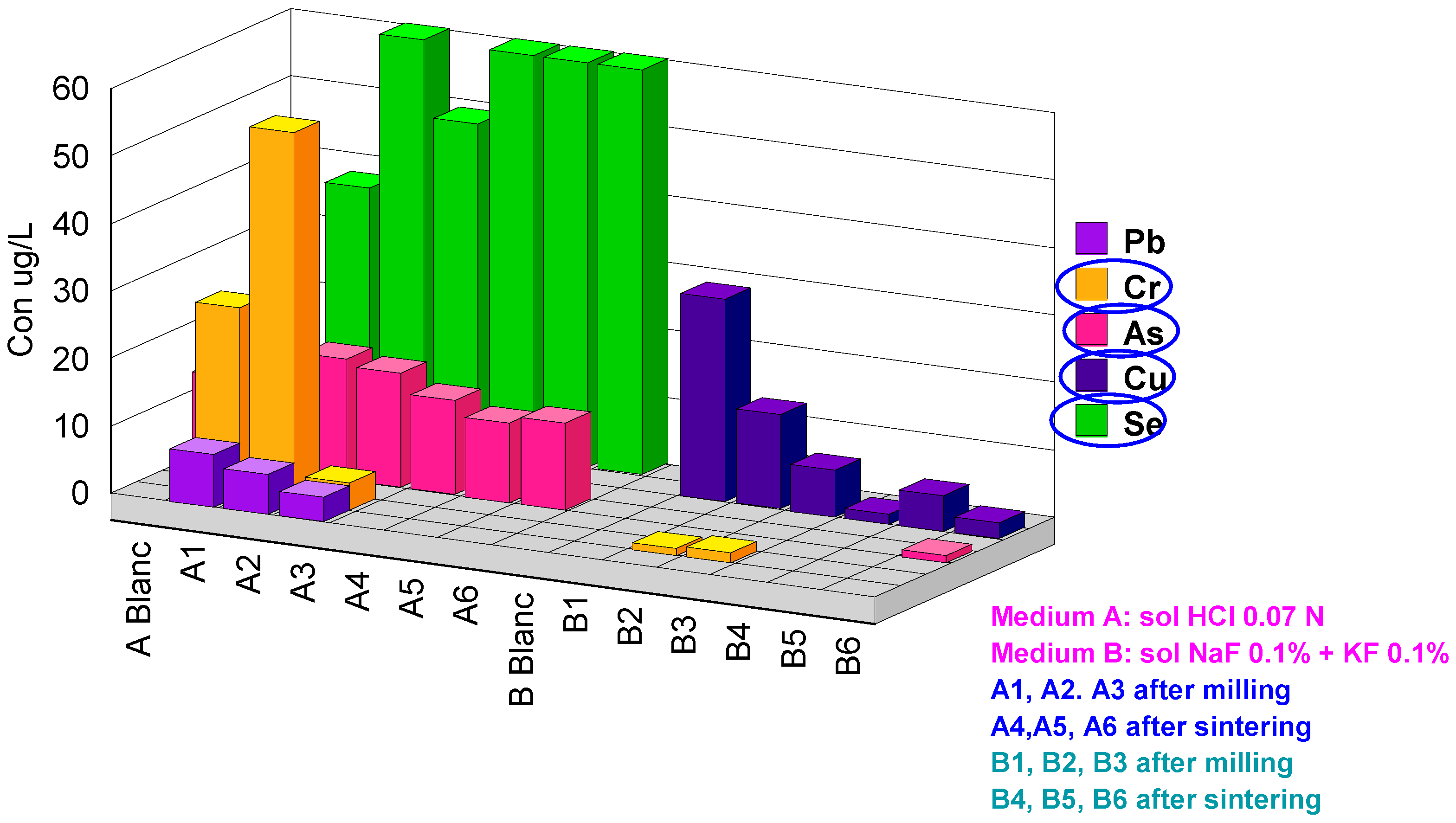
| Code | Hf | Cr | Y | As | Pb | Al | Fe | Cu | Se | Zr | Sb | La |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABlanc | <0.2 | <1 | <0.2 | 13 | <0.2 | 19 | <20 | <0.2 | <1 | <0.2 | <0.2 | <0.2 |
| A1 | 1.9 | 28 | 6000 | 16 | 8.0 | 140 | 460 | <0.2 | 37 | 14 | <0.2 | 1.7 |
| A2 | 0.2 | 55 | 5800 | 18 | 6.1 | 110 | 585 | <0.2 | 63 | 4.2 | <0.2 | 1.5 |
| A3 | <0.2 | 4.2 | 5600 | 17 | 3.6 | 99 | 56 | <0.2 | 49 | 10 | <0.2 | 1.5 |
| A4 | <0.2 | <1 | 6.2 | 14 | <0.2 | <10 | <20 | <0.2 | 100 | <0.2 | <0.2 | <0.2 |
| A5 | <0.2 | <1 | 4.4 | 12 | <0.2 | <10 | <20 | <0.2 | 97 | <0.2 | <0.2 | <0.2 |
| A6 | <0.2 | <1 | 3.2 | 13 | <0.2 | <10 | 75 | <0.2 | 67 | <0.2 | <0.2 | <0.2 |
| BBlanc | <0.2 | <1 | <0.2 | <1 | <0.2 | <10 | <20 | <0.2 | <1 | 3.1 | <0.2 | <0.2 |
| B1 | 320 | <1 | <0.2 | <1 | <0.2 | <10 | <20 | 30 | <1 | 1900 | 0.2 | <0.2 |
| B2 | 400 | 1.2 | <0.2 | <1 | <0.2 | <10 | <20 | 14 | 4.2 | 2400 | 0.4 | <0.2 |
| B3 | 290 | 1.5 | <0.2 | <1 | <0.2 | <10 | <20 | <0.2 | <1 | 1700 | 0.2 | <0.2 |
| B4 | 150 | <1 | <0.2 | <1 | <0.2 | <10 | 24 | 0.5 | <1 | 960 | 0.2 | <0.2 |
| B5 | 180 | <1 | <0.2 | <1 | <0.2 | <10 | <20 | 5.4 | <1 | 1300 | 0.2 | <0.2 |
