Elucidation of Antimicrobial Activity of Non-Covalently Dispersed Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Purification of CNT Samples
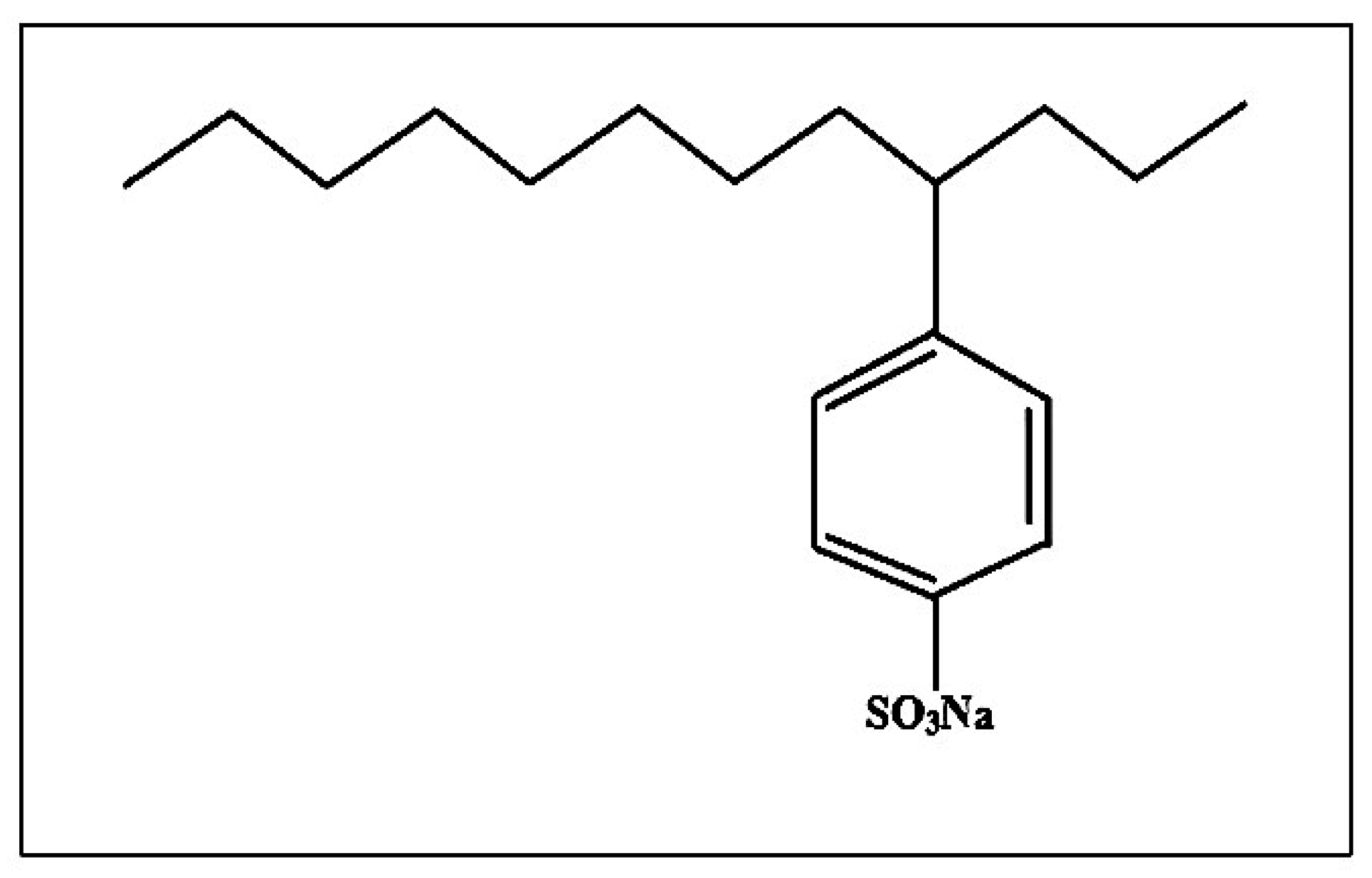
2.2. Preparation of Surfactant-Modified CNTs
2.3. Characterization of Surfactant-Modified CNTs
2.4. Preparation of Bacterial Cultures
2.5. Evaluation of Surfactant Biocompatibility
2.6. Treatment of Bacterial Cells with CNTs
2.7. Measurements of Optical Density (OD)
2.8. Determination of Viable Cell Number
2.9. SEM Imaging
2.10. Statistical Analysis
3. Results and Discussion
3.1. Biocompatibility of Surfactant
3.2. Antimicrobial Activity of CNTs
3.3. Microbial Viability Based on Concentration and Treatment Time
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Usui, Y.; Haniu, H.; Tsuruoka, S.; Saito, N. Carbon Nanotubes Innovate on Medical Technology. Med. Chem. 2012, 2, 1–6. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, Y.; Yan, B. Functionalized carbon nanotubes for potential medicinal applications. Drug Discov. Today 2010, 15, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Saifuddin, N.; Raziah, A.; Junizah, A. Carbon nanotubes: A review on structure and their interaction with proteins. J. Chem. 2013, 2013, 18. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Zodrow, K.R.; Kang, S.; Elimelech, M. Electronic-structure-dependent bacterial cytotoxicity of single-walled carbon nanotubes. ACS Nano 2010, 4, 5471–5479. [Google Scholar] [CrossRef] [PubMed]
- Smotlacha, J.; Pincak, R. Electronic Properties of Carbon Nanostructures. Recent Adv. Graphene Res. IntechOpen 2016, 32–56. [Google Scholar] [CrossRef]
- Rausch, J.; Zhuang, R.C.; Mäder, E. Surfactant assisted dispersion of functionalized multi-walled carbon nanotubes in aqueous media. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1038–1046. [Google Scholar] [CrossRef]
- Alsehlawi, Z.S.; Abdulridha, W.M.; Abd, A.N.; Habubi, N.F. Synergistic Activity of Multi-Walled Carbon Nanotubes Suspension against Clinical Isolates of Pathogenic Bacteria. J. Pharm. Sci. Res. 2018, 10, 718–722. [Google Scholar]
- Mocan, T.; Matea, C.T.; Pop, T.; Mosteanu, O.; Buzoianu, A.D.; Suciu, S.; Mocan, L. Carbon nanotubes as anti-bacterial agents. Cell. Mol. Life Sci. 2017, 74, 3467–3479. [Google Scholar] [CrossRef]
- Oyelami, A.O.; Semple, K.T. Impact of carbon nanomaterials on microbial activity in soil. Soil Biol. Biochem. 2015, 86, 172–180. [Google Scholar] [CrossRef]
- Bai, Y.; Park, I.S.; Ho, M. Aqueous dispersion of surfactant-modified multiwalled carbon nanotubes and their application as an antibacterial agent. Carbon 2011, 49, 3663–3671. [Google Scholar] [CrossRef]
- Khazaee, M.; Ye, D.; Majumder, A.; Baraban, L.; Opitz, J.; Cuniberti, G. Non-covalent modified multi-walled carbon nanotubes: Dispersion capabilities and interactions with bacteria. Biomed. Phys. Eng. Express 2016, 2, 055008. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, G.R.; Yoon, D.J.; Sin, C.H.; Park, I.S.; Bea, T.S.; Lee, M.H. The Effect of Dispersed MWCNTs Using SDBS Surfactant on Bacterial Growth. Int. J. Biotechnol. Bioeng. 2012, 6, 921–924. [Google Scholar] [CrossRef]
