Detailed Molecular and Structural Analysis of Dual Emitter IrQ(ppy)2 Complex
Abstract
1. Introduction
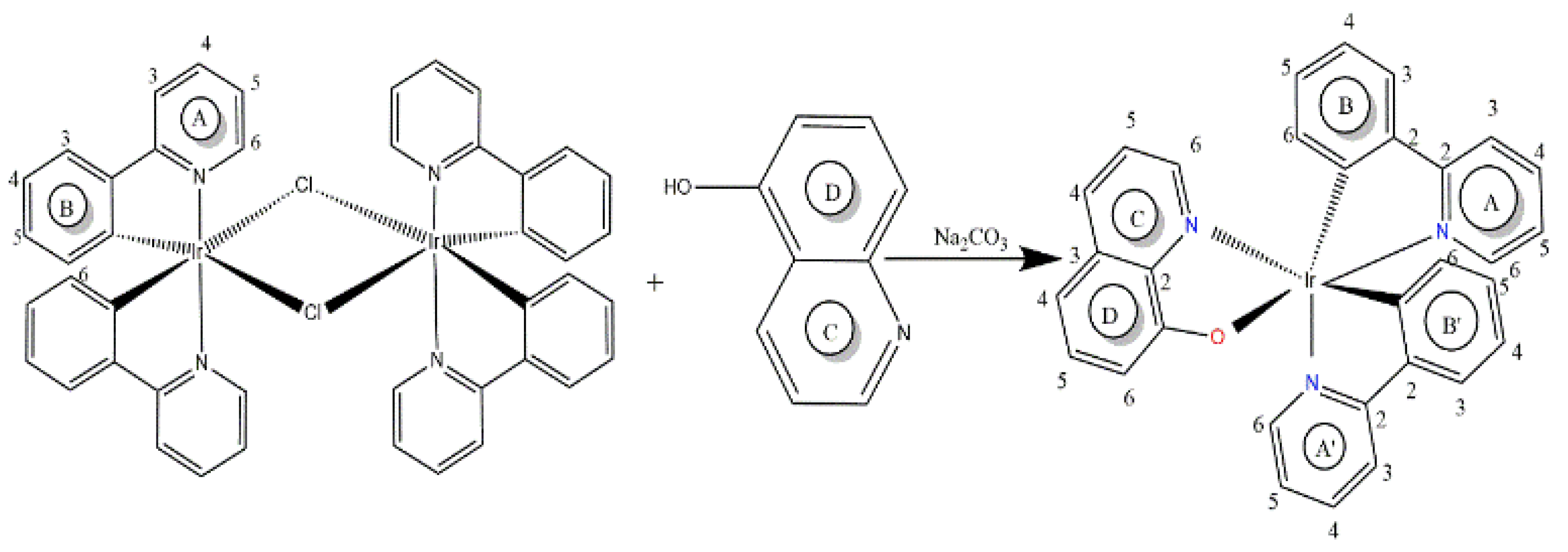
2. Materials and Methods
3. Results and Discussion
3.1. EXAFS Measurements
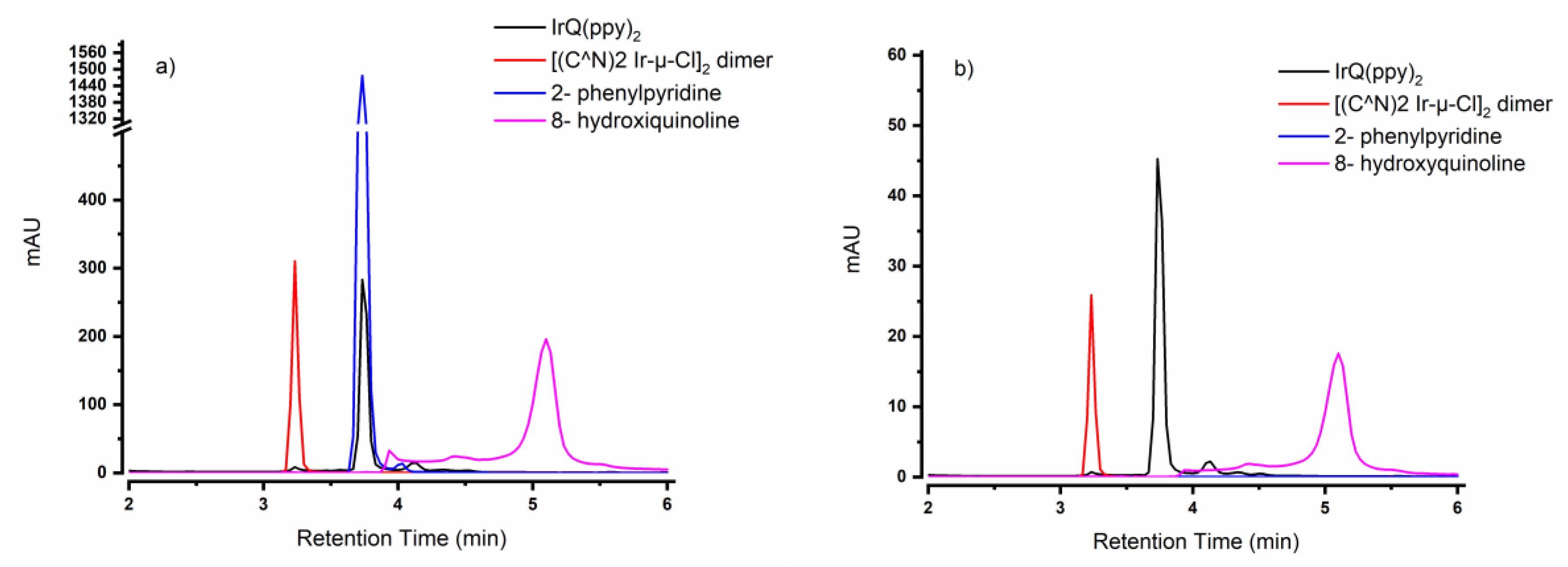
3.2. High-Performance Liquid Chromatography
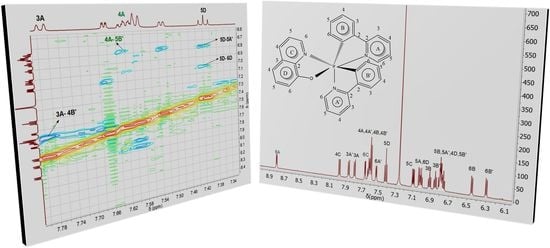
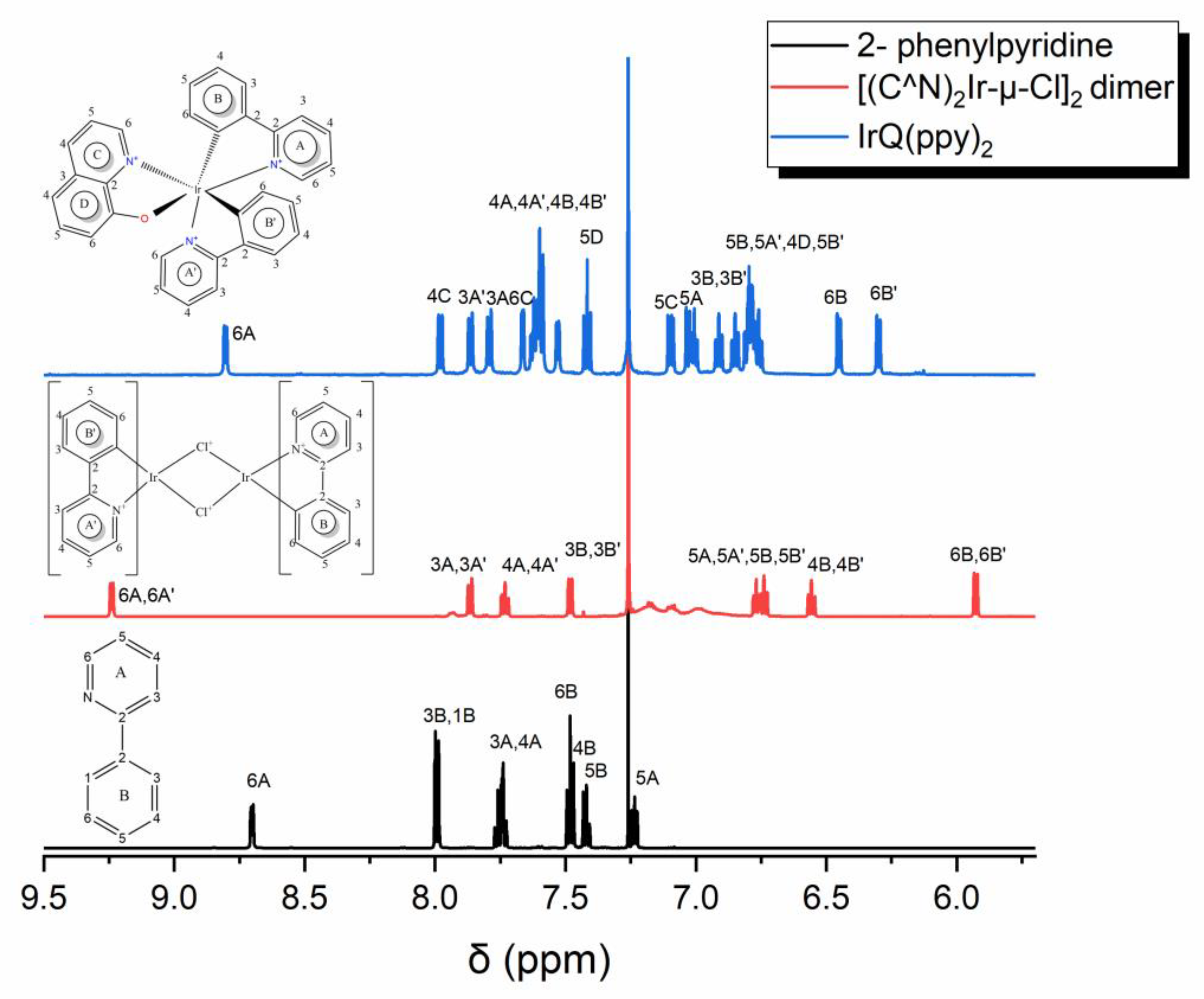
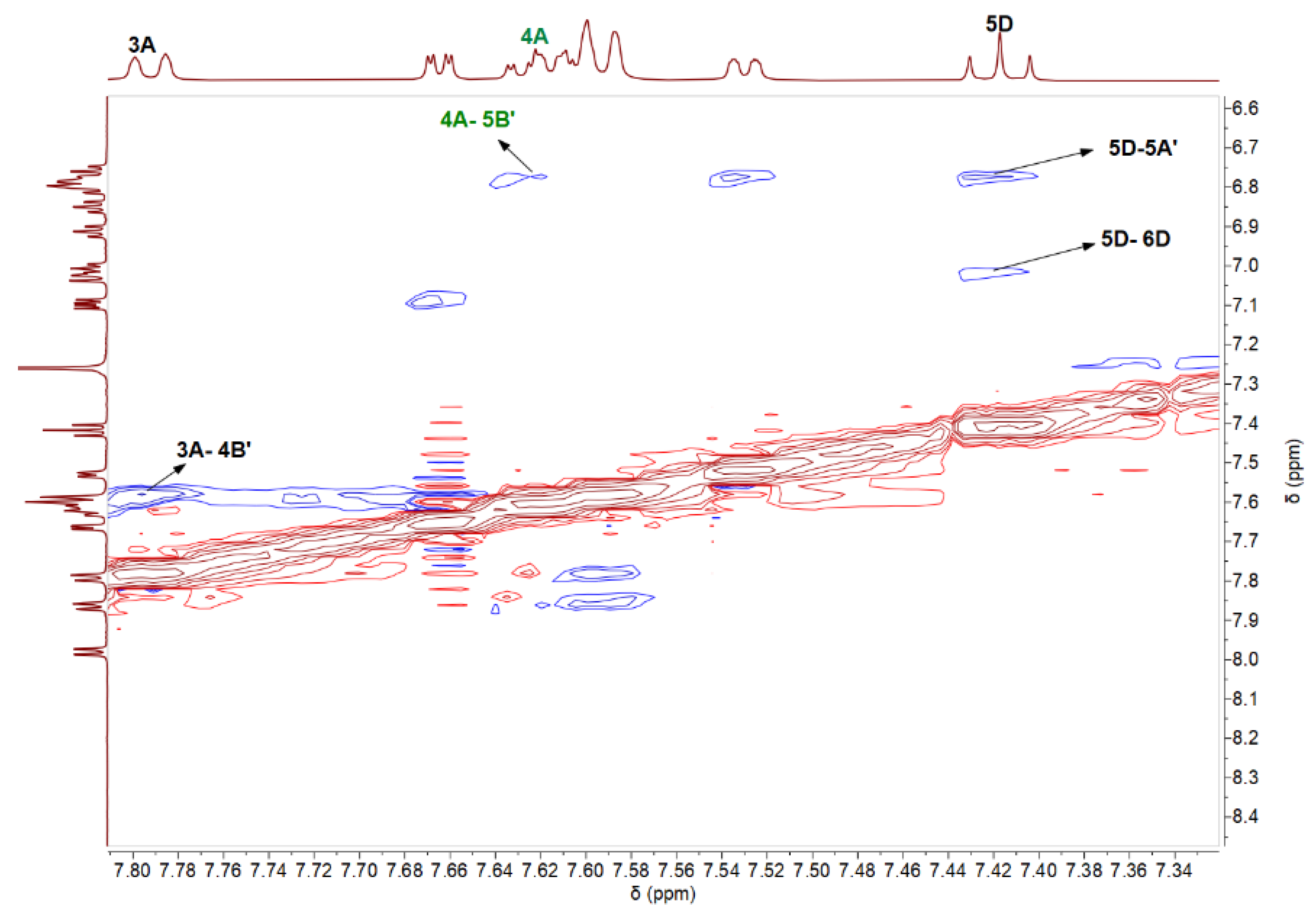
3.3. Nuclear Magnetic Resonance
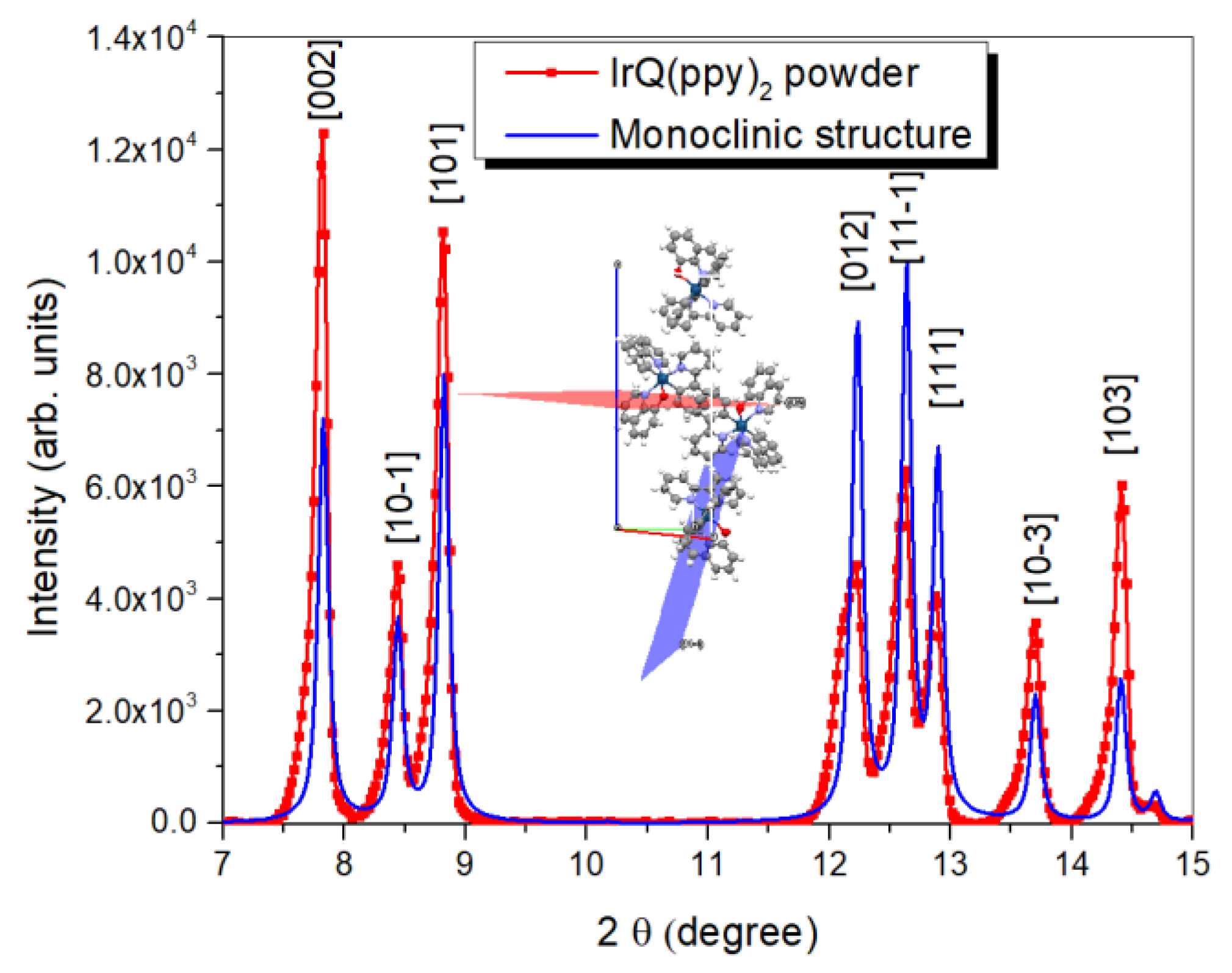
3.4. X-ray Diffraction Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yersin, H. Highly Efficient OLEDs with Phosphorescent Materials; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008; p. 458. [Google Scholar]
- Baldo, M.A.; Thompson, M.E.; Forrest, S.R. Phosphorescent materials for application to organic light emitting devices. Pure Appl. Chem. 1999, 71, 2095. [Google Scholar] [CrossRef]
- Park, Y.S.; Lee, S.; Kim, K.H.; Kim, S.Y.; Lee, J.H.; Kim, J.J. Exciplex-Forming Co-host for Organic Light-Emitting Diodes with Ultimate Efficiency. Adv. Funct. Mater. 2013, 23, 4914. [Google Scholar] [CrossRef]
- Hu, Y.X.; Xia, X.; He, W.Z.; Chi, H.J.; Dong, Y.; Xiao, G.Y.; Lv, Y.L.; Li, X.; Zhang, D.Y. Novel Ir(III) complexes ligated with 2-(2,6-difluoropyridin-3-yl)benzo[d] thiazole for highly efficient OLEDs with mild efficiency roll-off. Dyes Pigments 2019, 166, 254. [Google Scholar] [CrossRef]