| B6 | 180 | <1 | <0.2 | 1.1 | <0.2 | <10 | <20 | 2.5 | <1 | 1300 | 0.3 | <0.2 |
| Element | Method | Blanc | C4 | C5 | ||||
|---|---|---|---|---|---|---|---|---|
| µg/L | µg/L | % (m/m) | ppm | µg/L | % (m/m) | ppm | ||
| Ni | ICP-OES | <20 | 646,000 | 10.4 | 103,508 | 634,000 | 10.6 | 10,6460 |
| Cr | ICP-OES | <5 | 142,000 | 2.27 | 22,753 | 135,000 | 2.27 | 22,669 |
| Co | ICP-OES | <10 | 4370 | 0.07 | 700 | 4150 | 0.07 | 697 |
| Fe | ICP-OES | <20 | 1900 | 0.03 | 304 | 1870 | 0.03 | 314 |
| Hg | ICP-MS | <0.5 | 570 | - | 91 | 610 | - | 102 |
| Cu | ICP-MS | <0.2 | 81 | - | 13 | 78 | - | 13 |
| Te | ICP-MS | <1 | 40 | - | 6.4 | 52 | - | 8.7 |
| As | ICP-MS | <1 | 22.0 | - | 3.5 | 15.0 | - | 2.6 |
| Ba | ICP-MS | <0.2 | 10.0 | - | 1.6 | 8.8 | - | 1.5 |
| Se | ICP-MS | <1 | 4.1 | - | 0.7 | 4.5 | - | 0.7 |
| Pb | ICP-MS | <0.2 | 3.0 | - | 0.5 | 3.0 | - | 0.5 |
| Cd | ICP-MS | <0.2 | 1.9 | - | 0.3 | 1.6 | - | 0.3 |
| Sb | ICP-MS | <0.2 | 3.5 | - | 0.6 | 1.7 | - | 0.3 |
| Element | Method | Blanc | C1 | C2 | C3 | |||
|---|---|---|---|---|---|---|---|---|
| µg/L | µg/L | µg/cm2 | µg/L | µg/cm2 | µg/L | µg/cm2 | ||
| Fe | ICP-OES | <20 | <20 | DL | <20 | DL | <20 | DL |
| Co | ICP-OES | <10 | <10 | DL | <10 | DL | <10 | DL |
