Preparation of Microcapsules Coating and the Study of Their Bionic Anti-Fouling Performance
Abstract
1. Introduction
2. Experiments
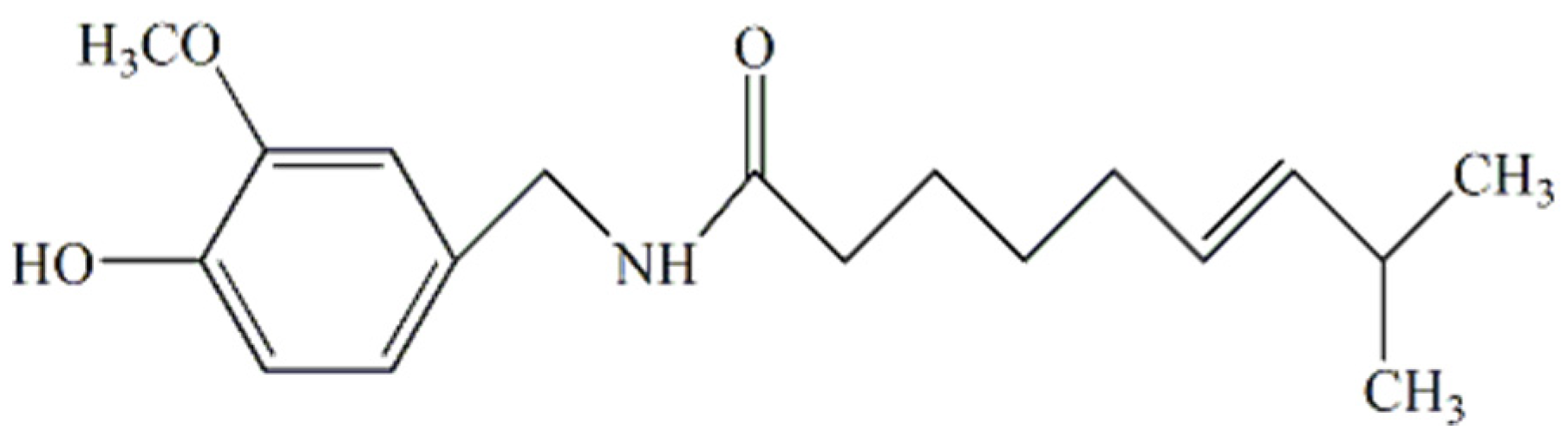
2.1. Materials
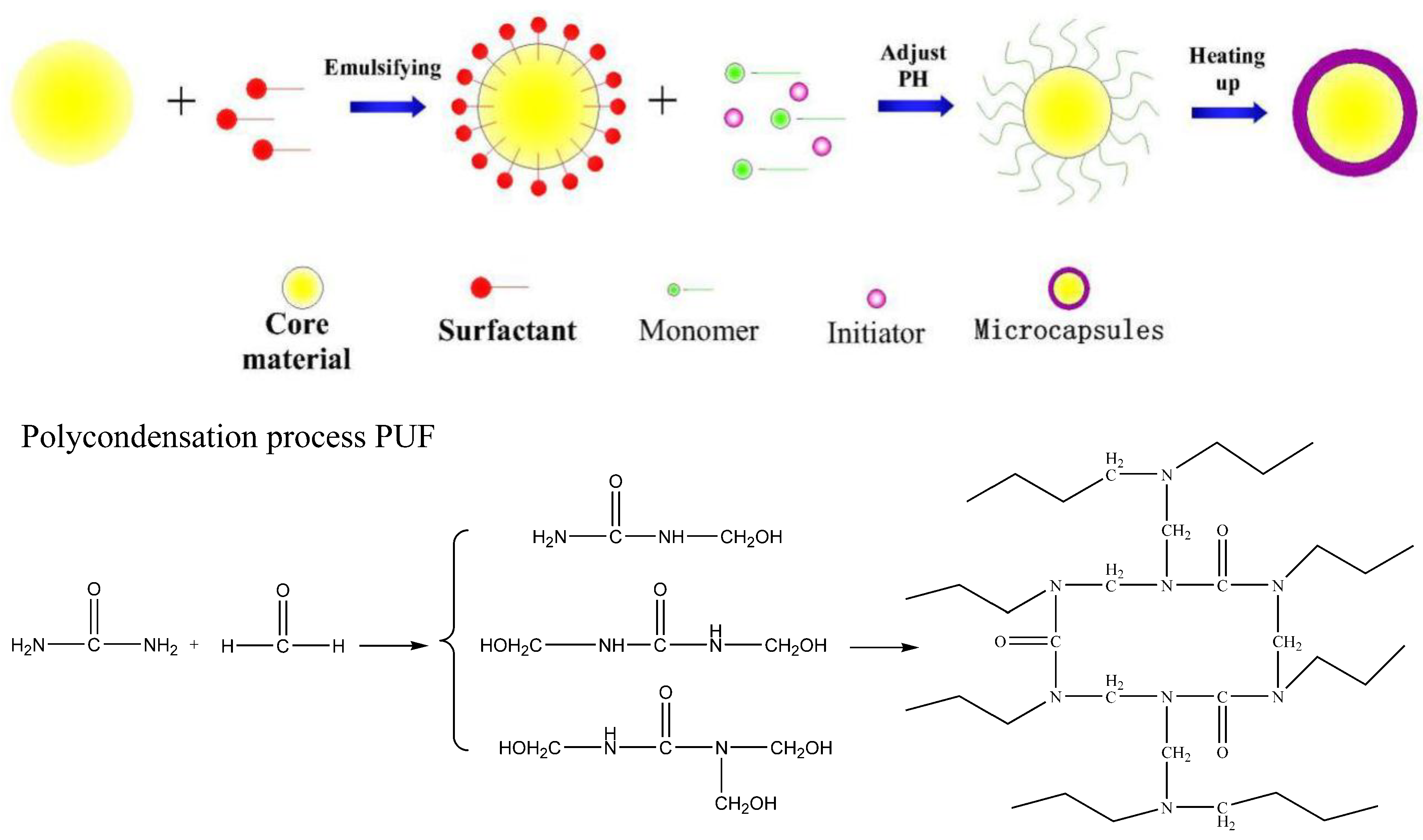
2.2. Preparation of the Microcapsules (MOCs)
2.3. Fabrication of the Coatings
2.3.1. Electrochemical Impedance Spectroscopy (EIS) Testing Plate
2.3.2. Plate for Real-Sea Anti-Fouling Properties Testing
2.4. Characterization
2.4.1. Optical and Scanning Electron Microscopy
2.4.2. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
2.4.3. Fourier Transform Infrared (FTIR) Analysis
2.4.4. Soxhlet Extraction for the Core Material
2.4.5. Water Contact Angle Measurements
2.4.6. Electrochemical Tests
2.4.7. Real-Sea Tests of the Coatings
3. Results and Discussion
3.1. Formation Mechanism
3.2. Characterization of the Microcapsules (MOCs)
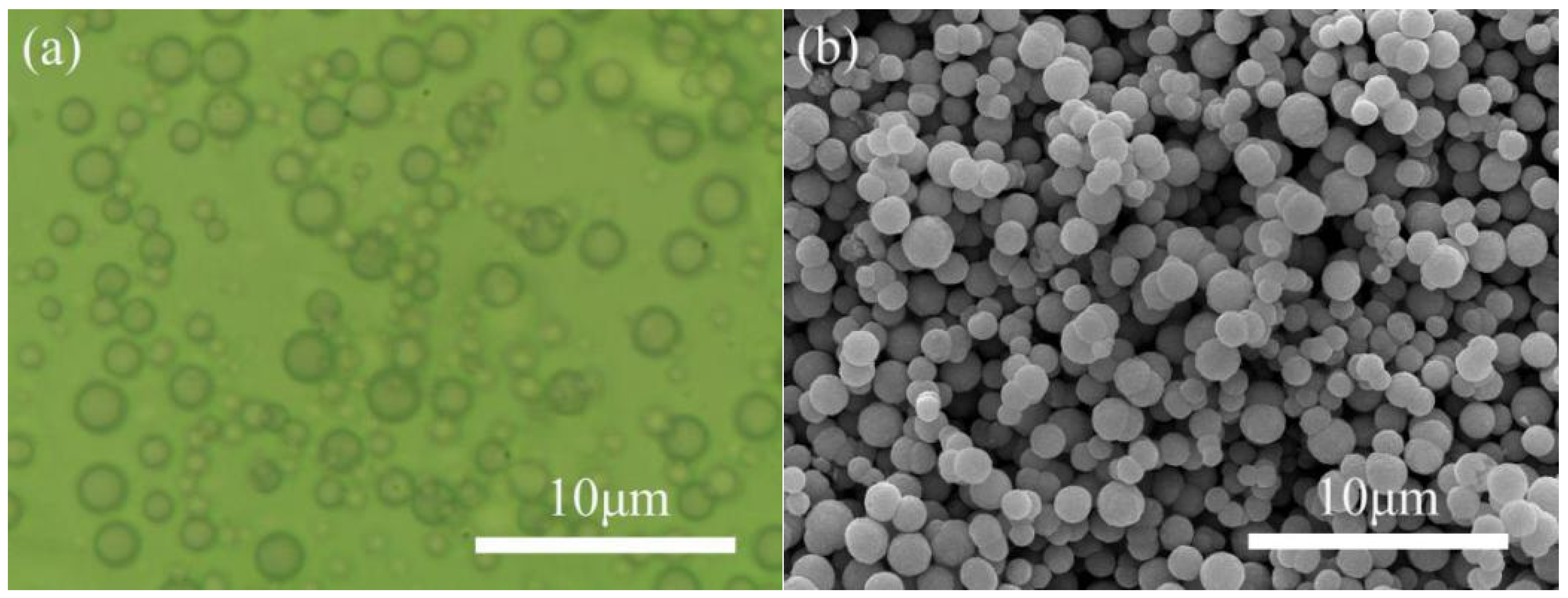
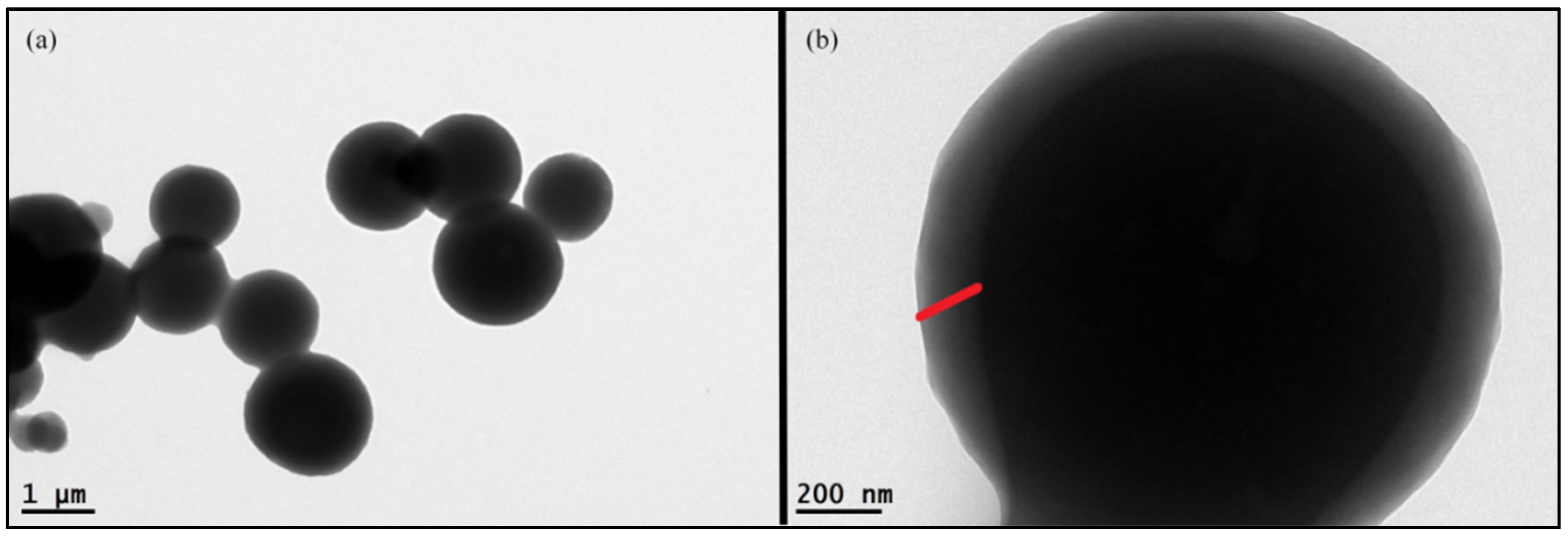
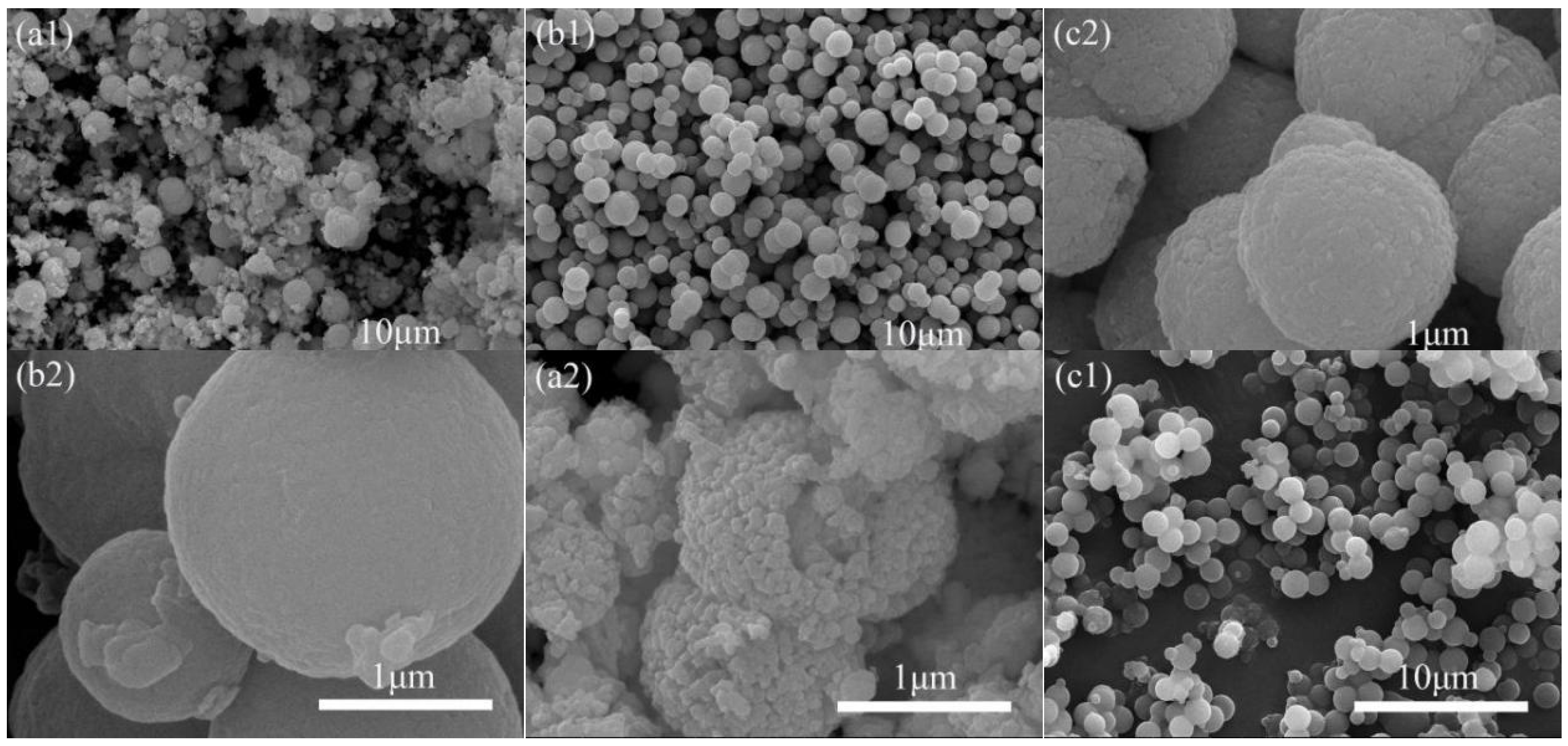
3.2.1. Morphology of the MOCs
3.2.2. Fourier Transform Infrared (FTIR) Spectroscopy
3.2.3. The Yield and the Content of Core Material for Microcapsule
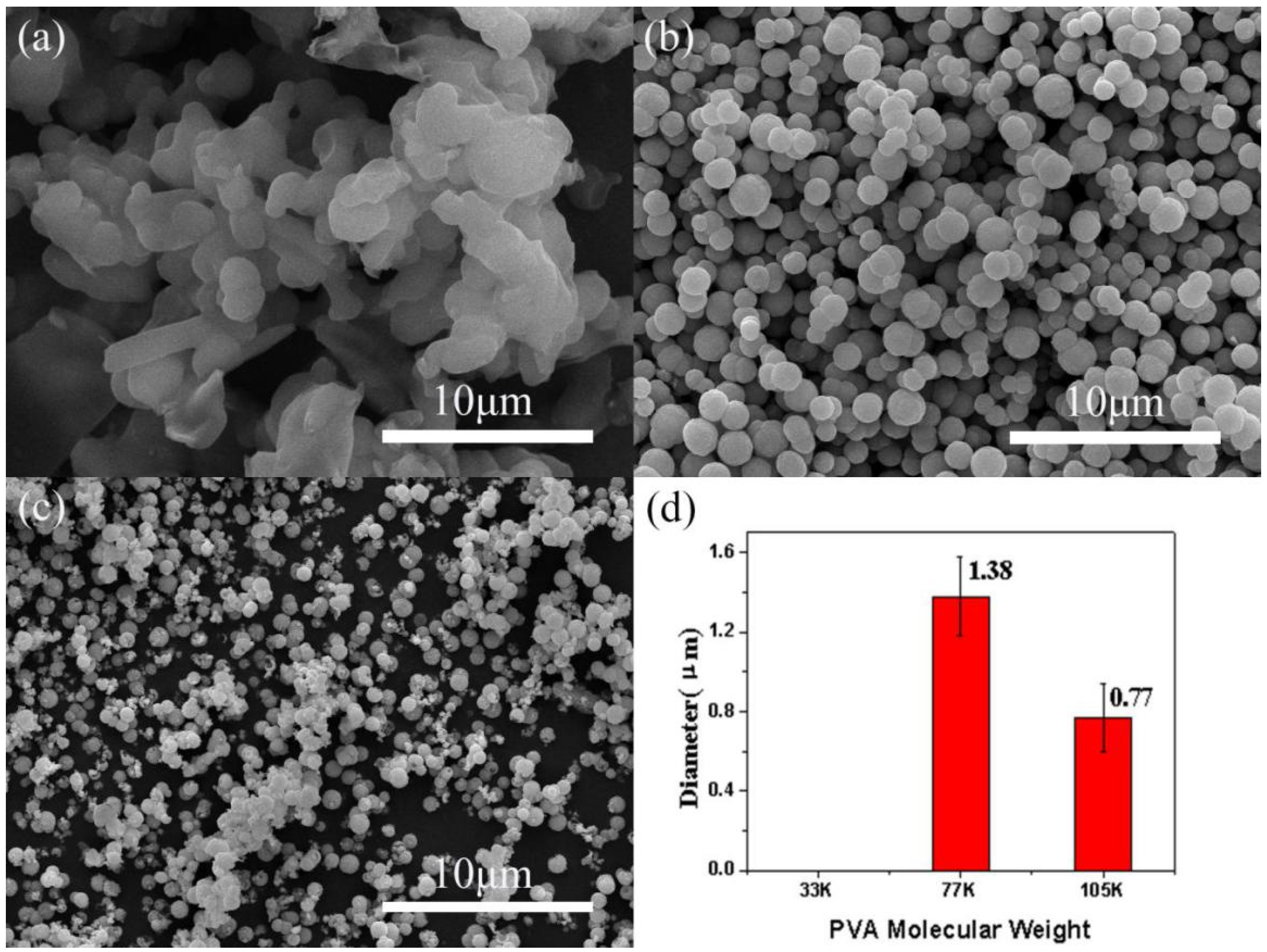
3.2.4. Effect of the Molecular Weight of the PVA Stabilizer
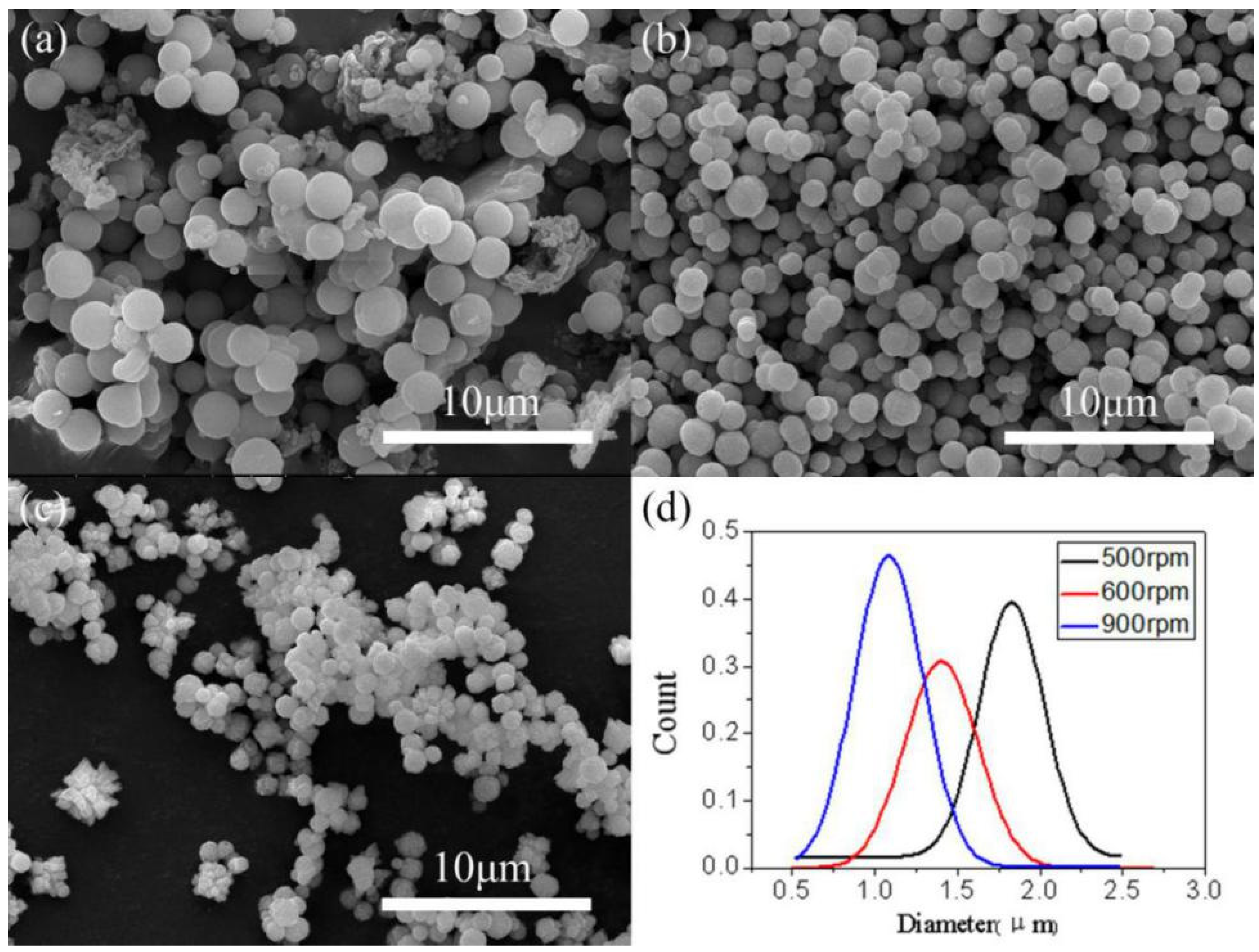
3.2.5. Effect of the Stirring Rate
3.2.6. Effect of Temperature
3.3. The Anti-Fouling Performance of the Microcapsule’s Coatings
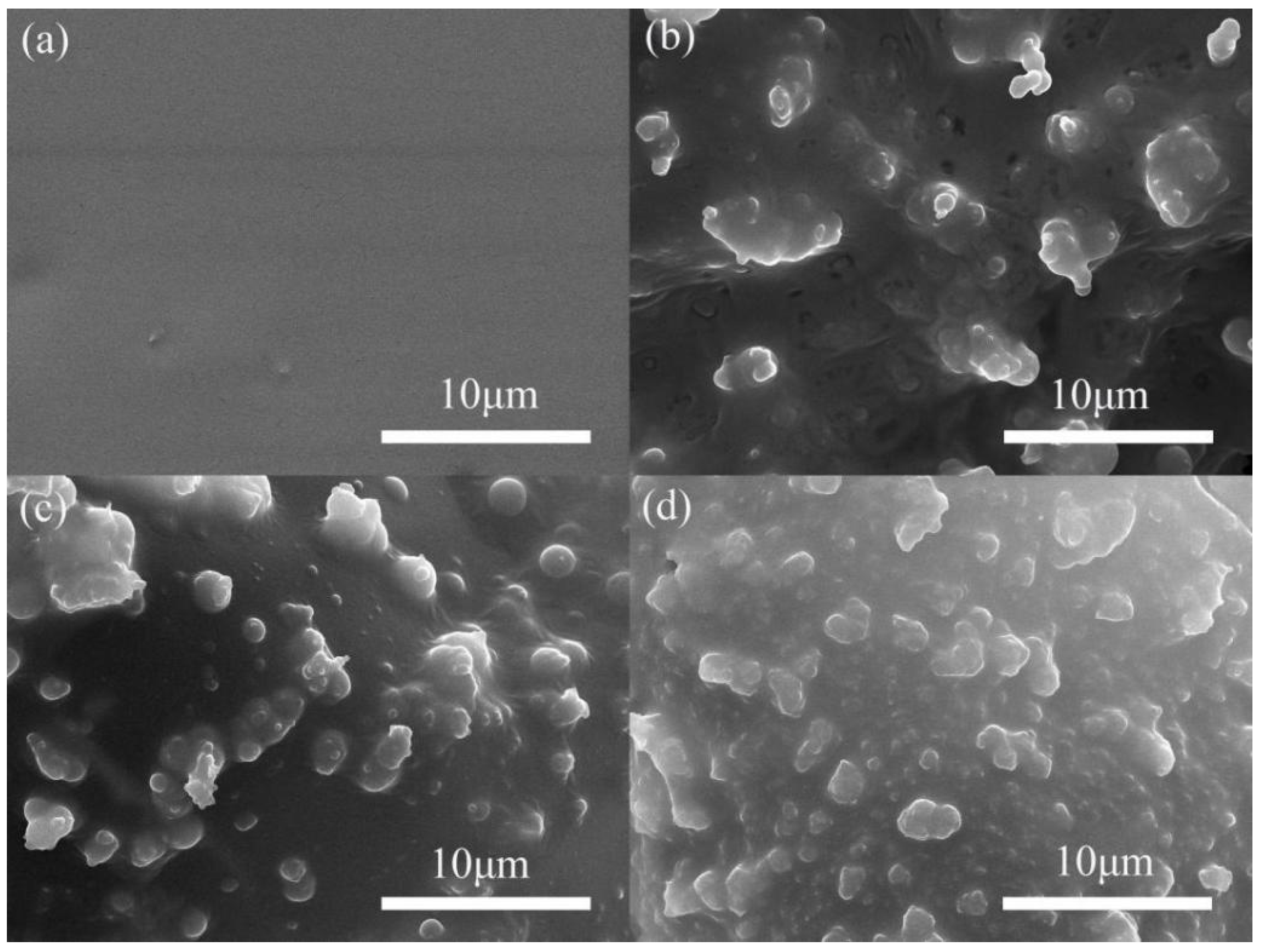
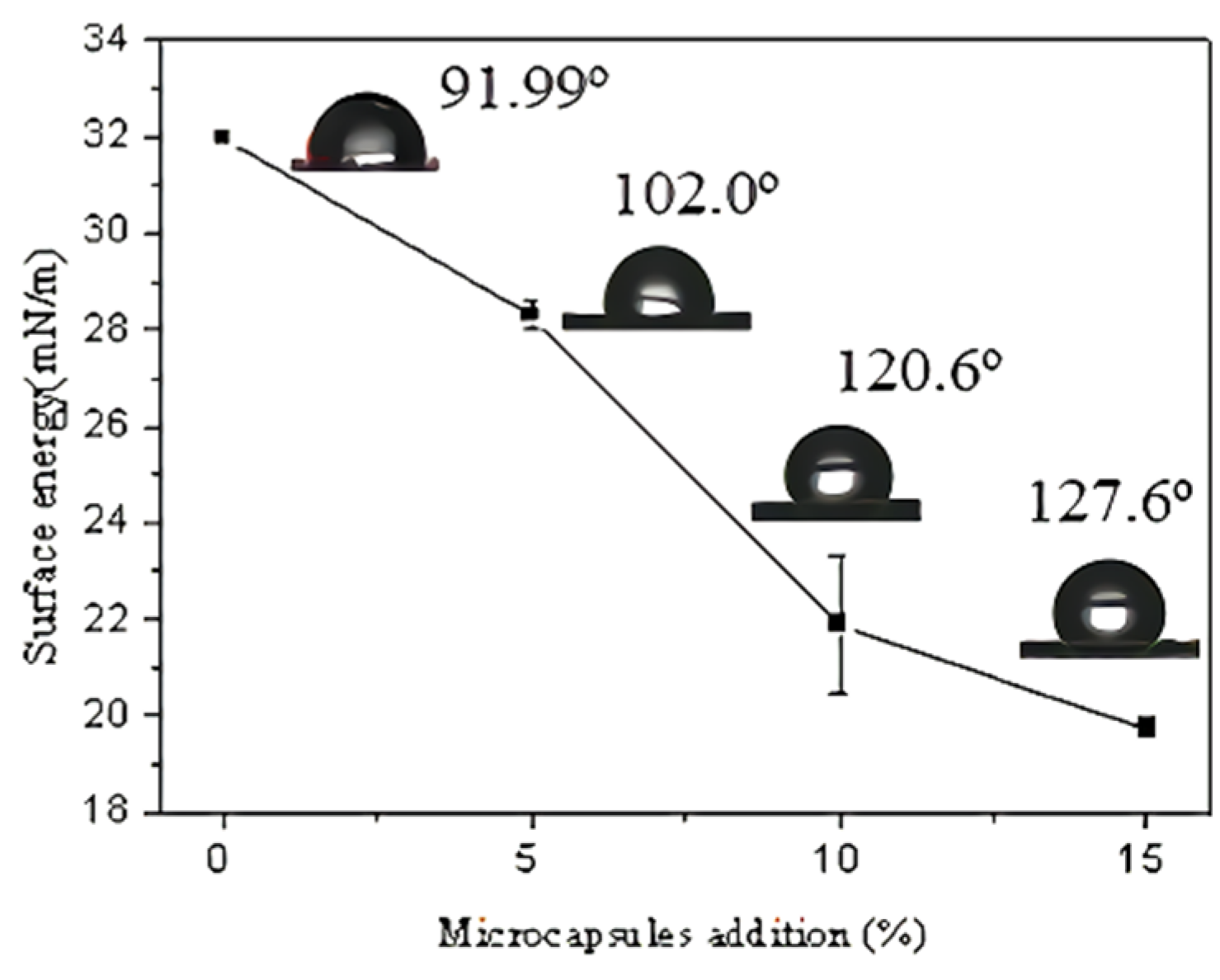
3.3.1. Contact Angles and Slow-Release Effect of the Microcapsules Coating
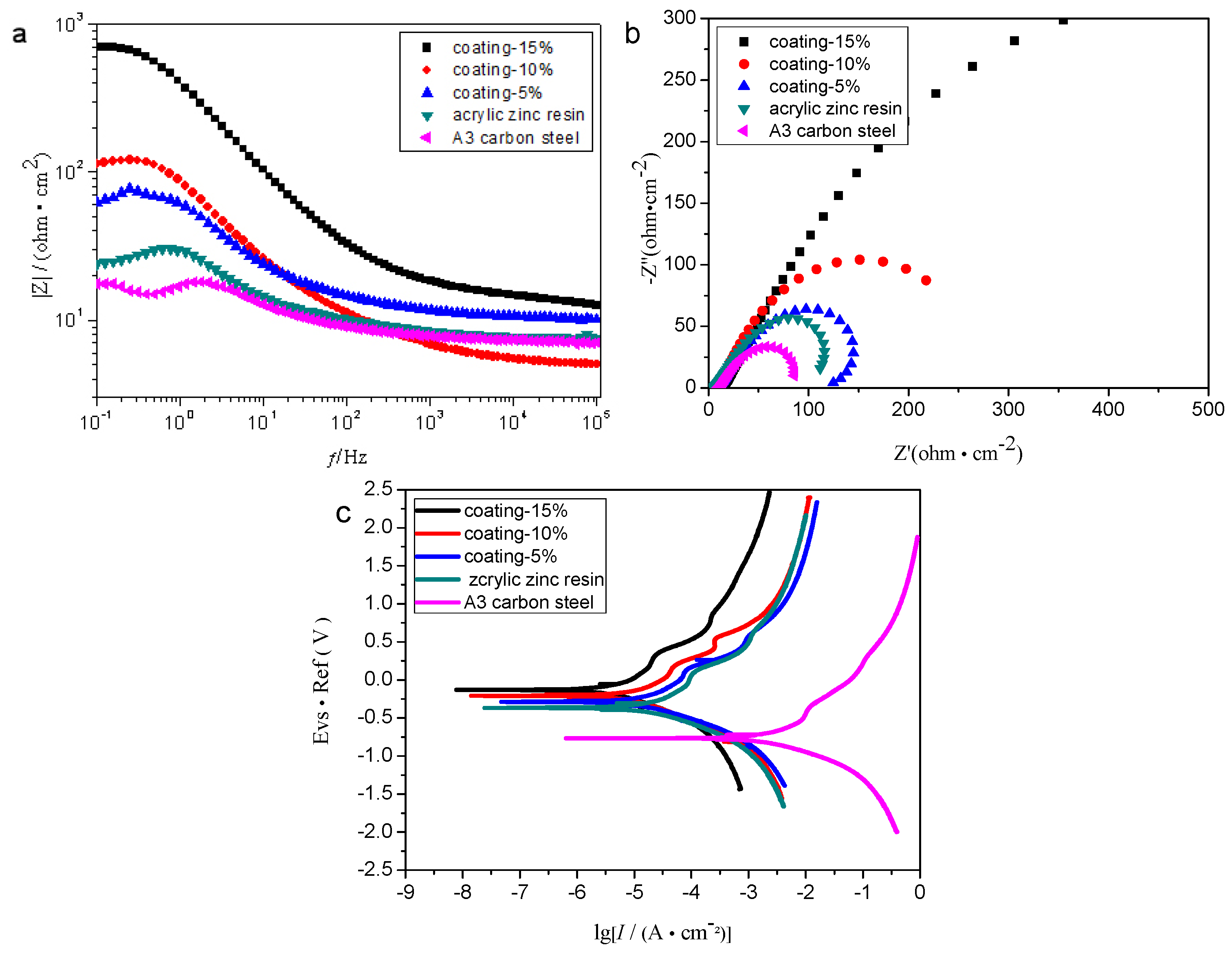
