Preparation of Silver/Chitosan Nanofluids Using Selected Plant Extracts: Characterization and Antimicrobial Studies against Gram-Positive and Gram-Negative Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Leaves Extraction
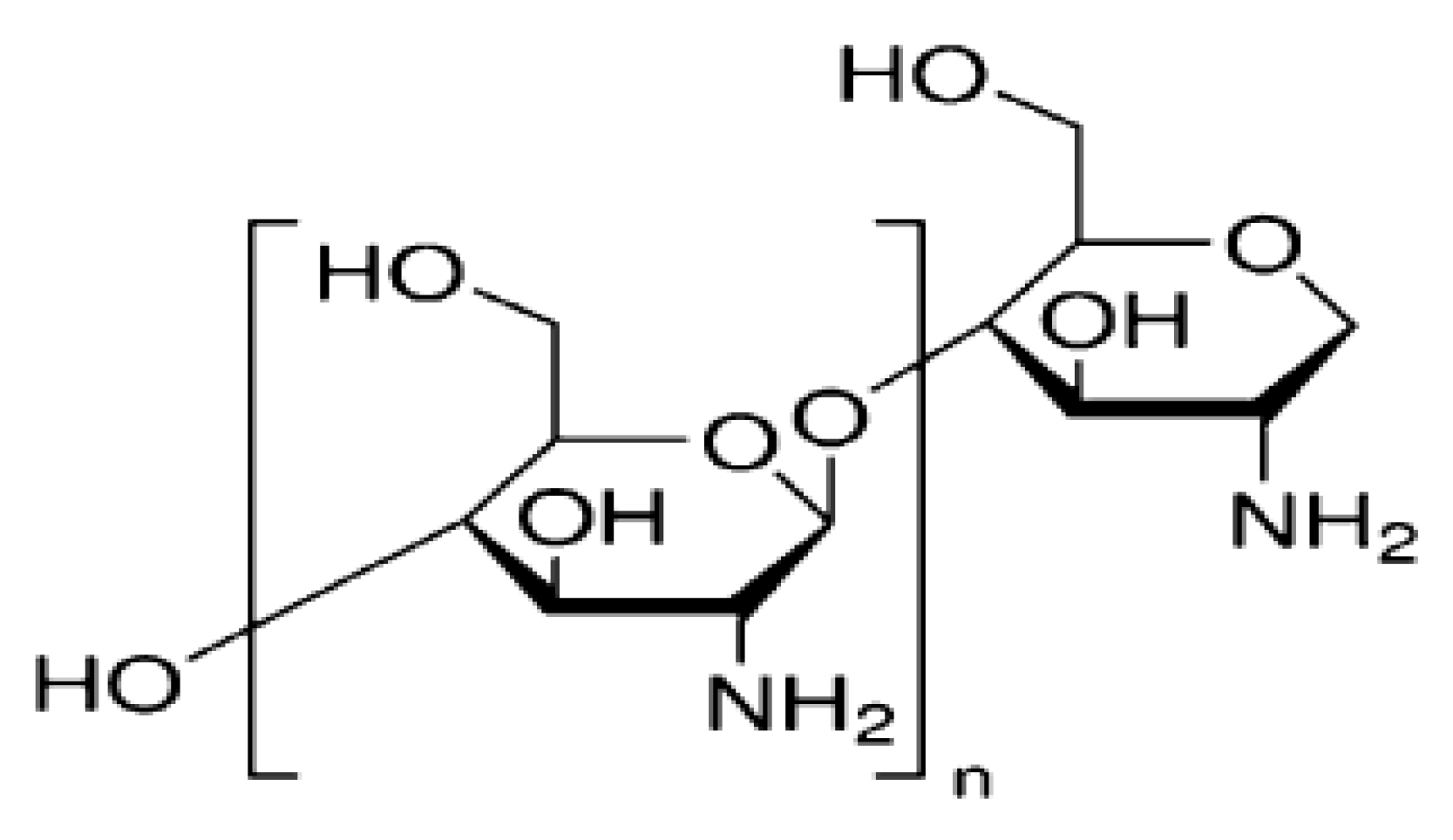
2.3. Preparation of AgNPs/Chitosan Nanofluids
2.4. Characterization
2.5. Bacteria Cultures
2.6. Antibacterial Activity by ‘cup-plating’
2.7. Assessment of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
3. Results and Discussion
3.1. Physical Appearance of Synthesized AgNPs/Chitosan Nanofluids
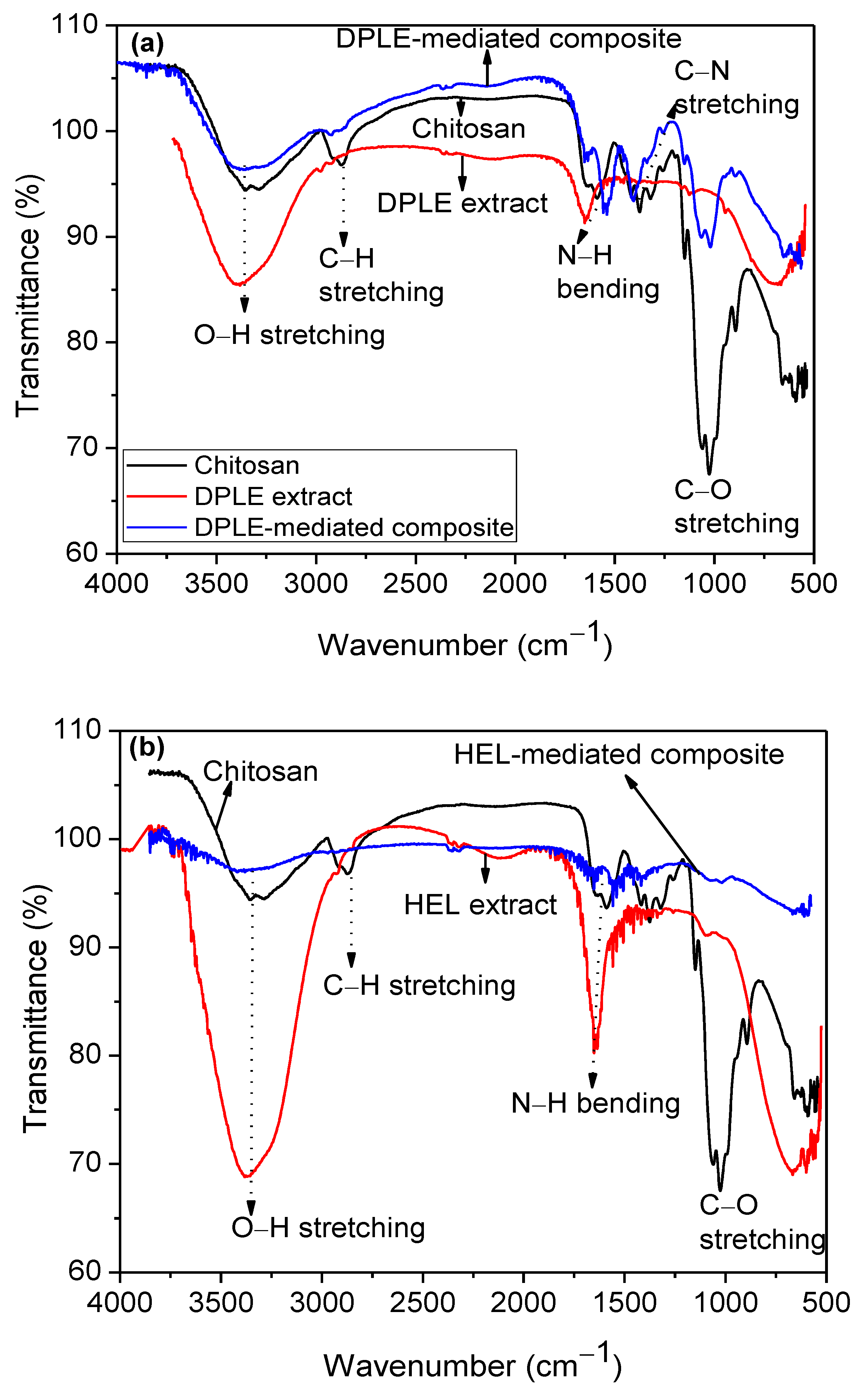
3.2. FTIR Studies
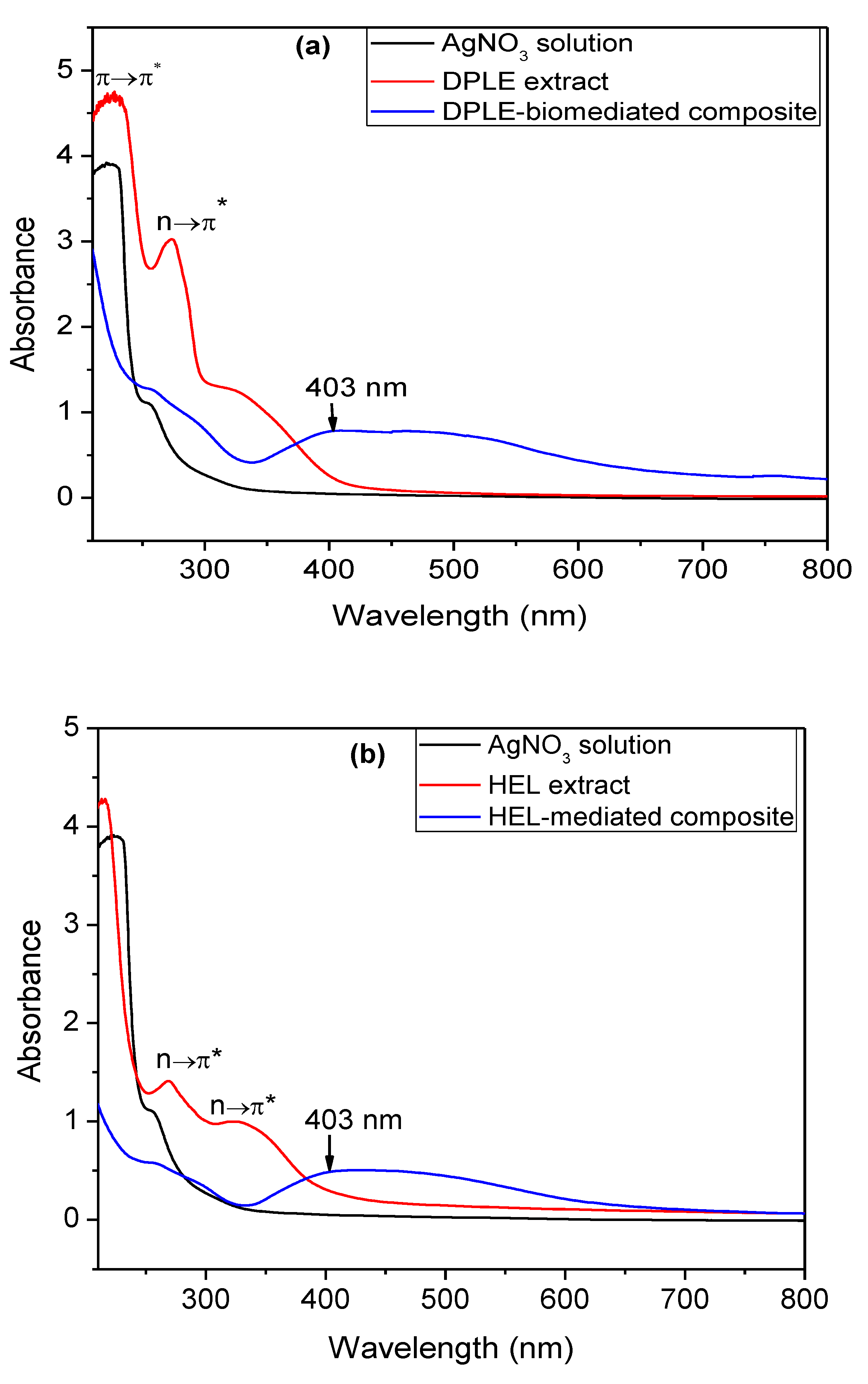
3.3. UV-vis Studies
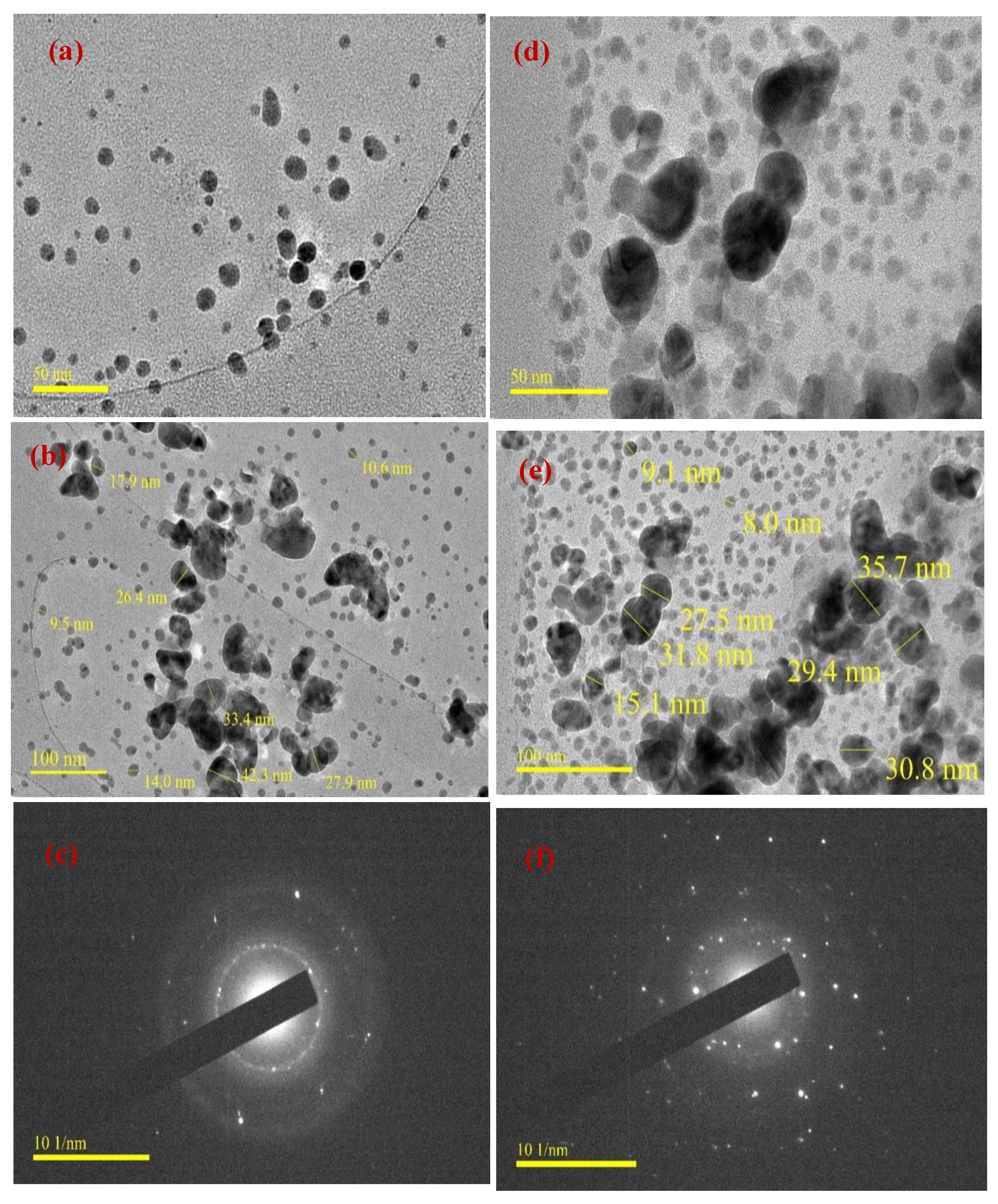
3.4. TEM Studies
3.5. Zeta Potential (ZP) and Polydispersity Index (PDI) Studies
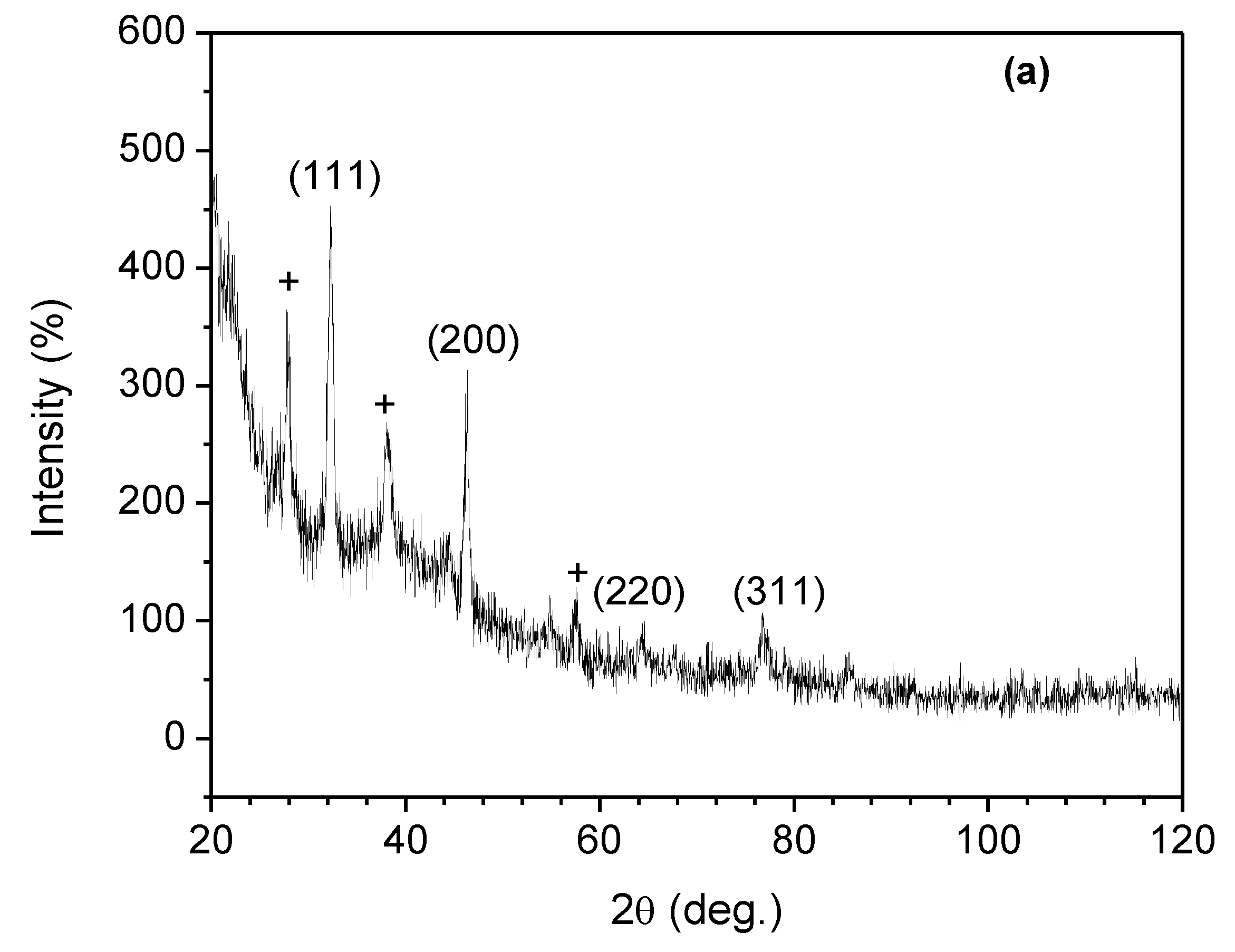
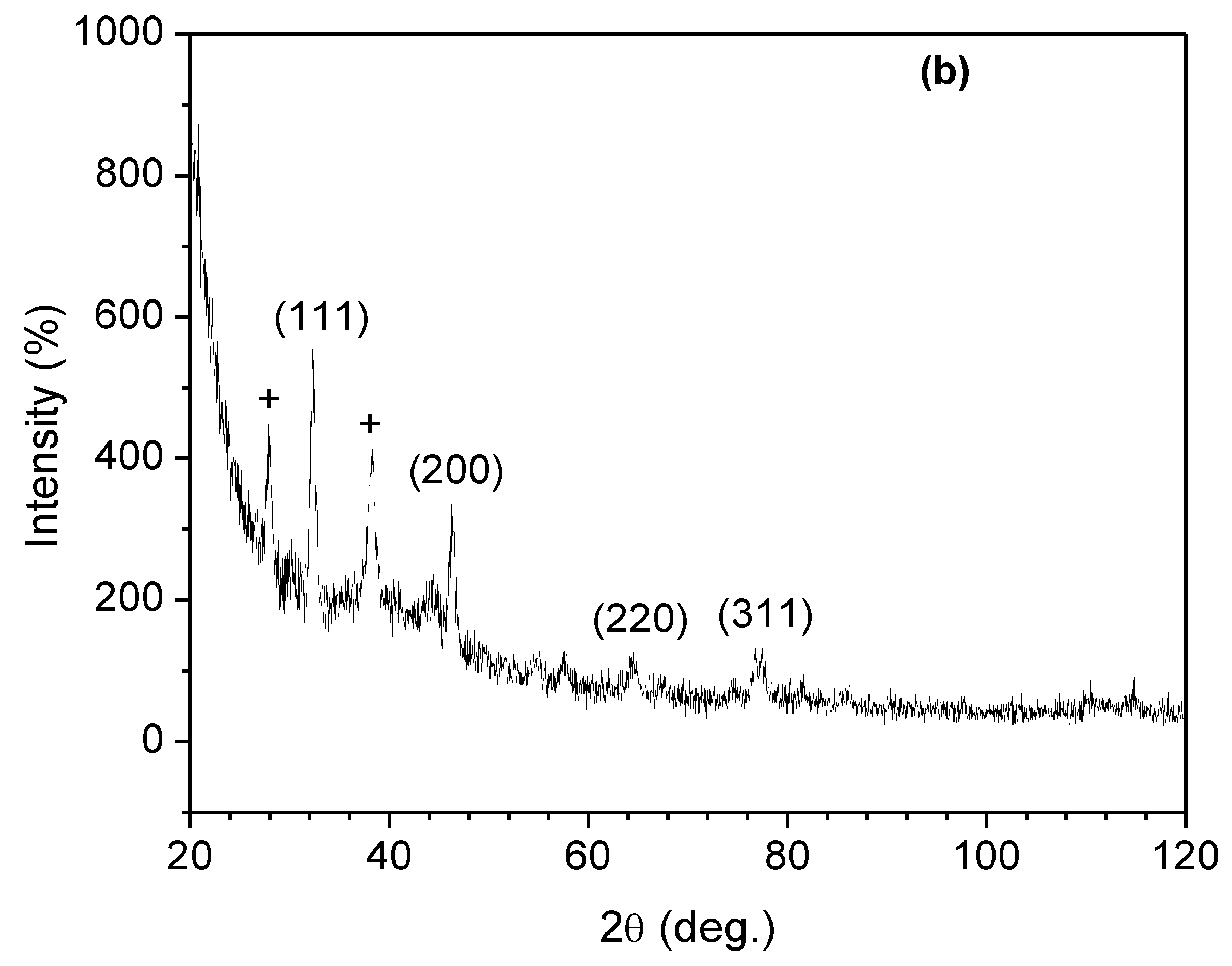
3.6. XRD Studies
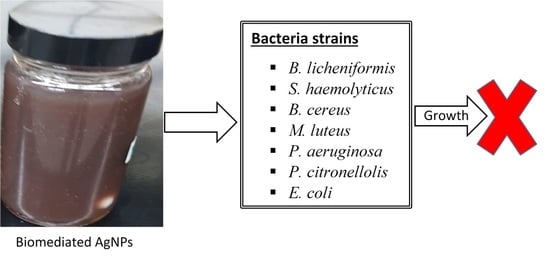
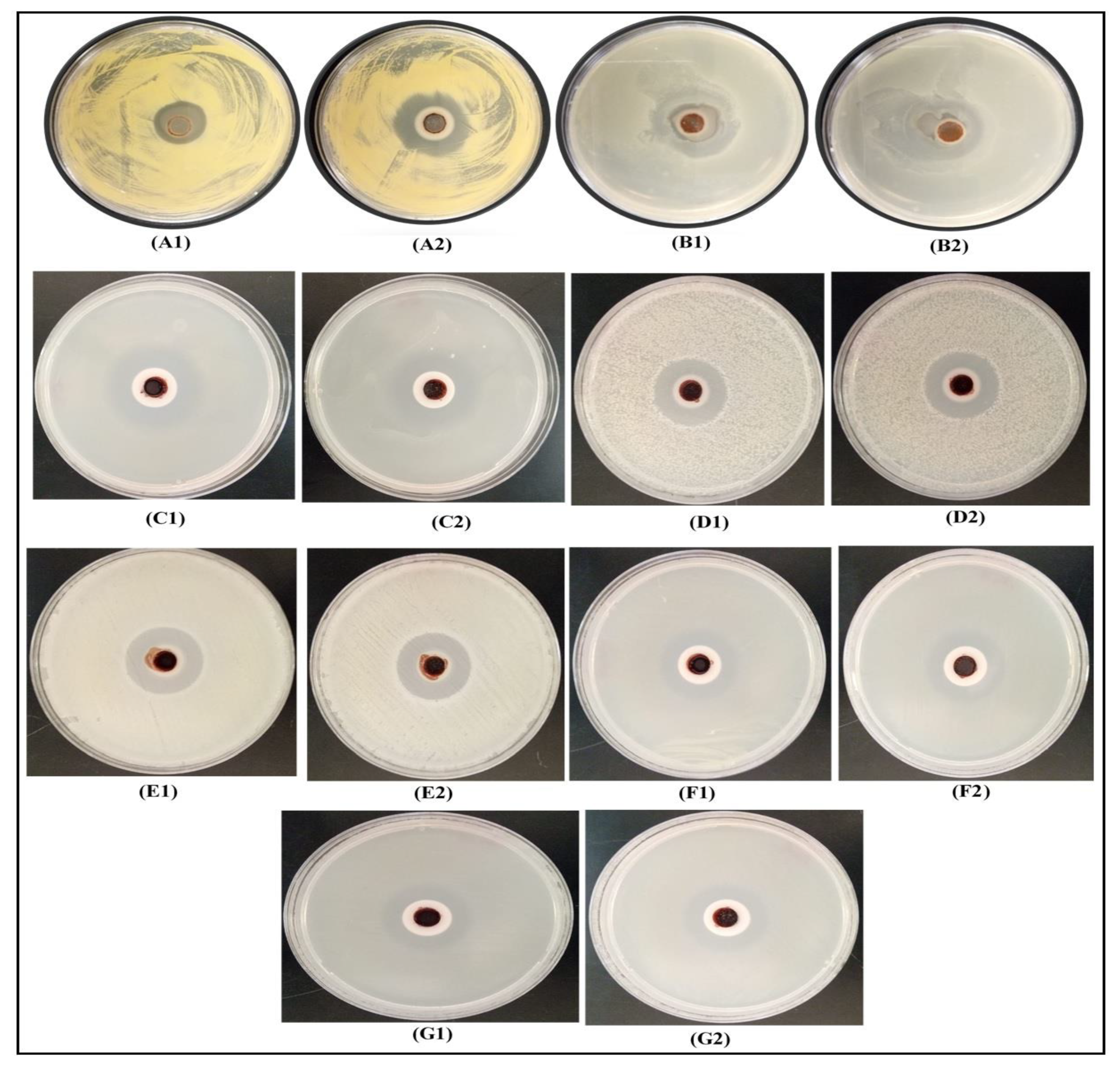
3.7. Antimicrobial Studies
3.8. A Comparison of the Antimicrobial Performance of DPLE- amd HEL-mediated Composites with the Individual Components (HEL, DPLE Extracts, AgNPs, and Chitosan Solution)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.; Jiang, Y.; Zhu, J.; Huang, J.; Zhang, H. Inhibition of bacterial adhesion and biofilm formation of sulfonated chitosan against Pseudomonas aeruginosa. Carbohydr. Polym. 2019, 206, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Vena, A.; Croxatto, A.; Righi, E.; Guery, B. How to manage Pseudomonas aeruginosa infections. Drugs Context 2018, 7, 212527. [Google Scholar] [CrossRef] [PubMed]
- Barras, F.; Aussel, L.; Ezraty, B. Silver and antibiotic, new facts to an old story. Antibiotics 2018, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B.G. Silver in Healthcare: Its Antimicrobial Efficacy and Safety in Use (Issues in Toxicology), 1st ed.; Royal Society of Chemistry: London, UK, 2010. [Google Scholar]
- Ghaffari-Moghaddam, M.; Hadi-Dabanlou, R. Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Crataegus douglasii fruit extract. J. Ind. Eng. Chem. 2014, 20, 739–744. [Google Scholar] [CrossRef]