| Cu | ICP-OES | <20 | <20 | DL | <20 | DL | <20 | DL |
| Ni | ICP-OES | <20 | 49 | 0.29 | 48 | 0.28 | 57 | 0.34 |
| As | ICP-MS | <1 | <1 | DL | <1 | DL | <1 | DL |
| Ba | ICP-MS | 4.4 | 6.3 | 0.037 | 5.4 | 0.032 | 5.4 | 0.032 |
| Cd | ICP-MS | <0.2 | <0.2 | DL | <0.2 | DL | <0.2 | DL |
| Cr | ICP-MS | <1 | 4.1 | 0.024 | 4.0 | 0.024 | 4.4 | 0.026 |
| Hg | ICP-MS | <0.5 | <0.5 | DL | <0.5 | DL | <0.5 | DL |
| Pb | ICP-MS | <0.5 | 0.9 | 0.005 | 0.9 | 0.005 | 0.9 | 0.005 |
| Sb | ICP-MS | <0.2 | <0.2 | DL | <0.2 | DL | <0.2 | DL |
| Se | ICP-MS | <1 | <1 | DL | <1 | DL | <1 | DL |
| Te | ICP-MS | <1 | <1 | DL | <1 | DL | <1 | DL |
| Element | Method | Blanc | C4 | C5 | C6 | |||
|---|---|---|---|---|---|---|---|---|
| µg/L | µg/L | µg/cm2 | µg/L | µg/cm2 | µg/L | µg/cm2 | ||
| Fe | ICP-OES | <20 | <20 | DL | <20 | DL | <20 | DL |
| Co | ICP-OES | <10 | <10 | DL | <10 | DL | <10 | DL |
| Cu | ICP-OES | <20 | <20 | DL | <20 | DL | <20 | DL |
| Ni | ICP-OES | <20 | 433 | 2.6 | 407 | 2.4 | 421 | 2.5 |
| As | ICP-MS | <1 | <1 | DL | <1 | DL | <1 | DL |
| Ba | ICP-MS | <0.2 | 1.3 | 0.008 | 1.2 | 0.007 | 6.2 | 0.036 |
| Cd | ICP-MS | <0.2 | <0.2 | DL | <0.2 | DL | <0.2 | DL |
| Cr | ICP-MS | <1 | 15.0 | 0.09 | 17.0 | 0.10 | 14.0 | 0.08 |