3.3.2. Electrochemical Tests (EIS)
3.3.3. Real-Sea Tests of the Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gogoi, P.; Boruah, M.; Bora, C.; Dolui, S.K. Jatropha curcas oil based alkyd/epoxy resin/expanded graphite (EG) reinforced bio-composite: Evaluation of the thermal, mechanical and flame retardancy properties. Prog. Org. Coat. 2014, 77, 87–93. [Google Scholar] [CrossRef]
- Liu, K.S.; Tian, Y.; Jiang, L. Bio-inspired superoleophobic and smart materials: Design, fabrication, and application. Progr. Mater. Sci. 2013, 58, 503–564. [Google Scholar] [CrossRef]
- Amini, S.; Kolle, S.; Petrone, L.; Ahanotu, O.; Sunny, S.; Sutanto, C.N.; Hoon, S.; Cohen, L.; Weaver, J.C.; Aizenberg, J. Preventing mussel adhesion using lubricant-infused materials. Science 2017, 357, 668–673. [Google Scholar] [CrossRef]
- Wen, L.P.; Tian, Y.; Jiang, L. Bioinspired super-wettability from fundamental research to practical applications. Angew. Chem. Int. Edit. 2015, 54, 3387–3399. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Wu, Z.H.; Wang, Y.; Wang, C.Y.; Xu, Y. Mini-Review: Antifouling Natural Products from Marine Microorganisms and Their Synthetic Analogs. Mar. Drugs 2017, 15, 266. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Ye, R.; Xu, Y.; Gao, Z.M.; Au, D.W.T.; Qian, P.Y. Comparative safety of the anti-fouling compound butenolide and 4,5-dichloro-2-n-octyl-4-isothiazolin-3-one (DCOIT) to the marine medaka (Oryzias melastigma). Aquat. Toxicol. 