- Park, Y.W.; Kim, Y.M.; Choi, J.H.; Park, T.H.; Jeong, J.W.; Cho, M.J.; Choi, D.H.; Ju, B.K. Electroluminescence Property of Highly Soluble Ir(III) Complex Utilized by Various Hole Blocking Layers in Polymer Light Emitting Diodes. J. Nanosci. Nanotechnol. 2010, 10, 3250. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, H.; Xu, H.; Li, J.; Wu, Y.; Du, X.; Xu, B. Synthesis, structure, photophysical and electroluminescent properties of a blue-green self-host phosphorescent iridium(III) complex. Mater. Chem. Phys. 2015, 162, 392. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, X.; Liu, B.; Dang, J.; Li, Y.; Zhou, G.; Wu, Z.; Wong, W.Y. Towards high performance solution-processed orange organic light-emitting devices: Precisely adjusting properties of Ir(III) complexes by reasonably engineering the asymmetric configuration with second functionalized cyclometalating ligands. Mater. Chem. C 2019, 7, 8836. [Google Scholar] [CrossRef]
- Yang, H.; Guo, H.; Wu, L. Green phosphorescent organic light-emitting devices based on different electron transport layers combining with fluorescent sub-monolayer. Opt. Lett. 2017, 13, 0116. [Google Scholar] [CrossRef]
- Ciobotaru, I.C.; Polosan, S.; Ciobotaru, C.C. Organometallic compounds for photovoltaic applications. Inorg. Chim. Acta 2018, 483, 448. [Google Scholar] [CrossRef]
- Lowry, M.S.; Goldsmith, J.I.; Slinker, J.D.; Rohl, R.; Pascal, R.A.; Malliaras, G.G.; Bernhard, S. Single-Layer Electroluminescent Devices and Photoinduced Hydrogen Production from an Ionic Iridium(III) Complex. Chem. Mater. 2005, 17, 5712. [Google Scholar] [CrossRef]
- Polosan, S.; Ciobotaru, C.C.; Ciobotaru, I.C.; Tsuboi, T. Crystallization properties of IrQ(ppy)2 organometallic complex films. J. Mater. Res. 2017, 32, 1735. [Google Scholar] [CrossRef]