| Hg | ICP-MS | <0.5 | <0.5 | DL | <0.5 | DL | <0.5 | DL |
| Pb | ICP-MS | <0.2 | 0.4 | 0.002 | 0.6 | 0.004 | 0.5 | 0.003 |
| Sb | ICP-MS | <0.2 | <0.2 | DL | <0.2 | DL | <0.2 | DL |
| Se | ICP-MS | <1 | <1 | DL | <1 | DL | <1 | DL |
| Te | ICP-MS | <1 | <1 | DL | <1 | DL | <1 | DL |
| Element | Lower Measurement Limit [mg/L] | 4h | 18h |
|---|---|---|---|
| Ni | 2 | Negative | Negative |
| Co | 2 | Negative | Negative |
| Cu | 2 | Negative | Negative |
| Fe | 3 | Negative | Negative |
| Description | Code | Mass [g] |
|---|---|---|
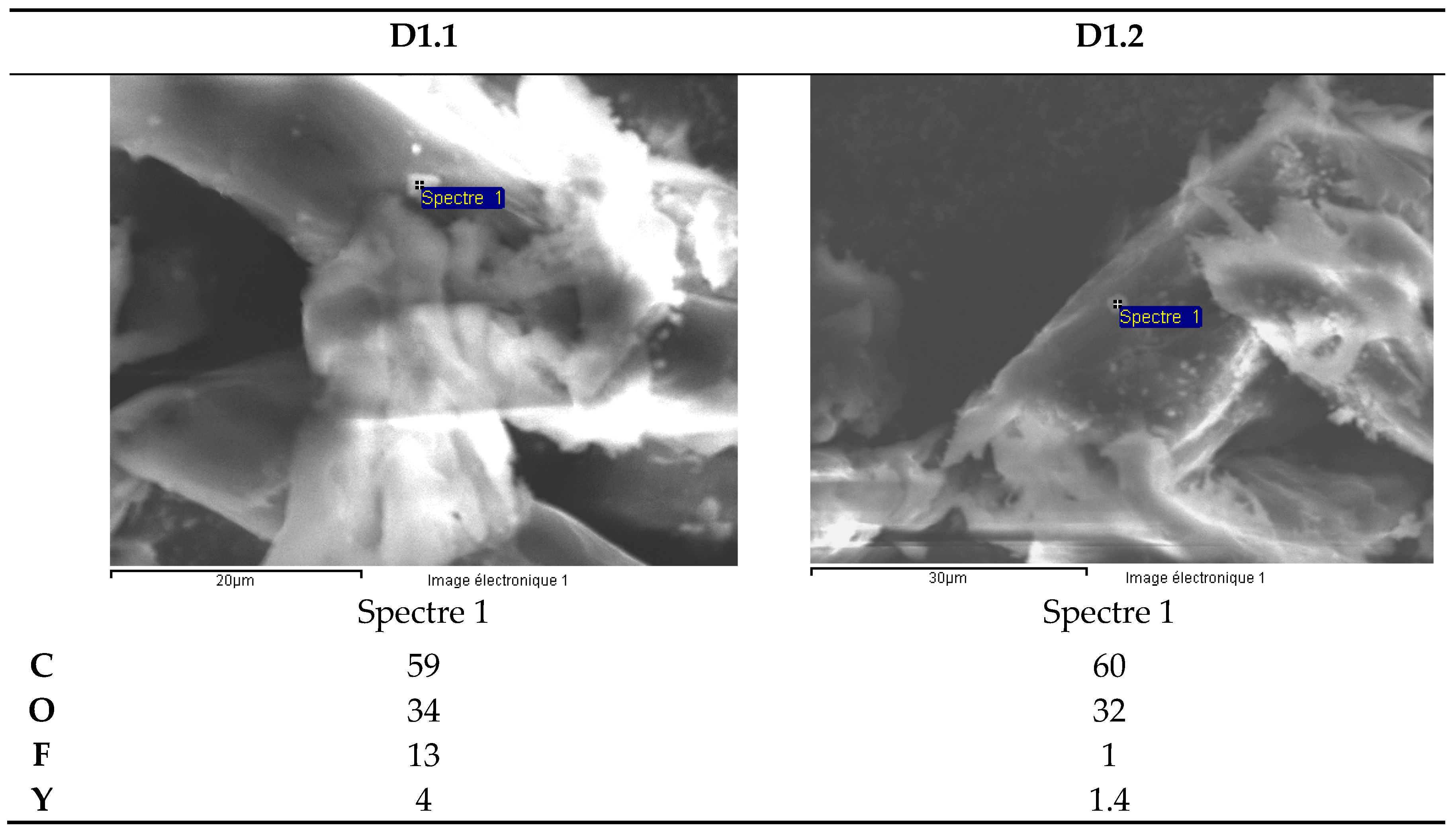
| White ceramic watch link | D1.1 | 0.03715 |
| D1.2 | 0.03398 | |
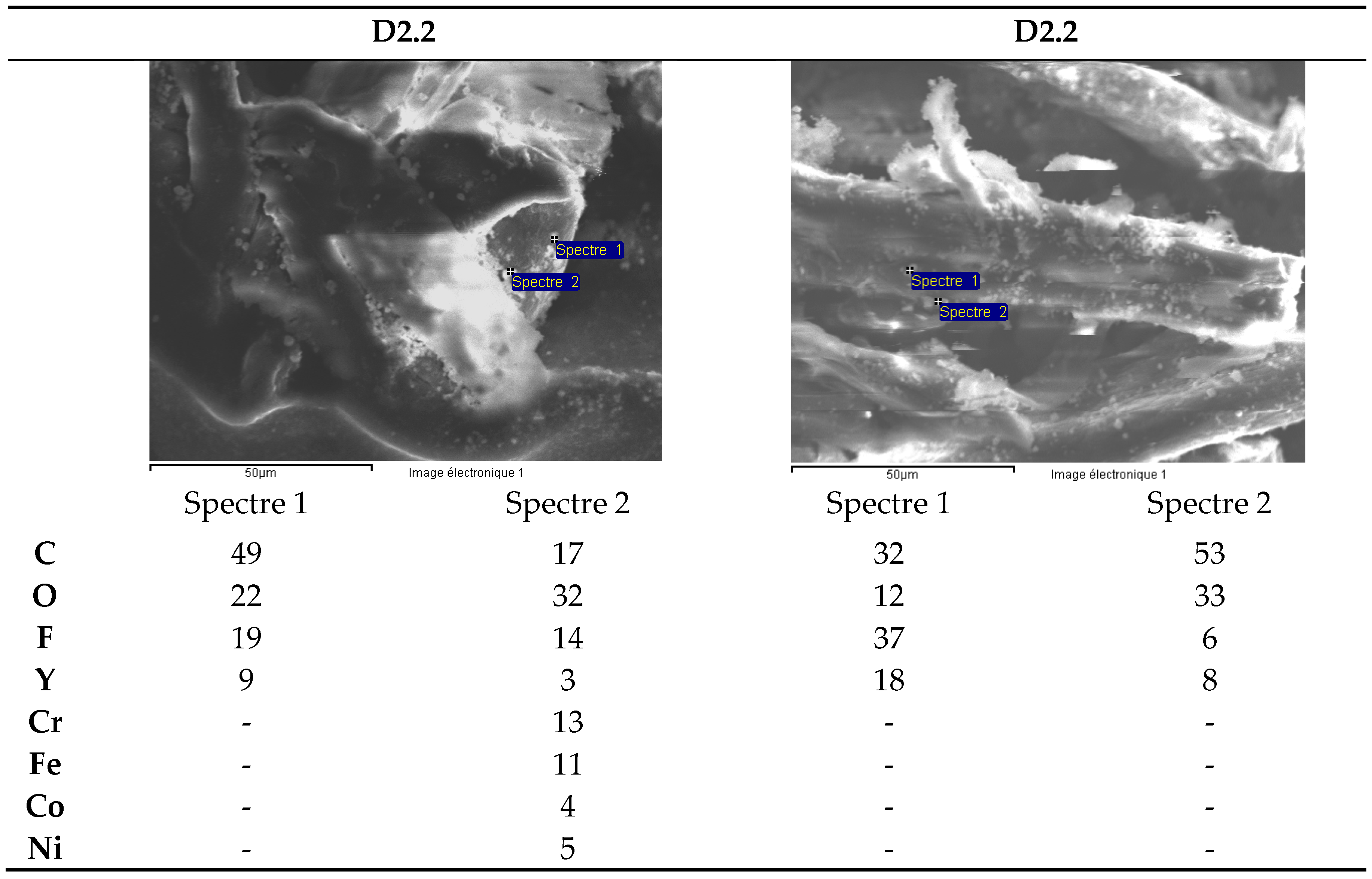
| Black ceramic watch band | D2.1 | 0.01888 |
| D2.2 | 0.03888 |
| White Ceramic Watch Link D1 | Black Ceramic Watch Band D2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D1.1 | µg/L | mg/kg | % | D1.2 | µg/L | mg/kg | % | D2.1 | µg/L | mg/kg | % | D2.2 | µg/L | mg/kg | % |
| Hf | 12,000 | 16,151 | 1.62 | Hf | 9900 | 14,567 | 1.46 | Hf | 5700 | 15,095 | 1.51 | Hf | 12,000 | 15,432 | 1.54 |
| Cr | 7.3 | 10 | - | Cr | 5.4 | 8 | - | Cr | 390 | 1033 | 0.10 | Cr | 790 | 1016 | 0.10 |
| Ni | <20 | DL | DL | Ni | <20 | DL | DL | Ni | 80 | 212 | 0.02 | Ni | 137 | 176 | 0.02 |
