How Reproducible are Electrochemical Impedance Spectroscopic Data for Dye-Sensitized Solar Cells?
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Effect of the Preirradiation of DSCs
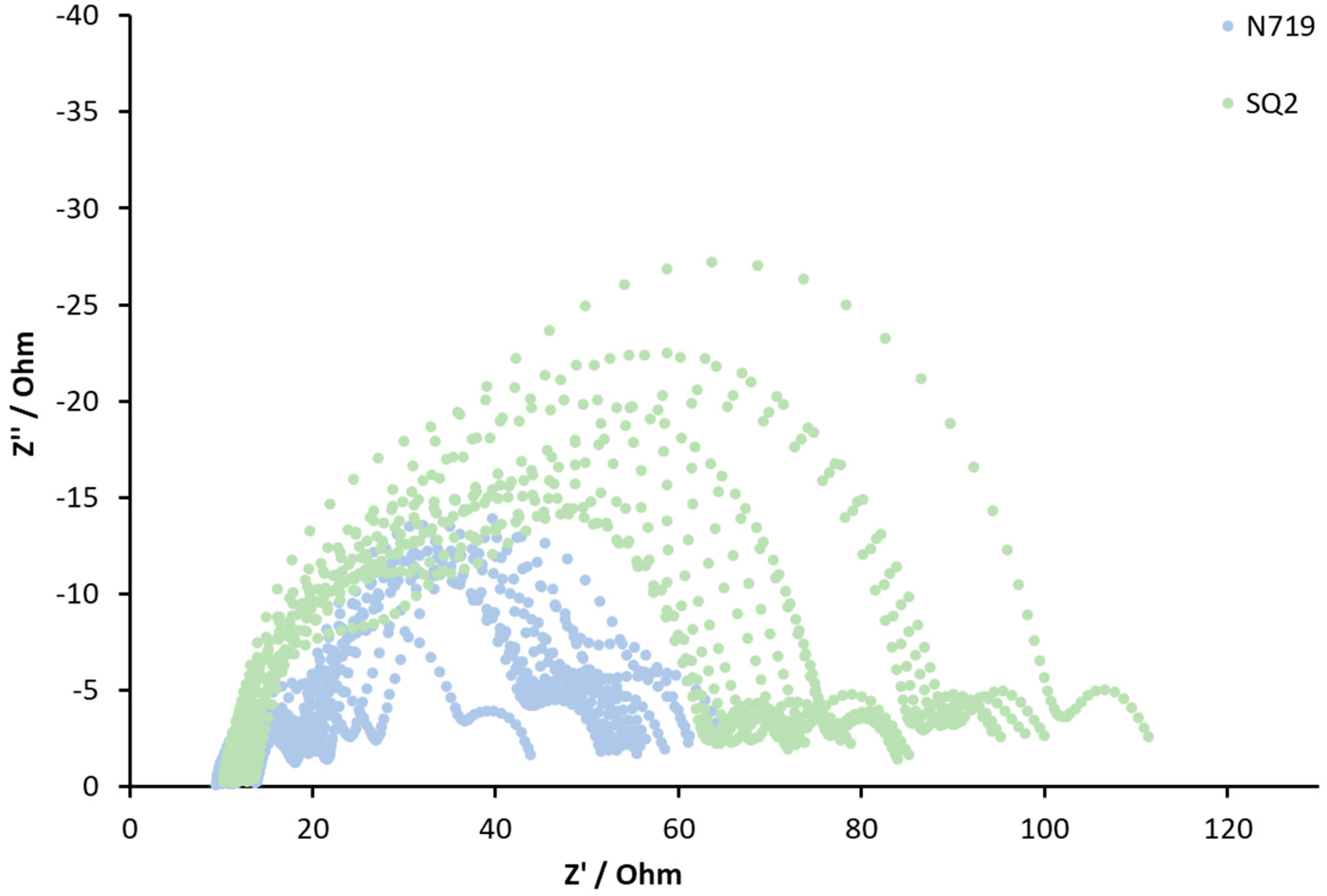
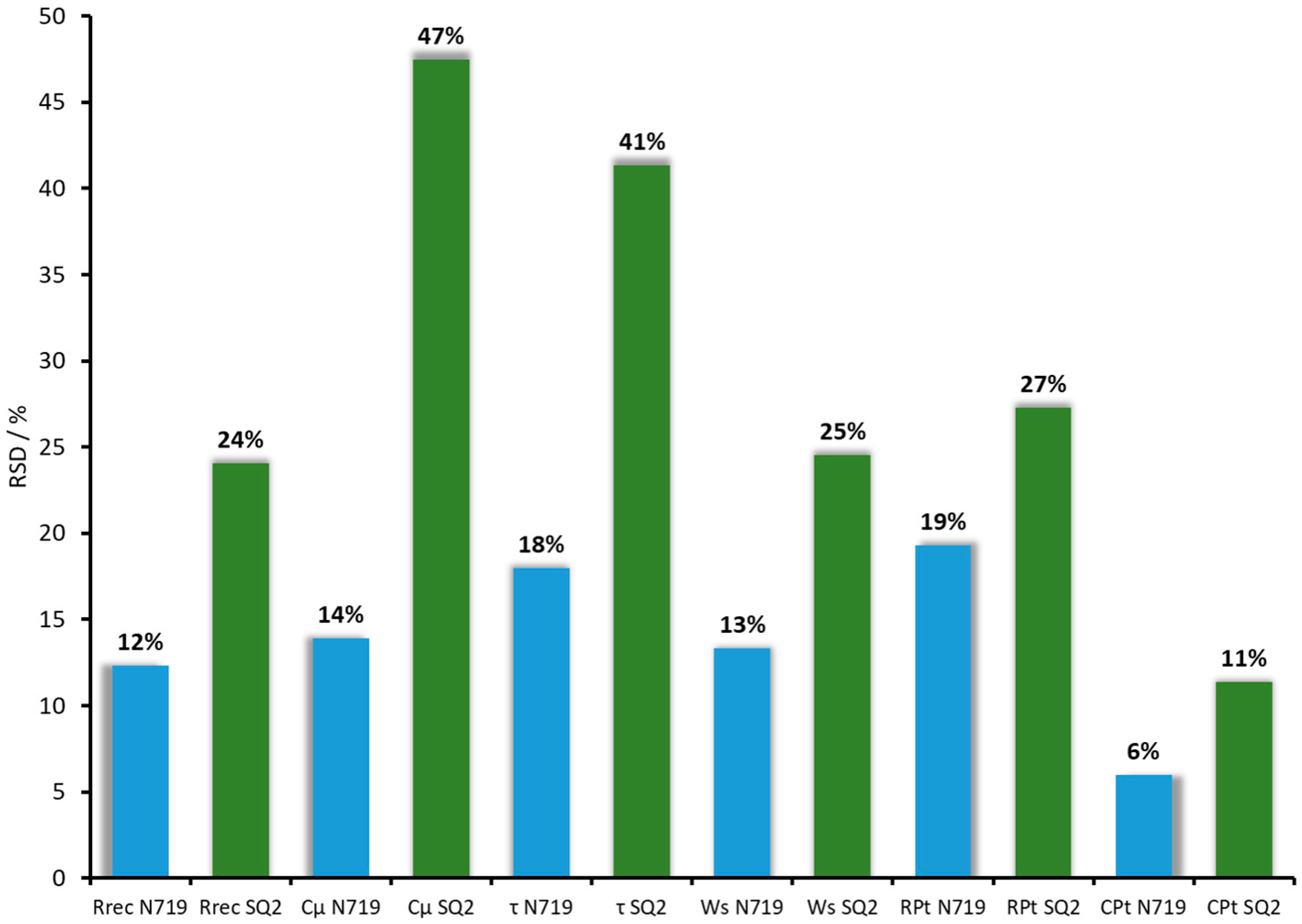
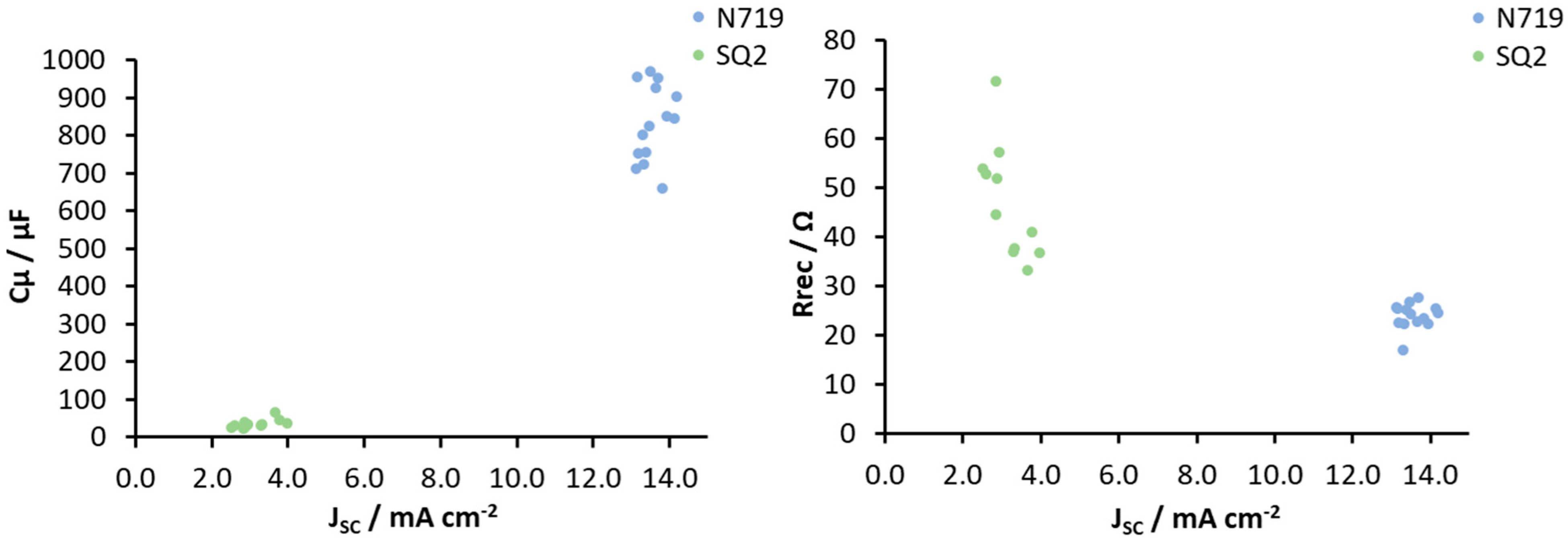
3.2. General Reproducibility of DSCs in Terms of EIS Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bozic-Weber, B.; Constable, E.C.; Housecroft, C.E. Light Harvesting with Earth Abundant d-Block Metals: Development of Sensitizers in Dye-Sensitized Solar Cells (DSCs). Coord. Chem. Rev. 2013, 257, 3089–3106. [Google Scholar] [CrossRef]
- Snaith, H.J. The perils of solar cell efficiency measurements. Nat. Photon. 2012, 6, 337–340. [Google Scholar] [CrossRef]
- Snaith, H.J. How should you measure your excitonic solar cells? Energy Environ. Sci. 2012, 5, 6513. [Google Scholar] [CrossRef]
- Pazoki, M.; Cappel, U.B.; Johansson, E.M.J.; Hagfeldt, A.; Boschloo, G. Characterization techniques for dye-sensitized solar cells. Energy Environ. Sci. 2017, 10, 672–709. [Google Scholar] [CrossRef]
- Liberatore, M.; Decker, F.; Burtone, L.; Zardetto, V.; Brown, T.M.; Reale, A.; Di Carlo, A. Using EIS for diagnosis of dye-sensitized solar cells performance. J. Appl. Electrochem. 2009, 39, 2291–2295. [Google Scholar] [CrossRef]