- Katz, H.E.; Bao, Z. The Physical Chemistry of Organic Field-Effect Transistors. J. Phys. Chem. B 2000, 104, 671. [Google Scholar] [CrossRef]
- Qiao, X.; Tao, Y.; Wang, Q.; Ma, D.; Yang, C.; Wang, L.; Qin, J.; Wang, F. Controlling charge balance and exciton recombination by bipolar host in single-layer organic light-emitting diodes. J. Appl. Phys. 2010, 108, 034508. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Zhong, G.Y.; Cao, X.A. Concentration quenching of electroluminescence in neat Ir(ppy)3 organic light-emitting diodes. J. Appl. Phys. 2010, 108, 083107. [Google Scholar] [CrossRef]
- Huh, J.S.; Moon, C.K.; Kim, J.J. Dependence of apparent emitting dipole orientation of an Ir(III) complex on doping concentration, film thickness, and excitation condition. Org. Electron. 2019, 74, 299. [Google Scholar] [CrossRef]
- Xie, L.S.; Alexandrov, E.V.; Skorupskii, G.; Proserpio, D.M.; Dinca, M. Diverse p–p stacking motifs modulate electrical conductivity in tetrathiafulvalene-based metal–organic frameworks. Chem. Sci. 2019, 10, 8558. [Google Scholar] [CrossRef]
- Schneebeli, S.T.; Kamenetska, M.; Cheng, Z.; Skouta, R.; Friesner, R.A.; Venkataraman, L.; Breslow, R. Single-Molecule Conductance through Multiple π-π-Stacked Benzene Rings Determined with Direct Electrode-to-Benzene Ring Connections. J. Am. Chem. Soc. 2011, 133, 2136. [Google Scholar] [CrossRef]
- Polosan, S.; Ciobotaru, I.C.; Ciobotaru, C.C. Organometallic Coatings for Electroluminescence Applications. Coatings 2020, 10, 277. [Google Scholar] [CrossRef]
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913. [Google Scholar] [CrossRef]