- Shi, B.; Zhuang, X.; Yan, X.; Lu, J.; Tang, H. Adsorption of atrazine by natural organic matter and surfactant dispersed carbon nanotubes. J. Env. Sci. 2010, 22, 1195–1202. [Google Scholar] [CrossRef]
- Ryabenko, A.G.; Dorofeeva, T.V.; Zvereva, G.I. UV–VIS–NIR spectroscopy study of sensitivity of single-wall carbon nanotubes to chemical processing and Van-der-Waals SWNT/SWNT interaction. Verification of the SWNT content measurements by absorption spectroscopy. Carbon 2004, 42, 1523–1535. [Google Scholar] [CrossRef]
- Yu, J.; Grossiord, N.; Koning, C.E.; Loos, J. Controlling the dispersion of multi-wall carbon nanotubes in aqueous surfactant solution. Carbon 2007, 45, 618–623. [Google Scholar] [CrossRef]
- Ding, L.; Wang, H.; Liu, D.; Zeng, X.A.; Mao, Y. Bacteria Capture and Inactivation with Functionalized Multi-Walled Carbon Nanotubes (MWCNTs). J. Nanosci. Nanotechnol. 2020, 20, 2055–2062. [Google Scholar] [CrossRef]
- Baek, S.; Joo, S.H.; Toborek, M. Antibacterial effects of graphene- and carbon nanotube-based nanohybrids on Escherichia coli: Implications for treating multidrug-resistant bacteria. J. Environ. Manag. 2019, 247, 214–223. [Google Scholar] [CrossRef]
- Maas, M. Carbon Nanomaterials as Antibacterial Colloids. Materials 2016, 9, 617. [Google Scholar] [CrossRef]
- Helian, L.; Yanhua, Q. Dispersion, sedimentation and aggregation of multi-walled carbon nanotubes as affected by single and binary mixed surfactants. R. Soc. Open Sci. 2019, 6, 190241. [Google Scholar] [CrossRef]
- Bai, Y.; Gao, J.; Wang, C.; Zhang, R.; Ma, W. Mixed Surfactant Solutions for the Dispersion of Multiwalled Carbon Nanotubes and the Study of Their Antibacterial Activity. J. Nanosci. Nanotechnol. 2016, 16, 2239–2245. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.R.; Yang, L. Inactivation of Bacterial Pathogens by Carbon Nanotubes in Suspensions. Langmuir 2009, 25, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Olivi, M.; Zanni, E.; De Bellis, G.; Talora, C.; Sarto, M.S.; Palleschi, C.; Flahaut, E.; Monthioux, M.; Rapino, S.; Uccelletti, D.; et al. Inhibition of microbial growth by carbon nanotube networks. Nanoscale 2013, 5, 9023. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.F.; Wu, W.L.; Du, Y.; Chin, C.J.M.; Lin, C.C. Inactivation of Escherichia coli planktonic cells by multi-walled carbon nanotubes in suspensions: Effect of surface functionalization coupled with medium nutrition level. J. Hazard. Mater. 2016, 318, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zheng, X.; Chen, A.; Chen, Y.; He, G.; Chen, H. Hydroxyl functionalization of single-walled carbon nanotubes causes inhibition to the bacterial denitrification process. Chem. Eng. J. 2015, 279, 47–55. [Google Scholar] [CrossRef]
- Anju, V.; Paramanantham, P.; SB, S.L.; Sharan, A.; Syed, A.; Bahkali, N.A.; Alsaedi, M.H.; Kaviyarasu, K.; Busi, S. Antimicrobial photodynamic activity of toluidine blue-carbon nanotube conjugate against Pseudomonas aeruginosa and Staphylococcus aureus - understanding the mechanism of action. Photodiagnosis Photodyn. 2019, 27, 305–316. [Google Scholar] [CrossRef]