- Fabregat-Santiago, F.; Bisquert, J.; Palomares, E.; Otero, L.; Kuang, D.; Zakeeruddin, S.M.; Grätzel, M. Correlation between Photovoltaic Performance and Impedance Spectroscopy of Dye-Sensitized Solar Cells Based on Ionic Liquids. J. Phys. Chem. C 2007, 111, 6550–6560. [Google Scholar] [CrossRef]
- Tian, H.; Boschloo, G.; Hagfeldt, A. Molecular Devices for Solar Energy Conversion and Storage; Springer: Singapore, 2018; pp. 353–384. [Google Scholar] [CrossRef]
- Halme, J.; Vahermaa, P.; Miettunen, K.; Lund, P. Device physics of dye solar cells. Adv. Mater. 2010, 22, E210–E234. [Google Scholar] [CrossRef]
- Bhatt, P.; Pandey, K.; Yadav, P.; Tripathi, B.; Kumar, M. Impedance Spectroscopic Investigation of the Degraded Dye-Sensitized Solar Cell due to Ageing. Int. J. Photoenergy 2016, 2016, 8523150. [Google Scholar] [CrossRef]
- Ho, P.; Bao, L.Q.; Ahn, K.-S.; Cheruku, R.; Kim, J.H. P-Type dye-sensitized solar cells: Enhanced performance with a NiO compact blocking layer. Synth. Met. 2016, 217, 314–321. [Google Scholar] [CrossRef]
- Wang, Q.; Ito, S.; Grätzel, M.; Fabregat-Santiago, F.; Mora-Seró, I.; Bisquert, J.; Bessho, T.; Imai, H. Characteristics of High Efficiency Dye-Sensitized Solar Cells. J. Phys. Chem. B 2006, 110, 25210–25221. [Google Scholar] [CrossRef]