- Ciobotaru, C.C.; Polosan, S.; Ciobotaru, I.C. Electroluminescence Properties of IrQ(ppy)2 Dual-Emitter Organometallic Compound in Organic Light-Emitting Devices. J. Elec. Mater. 2018, 47, 1490. [Google Scholar] [CrossRef]
- Nonoyama, M.; Yamasaki, K. Rhodium (III) complexes of benzo[h]quinoline and 2-phenylpyridine. Inorg. Nucl. Chem. Lett. 1971, 7, 943. [Google Scholar] [CrossRef]
- Nonoyama, M. Benzo[h]quinolin-10-yl-N Iridium (III) Complexes. Bull. Chem. Soc. Jpn. 1974, 47, 767. [Google Scholar] [CrossRef]
- Nonoyama, M. Chelating C-metallation of some organic nitrogen compounds with hexachlorotetrakis (tri-n-butylphosphine)-dirhodium (II). J. Organomet. Chem. 1975, 92, 89. [Google Scholar] [CrossRef]
- Nonoyama, M. Synthesis of several bis (benzo[h]quinolin-10-yl-n) rhodium (III) complexes. J. Organomet. Chem. 1974, 82, 271. [Google Scholar] [CrossRef]
- Ciobotaru, I.C.; Polosan, S.; Ciobotaru, C.C. Dual emitter IrQ(ppy)2 for OLED applications: Synthesis and spectroscopic analysis. J. Lumin. 2014, 145, 259. [Google Scholar] [CrossRef]
- Yi, C.; Yang, C.J.; Liu, J.; Xu, M.; Wang, J.H.; Cao, Q.Y.; Gao, X.C. Red to near-infrared electrophosphorescence from an iridium complex coordinated with 2-phenylpyridine and 8-hydroxyquinoline. Inorg. Chim. Acta 2007, 360, 3493. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Zhao, H.; Li, J.; Miao, Y.; Wang, H.; Zhu, F.; Xu, B. Pyrene-based hyperbranched porous polymers with doped Ir(piq)2(acac) red emitter for highly efficient white polymer light-emitting diodes. Org. Electron. 2020, 76, 105487. [Google Scholar] [CrossRef]