- Gopinath, V.; MubarakAli, D.; Priyadarshini, S.; Priyadharsshini, N.M.; Thajuddin, N.; Velusamy, P. Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: A novel biological approach. Colloids Surf. B Biointerfaces 2012, 96, 69–74. [Google Scholar] [CrossRef]
- Umoren, S.A.; Nzila, A.M.; Sankaran, S.; Solomon, M.M.; Umoren, P.S. Green synthesis, characterization and antibacterial activities of silver nanoparticles from strawberry fruit extract. Pol. J. Chem. Technol. 2017, 19, 128–136. [Google Scholar]
- Ravishankar, R.V.; Jamuna, B.A. Nanoparticles and their potential application as antimicrobials. In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Méndez-Vila, A., Ed.; FORMATEX: Paris, France, 2011; pp. 197–209. [Google Scholar]
- Luksiene, Z. Nanoparticles and their potential application as antimicrobials in the food industry. In Nanotechnology in the Agri-Food Industry, Food Preservation; Grumezescu, A.M., Ed.; Academic Press: Cambridge, UK, 2017; pp. 567–601. [Google Scholar]
- Renu, S.; Shivashangari, K.S.; Ravikumar, V. Incorporated plant extract fabricated silver/poly-D,l-lactide-co-glycolide nanocomposites for antimicrobial based wound healing. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 228, 117673. [Google Scholar] [CrossRef]
- Gao, A.; Chen, H.; Hou, A.; Xie, K. Efficient antimicrobial silk composites using synergistic effects of violacein and silver nanoparticles. Mater. Sci. Eng. C 2019, 103, 109821. [Google Scholar] [CrossRef]
- Parida, D.; Simonetti, P.; Frison, R.; Bülbül, E.; Altenried, S.; Arroyo, Y.; Balogh-Michels, Z.; Caseri, W.; Ren, Q.; Hufenus, R.; et al. Polymer-assisted in-situ thermal reduction of silver precursors: A solventless route for silver nanoparticles-polymer composites. Chem. Eng. J. 2019, 389, 123983. [Google Scholar] [CrossRef]
- Rahimi, M.; Noruzi, E.B.; Sheykhsaran, E.; Ebadi, B.; Kariminezhad, Z.; Molaparast, M.; Mehrabani, M.G.; Mehramouz, B.; Yousefi, M.; Ahmadi, R.; et al. Carbohydrate polymer-based silver nanocomposites: Recent progress in the antimicrobial wound dressings. Carbohydr. Polym. 2019, 231, 115696. [Google Scholar] [CrossRef]