2014, 149, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Sun, J.; Zhang, H.M.; Au, D.W.T.; Lam, P.K.S.; Zhang, W.P.; Bajic, V.B.; Qiu, J.W.; Qian, P.Y. Hepatic Proteomic Responses in Marine Medaka (Oryzias melastigma) Chronically Exposed to anti-fouling Compound Butenolide [5-octylfuran-2(5H)-one] or 4,5-Dichloro-2-N-Octyl-4-Isothiazolin-3-One (DCOIT). Environ. Sci. Technol. 2015, 49, 1851–1859. [Google Scholar] [CrossRef]
- Chen, L.G.; Xu, Y.; Wang, W.X.; Qian, P.Y. Degradation kinetics of a potent anti-fouling agent, butenolide, under various environmental conditions. Chemosphere 2015, 119, 1075–1083. [Google Scholar] [CrossRef]
- Ma, C.F.; Xu, L.G.; Xu, W.T.; Zhang, G.Z. Degradable polyurethane for marine anti-biofouling. J. Mater. Chem. B 2013, 1, 3099–3106. [Google Scholar] [CrossRef]
- Yao, J.H.; Chen, S.S.; Ma, C.F.; Zhang, G.Z. Marine anti-biofouling system with poly (ε-caprolactone)/clay composite as carrier of organic antifoulant. J. Mater. Chem. B 2014, 2, 5100–5106. [Google Scholar] [CrossRef]
- Qian, P.Y.; Li, Z.R.; Xu, Y.; Li, Y.X.; Fusetani, N. Mini-review: Marine natural products and their synthetic analogs as anti-fouling compounds: 2009-2014. Biofouling 2015, 31, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.; Abel, P.D.; Arnold, D.W.; Miline, A. Cost–benefit analysis of the use of TBT: The case for a treatment approach. Sci. Total Environ. 2000, 258, 5–19. [Google Scholar] [CrossRef]
- Pelletier, E.; Bonnet, C.; Lemarchand, K. Biofouling Growth in Cold Estuarine Waters and Evaluation of Some Chitosan and Copper Anti-Fouling Paints. Int. J. Mol. Sci. 2009, 10, 3209–3223. [Google Scholar] [CrossRef] [PubMed]
- Champ, M.A. Economic and environmental impacts on ports and harbors from the convention to ban harmful marine anti-fouling systems. Mar. Pollut. Bull. 2003, 46, 935–940. [Google Scholar] [CrossRef]