- Liu, J.; Ma, D.G.; Bai, J. Synthesis of a new iridium complex and its yellow efficient electroluminescence with low efficiency roll-off by exhaustive optimization of device. J. Lumin. 2019, 215, 116655. [Google Scholar] [CrossRef]
- Su, N.; Shen, C.Z.; Zheng, Y.X. Room temperature fast synthesis four-membered red iridium(III) complexes containing IreSePeS structures for OLEDs. J. Organom. Chem. 2019, 896, 188. [Google Scholar] [CrossRef]
- Pastor, A.; Martínez-Viviente, E. NMR spectroscopy in coordination supramolecular chemistry: A unique and powerful methodology. Coord. Chem. Rev. 2008, 252, 2314. [Google Scholar] [CrossRef]
- Teng, Q.; Huynh, H.V. A unified ligand electronic parameter based on 13C NMR spectroscopy of N-Heterocyclic carbene complexes. Dalton Trans. 2016, 46, 614. [Google Scholar] [CrossRef]
- Martschitsch, C.; Schubert, U. An NMR study on the reaction of [(k2 -P,N)- Ph2PCH2CH2NMe2]PtMe2 with activated chlorocarbons: C–Cl and C–C activation, followed by C–N cleavage of the phosphinoalkylamine ligand and formation of an ylide complex. Monatsh. Chem. 2014, 145, 577. [Google Scholar] [CrossRef]
- Gavriluta, A.; Büchel, G.E.; Freitag, L.; Novitchi, G.; Tommasino, J.B.; Jeanneau, E.; Kuhn, P.S.; González, L.; Arion, V.B.; Luneau, D. Mechanism Elucidation of the cis–trans Isomerization of an Azole Ruthenium–Nitrosyl Complex and Its Osmium Counterpart. Inorg. Chem. 2013, 52, 6260. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.C.G.A.; Santos, C.M.M.; Silva, A.M.S. Advanced NMR techniques for structural characterization of heterocyclic structures. In Recent Research Developments in Heterocyclic Chemistry, 1st ed.; Pinho e Melo, T.M.V.D., Ed.; Research Signpost: Kerala, India, 2007; Volume 37, pp. 397–475. [Google Scholar]