- Umoren, S.A.; Banera, M.J.; Alonso-Garcia, T.; Gervasi, C.A.; Mirífico, M.V. Inhibition of mild steel corrosion in HCl solution using chitosan. Cellulose 2013, 20, 2529–2545. [Google Scholar] [CrossRef]
- Solomon, M.M.; Gerengi, H.; Kaya, T.; Kaya, E.; Umoren, S.A. Synergistic inhibition of St37 steel corrosion in 15% H2SO4 solution by chitosan and iodide ion additives. Cellulose 2017, 24, 931–950. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Suh, J.-K.F.; Matthew, H.W.T. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [PubMed]
- Goy, R.C.; Morais, S.T.B.; Assis, O.B.G. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. Coli and S. aureus growth. Braz. J. Pharmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Li, Q.; Shen, Y.; Ge, Z.; Zhang, W.; Chen, S. Enhanced water-solubility, antibacterial activity and biocompatibility upon introducing sulfobetaine and quaternary ammonium to chitosan. Carbohydr. Polym. 2016, 143, 246–253. [Google Scholar] [CrossRef]
- Zayed, M.F.; Mahfoze, R.A.; El-kousy, S.M.; Al-Ashkar, E.A. In-vitro antioxidant and antimicrobial activities of metal nanoparticles biosynthesized using optimized Pimpinella anisum extract. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 1241672. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Nakkala, J.R.; Bhagat, E.; Suchiang, K.; Sadras, S.R. Comparative study of antioxidant and catalytic activity of silver and gold nanoparticles synthesized from costus pictus leaf extract. J. Mater. Sci. Technol. 2015, 31, 986–994. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M. Synthesis of monodispersed silver nanoparticles using Hibiscus cannabinus leaf extract and its antimicrobial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 101, 184–190. [Google Scholar] [CrossRef]
- Chowdhury, I.H.; Ghosh, S.; Roy, M.; Naskar, M.K. Green synthesis of water-dispersible silver nanoparticles at room temperature using green carambola (star fruit) extract. J. Sol-Gel Sci. Technol. 2014, 73, 199–207. [Google Scholar] [CrossRef]
