Organic Acid Regulated Self-Assembly and Photophysical Properties of Perylene Bisimide Derivatives
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis and Characterization
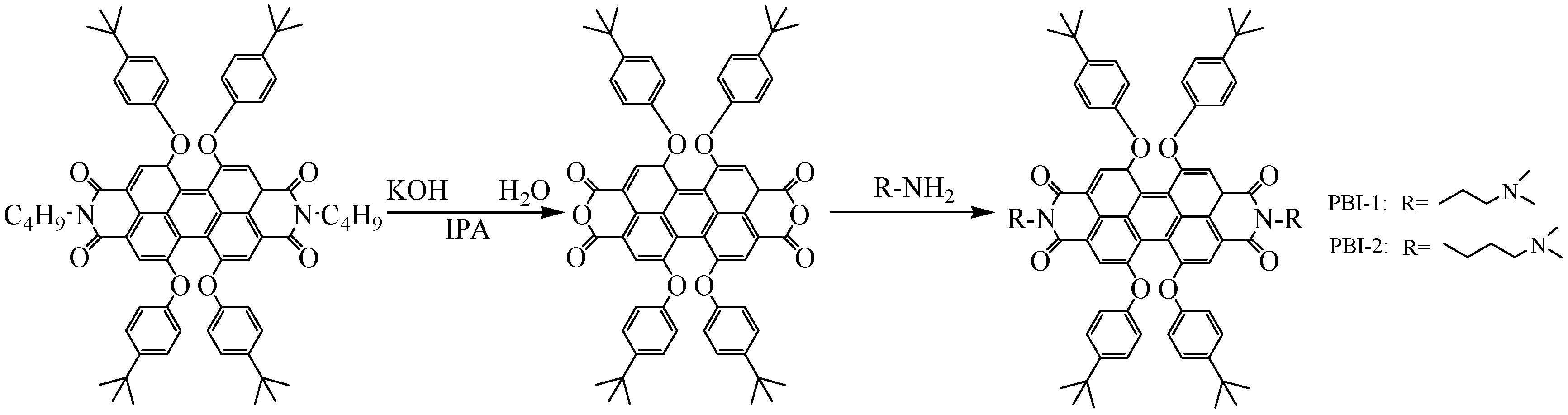
2.2.1. Synthesis of PBI-R
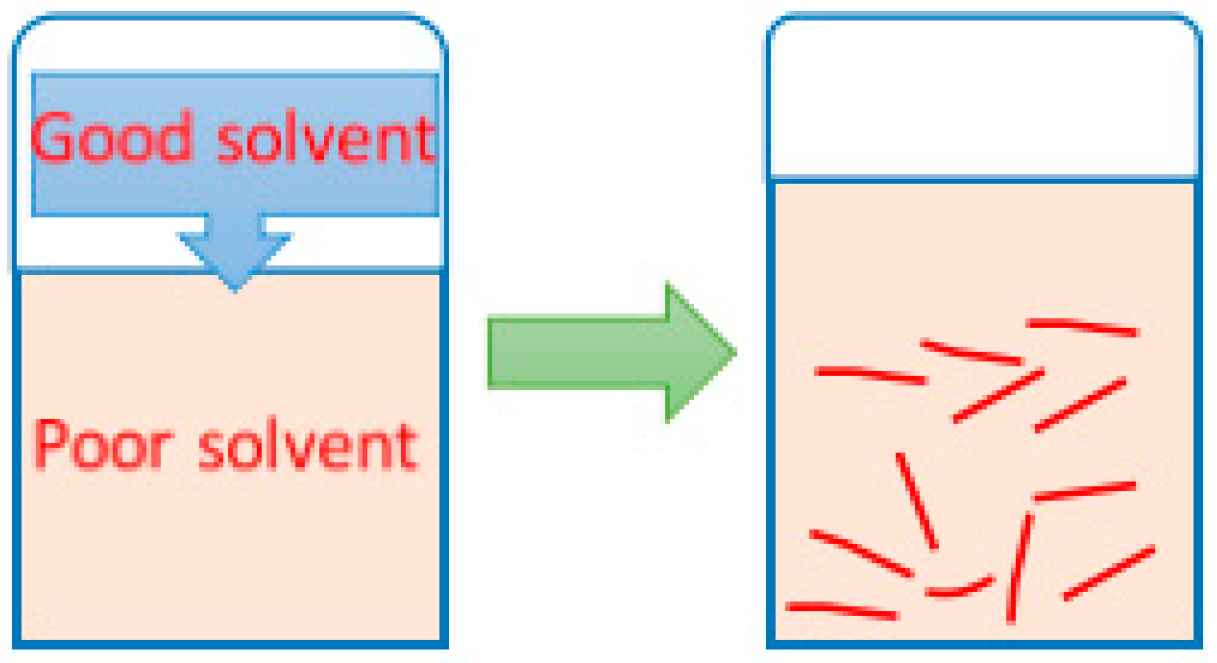
2.2.2. Self-Assembly of PBI-R
2.3. Characterization
3. Results and Discussion
3.1. Chemical Structures Dependence
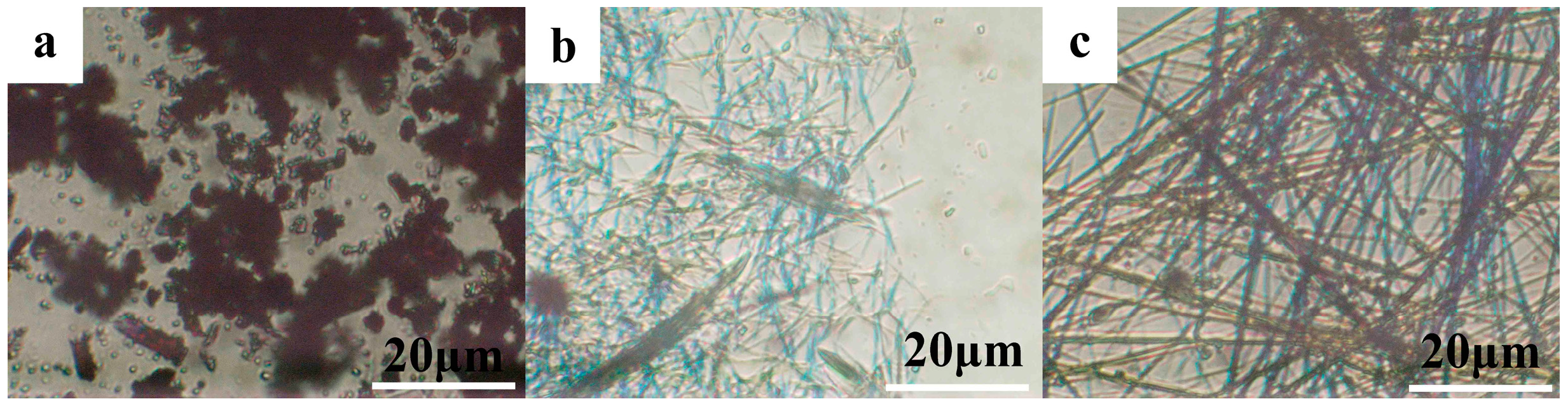
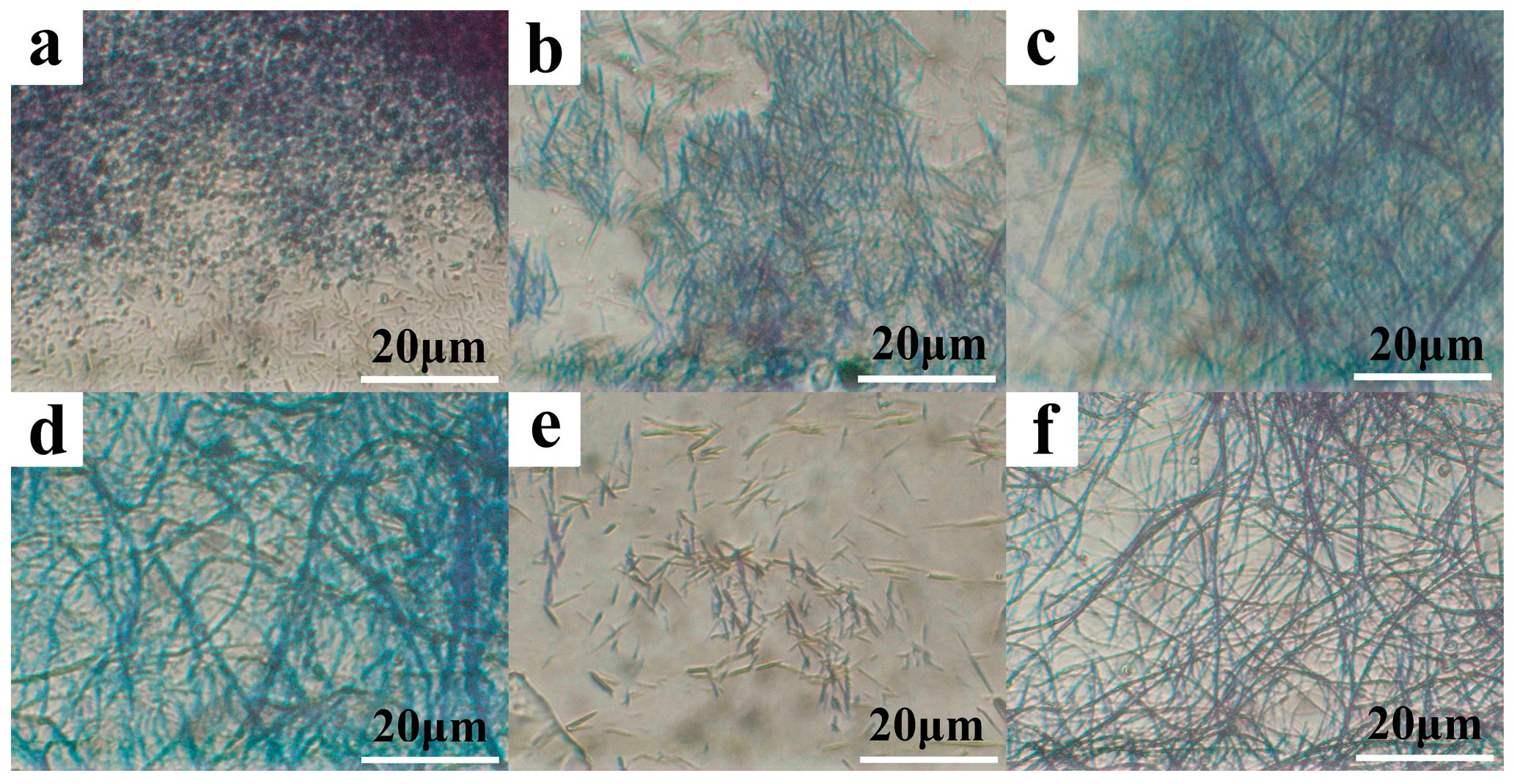
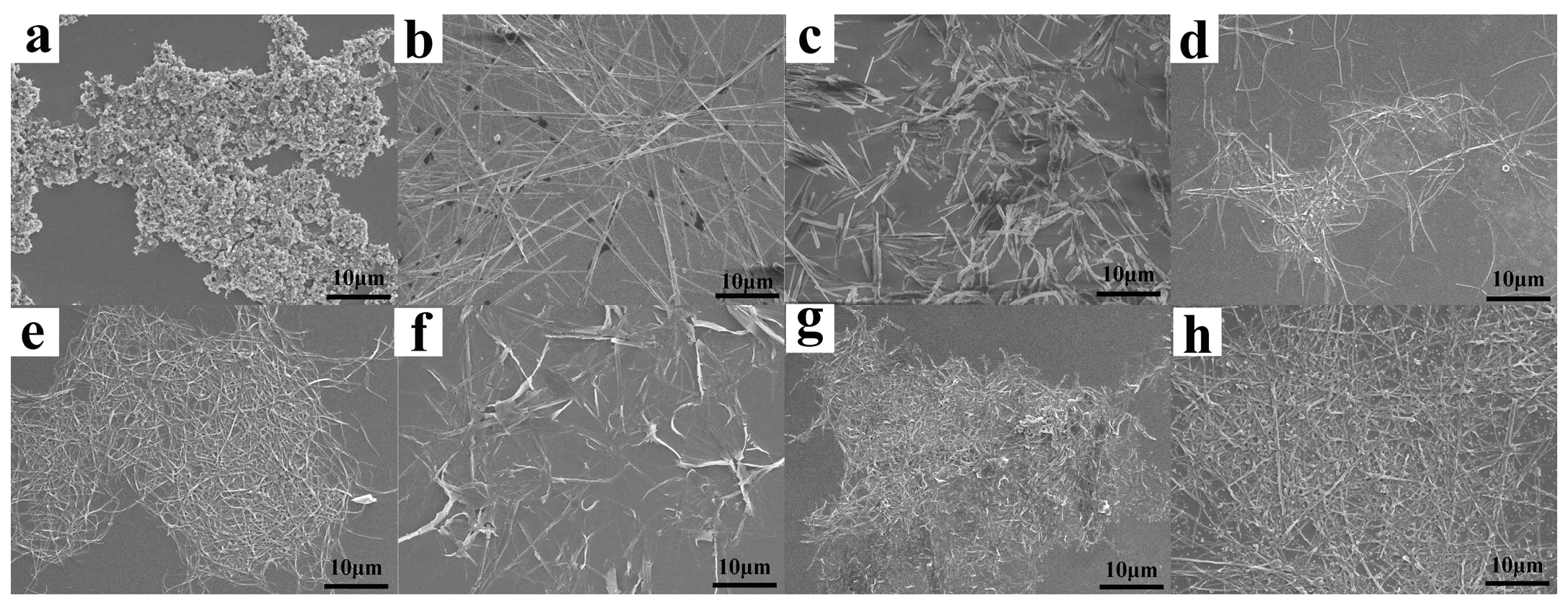
3.2. Morphologies of PBI-1 Aggregates
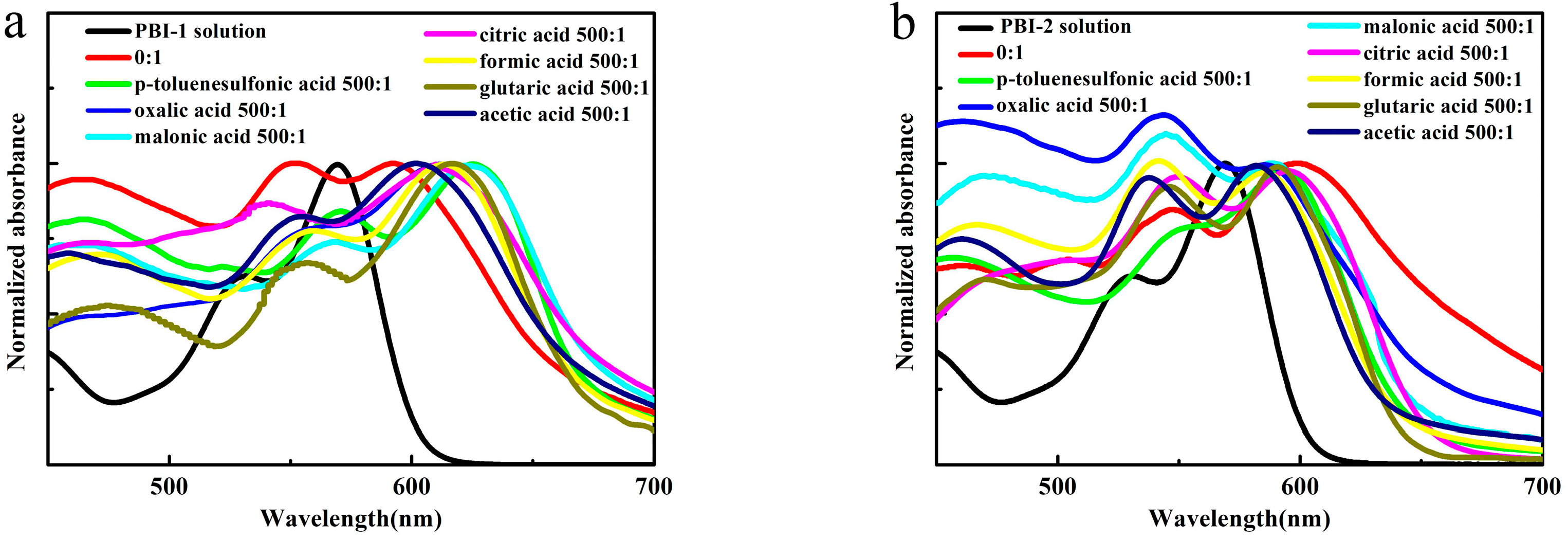
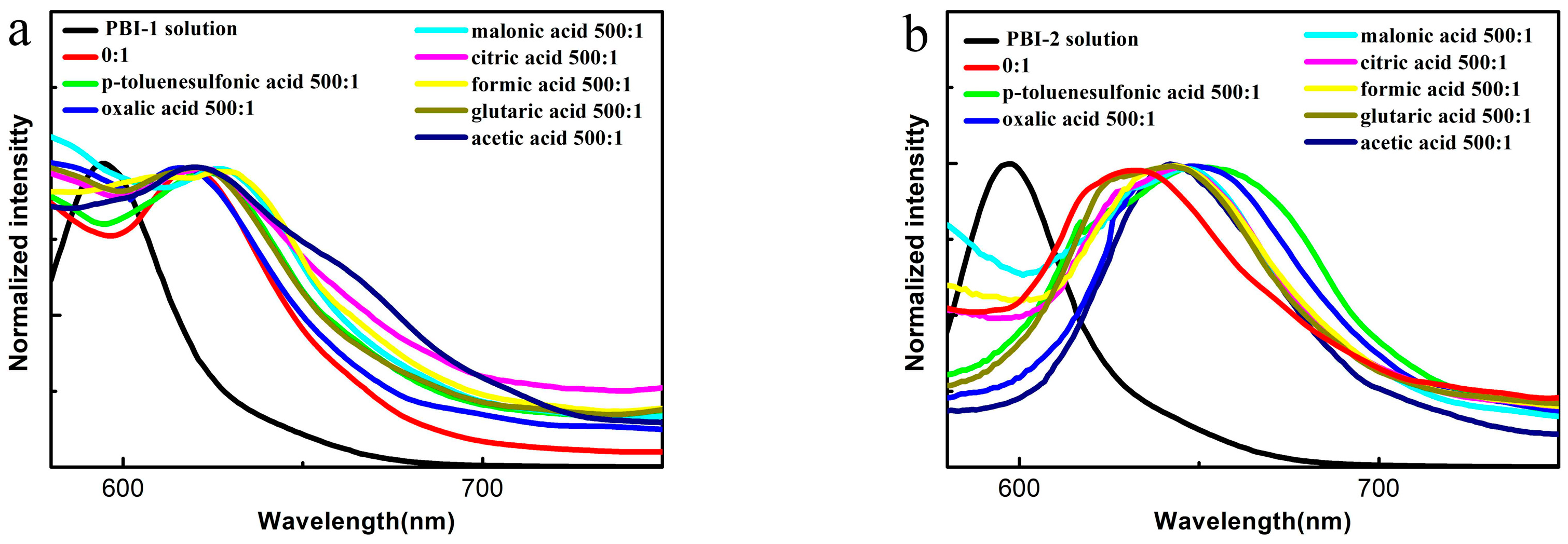
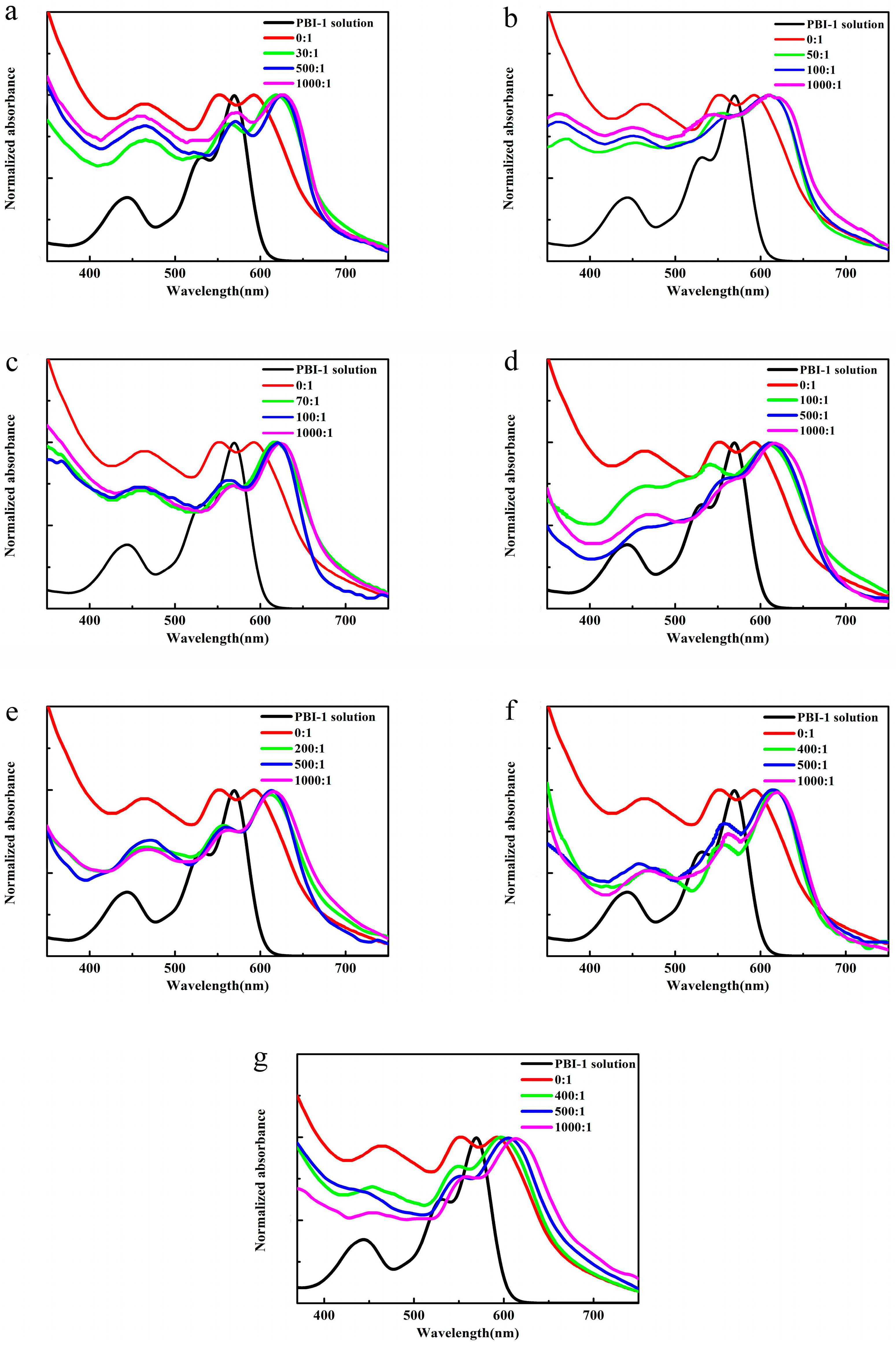
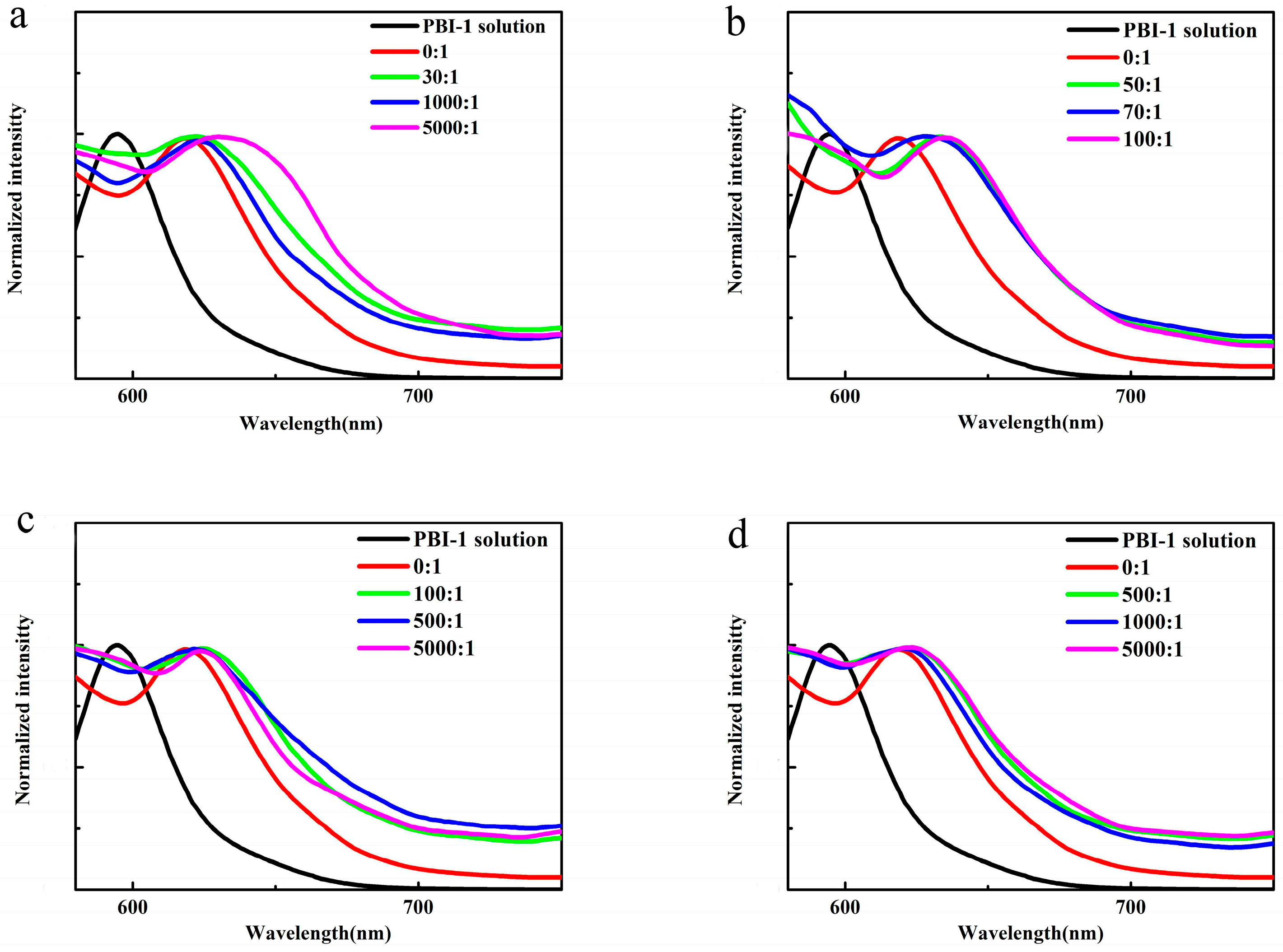
3.3. Photophysical Properties of PBI-1 Aggregates
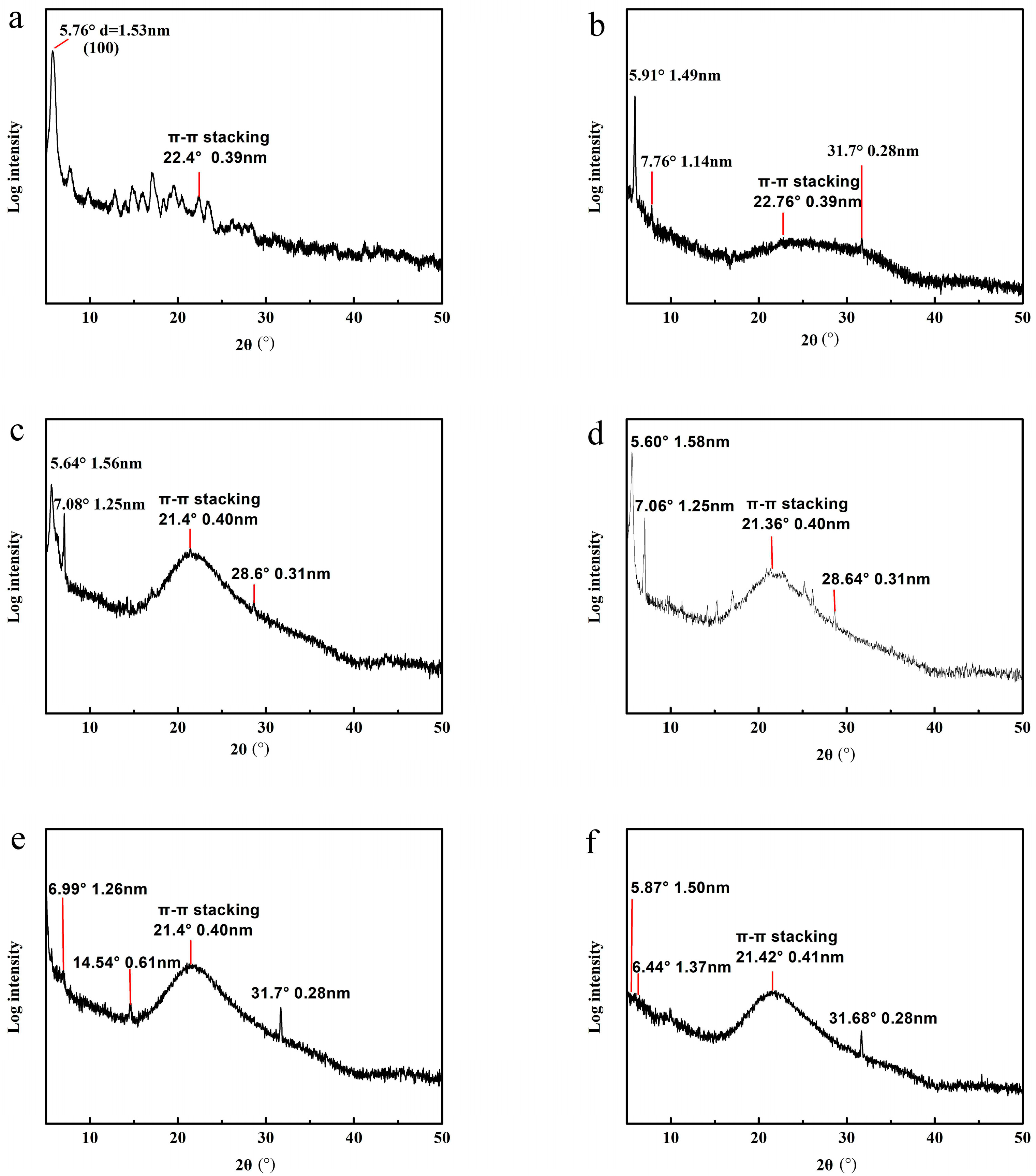
3.4. Molecular Stacking of PBI-1 Aggregates
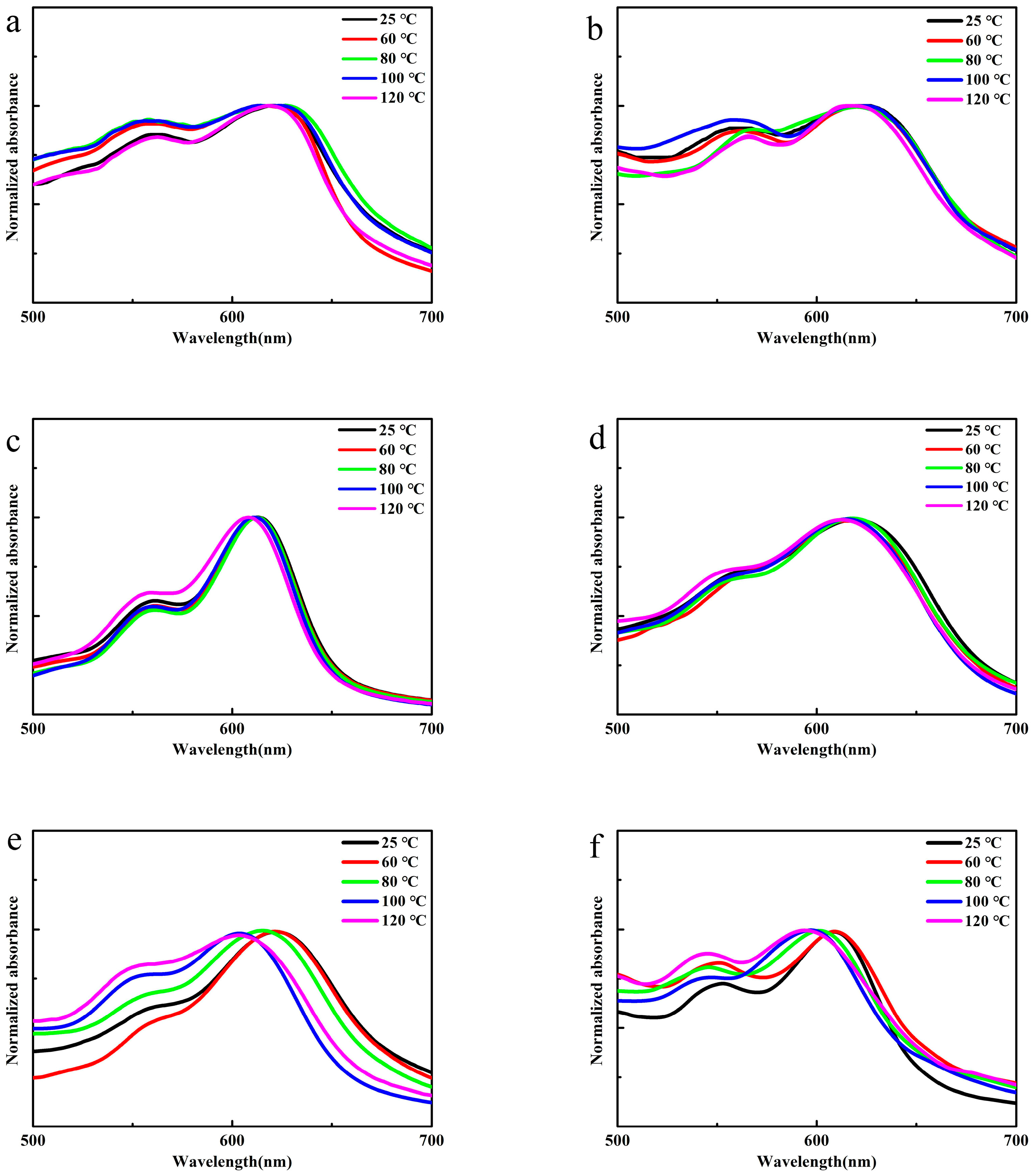
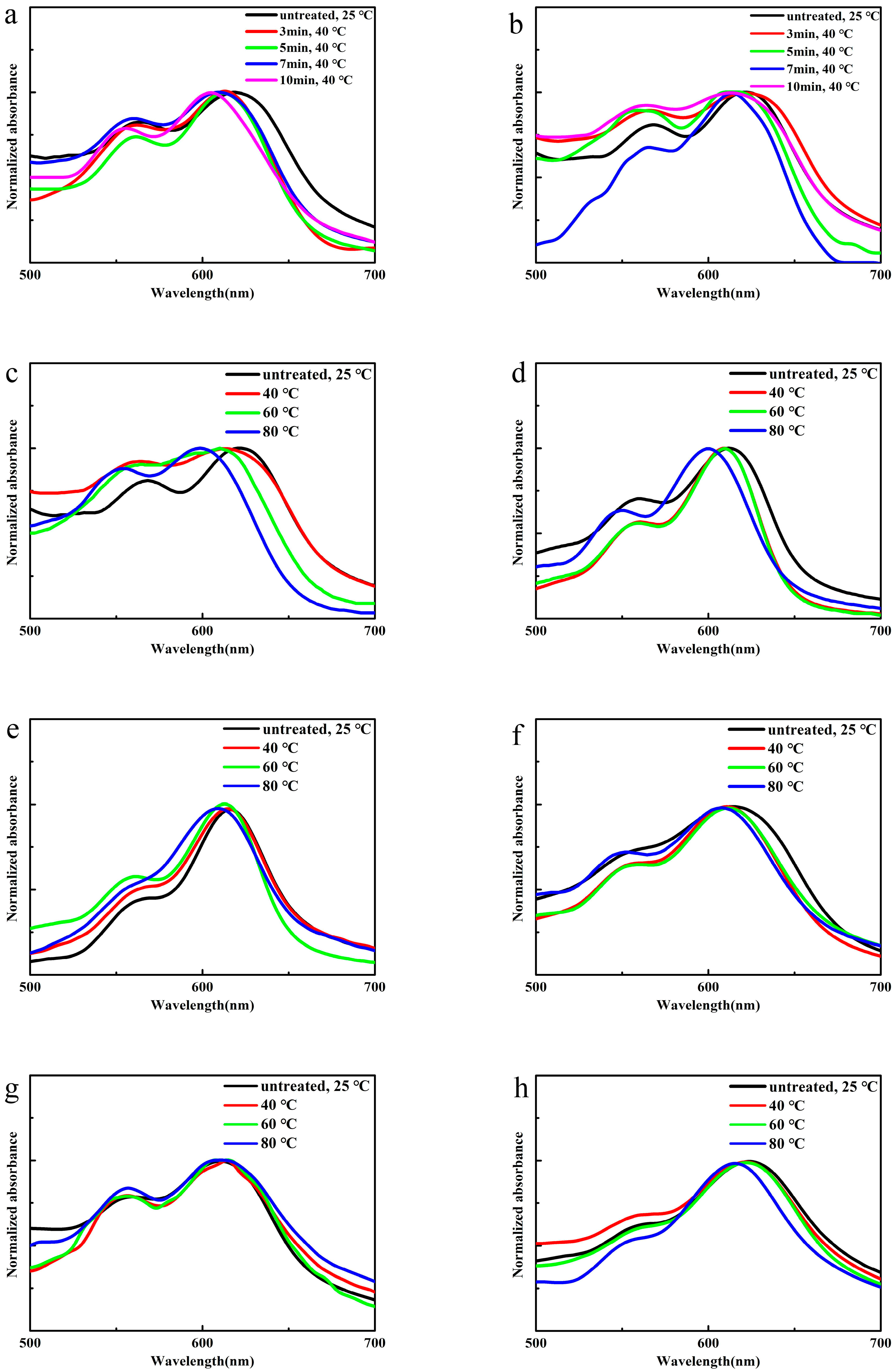
3.5. Thermal Sensitivity of PBI-1 Aggregates
3.6. Gas Sensitivity of PBI-1 Aggregates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mateos-Timoneda, M.A.; Crego-Calama, M.; Reinhoudt, D.N. Supramolecular chirality of self-assembled systems in solution. Chem. Soc. Rev. 2004, 33, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Qu, J.; Müllen, K.; Webber, S.E. Molecular layer-by-layer self-assembly of water-soluble perylene diimides through π− π and electrostatic interactions. Langmuir 2006, 22, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Willerich, I.; Gröhn, F. Molecular structure encodes nanoscale assemblies: Understanding driving forces in electrostatic self-assembly. J. Am. Chem. Soc. 2011, 133, 20341–20356. [Google Scholar] [CrossRef]