- Trojer, M.A.; Nordstierna, L.; Bergek, J.; Blanck, H.; Holmberg, K.; Nyden, M. Use of microcapsules as controlled release devices for coatings. Adv. Colloid Interface Sci. 2015, 222, 18–43. [Google Scholar] [CrossRef]
- Tamás, S.; Lívia, M.N.; János, B.; Lajos, N.; Judit, T. Self-healing microcapsules and slow release microspheres in paints. Prog. Org. Coat. 2011, 72, 52–57. [Google Scholar]
- Hao, X.P.; Wang, W.H.; Yang, Z.Q.; Yue, L.F.; Sun, H.Y.; Wang, H.F.; Guo, Z.H.; Cheng, F.; Chen, S.G. pH responsive antifouling and antibacterial multilayer films with Self-healing performance. Chem. Eng. J. 2019, 356, 130–141. [Google Scholar] [CrossRef]
- Chang, K.C.; Ji, W.F.; Lai, M.C.; Hsiao, Y.R.; Hsu, C.H.; Chuang, T.L.; Yen, W.; Yeh, J.M.; Liu, W.R. Synergistic effects of hydrophobicity and gas barrier properties on the anticorrosion property of PMMA nanocomposite coatings embedded with graphene nanosheets. Polym. Chem. 2014, 5, 1049–1056. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-inhibiting coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Chen, J.J.; Li, Y.C.; Creamer, A.E.; Chen, H. Slow-release fertilizer encapsulated by graphene oxide films. Chem. Eng. J. 2014, 255, 107–113. [Google Scholar] [CrossRef]
- Chae, H.R.; Lee, J.W.; Lee, C.H.; Kim, I.C.; Park, P.K. Graphene oxide-embedded thin-film composite reverse osmosis membrane with high flux, anti-biofouling, and chlorine resistance. J. Membr. Sci. 2015, 483, 128–135. [Google Scholar] [CrossRef]