- Jha, D.; Thiruveedula, P.K.; Pathak, R.; Kumar, B.; Gautam, H.K.; Agnihotri, S.; Sharma, A.K.; Kumar, P. Multifunctional biosynthesized silver nanoparticles exhibiting excellent antimicrobial potential against multi-drug resistant microbes along with remarkable anticancerous properties. Mater. Sci. Eng. C 2017, 80, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Lateef, A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A.; Akinboro, A.; Oladipo, I.C.; Azeez, L.; Ajibade, S.E.; Ojo, S.A.; Gueguim-Kana, E.B.; et al. Biogenic synthesis of silver nanoparticles using a pod extract of Cola nitida: Antibacterial and antioxidant activities and application as a paint additive. Integr. Med. Res. 2016, 10, 551–562. [Google Scholar] [CrossRef]
- Pirtarighat, S.; Ghannadnia, M.; Baghshahi, S. Biosynthesis of silver nanoparticles using Ocimum basilicum cultured under controlled conditions for bactericidal application. Mater. Sci. Eng. C 2019, 98, 250–255. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information (NCBI). Genomic sequence. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 3 December 2019).
- Rios, J.L.; Recio, M.C.; Villar, A. Screening methods for natural products with antimicrobial activity: A review of the literature. J. Ethnopharmacol. 1988, 23, 127–149. [Google Scholar] [CrossRef]
- Solomon, M.M.; Umoren, S.A. In-situ preparation, characterization and anticorrosion property of polypropylene glycol/silver nanoparticles composite for mild steel corrosion in acid solution. J. Colloid Interface Sci. 2016, 462, 29–41. [Google Scholar] [CrossRef]
- Al Aboody, M.S. Silver/silver chloride (Ag/AgCl) nanoparticles synthesized from Azadirachta indica lalex and its antibiofilm activity against fluconazole resistant Candida tropicalis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2107–2113. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef]
- Alfawaz, M.A. Chemical composition of hummayd (Rumex vesicarius) grown in Saudi Arabia. J. Food Compos. Anal. 2006, 19, 552–555. [Google Scholar] [CrossRef]
- Derrouiche, I.; Ben Marzoug, I.; Sakli, F.; Roudesli, S. Study of extraction and characterization of ultimate Date Palm fibers. Adv. Mater. 2015, 4, 7–14. [Google Scholar] [CrossRef]
- Nadia, K.J.; Al-Dawah, N.K.; Ibrahim, S.L. Phytochemical characteristics of Date Palm (Phoenix dactylifera L.) leaves extract. Kufa J. Vet. Med. Sci. 2013, 4, 90–95. [Google Scholar]