- Fabregat-Santiago, F.; Bisquert, J.; Garcia-Belmonte, G.; Boschloo, G.; Hagfeldt, A. Influence of electrolyte in transport and recombination in dye-sensitized solar cells studied by impedance spectroscopy. Sol. Energy Mater. Sol. Cells 2005, 87, 117–131. [Google Scholar] [CrossRef]
- Sacco, A. Electrochemical impedance spectroscopy: Fundamentals and application in dye-sensitized solar cells. Renew. Sustain. Energy Rev. 2017, 79, 814–829. [Google Scholar] [CrossRef]
- Wei-Qing, L.; Zhong-Guan, L.; Dong-Xing, K.; Lin-Hua, H.; Song-Yuan, D. Wide frequency range diagnostic impedance behavior of the multiple interfaces charge transport and transfer processes in dye-sensitized solar cells. Electrochim. Acta 2013, 88, 395–403. [Google Scholar] [CrossRef]
- Bisquert, J. A variable series resistance mechanism to explain the negative capacitance observed in impedance spectroscopy measurements of nanostructured solar cells. Phys. Chem. Chem. Phys. 2011, 13, 4679–4685. [Google Scholar] [CrossRef] [PubMed]
- Fabregat-Santiago, F.; Garcia-Belmonte, G.; Mora-Seró, I.; Bisquert, J. Characterization of nanostructured hybrid and organic solar cells by impedance spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 9083–9118. [Google Scholar] [CrossRef] [PubMed]
- Bisquert, J. Chemical capacitance of nanostructured semiconductors: Its origin and significance for nanocomposite solar cells. Phys. Chem. Chem. Phys. 2003, 5, 5360–5364. [Google Scholar] [CrossRef]