- Bers, A.V.; Wahl, M. The influence of natural surface microtopographies on fouling. Biofouling 2004, 20, 43–51. [Google Scholar] [CrossRef]
- Schumacher, J.F.; Carman, M.L.; Estes, T.G.; Feinberg, A.W.; Wilson, L.H.; Callow, M.E.; Callow, J.A.; Finlay, J.A.; Brennan, A.B. Engineered antifouling microtopographies-effect of feature size, geometry, and roughness on settlement of zoospores of the green alga Ulva. Biofouling 2007, 23, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Magin, C.M.; Long, C.J.; Cooper, S.P.; Ista, L.K.; Lopez, G.P.; Brennan, A.B. Engineered antifouling microtopographies: The role of Reynolds number in a model that predicts attachment of zoospores of Ulva and cells of Cobetia marina. Biofouling 2010, 26, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Domel, A.G.; Saadat, M.; Weaver, J.C.; Haj-Hariri, H.; Bertoldi, K.; Lauder, G.V. Shark skin-inspired designs that improve aerodynamic performance. J. R. Soc. Interf. 2018, 15, 139. [Google Scholar] [CrossRef]
- Nair, B.R.; Francis, D.J. Kinetics and mechanism of urea-formaldehyde reaction. Polymer 1983, 24, 626–630. [Google Scholar] [CrossRef]
- Lang, S.N.; Zhou, Q.X. Synthesis and characterization of poly(urea-formaldehyde) microcapsules containing linseed oil for self-healing coating development. Prog. Org. Coat. 2017, 105, 99–110. [Google Scholar] [CrossRef]
- Li, R.; Yan, X.F.; Yu, L.M.; Dong, L.; Zhu, F.Y. Dependence of Micro/Nano-Cu2O Structures: Controlled Morphology Synthesis, and Photocatalytic and Antifouling Property. Chin. J. Inorg. Chem. 2014, 30, 2258–2269. [Google Scholar]