| Co | <10 | DL | DL | Co | <10 | DL | DL | Co | 111 | 294 | 0.03 | Co | 194 | 249 | 0.02 |
| Y | 1500 | 2019 | 0.20 | Y | 420 | 618 | 0.06 | Y | 680 | 1801 | 0.18 | Y | 350 | 450 | 0.05 |
| Sb | <0.4 | DL | DL | Sb | <0.4 | DL | DL | Sb | <0.4 | DL | DL | Sb | <0.4 | DL | DL |
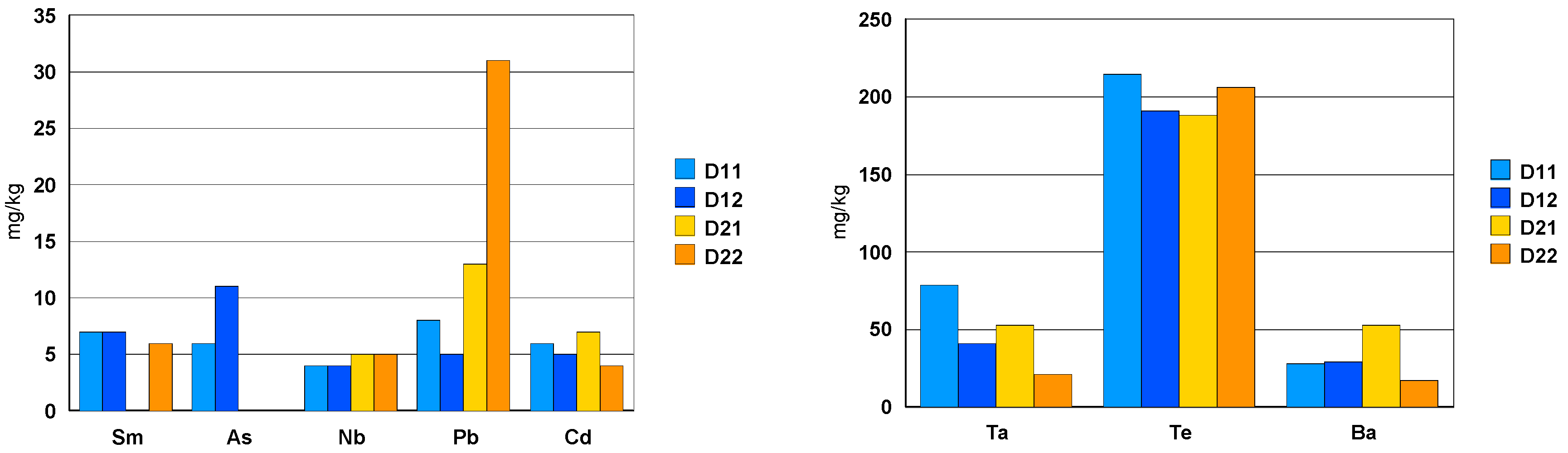
| Ta | 59 | 79 | 0.008 | Ta | 28 | 41 | 0.004 | Ta | 20 | 53 | 0.005 | Ta | 16 | 21 | 0.002 |
| Te | 160 | 215 | 0.02 | Te | 130 | 191 | 0.02 | Te | 71 | 188 | 0.02 | Te | 160 | 206 | 0.02 |
| Ba | 21 | 28 | - | Ba | 20 | 29 | - | Ba | 20 | 53 | 0.01 | Ba | 13 | 17 | - |
| Sm | 5.0 | 7 | - | Sm | 4.6 | 7 | - | Sm | <2 | DL | DL | Sm | 4.4 | 6 | - |
| As | 4.5 | 6 | - | As | 7.5 | 11 | - | As | <2 | DL | DL | As | <2 | DL | DL |
| Nb | 3.0 | 4 | - | Nb | 2.4 | 4 | - | Nb | 1.8 | 5 | - | Nb | 3.6 | 5 | - |
| Pb | 5.7 | 8 | - | Pb | 3.5 | 5 | - | Pb | 4.9 | 13 | - | Pb | 24 | 31 | - |
| Hg | <1 | DL | DL | Hg | <1 | DL | DL | Hg | <1 | DL | DL | Hg | <1 | DL | DL |
| Cd | 4.5 | 6 | - | Cd | 3.1 | 5 | - | Cd | 2.7 | 7 | - | Cd | 2.9 | 4 | - |
| Cu | <20 | DL | DL | Cu | <20 | DL | DL | Cu | <20 | DL | DL | Cu | <20 | DL | DL |