- Sukul, P.K.; Asthana, D.; Mukhopadhyay, P.; Summa, D.; Muccioli, L.; Zannoni, C.; Beljonne, D.; Rowan, A.E.; Malik, S. Assemblies of perylene diimide derivatives with melamine into luminescent hydrogels. Chem. Commun. 2011, 47, 11858–11860. [Google Scholar] [CrossRef] [PubMed]
- Tuccitto, N.; Delfanti, I.; Torrisi, V.; Scandola, F.; Chiorboli, C.; Stepanenko, V.; Würthner, F.; Licciardello, A. Supramolecular self-assembled multilayers of terpyridine-functionalized perylene bisimide metal complexes. Phys. Chem. Chem. Phys. 2009, 11, 4033–4038. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fimmel, B.; Würthner, F. Solvent and substituent effects on aggregation constants of perylene bisimide π-stacks–a linear free energy relationship analysis. Org. Biomol. Chem. 2012, 10, 5845–5855. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Slattum, P.; Wang, C.; Zang, L. Self-assembly of perylene imide molecules into 1D nanostructures: Methods, morphologies, and applications. Chem. Rev. 2015, 115, 11967–11998. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, Y.; Chi, Y.; Yu, H.; Li, Y.; Jiang, T.; Wei, X.; Shi, J. Self-assembly, optical and electrical properties of perylene diimide dyes bearing unsymmetrical substituents at bay position. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Ma, W.; Luo, Y.; Nian, L.; Wang, J.; Wen, X.; Liu, L.; Hanif, M.; Xie, Z.; Ma, Y. Construction of layered structure of anion–cations to tune the work function of aluminum-doped zinc oxide for inverted polymer solar cells. Acs Appl. Mater. Interfaces 2018, 10, 10513–10519. [Google Scholar] [CrossRef]
- Yu, C.; Xu, Y.; Liang, S.; Jiang, X.; Feng, G.; Li, C.; Li, W. Ethynyl-linked perylene bisimide based electron acceptors for non-fullerene organic solar cells. Chin. Chem. Lett. 2018, 29, 325–327. [Google Scholar] [CrossRef]