- Galhenage, P.T.; Hoffffman, D.; Silbert, D.S.; Stafslien, J.S.; Daniels, J.; Miljkovic, T.; Finlay, A.J.; Franco, C.S.; Clare, S.A.; Nedved, T.B.; et al. Fouling-Release Performance of Silicone Oil-Modified Siloxane-Polyurethane Coatings. ACS Appl. Mater. Interf. 2016, 8, 29025–29036. [Google Scholar] [CrossRef]
- Liu, C.; Xie, Q.Y.; Ma, C.F.; Zhang, G.Z. Fouling Release Property of Polydimethylsiloxane-Based Polyurea with Improved Adhesion to Substrate. Ind. Eng. Chem. Res. 2016, 55, 6671–6676. [Google Scholar] [CrossRef]
- Xie, Q.Y.; Pan, J.S.; Ma, C.F.; Zhang, G.Z. Dynamic surface antifouling: Mechanism and systems. Soft Matter 2019, 15, 1087–1107. [Google Scholar] [CrossRef]
- Lu, Z.W.; Chen, Z.; Guo, Y.; Ju, Y.Y.; Liu, Y.; Feng, R.; Xiong, C.X.; Ober, C.K.; Dong, L.J. Flexible Hydrophobic Antifouling Coating with Oriented Nanotopography and Nonleaking Capsaicin. ACS Appl. Mater. Inter. 2018, 10, 9718–9726. [Google Scholar] [CrossRef]
- Asua, J.M. Miniemulsion polymerization. Prog. Polym. Sci. 2002, 27, 1283–1346. [Google Scholar] [CrossRef]
- Landfester, K. Polyreactions in Miniemulsions. Prog. Polym. Sci. 2002, 27, 689–757. [Google Scholar] [CrossRef]
- Chen, M.; Liu, J.; Liu, Y.; Guo, C.; Yang, Z.; Wu, H. Preparation and characterization of alginate-N-2-hydroxypropyl trimethyl ammonium chloride chitosan microcapsules loaded with patchouli oil. RSC Adv. 2015, 5, 14522–14530. [Google Scholar] [CrossRef]
- Liu, Y.; Suo, X.K.; Wang, Z.; Gong, Y.F.; Wang, X.; Li, H. Developing polyimide-copper antifouling coatings with capsule structures for sustainable release of copper. Mater. Des. 2017, 130, 285–293. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.Q.; Meng, Q.H.; Ding, C.H.; Jiang, H.; Fang, Y.Z. A biomimetic nano hybrid coating based on the lotus effect and its anti-biofouling behaviors. Appl. Surf. Sci. 2014, 315, 407–414. [Google Scholar] [CrossRef]