| Se | <2 | DL | DL | Se | <2 | DL | DL | Se | <2 | DL | DL | Se | <2 | DL | DL |
| Element | Reagent | Detection Limit (Semi-Quantitative) |
|---|---|---|
| Ni | Diméthylglyoxime | 10 mg/L |
| Co | Thiocyanate | 10 mg/L |
| Fe | 2,2′-bipyridine | 3 mg/L |
| Cu | 2,2′-biquinoléine | 10 mg/L |
| Code | Mass [g] |
|---|---|
| E1 | 0.10807 |
| E2 | 0.12027 |
| Black Zirconia Ceramic Bracelet Components | |||||||
|---|---|---|---|---|---|---|---|
| E1 | μg/L | mg/kg | % | E2 | μg/L | mg/kg | % |
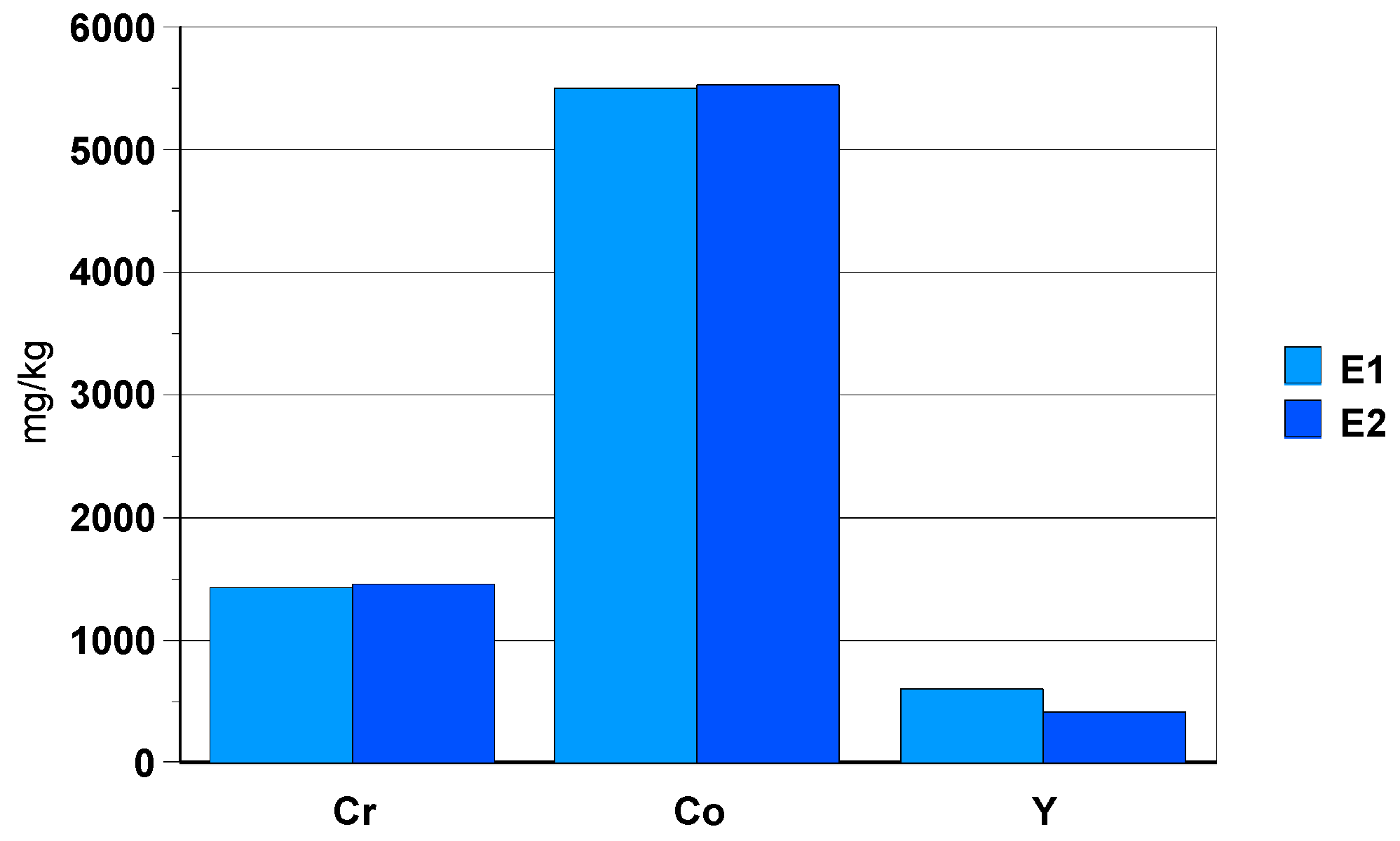
| Hf | 64,000 | 29,610 | 3.0 | Hf | 74,000 | 30,764 | 3.08 |
| Cr | 3100 | 1434 | 0.14 | Cr | 3500 | 1455 | 0.15 |
| Ni | <80 | DL | DL | Ni | <80 | DL | DL |
| Co | 11,900 | 5506 | 0.55 | Co | 13,300 | 5529 | 0.55 |
| Fe | 8810 | 4053 | 0.41 | Fe | 9925 | 4163 | 0.42 |
| Y | 1300 | 601 | 0.06 | Y | 990 | 412 | 0.04 |
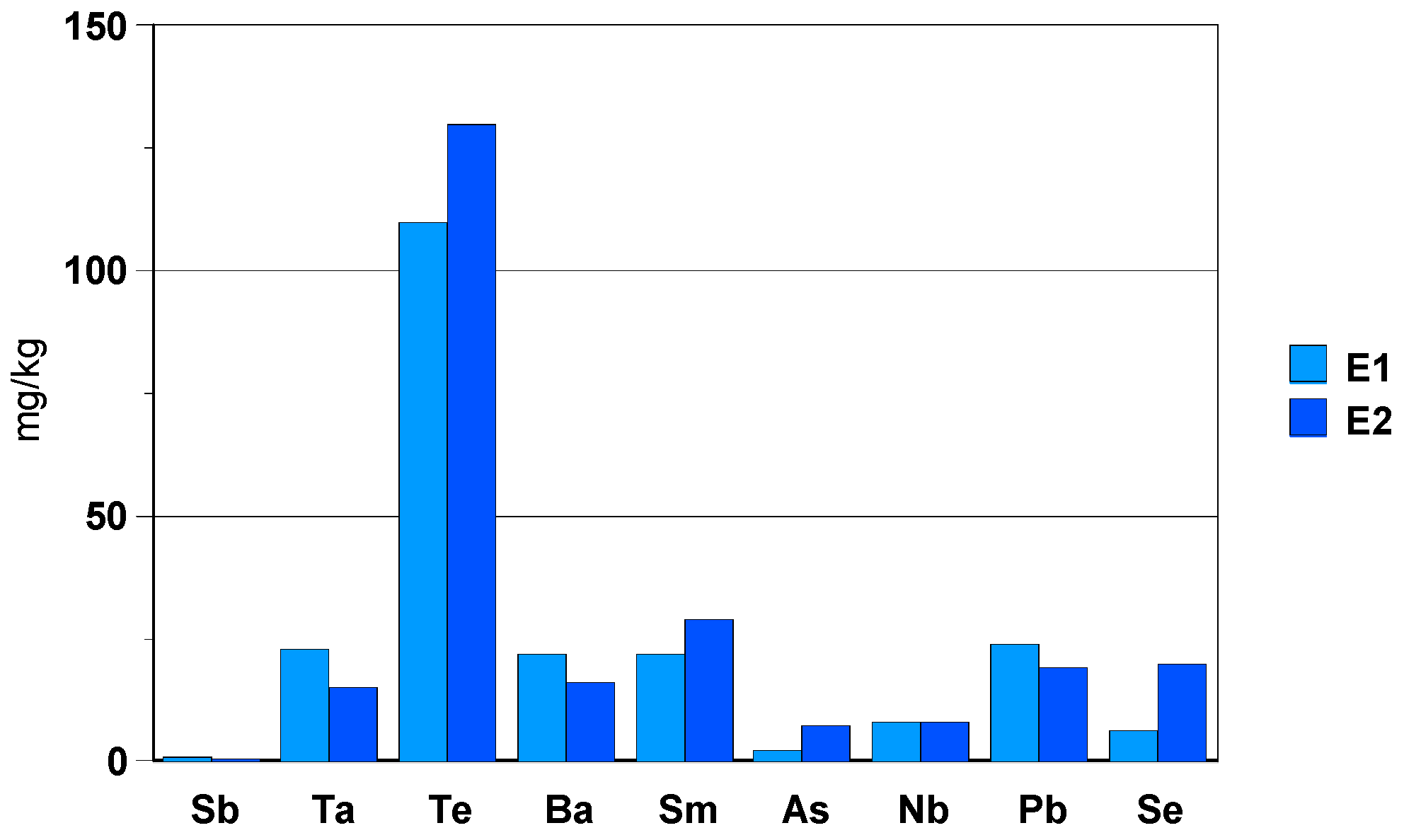
| Sb | 0.9 | 0.4 | - | Sb | 0.5 | 0.2 | - |
| Ta | 23 | 11 | - | Ta | 15 | 6 | - |
| Te | 110 | 51 | - | Te | 130 | 54 | - |
| Ba | 22 | 10 | - | Ba | 16 | 7 | - |
| Sm | 22 | 10 | - | Sm | 29 | 12 | - |
| As | 2.3 | 1 | - | As | 7.3 | 3 | - |
| Nb | 7.8 | 4 | - | Nb | 8.0 | 3 | - |
| Pb | 24 | 11 | - | Pb | 19 | 8 | - |
| Hg | ≤1 | DL | DL | Hg | ≤1 | DL | DL |