- Zhou, F.; Wang, H.; Dai, Q. IOP Conference Series: Earth and Environmental Science Related content The Corrosion Inhibition of Imidazoline on the Surface of X65 Carbon Steel in Oxygen Environment Study on the compound of Imidazoline Corrosion Inhibitor. IOP Conf. Ser. Earth Environ. Sci. 2018, 153, 52001. [Google Scholar] [CrossRef]
- Solomon, M.M.; Umoren, S.A.; Obot, I.B.; Sorour, A.A.; Gerengi, H. Exploration of Dextran for Application as Corrosion Inhibitor for Steel in Strong Acid Environment: Effect of Molecular Weight, Modification, and Temperature on Efficiency. ACS Appl. Mater. Interfaces 2018, 10, 28112–28129. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.M.; Gerengi, H.; Umoren, S.A. Carboxymethyl Cellulose/Silver Nanoparticles Composite: Synthesis, Characterization and Application as a Benign Corrosion Inhibitor for St37 Steel in 15% H2SO4 Medium. ACS Appl. Mater. Interfaces 2017, 9, 6376–6389. [Google Scholar] [CrossRef] [PubMed]
- Philip, D. Honey mediated green synthesis of gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.M.A.; Fageer, A.S.M.; Eltayeb, M.M.; Mohamed Ahmed, I.A. Chemical composition, antioxidant capacity, and mineral extractability of Sudanese date palm (Phoenix dactylifera L.) fruits. Food Sci. Nutr. 2014, 2, 478–489. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M. Protective polymeric films for industrial substrates: A critical review on past and recent applications with conducting polymers and polymer composites/nanocomposites. Prog. Mater. Sci. 2019, 104, 380–450. [Google Scholar] [CrossRef]
- Aiad, I.; El-Sukkary, M.M.; Soliman, E.A.; El-Awady, M.Y.; Shaban, S.M. In situ and green synthesis of silver nanoparticles and their biological activity. J. Ind. Eng. Chem. 2014, 20, 3430–3439. [Google Scholar] [CrossRef]
- Kane, S.N.; Mishra, A.; Dutta, A.K. Preface: International Conference on Recent Trends in Physics (ICRTP 2016). J. Phys. Conf. Ser. 2016, 755, 011001. [Google Scholar]
- Saeb, A.T.M.; Alshammari, A.S.; Al-Brahim, H.; Al-Rubeaan, K.A. Production of silver nanoparticles with strong and stable antimicrobial activity against highly pathogenic and multidrug resistant bacteria. Sci. World J. 2014, 2014, 704708. [Google Scholar] [CrossRef] [PubMed]