- Klein, Y.M.; Willgert, M.; Perscimone, A.; Constable, E.C.; Housecroft, C.E. Positional isomerism makes a difference: Phosphonic acid anchoring ligands with thienyl spacers in copper(I)-based dye-sensitized solar cells. Dalton Trans. 2016, 45, 4659–4672. [Google Scholar] [CrossRef]
- Sarker, S.; Seo, H.W.; Kim, D.M. Electrochemical impedance spectroscopy of dye-sensitized solar cellswith thermally degraded N719 loaded TiO2. Chem. Phys. Lett. 2013, 585, 193–197. [Google Scholar] [CrossRef]
- Wei, L.; Yang, Y.; Fan, R.; Wang, P.; Li, L.; Yu, J.; Yang, B.; Cao, W. Enhance the performance of dye-sensitized solar cells by co-sensitization of 2,6-bis(iminoalkyl)pyridine and N719. RSC Adv. 2013, 3, 25908–25916. [Google Scholar] [CrossRef]
- Malzner, F.J.; Willgert, M.; Constable, E.C.; Housecroft, C.E. The way to panchromatic copper(I)-based dyesensitized solar cells: Co-sensitization with the organic dye SQ2. J. Mater. Chem. A. 2017, 5, 13717–13729. [Google Scholar] [CrossRef]
- Han, L.; Koide, N.; Chiba, Y.; Islam, A.; Mitate, T. Modeling of an equivalent circuit for dye-sensitized solar cells: Improvement of efficiency of dye-sensitized solar cells by reducing internal resistance. Comptes Rendus Chim. 2006, 9, 645–651. [Google Scholar] [CrossRef]