| Cd | ≤8 | DL | DL | Cd | ≤8 | DL | DL |
| Cu | ≤80 | DL | DL | Cu | ≤80 | DL | DL |
| Se | 6.4 | 3 | - | Se | 19.8 | 8 | - |
| Sample | Mass [mg] | Dissolution Time [h] | Color of Precipitates |
|---|---|---|---|
| F1 | 8180 | 16 (ultrasound) | blackish |
| F2 | 24,923 | 40 (ultrasound) | white |
| F3 | 16,134 | 160 (ultrasound) | white |
| F1 | μg/L | mg/kg | % | F2 | μg/L | mg/kg | % | F3 | μg/L | mg/kg | % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hf | 12,000 | 14,670 | 1.47 | Hf | 46,000 | 18,457 | 1.85 | Hf | 27,000 | 10,833 | 1.08 |
| Cr | 1400 | 1711 | 0.17 | Cr | 3.9 | 2 | - | Cr | 3.5 | 1 | - |
| Ni | 1180 | 1443 | 0.14 | Ni | <20 | - | - | Ni | <20 | - | - |
| Co | 820 | 1002 | 0.10 | Co | <10 | - | - | Co | 9610 | 3856 | 0.39 |
| Y | 440 | 538 | 0.05 | Y | 2100 | 843 | 0.08 | Y | 1500 | 602 | 0.06 |
| Sb | 110 | 134 | 0.01 | Sb | <0.4 | - | Sb | <0.4 | - | - | |
| Ta | 67 | 82 | 0.01 | Ta | 160 | 64 | 0.01 | Ta | 76 | 30 | - |
| Te | 32.0 | 39 | - | Te | 120 | 48 | - | Te | 73 | 29 | - |
| Ba | 7.7 | 9 | - | Ba | 12 | 5 | - | Ba | 8.0 | 3 | - |
| Sm | 6.0 | 7 | - | Sm | 8.6 | 3 | - | Sm | 6.2 | 2 | - |
| As | 4.5 | 6 | - | As | <2 | - | - | As | 2.4 | 1 | - |
| Nb | 2.6 | 3 | - | Nb | 8.2 | 3 | - | Nb | 6.0 | 2 | - |