- Atiyah, A.A.; Haider, A.J.; Dhahi, R.M. Cytotoxicity properties of functionalised carbon nanotubes on pathogenic bacteria. Iet Nanotechnol. 2019, 13, 597–601. [Google Scholar] [CrossRef]
- Lohan, S.; Raza, K.; Singla, S.; Chhibber, S.; Wadhwa, S.; Katare, O.P.; Kumar, P.; Singh, B. Studies on Enhancement of Anti-microbial Activity of Pristine MWCNTs Against Pathogens. AAPS PharmSciTech 2016, 17, 1042–1048. [Google Scholar] [CrossRef][Green Version]
- Marković, Z.; Jovanović, S.; Kleut, D.; Romčević, N.; Jokanović, V.; Trajković, V.; Todorović-Marković, B. Comparative study on modification of single wall carbon nanotubes by sodium dodecylbenzene sulfonate and melamine sulfonate super plasticiser. Appl. Surf. Sci. 2009, 255, 6359–6366. [Google Scholar] [CrossRef]
- Vaisman, L.; Wagner, H.D.; Marom, G. The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 2006, 128–130, 37–46. [Google Scholar] [CrossRef]
- Lin, D.; Liu, N.; Yang, K.; Xing, B.; Wu, F. Different stabilities of multiwalled carbon nanotubes in fresh surface water samples. Env. Pollut. 2010, 158, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Strano, M.S.; Moore, V.C.; Miller, M.K.; Allen, M.J.; Haroz, E.H.; Kittrell, C.; Hauge, R.H.; Smalley, R.E. The role of surfactant adsorption during ultrasonication in the dispersion of single-walled carbon nanotubes. J. Nanosci. Nanotechnol. 2003, 3, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, B.; Poorgholami-Bejarpasi, N.; Nayeri, N. Dispersion of carbon nanotubes using mixed surfactants: Experimental and molecular dynamics simulation studies. J. Phys. Chem. B 2014, 118, 3094–3103. [Google Scholar] [CrossRef] [PubMed]
- Poorsargol, M.; Alimohammadian, M.; Sohrabi, B.; Dehestani, M. Dispersion of graphene using surfactant mixtures: Experimental and molecular dynamics simulation studies. Appl. Surf. Sci. 2019, 464, 440–450. [Google Scholar] [CrossRef]
- Yang, C.; Mamouni, J.; Tang, Y.; Yang, L. Antimicrobial activity of single-walled carbon nanotubes: Length effect. Langmuir 2010, 26, 16013–16019. [Google Scholar] [CrossRef]
- Dong, L.; Henderson, A.; Field, C. Antimicrobial Activity of Single-Walled Carbon Nanotubes Suspended in Different Surfactants. J. Nanotechnol. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial Activity of Carbon-Based Nanoparticles. Adv. Pharm. Bull. 2015, 5, 19–23. [Google Scholar] [CrossRef]
- Chung, H.; Son, Y.; Yoon, T.K.; Kim, S.; Kim, W. The effect of multi-walled carbon nanotubes on soil microbial activity. Ecotoxicol. Env. Saf. 2011, 74, 569–575. [Google Scholar] [CrossRef]
- Jin, L.; Son, Y.; Yoon, T.K.; Kang, Y.J.; Kim, W.; Chung, H. High concentrations of single-walled carbon nanotubes lower soil enzyme activity and microbial biomass. Ecotoxicol. Env. Saf. 2013, 88, 9–15. [Google Scholar] [CrossRef]