- Regar, R.; Mishra, R.; Singhal, R.; Sharma, G.D.; Sankar, J. NIR absorbing ortho-π-extended perylene bisimide as a promising material for bulk heterojunction organic solar cells. J. Mater. Chem. A 2019, 7, 3012–3017. [Google Scholar] [CrossRef]
- Xia, D.; Li, C.; Li, W. Crystalline conjugated polymers for organic solar cells: From donor, acceptor to single-component. Chem. Rec. 2019, 19, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.B.; Erten, S.; Günes, S.; Zafer, C.; Turkmen, G.; Kuban, B.; Teoman, Y.; Sariciftci, N.; Icli, S. Soluble derivatives of perylene and naphthalene diimide for n-channel organic field-effect transistors. Org. Electron. 2006, 7, 480–489. [Google Scholar] [CrossRef]
- Gawrys, P.; Boudinet, D.; Zagorska, M.; Djurado, D.; Verilhac, J.-M.; Horowitz, G.; Pécaud, J.; Pouget, S.; Pron, A. Solution processible naphthalene and perylene bisimides: Synthesis, electrochemical characterization and application to organic field effect transistors (OFETs) fabrication. Synth. Met. 2009, 159, 1478–1485. [Google Scholar] [CrossRef]
- Cherpak, V.; Stakhira, P.; Volynyuk, D.; Politanskiy, L.; Kus, N.; Reghu, R.; Grazulevicius, J. Ambipolar conductivity in organic field-effect transistors based on 1, 7-bis (9-ethyl-3-carbazolyl) N, N′-2-ethyl hexyl perylene bisimide under the light illumination. Opt. Mater. 2014, 36, 1511–1514. [Google Scholar] [CrossRef]
- Kucinska, M.; Frac, I.; Ulanski, J.; Makowski, T.; Nosal, A.; Gazicki-Lipman, M. The role of surface morphology in a performance of top-gate OFETs prepared from a solution processable derivative of perylene bisimide. Synth. Met. 2019, 250, 12–19. [Google Scholar] [CrossRef]
- Tsai, H.-Y.; Chen, K.-Y. Synthesis and optical properties of novel asymmetric perylene bisimides. J. Lumin. 2014, 149, 103–111. [Google Scholar] [CrossRef]