- Hetterscheid, D.G.H.; Reek, J.N.H. Me2–NHC based robust Ir catalyst for efficient water oxidation. Chem. Commun. 2011, 47, 2712. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Beasley, C.A.; Murray, R.W. Efficient Electro-Oxidation of Water near Its Reversible Potential by a Mesoporous IrOx Nanoparticle Film. J. Phys. Chem. C 2009, 113, 12958. [Google Scholar] [CrossRef]
- Taguchi, T.; Harada, J.; Kiku, A.; Tohji, K.; Shinoda, K. Development of a new in-laboratory XAFS apparatus based on new concept. J. Synchrotron Radiat. 2001, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, T.; Harada, J.; Tohji, K.; Shinoda, K. An innovated laboratory XAFS apparatus. Adv. X-ray Anal. 2002, 45, 397. [Google Scholar]
- Taguchi, T.; Ozawa, T.; Yashiro, H. REX2000: Yet another XAFS analysis package. Phys. Scr. 2005, 2005, 205. [Google Scholar] [CrossRef]
- Rehr, J.J.; Mustre de Leon, J.; Zabinsky, S.I.; Albers, R.C. Theoretical x-ray absorption fine structure standards. J. Am. Chem. Soc. 1991, 113, 5135. [Google Scholar] [CrossRef]
- Hoare, R.J.; Mills, O.S. Carbon compounds of the transition metals. Part XXV. Crystal and molecular structure of acetatobis(phenylazophenyl-2C,N′)rhodium(II). J. Chem. Soc.-Dalton Trans. 1972, 19, 2138–2141. [Google Scholar] [CrossRef]
- Patrick, J.M.; White, A.H.; Bruce, M.I.; Beatson, M.J.; Black, D.S.; Deacon, G.B.; Thomas, N.C. Crystal structure of bis(benzo[h]quinolin-10-yl-C10,N)dicarbonylruthenium(II). J. Chem. Soc.-Dalton Trans. 1983, 9, 2121–2123. [Google Scholar] [CrossRef]