- Yeoh, M.E.; Chan, K.Y. Short-term light soaking effect on dye-sensitized solar cells. J. Phys. Conf. Ser. 2019, 1349, 012042. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Tran, H.M.; Nguyen, T.T.P. Application of Electrochemical Impedance Spectroscopy in Characterization of Mass- and Charge Transfer Processes in Dye-Sensitized Solar Cells. ECS Trans. 2013, 50, 49–58. [Google Scholar] [CrossRef]
- Bisquert, J.; Fabregat-Santiago, F.; Mora-Seró, I.; Garcia-Belmonte, G.; Giménez, S. Electron Lifetime in Dye-Sensitized Solar Cells: Theory and Interpretation of Measurements. J. Phys. Chem. C 2009, 113, 17278–17290. [Google Scholar] [CrossRef]
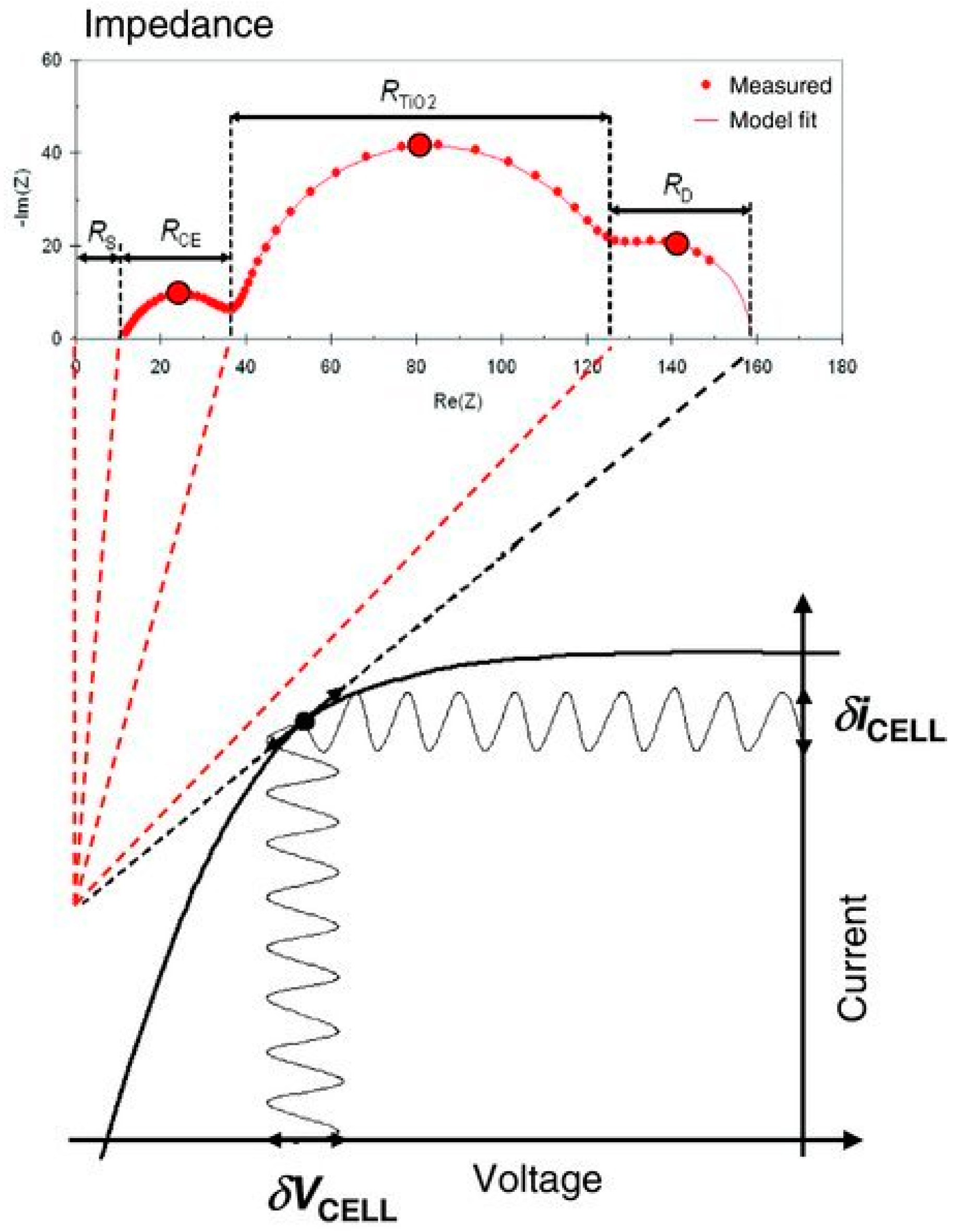
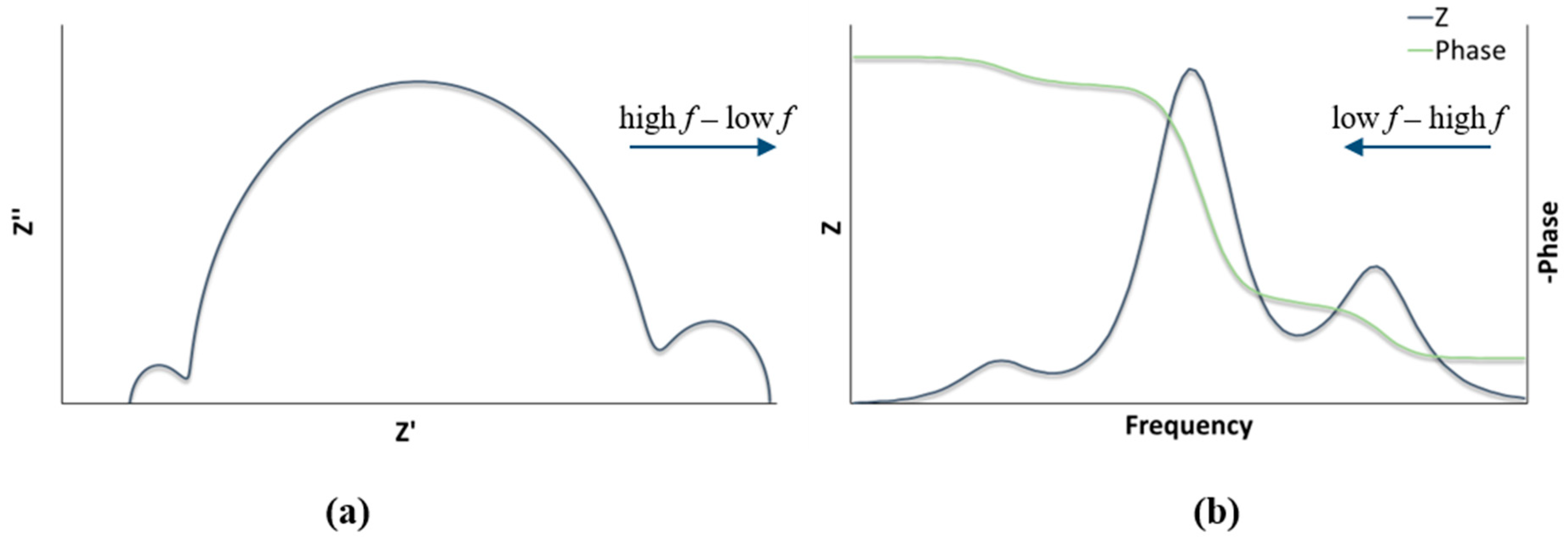
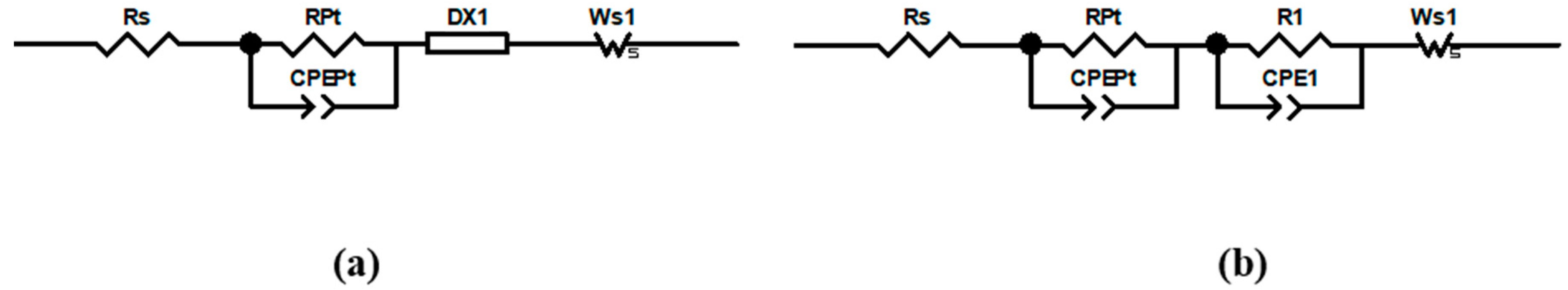
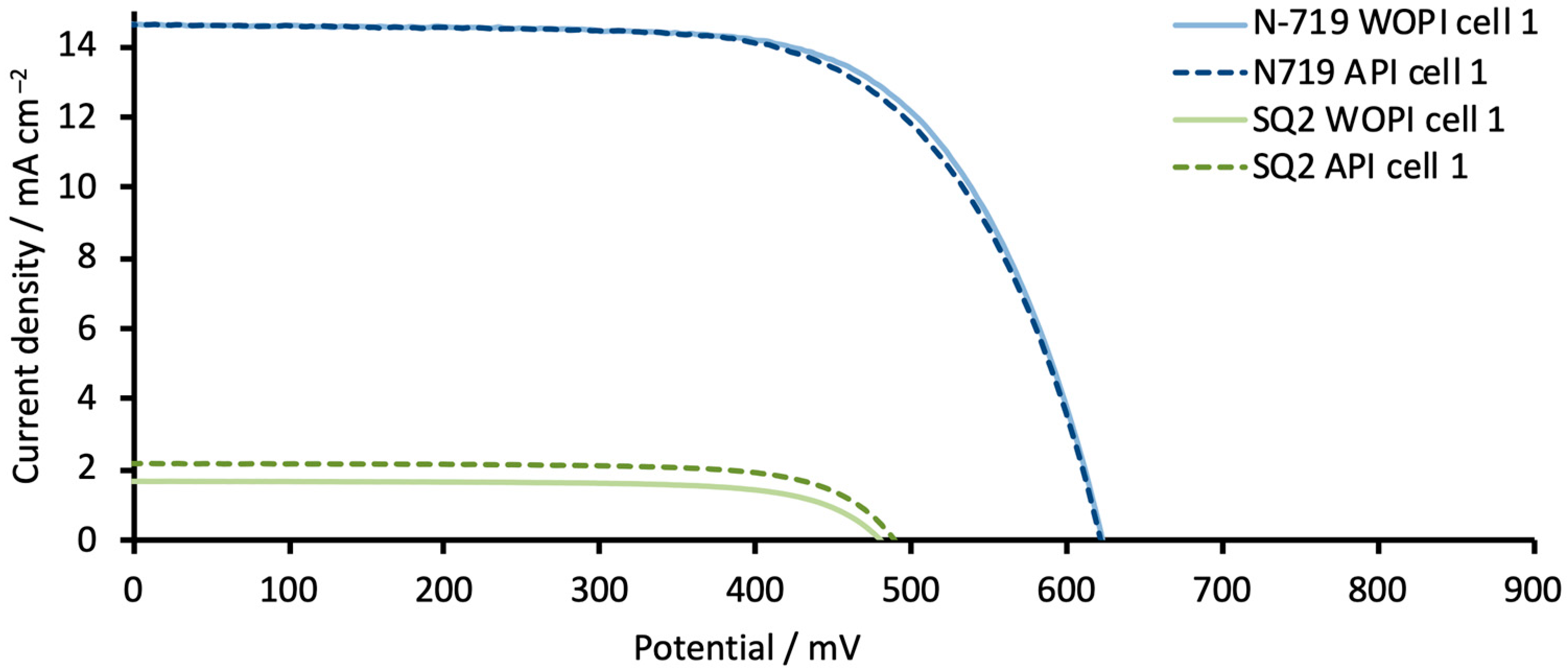
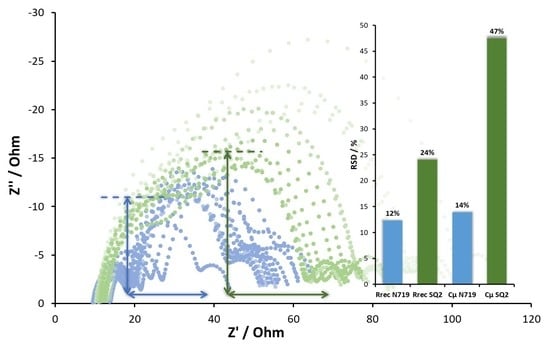
| DSC 1 | Rrec (Ω) | Cμ (μF) | τ (ms) | Ws (Ω) | Rs (Ω) | RPt (Ω) | CPt (μF) |
|---|---|---|---|---|---|---|---|
| N719 WOPI 0 min | 26 | 845 | 22 | 11 | 15 | 7 | 4 |
| N719 WOPI 15 min | 22 | 905 | 20 | 11 | 13 | 8 | 4 |
| N719 WOPI 30 min | 19 | 955 | 18 | 10 | 13 | 8 | 4 |
| N719 WOPI 45 min | 22 | 981 | 22 | 10 | 12 | 8 | 5 |
| N719 WOPI 60 min | 22 | 970 | 21 | 10 | 12 | 8 | 5 |
| SQ2 WOPI 15 min | 185 | 18 | 3 | 15 | 12 | 32 | 9 |
| SQ2 WOPI 30 min | 136 | 20 | 3 | 11 | 12 | 23 | 6 |
| SQ2 WOPI 45 min | 107 | 22 | 2 | 11 | 11 | 20 | 6 |
| SQ2 WOPI 60 min | 94 | 23 | 2 | 10 | 11 | 19 | 5 |
| DSC | Rrec (Ω) | Cμ (μF) | τ (ms) | Ws (Ω) | Rs (Ω) | RPt (Ω) | CPt (μF) |
