There Is a Future for N-Heterocyclic Carbene Iron(II) Dyes in Dye-Sensitized Solar Cells: Improving Performance through Changes in the Electrolyte
Abstract
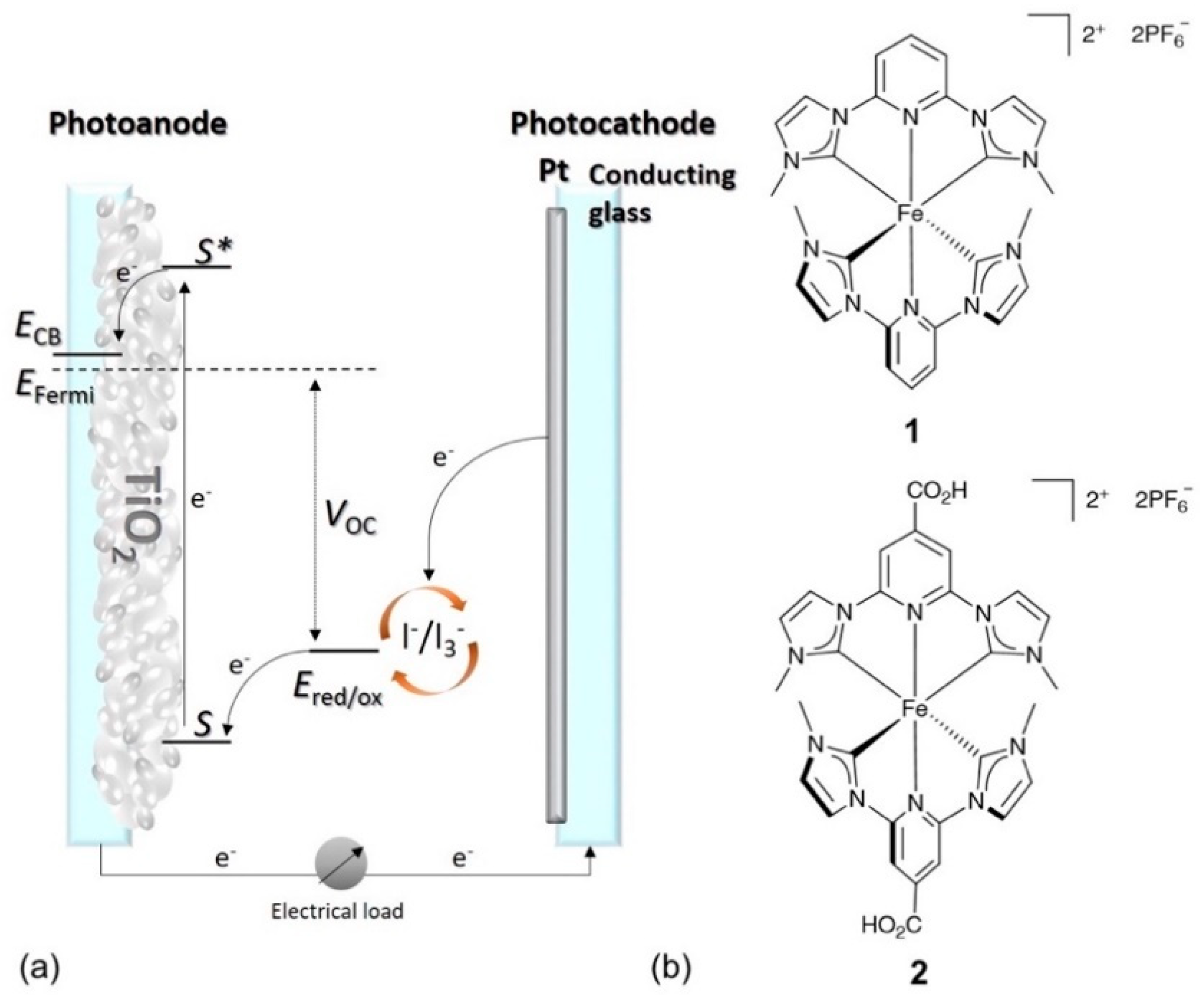
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Influence of Lithium Salts on the Performance of the Fe(II)-NHC DSCs
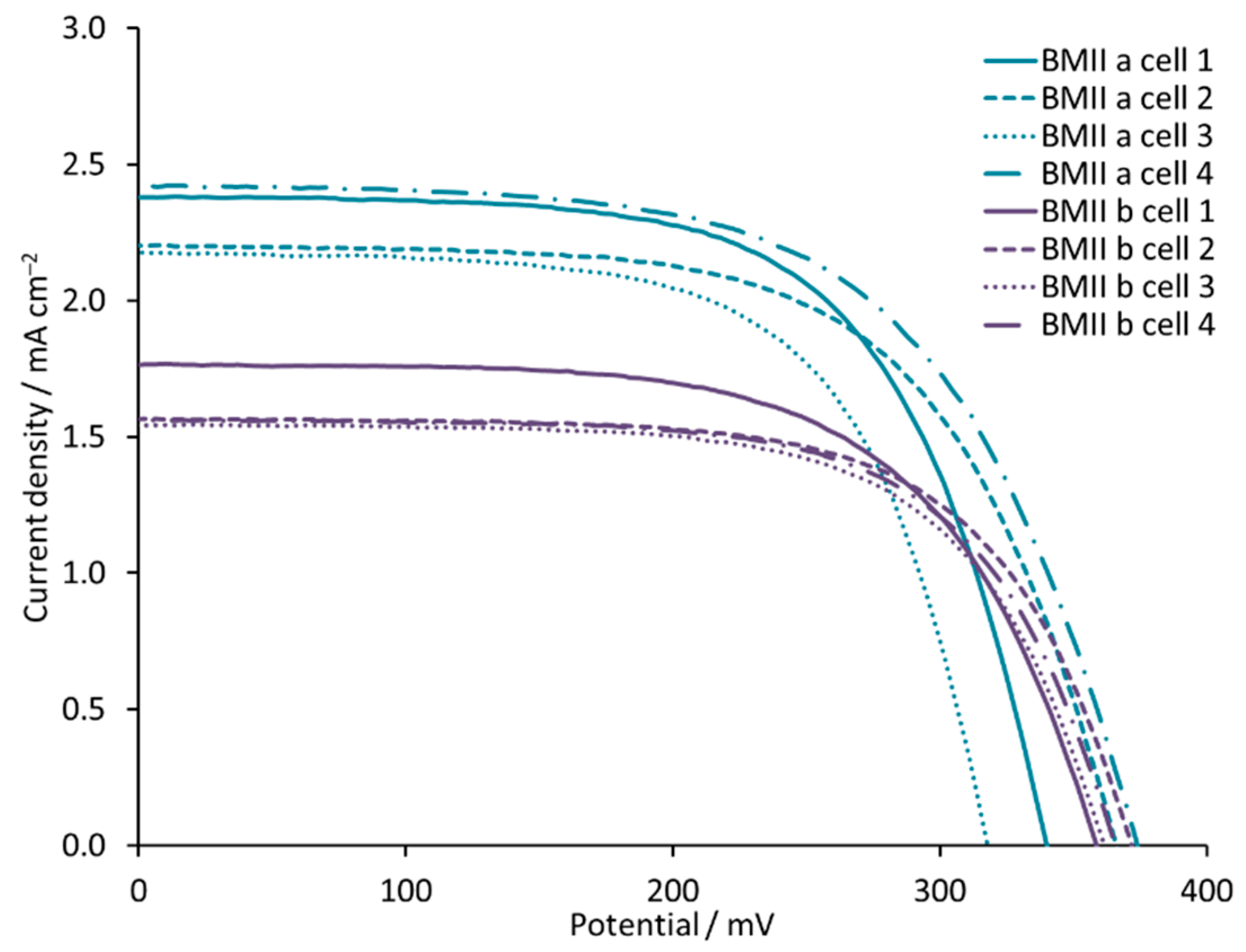
3.2. Influence of the Structure of the Ionic Liquid
3.3. Influence of Ionic Liquid Counterions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Wang, Z.M. (Eds.) High-Efficiency Solar Cells. Physics, Materials, and Devices; Springer: Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Battaglia, C.; Cuevas, A.; De Wolf, S. High-efficiency Crystalline Silicon Solar Cells: Status and Perspectives. Energy Environ. Sci. 2016, 9, 1552–1576. [Google Scholar] [CrossRef]
- Luceño-Sánchez, J.A.; Díez-Pascual, A.M.; Peña Capilla, R. Materials for Photovoltaics: State of Art and Recent Developments. Int. J. Mol. Sci. 2019, 20, 976. [Google Scholar] [CrossRef] [PubMed]
- Kalyanasundaram, K. Dye Sensitized Solar Cells; EPFL Press: Lausanne, Switzerland, 2010; ISBN 9780429156359. [Google Scholar]
- Parisi, M.L.; Maranghi, S.; Basosi, R. The Evolution of the Dye Sensitized Solar Cells from Grätzel Prototype to Up-scaled Solar Applications: A Life Cycle Assessment Approach. Renew. Sustain. Energy Rev. 2014, 39, 124–138. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-sensitized Solar Cells: Fundamentals and Current Status. Nanoscale. Res. Lett. 2018, 13, 381. [Google Scholar] [CrossRef]
- GCell. Smart. Sustainable, Solar. Available online: https://gcell.com/dye-sensitized-solar-cells/advantages-of-dscc/flexible-solar-cells (accessed on 26 July 2019).
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Wu, R.J.; Lin, L.Y.; Chang, W.C. Novel synthesis of popcorn-like TiO2 light scatterers using a facile solution method for efficient dye-sensitized solar cells. J. Power Sources 2019, 413, 384–390. [Google Scholar] [CrossRef]