- Sprouse, S.; King, K.A.; Spellane, P.J.; Watts, R.J. Photophysical effects of metal-carbon. sigma. bonds in ortho-metalated complexes of iridium(III) and rhodium(III). J. Am. Chem. Soc. 1984, 106, 6647. [Google Scholar] [CrossRef]
- Feiters, M.C.; Longo, A.; Banerjee, D.; van der Ham, C.J.M.; Hetterscheid, D.G.H. Validation of EXAFS Analysis of Iridium Compounds. J. Phys. Conf. Ser. 2016, 712, 012059. [Google Scholar] [CrossRef]
- Graber, S. From STM to LEECs: Syntheses and Applications of Multifunctional Bipyridine Ligands and their Iridium(III) Complexes. Ph.D. Thesis, Philosophisch-Naturwissenschaftlichen Fakultat der Universitat Basel, Basel, Switzerland, 2009. [Google Scholar]
- Kappaun, S.; Eder, S.; Sax, S.; Mereiter, K.; List, E.J.W.; Slugovc, C. Organoiridium Quinolinolate Complexes: Synthesis, Structures, Thermal Stabilities and Photophysical Properties. Eur. J. Inorg. Chem. 2007, 16, 4207. [Google Scholar] [CrossRef]
- Ding, J.; Gao, J.; Fu, Q.; Cheng, Y.; Ma, D.; Wang, L. Highly efficient phosphorescent bis-cyclometalated iridium complexes based on quinoline ligands. Synth. Met. 2005, 155, 539. [Google Scholar] [CrossRef]
- You, Y.; Park, S.Y. Inter-Ligand Energy Transfer and Related Emission Change in the Cyclometalated Heteroleptic Iridium Complex: Facile and Efficient Color Tuning over the Whole Visible Range by the Ancillary Ligand Structure. J. Am. Chem. Soc. 2005, 127, 12438. [Google Scholar]
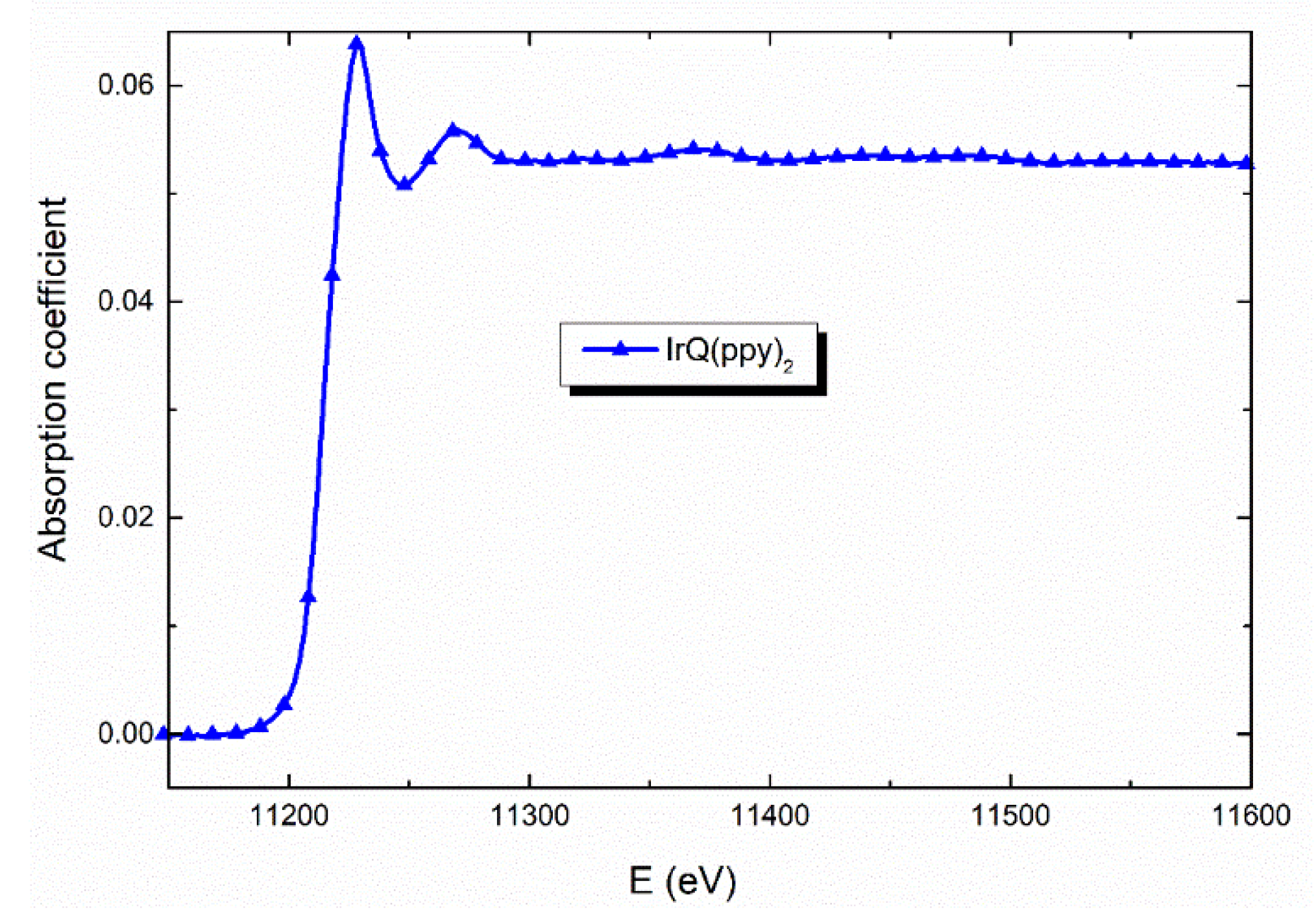
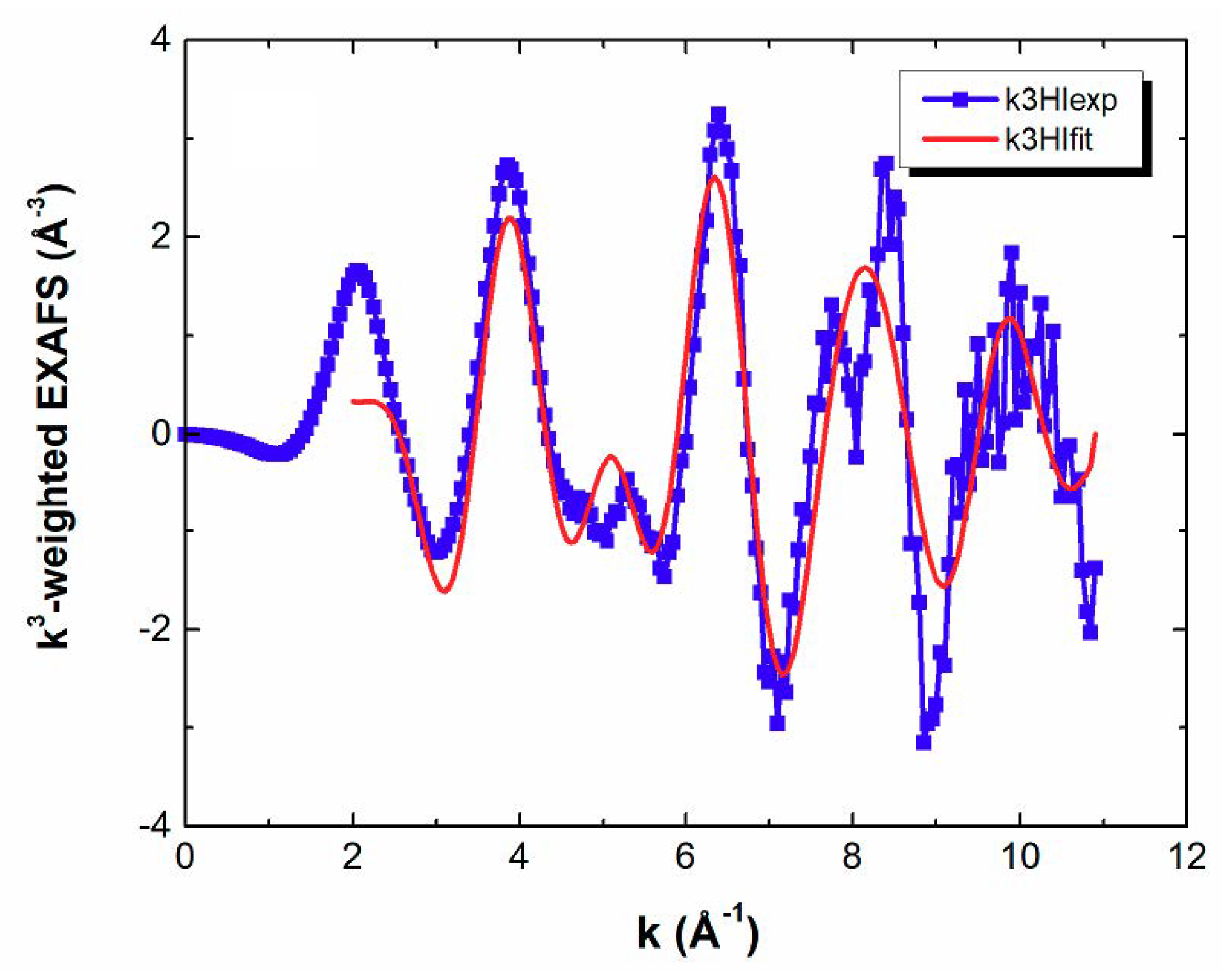