|---|---|---|---|---|---|---|---|
| N719 API 0 min | 19 | 976 | 19 | 11 | 11 | 9 | 5 |
| N719 API 15 min | 23 | 948 | 22 | 10 | 11 | 9 | 5 |
| N719 API 30 min | 20 | 991 | 20 | 10 | 11 | 9 | 5 |
| N719 API 45 min | 20 | 1007 | 20 | 10 | 11 | 9 | 5 |
| N719 API 60 min | 22 | 990 | 21 | 10 | 13 | 9 | 5 |
| SQ2 API 0 min | 65 | 37 | 2 | 10 | 10 | 17 | 5 |
| SQ2 API 15 min | 62 | 37 | 2 | 9 | 10 | 18 | 5 |
| SQ2 API 30 min | 56 | 39 | 2 | 9 | 10 | 17 | 5 |
| SQ2 API 45 min | 48 | 42 | 2 | 9 | 10 | 16 | 5 |
| SQ2 API 60 min | 52 | 42 | 2 | 9 | 10 | 17 | 5 |
| DSC | Rrec (Ω) | Cμ (μF) | α | τ (ms) | Ws (Ω) | Rs (Ω) | RPt (Ω) | CPt (μF) |
|---|---|---|---|---|---|---|---|---|
| N719 cell 1 | 25 | 756 | 0.92 | 19 | 12 | 12 | 13 | 4 |
| N719 cell 2 | 22 | 724 | 0.95 | 16 | 11 | 11 | 9 | 4 |
| N719 cell 3 | 24 | 902 | 0.94 | 22 | 9 | 14 | 8 | 4 |
| N719 cell 4 | 22 | 852 | 0.94 | 19 | 10 | 11 | 9 | 4 |
| N719 cell 5 | 26 | 847 | 0.92 | 22 | 11 | 10 | 9 | 4 |
| N719 cell 6 | 24 | 971 | 0.94 | 24 | 9 | 11 | 7 | 5 |
| N719 cell 7 | 25 | 955 | 0.94 | 24 | 14 | 14 | 8 | 4 |
| N719 cell 8 | 23 | 927 | 0.93 | 21 | 11 | 10 | 10 | 4 |
| N719 cell 9 | 28 | 951 | 0.94 | 26 | 11 | 9 | 8 | 5 |
| N719 cell 10 | 27 | 826 | 0.93 | 22 | 11 | 14 | 13 | 4 |
| N719 cell 11 | 22 | 753 | 0.94 | 17 | 10 | 11 | 9 | 4 |
| N719 cell 12 | 17 | 801 | 0.95 | 14 | 9 | 12 | 6 | 4 |