| Pb | 2.4 | 3 | - | Pb | 2.1 | 1 | - | Pb | 1.3 | 1 | - |
| Hg | 2.4 | 3 | - | Hg | 1.8 | 1 | - | Hg | <1 | - | - |
| Cd | 2.3 | 3 | - | Cd | 3.5 | 1 | - | Cd | 2 | 1 | - |
| Cu | <20 | - | - | Cu | <20 | - | - | Cu | <20 | - | - |
| Se | <2 | - | - | Se | <2 | - | - | Se | <2 | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reclaru, L.; Ardelean, L.C.; Miu, C.A.; Grecu, A.F. Are Zirconia Bioceramics and Ceramics Intended to Come in Contact with Skin Inert? Materials 2020, 13, 1697. https://doi.org/10.3390/ma13071697
Reclaru L, Ardelean LC, Miu CA, Grecu AF. Are Zirconia Bioceramics and Ceramics Intended to Come in Contact with Skin Inert? Materials. 2020; 13(7):1697. https://doi.org/10.3390/ma13071697
Chicago/Turabian StyleReclaru, Lucien, Lavinia Cosmina Ardelean, Catalin Adrian Miu, and Alexandru Florian Grecu. 2020. "Are Zirconia Bioceramics and Ceramics Intended to Come in Contact with Skin Inert?" Materials 13, no. 7: 1697. https://doi.org/10.3390/ma13071697
APA StyleReclaru, L., Ardelean, L. C., Miu, C. A., & Grecu, A. F. (2020). Are Zirconia Bioceramics and Ceramics Intended to Come in Contact with Skin Inert? Materials, 13(7), 1697. https://doi.org/10.3390/ma13071697