- Kozma, E.; Mróz, W.; Galeotti, F. A polystyrene bearing perylene diimide pendants with enhanced solid state emission for white hybrid light-emitting diodes. Dye. Pigment. 2015, 114, 138–143. [Google Scholar] [CrossRef]
- Zong, L.; Gong, Y.; Yu, Y.; Xie, Y.; Xie, G.; Peng, Q.; Li, Q.; Li, Z. New perylene diimide derivatives: Stable red emission, adjustable property from ACQ to AIE, and good device performance with an EQE value of 4.93%. Sci. Bull. 2018, 63, 108–116. [Google Scholar] [CrossRef]
- Yan, L.; Ye, Z.; Peng, C.; Zhang, S. A new perylene diimide-based fluorescent chemosensor for selective detection of ATP in aqueous solution. Tetrahedron 2012, 68, 2725–2727. [Google Scholar] [CrossRef]
- Pramanik, B.; Mondal, J.H.; Singha, N.; Ahmed, S.; Mohanty, J.; Das, D. A Viologen–perylenediimide conjugate as an efficient base sensor with solvatochromic property. Chem. Phys. Chem. 2017, 18, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Liang, X.-M.; Wu, N.; Li, P.; Chai, Q.; Fu, Y. A new perylene-based fluorescent pH chemosensor for strongly acidic condition. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2019, 216, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Wang, Y.; Ma, P.; Li, S.; Fan, F.; Cui, K.; Ge, S.; Zhang, Y.; Yu, J. Low-power and high-performance trimethylamine gas sensor based on nn heterojunction microbelts of perylene diimide/CdS. Anal. Chem. 2019, 91, 5591–5598. [Google Scholar] [CrossRef]
- Sukul, P.K.; Datta, A.; Malik, S. Light harvesting and amplification of emission of donor perylene–acceptor perylene aggregates in aqueous medium. Chem. A Eur. J. 2014, 20, 3019–3022. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, K.; Datar, A.; Naddo, T.; Huang, J.; Oitker, R.; Yen, M.; Zhao, J.; Zang, L. Effect of side-chain substituents on self-assembly of perylene diimide molecules: Morphology control. J. Am. Chem. Soc. 2006, 128, 7390–7398. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, X.; Ji, H.-F. Solvent effect on the self-assembled structure of an amphiphilic perylene diimide derivative. J. Phys. Chem. B 2008, 112, 7196–7202. [Google Scholar] [CrossRef]