- Essien, E.A.; Kavaz, D.; Solomon, M.M. Olive leaves extract mediated zero-valent iron nanoparticles: Synthesis, characterization, and assessment as adsorbent for nickel (II) ions in aqueous medium. Chem. Eng. Commun. 2018, 205, 1568–1582. [Google Scholar] [CrossRef]
- Ardani, H.K.; Imawan, C.; Handayani, W.; Djuhana, D.; Harmoko, A.; Fauzia, V. Enhancement of the stability of silver nanoparticles synthesized using aqueous extract of Diospyros discolor Willd. leaves using polyvinyl alcohol. In IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing: Bristol, UK, 2017; Volume 188, p. 012056. [Google Scholar]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Soliman, T.S.; Vshivkov, S.A. Effect of Fe nanoparticles on the structure and optical properties of polyvinyl alcohol nanocomposite films. J. Non-Cryst. Solids 2019, 519, 119452. [Google Scholar] [CrossRef]
- Baygar, T.; Sarac, N.; Ugur, A.; Karaca, I.R. Antimicrobial characteristics and biocompatibility of the surgical sutures coated with biosynthesized silver nanoparticles. Bioorg. Chem. 2019, 86, 254–258. [Google Scholar] [CrossRef]
- Ravindra, S.; Murali Mohan, Y.; Narayana Reddy, N.; Mohana Raju, K. Fabrication of antibacterial cotton fibres loaded with silver nanoparticles via Green Approach. Colloids Surf. A Physicochem. Eng. Asp. 2010, 367, 31–40. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ejaz, M.; Jacob, H.; Ahmed, J. Deciphering the potential of guar gum/Ag-Cu nanocomposite films as an active food packaging material. Carbohydr. Polym. 2017, 157, 65–71. [Google Scholar] [CrossRef]
- Gupta, A.P.; Kumar Verma, D. Preparation of carboxymethyl guar gum/silver nanocomposites film and its optical and antimicrobial properties. Int. J. Adv. Res. 2015, 3, 224–229. [Google Scholar]
- Murthy, P.S.K.; Murali Mohan, Y.; Varaprasad, K.; Sreedhar, B.; Mohana Raju, K. First successful design of semi-IPN hydrogel-silver nanocomposites: A facile approach for antibacterial application. J. Colloid Interface Sci. 2008, 318, 217–224. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Pina, C.; Tavaria, F.K.; Pintado, M.M. Evaluation and insights into chitosan antimicrobial activity against anaerobic oral pathogens. Anaerobe 2012, 18, 305–309. [Google Scholar] [CrossRef]