| N719 cell 13 | 24 | 660 | 0.94 | 16 | 10 | 10 | 9 | 4 |
| N719 cell 14 | 26 | 711 | 0.93 | 18 | 10 | 10 | 10 | 4 |
| N719 cell 15 | 30 | 604 | 0.93 | 18 | 9 | 11 | 9 | 4 |
| Parameter | Rrec (Ω) | Cμ (μF) | τ (ms) | Ws (Ω) | RPt (Ω) | CPt (μF) |
|---|---|---|---|---|---|---|
| Maximum (max) | 30 | 971 | 26 | 14 | 13 | 5 |
| Minimum (min) | 17 | 604 | 14 | 9 | 6 | 4 |
| Average | 24 | 816 | 20 | 11 | 9 | 4 |
| SD | 3 | 113 | 4 | 1 | 2 | 0.3 |
| Relative standard deviation (RSD) (%) | 12 | 14 | 18 | 13 | 19 | 6 |
| DSC | Rrec (Ω) | Cμ (μF) | α | τ (ms) | Ws (Ω) | Rs (Ω) | RPt (Ω) | CPt (μF) |
|---|---|---|---|---|---|---|---|---|
| SQ2 cell 1 | 45 | 39 | 0.81 | 2 | 8 | 11 | 20 | 4 |
| SQ2 cell 2 | 53 | 30 | 0.80 | 2 | 2 | 12 | 12 | 5 |
| SQ2 cell 3 | 54 | 26 | 0.79 | 1 | 8 | 10 | 12 | 5 |
| SQ2 cell 4 | 37 | 32 | 0.82 | 1 | 11 | 11 | 19 | 5 |
| SQ2 cell 5 | 52 | 27 | 0.81 | 1 | 11 | 11 | 26 | 5 |
| SQ2 cell 6 | 37 | 36 | 0.82 | 1 | 9 | 10 | 16 | 4 |
| SQ2 cell 7 | 72 | 23 | 0.80 | 2 | 11 | 13 | 17 | 5 |
| SQ2 cell 8 | 38 | 35 | 0.82 | 1 | 10 | 11 | 14 | 5 |
| SQ2 cell 9 | 33 | 66 | 0.83 | 2 | 9 | 11 | 21 | 4 |
| SQ2 cell 10 | 57 | 35 | 0.81 | 2 | 10 | 10 | 20 | 5 |
| SQ2 cell 11 | 41 | 45 | 0.82 | 2 | 11 | 12 | 19 | 5 |
| SQ2 cell 12 | 34 | 93 | 0.86 | 3 | 9 | 13 | 24 | 4 |
| SQ2 cell 13 | 45 | 59 | 0.88 | 3 | 10 | 11 | 30 | 4 |