- Ke, D.; Zhan, C.; Xu, S.; Ding, X.; Peng, A.; Sun, J.; He, S.; Li, A.D.; Yao, J. Self-assembled hollow nanospheres strongly enhance photoluminescence. J. Am. Chem. Soc. 2011, 133, 11022–11025. [Google Scholar] [CrossRef]
- Ariga, K.; Hill, J.P.; Lee, M.V.; Vinu, A.; Charvet, R.; Acharya, S. Challenges and breakthroughs in recent research on self-assembly. Sci. Technol. Adv. Mater. 2008, 9, 014109. [Google Scholar] [CrossRef]
- Cui, L.; Jiao, Y.; Wang, A.; Zhao, L.; Dong, Q.; Yan, X.; Bai, S. Regulating morphologies and near-infrared photothermal conversion of perylene bisimide via sequence-dependent peptide self-assembly. Chem. Commun. 2018, 54, 2208–2211. [Google Scholar] [CrossRef]
- Moghaddam, R.S.; Draper, E.R.; Wilson, C.; Heidari, H.; Adams, D.J. Effect of electric field on the electrical properties of a self-assembled perylene bisimide. Rsc Adv. 2018, 8, 34121–34125. [Google Scholar] [CrossRef]
- Nandi, M.; Maiti, B.; Banerjee, S.; De, P. Hydrogen bonding driven self-assembly of side-chain amino acid and fatty acid appended poly (methacrylate) s: Gelation and application in oil spill recovery. J. Polym. Sci. Part. A Polym. Chem. 2019, 57, 511–521. [Google Scholar] [CrossRef]
- Sukul, P.K.; Singh, P.K.; Maji, S.K.; Malik, S. Aggregation induced chirality in a self assembled perylene based hydrogel: Application of the intracellular pH measurement. J. Mater. Chem. B 2013, 1, 153–156. [Google Scholar] [CrossRef]