- Akasaka, T.; Watari, F. Capture of bacteria by flexible carbon nanotubes. Acta Biomater. 2009, 5, 607–612. [Google Scholar] [CrossRef]
- Urse, E.L.; Rosca, I.; Bahrin, L.G.; Clima, L.; Bejan, D.; Sardaru, M.C.; Marangoci, N.; Lozan, V.; Rotaru, A. Aqueous Dispersion of Single-Walled Carbon Nanotubes Using Tetra-Phenyl Bimesitylene Derivative via Noncovalent Modification and Improved Antimicrobial Activity. J. Nanosci. Nanotechnol. 2019, 19, 7960–7966. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, B.; Gao, D.; Guan, M.; Zheng, L.; Ouyang, H.; Feng, W. Broad-Spectrum Antibacterial Activity of Carbon Nanotubes to Human Gut Bacteria. Small 2013, 9, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.; Kaushal, R.; Tripathi, S.K.; Sharma, A.L.; Kaur, I.; Bharadwaj, L.M. Comparative study of carbon nanotube dispersion using surfactants. J. Colloid Interface Sci. 2008, 328, 421–428. [Google Scholar] [CrossRef]
- Crawford, R.J.; Webb, H.K.; Truong, V.K.; Hasan, J.; Ivanova, E.P. Surface topographical factors influencing bacterial attachment. Adv. Colloid Interface Sci. 2012, 9, 179–182. [Google Scholar] [CrossRef]
- Liu, S.; Wei, L.; Hao, L.; Fang, N.; Chang, M.W.; Xu, R.; Yang, Y.; Chen, Y. Sharper and faster “nano darts” kill more bacteria: A study of antibacterial activity of individually dispersed pristine single-walled carbon nanotube. ACS Nano 2009, 12, 3891–3902. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef]
- Dong, X.; Yang, L. Inhibitory effects of single-walled carbon nanotubes on biofilm formation from Bacillus anthracis spores. Biofouling 2014, 30, 1165–1174. [Google Scholar] [CrossRef]
- Rodrigues, D.F.; Elimelech, M. Toxic effects of single-walled carbon nanotubes in the development of E. coli biofilm. Env. Sci. Technol. 2010, 44, 4583–4589. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial effects of carbon nanotubes: Size does matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef]
- Deng, S.; Upadhyayula, V.K.K.; Smith, G.B.; Mitchell, M.C. Adsorption equilibrium and kinetics of microorganisms on single-walled carbon nanotubes. IEEE Sens. J. 2008, 8, 954–962. [Google Scholar] [CrossRef]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleemi, M.A.; Fouladi, M.H.; Yong, P.V.C.; Wong, E.H. Elucidation of Antimicrobial Activity of Non-Covalently Dispersed Carbon Nanotubes. Materials 2020, 13, 1676. https://doi.org/10.3390/ma13071676
Saleemi MA, Fouladi MH, Yong PVC, Wong EH. Elucidation of Antimicrobial Activity of Non-Covalently Dispersed Carbon Nanotubes. Materials. 2020; 13(7):1676. https://doi.org/10.3390/ma13071676
Chicago/Turabian StyleSaleemi, Mansab Ali, Mohammad Hosseini Fouladi, Phelim Voon Chen Yong, and Eng Hwa Wong. 2020. "Elucidation of Antimicrobial Activity of Non-Covalently Dispersed Carbon Nanotubes" Materials 13, no. 7: 1676. https://doi.org/10.3390/ma13071676
APA StyleSaleemi, M. A., Fouladi, M. H., Yong, P. V. C., & Wong, E. H. (2020). Elucidation of Antimicrobial Activity of Non-Covalently Dispersed Carbon Nanotubes. Materials, 13(7), 1676. https://doi.org/10.3390/ma13071676