- Ye, M.; Wen, X.; Wang, M.; Iocozzia, J.; Zhang, N.; Lin, C.; Lin, Z. Recent Advances in Dye-sensitized Solar Cells: From Photoanodes, Sensitizers and Electrolytes to Counter Electrodes. Mater. Today 2015, 18, 155–162. [Google Scholar] [CrossRef]
- Yan, W.; Huo, M.M.; Hu, R.; Wang, Y. Working Area Effects on the Energetic Distribution of Trap States and Charge Dynamics of Dye-sensitized Solar Cells. RSC Adv. 2019, 9, 1734–1740. [Google Scholar] [CrossRef]
- Jennings, J.R.; Liu, Y.; Safari-Alamuti, F.; Wang, Q. Dependence of Dye-sensitized Solar Cell Impedance on Photoelectrode Thickness. J. Phys. Chem. C 2012, 116, 1556–1562. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Wang, T.; Wang, C.; Ge, Z.; Liu, J.; Hao, X.; Du, N.; Xiao, H. Preparation and Photovoltaic Properties of Dye-sensitized Solar Cells Based on Zinc Titanium Mixed Metal Oxides. Colloid. Surf. A 2019, 568, 59–65. [Google Scholar] [CrossRef]
- Calogero, G.; Citro, I.; Crupi, C.; Carini, G.; Arigò, D.; Spinella, G.; Bartolotta, A.; Di Marco, G. Absorption Spectra, Thermal Analysis, Photoelectrochemical Characterization and Stability Test of Vegetable-based Dye-sensitized Solar Cells. Opt. Mater. 2019, 88, 24–29. [Google Scholar] [CrossRef]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.E.; Grätzel, M.; et al. Dye-sensitized Solar Cells for Efficient Power Generation under Ambient Lighting. Nat. Photonics 2017, 11, 372–378. [Google Scholar] [CrossRef]
- Aghazada, S.; Nazeeruddin, M.K. Ruthenium Complexes as Sensitizers in Dye-Sensitized Solar Cells. Inorganics 2018, 6, 52. [Google Scholar] [CrossRef]
- Mishra, A.; Fischer, M.K.; Bäuerle, P. Metal-Free Organic Dyes for Dye-Sensitized Solar Cells: From Structure: Property Relationships to Design Rules. Angew. Chem. Int. Ed. 2009, 48, 2474–2499. [Google Scholar] [CrossRef]
- Zhang, L.; Cole, J.M. Anchoring Groups for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interf. 2015, 7, 3427–3455. [Google Scholar] [CrossRef]