- Han, D.; Han, J.; Huo, S.; Qu, Z.; Jiao, T.; Liu, M.; Duan, P. Proton triggered circularly polarized luminescence in orthogonal-and co-assemblies of chiral gelators with achiral perylene bisimide. Chem. Commun. 2018, 54, 5630–5633. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, N.; Gan, H.; Liu, H.; Li, H.; Li, Y.; He, X.; Huang, C.; Cui, S.; Wang, S. Synthesis and characterization of 3, 5-bis (2-hydroxyphenyl)-1, 2, 4-triazole functionalized tetraaryloxy perylene bisimide and metal-directed self-assembly. J. Org. Chem. 2005, 70, 9686–9692. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Long, T.; Wang, Y.; Yang, X. Self-assembly, protonation-dependent morphology, and photophysical properties of perylene bisimide with tertiary amine groups. Dye. Pigment. 2020, 173, 107896. [Google Scholar] [CrossRef]
- Zang, L.; Che, Y.; Moore, J.S. One-dimensional self-assembly of planar π-conjugated molecules: Adaptable building blocks for organic nanodevices. Acc. Chem. Res. 2008, 41, 1596–1608. [Google Scholar] [CrossRef]
- Li, A.D.; Wang, W.; Wang, L.Q. Folding versus self-assembling. Chem. A Eur. J. 2003, 9, 4594–4601. [Google Scholar] [CrossRef]
- Zhong, L.; Xing, F.; Shi, W.; Yan, L.; Xie, L.; Zhu, S. Synthesis, spectra, and electron-transfer reaction of aspartic acid-functionalized water-soluble perylene bisimide in aqueous solution. ACS Appl. Mater. Interfaces 2013, 5, 3401–3407. [Google Scholar] [CrossRef]
- Xu, Z.; He, B.; Wei, W.; Liu, K.; Yin, M.; Yang, W.; Shen, J. Highly water-soluble perylenediimide-cored poly (amido amine) vector for efficient gene transfection. J. Mater. Chem. B 2014, 2, 3079–3086. [Google Scholar] [CrossRef]
- Yang, L.; Shi, M.; Wang, M.; Chen, H. Synthesis, electrochemical, and spectroscopic properties of soluble perylene monoimide diesters. Tetrahedron 2008, 64, 5404–5409. [Google Scholar] [CrossRef]