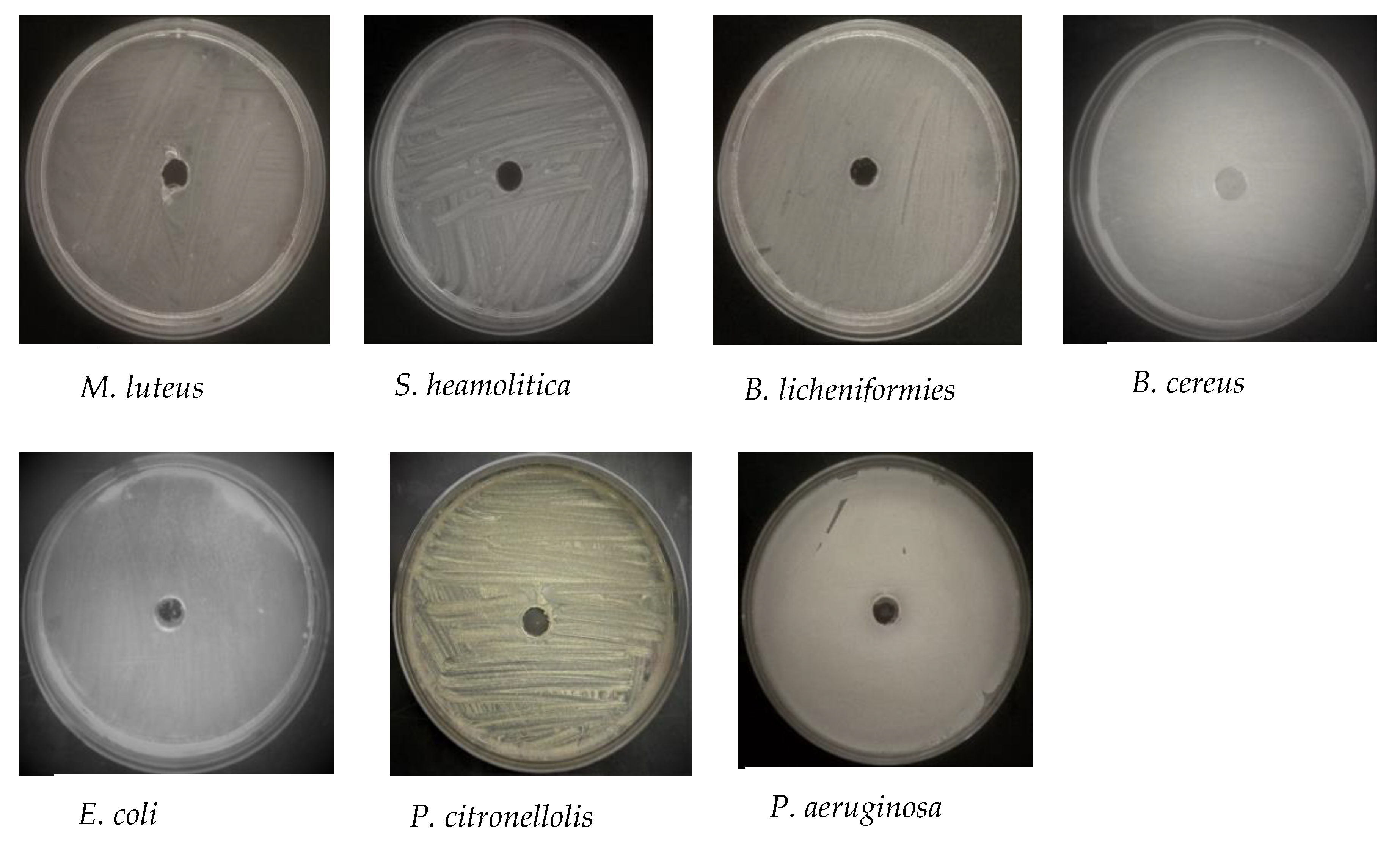
| Type | Bacterium | Diameter of Inhibition (in mm) | |
|---|---|---|---|
| DPLE-Mediated Composite | HEL-Mediated Composite | ||
| Gram negative | P. aeruginosa | 10.0 ± 2.0 | 12.0 ± 1.7 |
| P. citronellolis | 9.0 ± 1.4 | 11.0 ± 1.4 | |
| E. coli | 10.0 ± 2.8 | 12.5 ± 0.7 | |
| Gram positive | B. licheniformis | 7.0 ± 1.4 | 7.5 ± 0.7 |
| S. haemolyticus | 6.0 ± 1.4 | 6.5 ± 1.4 | |
| B. cereus | 11.0 ± 1.4 | 10.0 ± 0.0 | |
| M. luteus | 10.0 ± 0.0 | 14.0 ± 1.4 | |
| Bacterium | DPLE-Bionanofluid (%) | HEL-Bionanofluid (%) | Controls | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 1 | 0.1 | 0.01 | 0.001 | 10 | 1 | 0.1 | 0.01 | 0.001 | +ve | -ve | |
| P. aeruginosa | - (0) | - (0) | + (0.5) | ++ (0.8) | +++ (184) | - (0) | - (0) | - (0) | + (0.36) | +++ (1.1) | ++++ (6800) | - (0) |
| B. licheniformis | - (0) | - (0) | + (0.06) | ++ (128) | +++ (13600) | - (0) | - (0) | - (0) | + (84) | ++ (520) | ++++ (17000) | - (0) |
| E. coli | - (0) | - (0) | + (0.7) | ++ (1.4) | +++ (7400) | - (0) | - (0) | - (0) | + (0.6) | ++ (5600) | ++++ (19000) | - (0) |
| B. licheniformis | - (0) | - (0) | + (0.006) | ++ (128) | +++ (13600) | - (0) | - (0) | + (0) | + (84) | ++ (520) | ++++ (17000) | - (0) |
| S. haemolyticus | - (0) | - (0) | + (0.03) | ++ (72) | +++ (68) | - (0) | - (0) | + (0.34) | + (80) | ++ (94) | ++++ (820000) | - (0) |
| B. substilis | - (0) | - (0) | + (0.009) | ++ (0.18) | +++ (38) | - (0) | - (0) | - (0.11) | + (1.78) | ++ (44) | ++++ (23600) | - (0) |
| M. aloeverae | - (0) | - (0) | + (0.0018) | ++ (1.7) | +++ (8000) | - (0) | - (0) | - (0) | + (3.8) | ++ (11000) | ++++ (36000) | - (0) |
| Bacterium | DPLE-Mediated Composite (%) | HEL-Mediated Composite (%) | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| P. aeruginosa | 1.0 | 1.0 | 0.1 | 0.1 |
| P. citronellolis | 1.0 | 1.0 | 1.0 | 1.0 |
| E. coli | 1.0 | 1.0 | 0.1 | 0.1 |
| B. licheniformis | 1.0 | 1.0 | 1.0 | 1.0 |
| S. haemolyticus | 1.0 | 1.0 | 0.1 | 0.1 |
| B. cereus | 1.0 | 1.0 | 0.1 | 0.1 |