- Cole, J.M.; Pepe, G.; Al Bahri, O.K.; Cooper, C.B. Cosensitization in Dye-Sensitized Solar Cells. Chem. Rev. 2019, 119, 7279–7327. [Google Scholar] [CrossRef]
- Wang, J.; Liu, K.; Ma, L.; Zhan, X. Triarylamine: Versatile Platform for Organic, Dye-Sensitized, and Perovskite Solar Cells. Chem. Rev. 2016, 116, 14675–14725. [Google Scholar] [CrossRef]
- Sarkar, A.; Chakraborty, A.K.; Bera, S. NiS/rGO Nanohybrid: An Excellent Counter Electrode for Dye Sensitized Solar Cell. Solar Energy Mater. Solar Cells 2018, 182, 314–320. [Google Scholar] [CrossRef]
- Zhang, J.B.; Hao, Y.; Yang, L.; Mohammadi, H.; Vlachopoulos, N.; Sun, L.C.; Hagfeldt, A.; Sheibani, E. Electrochemically Polymerized Poly (3, 4-phenylenedioxythiophene) as Efficient and Transparent Counter Electrode for Dye Sensitized Solar Cells. Electrochim. Acta 2019, 300, 482–488. [Google Scholar] [CrossRef]
- Yun, S.; Hagfeldt, A. (Eds.) Counter Electrodes for Dye-Sensitized and Perovskite Solar Cells; Wiley-VCH: Weinheim, Germany, 2018; Volume 1–2, ISBN 978-3-527-41367-6. [Google Scholar]
- Boschloo, G. Improving the Performance of Dye-Sensitized Solar Cells. Front. Chem. 2019, 7, 77. [Google Scholar] [CrossRef]
- Wang, P.; Yang, L.; Wu, H.; Cao, Y.; Zhang, J.; Xu, N.; Chen, S.; Decoppet, J.D.; Zakeeruddin, S.M.; Grätzel, M. Stable and Efficient Organic Dye-Sensitized Solar Cell Based on Ionic Liquid Electrolyte. Joule 2018, 2, 2145–2153. [Google Scholar] [CrossRef]
- Wang, P.; Zakeeruddin, S.M.; Moser, J.E.; Grätzel, M. A New Ionic Liquid Electrolyte Enhances the Conversion Efficiency of Dye-Sensitized Solar Cells. J. Phys. Chem. B 2003, 107, 13280–13285. [Google Scholar] [CrossRef]