- Zheng, N.; Liu, J.; Li, W.G.; Xiao, W.; Li, Z.L. Preparation of Isocyanate-loaded Multi-wall Microcapsules and Application in Self-healing and Anticorrosive Coatings. Surf. Technol. 2019, 48, 262–269. [Google Scholar]
- Zheng, N.; Liu, J.; Li, W.G. TO/TMMP-TMTGE Double-Healing Composite Containing a Transesterifification Reversible Matrix and Tung Oil-Loaded Microcapsules for Active Self-Healing. Polymers 2019, 11, 1127. [Google Scholar] [CrossRef]
- Zampetti, E.; Pantalei, S.; Scalese, B.; Bearzotti, A.; De Cesare, F.; Spinella, C.; Macagnano, A. Biomimetic sensing layer based on electrospun conductive polymer webs. Biosens. Bioelectron. 2011, 26, 2460–2465. [Google Scholar] [CrossRef]
- Liu, K.S.; Yao, X.; Jiang, L. Recent developments in bio-inspired special wettability. Chem. Soc. Rev. 2010, 39, 3240–3255. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Hu, C.J.; Cui, J.Y.; Chen, S.C.; Wang, Y.Z. Concurrent Superhydrophobicity and Thermal Energy Storage of Microcapsule with Superior Thermal Stability and Durability. ACS Sustain. Chem. Eng. 2017, 5, 7759–7767. [Google Scholar] [CrossRef]
- Wu, G.; An, J.L.; Tang, X.Z.; Xiang, Y.; Yang, J.L. A Versatile Approach towards Multifunctional Robust Microcapsules with Tunable, Restorable, and Solvent-Proof Superhydrophobicity for Self-Healing and Self-Cleaning Coatings. Adv. Funct. Mater. 2014, 24, 6751–6761. [Google Scholar] [CrossRef]