| SQ2 cell 14 | 44 | 77 | 0.89 | 3 | 11 | 14 | 27 | 4 |
| SQ2 cell 15 | 34 | 78 | 0.85 | 3 | 10 | 12 | 17 | 4 |
| Parameter | Rrec (Ω) | Cμ (μF) | τ (ms) | Ws (Ω) | RPt (Ω) | CPt (μF) |
|---|---|---|---|---|---|---|
| max | 72 | 93 | 3 | 11 | 30 | 5 |
| min | 33 | 23 | 1 | 2 | 12 | 4 |
| average | 45 | 47 | 2 | 9 | 20 | 5 |
| SD | 11 | 22 | 1 | 2 | 5 | 1 |
| RSD (%) | 24 | 47 | 41 | 25 | 27 | 11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, M.; Bertrams, M.-S.; Constable, E.C.; Housecroft, C.E. How Reproducible are Electrochemical Impedance Spectroscopic Data for Dye-Sensitized Solar Cells? Materials 2020, 13, 1547. https://doi.org/10.3390/ma13071547
Becker M, Bertrams M-S, Constable EC, Housecroft CE. How Reproducible are Electrochemical Impedance Spectroscopic Data for Dye-Sensitized Solar Cells? Materials. 2020; 13(7):1547. https://doi.org/10.3390/ma13071547
Chicago/Turabian StyleBecker, Mariia, Maria-Sophie Bertrams, Edwin C. Constable, and Catherine E. Housecroft. 2020. "How Reproducible are Electrochemical Impedance Spectroscopic Data for Dye-Sensitized Solar Cells?" Materials 13, no. 7: 1547. https://doi.org/10.3390/ma13071547
APA StyleBecker, M., Bertrams, M.-S., Constable, E. C., & Housecroft, C. E. (2020). How Reproducible are Electrochemical Impedance Spectroscopic Data for Dye-Sensitized Solar Cells? Materials, 13(7), 1547. https://doi.org/10.3390/ma13071547