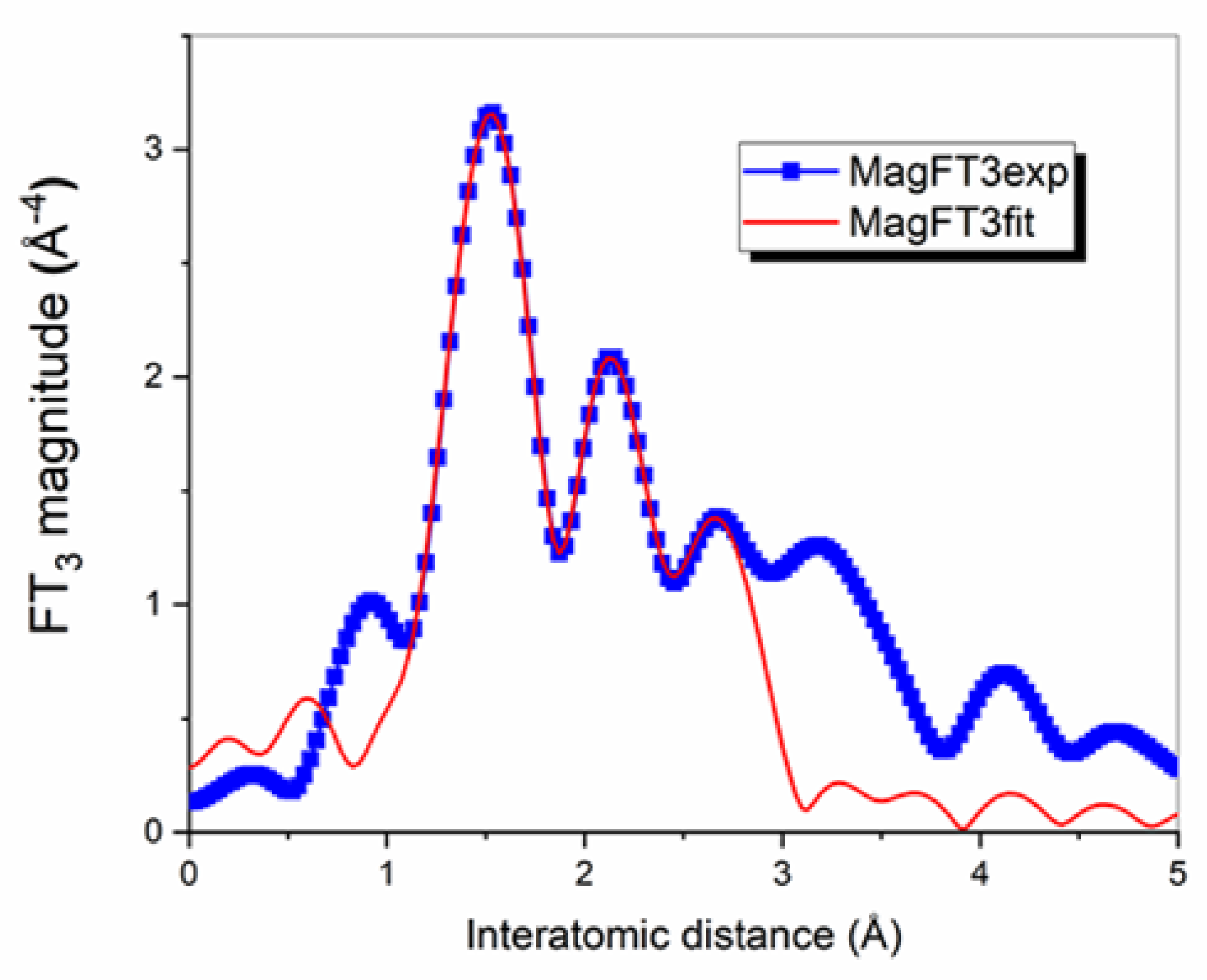
| Shells | Structural Model Based on XRD a | EXAFS b | ||
|---|---|---|---|---|
| N/R (Å) | N | R (Å) | σ2 (Å2) x10−3 | |
| 1st shell | 1 O/2.15 Å 1 N/2.13 Å 2 N/2.03 Å 2 C/2.03 Å | 6 (C,N,O) | 1.97 ± 0.02 Å | 10 ± 3 |
| 2nd shell | 11 C/2.98 Å | 5 C 6 C | 2.63 ± 0.02 Å 2.96 ± 0.03 Å | 8 ± 3 6 ± 2 |
| No of Sets | 1 H- δ (ppm) | ||
|---|---|---|---|
| 5 | 7.98 | 7.67 | 7.11 |
| 6 | 7.03 | 6.80 | 7.42 |
| No of Sets | 1 H- δ (ppm) | |||
|---|---|---|---|---|
| 1 | 8.80 | 7.00 | 7.62 | 7.79 |
| 2 | 7.86 | 7.63 | 6.79 | 7.53 |
| 3 | 6.45 | 6.79 | 7.60 | 6.92 |
| 4 | 6.30 | 6.77 | 7.60 | 6.85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciobotaru, I.C.; Crisan, D.N.; Šket, P.; Ciobotaru, C.C.; Polosan, S. Detailed Molecular and Structural Analysis of Dual Emitter IrQ(ppy)2 Complex. Materials 2020, 13, 1617. https://doi.org/10.3390/ma13071617
Ciobotaru IC, Crisan DN, Šket P, Ciobotaru CC, Polosan S. Detailed Molecular and Structural Analysis of Dual Emitter IrQ(ppy)2 Complex. Materials. 2020; 13(7):1617. https://doi.org/10.3390/ma13071617
Chicago/Turabian StyleCiobotaru, Iulia Corina, Daniel Nicolae Crisan, Primoz Šket, Constantin Claudiu Ciobotaru, and Silviu Polosan. 2020. "Detailed Molecular and Structural Analysis of Dual Emitter IrQ(ppy)2 Complex" Materials 13, no. 7: 1617. https://doi.org/10.3390/ma13071617
APA StyleCiobotaru, I. C., Crisan, D. N., Šket, P., Ciobotaru, C. C., & Polosan, S. (2020). Detailed Molecular and Structural Analysis of Dual Emitter IrQ(ppy)2 Complex. Materials, 13(7), 1617. https://doi.org/10.3390/ma13071617