- Xu, L.Q.; Wang, L.; Zhang, B.; Lim, C.H.; Chen, Y.; Neoh, K.-G.; Kang, E.-T.; Fu, G.D. Functionalization of reduced graphene oxide nanosheets via stacking interactions with the fluorescent and water-soluble perylene bisimide-containing polymers. Polymer 2011, 52, 2376–2383. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Said, A.I.; Toshkova, R.A.; Tzoneva, R.D.; Bojinov, V.B. A novel water-soluble perylenetetracarboxylic diimide as a fluorescent pH probe: Chemosensing, biocompatibility and cell imaging. Dye. Pigment. 2019, 160, 28–36. [Google Scholar] [CrossRef]
- Kazmaier, P.M.; Hoffmann, R. A theoretical study of crystallochromy. Quantum interference effects in the spectra of perylene pigments. J. Am. Chem. Soc. 1994, 116, 9684–9691. [Google Scholar] [CrossRef]
- Adachi, M.; Murata, Y.; Nakamura, S. Spectral similarity and difference of naphthalenetetracarboxylic dianhydride, perylenetetracarboxylic dianhydride, and their derivatives. J. Phys. Chem. 1995, 99, 14240–14246. [Google Scholar] [CrossRef]
- Görl, D.; Soberats, B.; Herbst, S.; Stepanenko, V.; Würthner, F. Perylene bisimide hydrogels and lyotropic liquid crystals with temperature-responsive color change. Chem. Sci. 2016, 7, 6786–6790. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wu, Q.; Sun, X.; Miao, H.; Deng, W.; Zhang, W.; Fan, Q.; Huang, W. J-Aggregate squaraine nanoparticles with bright NIR-II fluorescence for imaging guided photothermal therapy. Chem. Commun. 2018, 54, 13395–13398. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, Y.; Zhang, X.; Chen, M.; Guo, H.; Yang, F. Perylene bisimide with diphenylacrylonitrile on side-chain: Strongly fluorescent liquid crystal with large pseudo stokes shift based on AIE and FRET effect. Soft Matter 2018, 14, 6737–6744. [Google Scholar] [CrossRef]