| M. luteus | 1.0 | 1.0 | 0.1 | 0.1 |
| Type | Bacterium | Diameter of Inhibition (in mm) | |||||
|---|---|---|---|---|---|---|---|
| DPLE-Mediated Composite | HEL-Mediated Composite | DPLE Extract | HEL Extract | AgNPs | Chitosan Solution | ||
| Gram negative | P. aeruginosa | 10.0 ± 2.0 | 12.0 ± 1.7 | 0.0 ± 0.0 | 0.0 ± 0.0 | 5.0 ± 1.4 | 8.0 ± 1.4 |
| P. citronellolis | 9.0 ± 1.4 | 11.0 ± 1.4 | 1.0 ± 0.0 | 0.0 ± 0.0 | 5.0 ± 1.0 | 7.0 ± 1.4 | |
| E. coli | 10.0 ± 2.8 | 12.5 ± 0.7 | 0.0± 0.0 | 0.0 ± 0.0 | 4.0 ± 1.0 | 10.0 ± 2.0 | |
| Gram positive | B. licheniformis | 7.0 ± 1.4 | 7.5 ± 0.7 | 0.0 ± 0.0 | 1.0 ± 0.0 | 2.0 ± 0.0 | 8.0 ± 0.0 |
| S. haemolyticus | 6.0 ± 1.4 | 6.5 ± 1.4 | 0.0 ± 0.0 | 5.0 ± 1.0 | 2.0 ± 0.5 | 6.0 ± 1.0 | |
| B. cereus | 11.0 ± 1.4 | 10.0 ± 0.0 | 0.0 ±0.0 | 0.0 ± 0.0 | 4.0 ± 1.5 | 8.0 ± 1.4 | |
| M. luteus | 10.0 ± 0.0 | 14.0 ± 1.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 2.0 ± 0.0 | 12.0 ± 2.8 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umoren, S.A.; Solomon, M.M.; Nzila, A.; Obot, I.B. Preparation of Silver/Chitosan Nanofluids Using Selected Plant Extracts: Characterization and Antimicrobial Studies against Gram-Positive and Gram-Negative Bacteria. Materials 2020, 13, 1629. https://doi.org/10.3390/ma13071629
Umoren SA, Solomon MM, Nzila A, Obot IB. Preparation of Silver/Chitosan Nanofluids Using Selected Plant Extracts: Characterization and Antimicrobial Studies against Gram-Positive and Gram-Negative Bacteria. Materials. 2020; 13(7):1629. https://doi.org/10.3390/ma13071629
Chicago/Turabian StyleUmoren, Saviour A., Moses M. Solomon, Alexis Nzila, and Ime B. Obot. 2020. "Preparation of Silver/Chitosan Nanofluids Using Selected Plant Extracts: Characterization and Antimicrobial Studies against Gram-Positive and Gram-Negative Bacteria" Materials 13, no. 7: 1629. https://doi.org/10.3390/ma13071629
APA StyleUmoren, S. A., Solomon, M. M., Nzila, A., & Obot, I. B. (2020). Preparation of Silver/Chitosan Nanofluids Using Selected Plant Extracts: Characterization and Antimicrobial Studies against Gram-Positive and Gram-Negative Bacteria. Materials, 13(7), 1629. https://doi.org/10.3390/ma13071629