| Ws (g) | Wc (g) | WSDBS (g) | WPVA (g) | Wa (g) | Wr (g) | Yield (%) | Core Fraction (%) |
|---|---|---|---|---|---|---|---|
| 2.5 | 2.5 | 0.1 | 0.2 | 0.28 | 0.28 | 68.91 | 72.37 |
| Sample | Corrosion Potential (Ecorr) (V/SCE) | Corrosion Current Density (Icorr) (A/cm2) |
|---|---|---|
| A3 carbon steel | −0.770 | 8.612 × 10−5 |
| zinc acrylate resin | −0.371 | 1.084 × 10−6 |
| 5% microcapsule coating | −0.283 | 1.380 × 10−6 |
| 10% microcapsule coating | −0.194 | 6.589 × 10−7 |
| 15% microcapsule coating | −0.129 | 1.903 × 10−7 |
| Paint | Immersion Time | |||
|---|---|---|---|---|
| 0 Day | One Month | Two Months | Four Months | |
| blank |  |  |  |  |
| 5% microcapsules |  |  |  |  |
| 5% Copper pyrithione |  |  |  |  |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, G.; Guo, Z.; Wang, P.; Wang, A. Preparation of Microcapsules Coating and the Study of Their Bionic Anti-Fouling Performance. Materials 2020, 13, 1669. https://doi.org/10.3390/ma13071669
Li Y, Wang G, Guo Z, Wang P, Wang A. Preparation of Microcapsules Coating and the Study of Their Bionic Anti-Fouling Performance. Materials. 2020; 13(7):1669. https://doi.org/10.3390/ma13071669
Chicago/Turabian StyleLi, Yu, Guoqing Wang, Zehui Guo, Peiqing Wang, and Aimin Wang. 2020. "Preparation of Microcapsules Coating and the Study of Their Bionic Anti-Fouling Performance" Materials 13, no. 7: 1669. https://doi.org/10.3390/ma13071669
APA StyleLi, Y., Wang, G., Guo, Z., Wang, P., & Wang, A. (2020). Preparation of Microcapsules Coating and the Study of Their Bionic Anti-Fouling Performance. Materials, 13(7), 1669. https://doi.org/10.3390/ma13071669