- Che, Y.; Yang, X.; Liu, G.; Yu, C.; Ji, H.; Zuo, J.; Zhao, J.; Zang, L. Ultrathin n-type organic nanoribbons with high photoconductivity and application in optoelectronic vapor sensing of explosives. J. Am. Chem. Soc. 2010, 132, 5743–5750. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, K.; Yu, P.; Wang, X.; Lan, S.; Sun, K.; Yi, Y.; Li, Z. Impact of linear alkyl length on the assembly of twisted perylene bisimides: From molecular arrangement to nanostructures. Chem. An. Asian J. 2017, 12, 2827–2833. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Datar, A.; Balakrishnan, K.; Zang, L. Ultralong nanobelts self-assembled from an asymmetric perylene tetracarboxylic diimide. J. Am. Chem. Soc. 2007, 129, 7234–7235. [Google Scholar] [CrossRef]

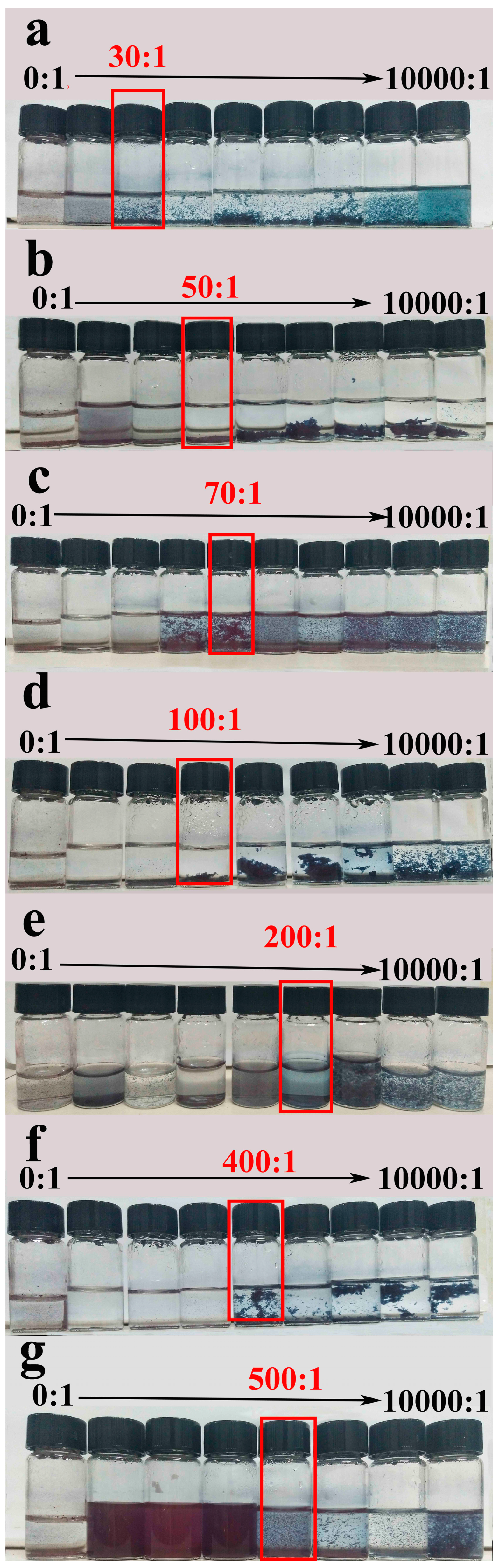

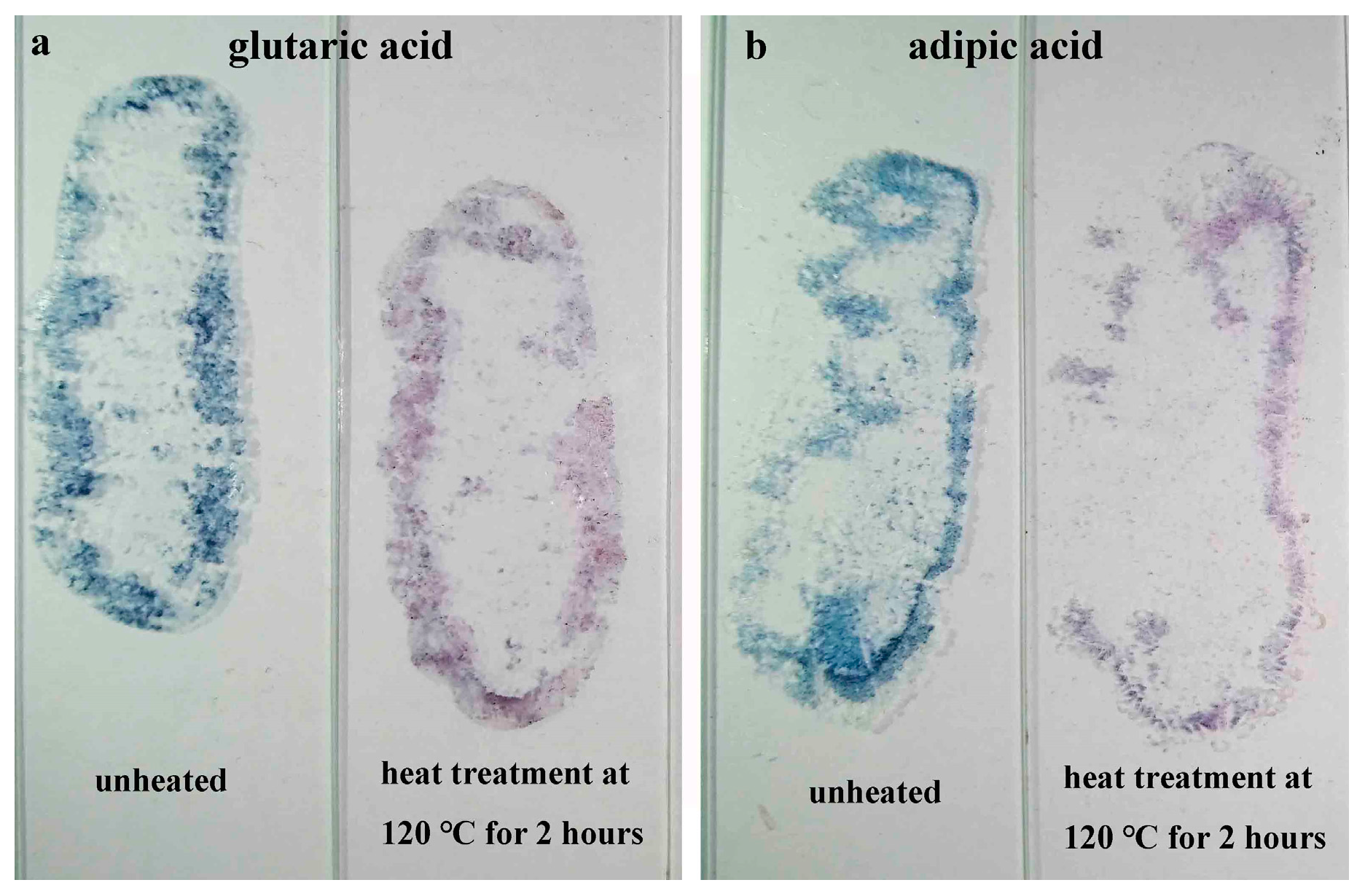
| p-Toluenesulfonic Acid | Oxalic Acid | Malonic Acid | Citric Acid | Formic Acid | Glutaric Acid | Acetic Acid | Adipic Acid | |
|---|---|---|---|---|---|---|---|---|
| pKa | −0.43 ± 0.50 | 1.23 | 2.83 | 3.14 | 3.75 | 4.31 | 4.74 | 4.43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yang, X.; Li, S.; Long, T.; Li, W. Organic Acid Regulated Self-Assembly and Photophysical Properties of Perylene Bisimide Derivatives. Materials 2020, 13, 1656. https://doi.org/10.3390/ma13071656
Wang Y, Yang X, Li S, Long T, Li W. Organic Acid Regulated Self-Assembly and Photophysical Properties of Perylene Bisimide Derivatives. Materials. 2020; 13(7):1656. https://doi.org/10.3390/ma13071656
Chicago/Turabian StyleWang, Ying, Xinguo Yang, Siyu Li, Tao Long, and Wei Li. 2020. "Organic Acid Regulated Self-Assembly and Photophysical Properties of Perylene Bisimide Derivatives" Materials 13, no. 7: 1656. https://doi.org/10.3390/ma13071656
APA StyleWang, Y., Yang, X., Li, S., Long, T., & Li, W. (2020). Organic Acid Regulated Self-Assembly and Photophysical Properties of Perylene Bisimide Derivatives. Materials, 13(7), 1656. https://doi.org/10.3390/ma13071656