- Fürer, S.O.; Bozic-Weber, B.; Schefer, T.; Wobill, C.; Constable, E.C.; Housecroft, C.E.; Willgert, M. Understanding why replacing I3−/I− by cobalt(II)/(III) electrolytes in bis(diimine)copper(I)-based dye-sensitized solar cells improves performance. J. Mater. Chem. A 2016, 4, 12995–13004. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, W.; Zhang, L.; Jiang, R.; Mijangos, E.; Saygili, Y.; Hammarström, L.; Hagfeldt, A.; Boschloo, G. A Small Electron Donor in Cobalt Complex Electrolyte Significantly Improves Efficiency in Dye-Sensitized Solar Cells. Nat. Commun. 2016, 7, 13934. [Google Scholar] [CrossRef]
- Sun, Z.; Liang, M.; Chen, J. Kinetics of Iodine-Free Redox Shuttles in Dye-Sensitized Solar Cells: Interfacial Recombination and Dye Regeneration. Acc. Chem. Res. 2015, 48, 1541–1550. [Google Scholar] [CrossRef]
- Saygili, Y.; Stojanovic, M.; Flores-Diaz, N.; Zakeeruddin, S.M.; Vlachopoulos, N.; Grätzel, M.; Hagfeldt, A. Metal Coordination Complexes as Redox Mediators in regenerative Dye-Sensitized Solar Cells. Inorganics 2019, 7, 30. [Google Scholar] [CrossRef]
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Fan, L.; Luo, G. Electrolytes in Dye-Sensitized Solar Cells. Chem. Rev. 2015, 115, 2136–2173. [Google Scholar] [CrossRef]
- Iftikhar, H.; Sonai, G.G.; Hashmi, S.G.; Nogueira, A.F.; Lund, P.D. Progress on Electrolytes Development in Dye-Sensitized Solar Cells. Materials 2019, 12, 1998. [Google Scholar] [CrossRef]
- Kroon, J.M.; Bakker, N.J.; Smit, H.J.P.; Liska, P.; Thampi, K.R.; Wang, P.; Zakeeruddin, S.M.; Grätzel, M.; Hinsch, A.; Hore, S.; et al. Nanocrystalline dye-sensitized solar cells having maximum performance. Prog. Photovolt. Res. Appl. 2007, 15, 1–18. [Google Scholar] [CrossRef]
- Chiba, Y.; Islam, A.; Watanabe, Y.; Komiya, R.; Koide, N.; Han, L. Dye-Sensitized Solar Cells with Conversion Efficiency of 11.1%. Jpn. J. Appl. Phys. 2006, 45, L638–L640. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Péchy, P.; Renouard, T.; Zakeeruddin, S.M.; Humphry-Baker, R.; Comte, P.; Liska, P.; Cevey, L.; Costa, E.; Shklover, V.; et al. Engineering of Efficient Panchromatic Sensitizers for Nanocrystalline TiO2-Based Solar Cells. J. Am. Chem. Soc. 2001, 123, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Bozic-Weber, B.; Constable, E.C.; Housecroft, C.E. Light Harvesting with Earth Abundant d-Block Metals: Development of Sensitizers in Dye-Sensitized Solar Cells (DSCs). Coord. Chem. Rev. 2013, 257, 3089–3106. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Constable, E.C. The Emergence of Copper(I)-Based Dye Sensitized Solar Cells. Chem. Soc. Rev. 2015, 44, 8386–8398. [Google Scholar] [CrossRef] [PubMed]
- Lazorski, M.S.; Castellano, F.N. Advances in the light conversion properties of Cu(I)-based photosensitizers. Polyhedron 2014, 82, 57–70. [Google Scholar] [CrossRef]
- Sandroni, M.; Pellegrin, Y.; Odobel, F. Heteroleptic Bis-diimine Copper(I) Complexes for Applications in Solar Energy Conversion. C. R. Chim. 2016, 19, 79–93. [Google Scholar] [CrossRef]
- Dragonetti, C.; Magni, M.; Colombo, A.; Fagnani, F.; Roberto, D.; Melchiorre, F.; Biagini, P.; Fantacci, S. Towards Efficient Sustainable Full-copper Dye-sensitized Solar Cells. Dalton Trans. 2019, 48, 9703–9711. [Google Scholar] [CrossRef]
- Wenger, O.S. Is Iron the New Ruthenium? Chem. Eur. J. 2019, 25, 6043–6052. [Google Scholar] [CrossRef]
- Duchanois, T.; Liu, L.; Pastore, M.; Monari, A.; Cebrián, C.; Trolez, Y.; Darari, M.; Magra, K.; Francés-Monerris, A.; Domenichini, E.; et al. NHC-Based Iron Sensitizers for DSSCs. Inorganics 2019, 6, 63. [Google Scholar] [CrossRef]
- Ferrere, S.; Gregg, B.A. Photosensitization of TiO2 by [FeII(2,2′-bipyridine-4,4′-dicarboxylic acid)2(CN)2]: Band Selective Electron Injection from Ultra-Short-Lived Excited States. J. Am. Chem. Soc. 1998, 120, 843–844. [Google Scholar] [CrossRef]
- Liu, Y.; Harlang, T.; Canton, S.E.; Chábera, P.; Suárez-Alcántara, K.; Fleckhaus, A.; Vithanage, D.A.; Göransson, E.; Corani, A.; Lomoth, R.; et al. Towards longer-lived metal-to-ligand charge transfer states of iron(II) complexes: An N-heterocyclic carbene approach. Chem. Commun. 2013, 49, 641–6414. [Google Scholar] [CrossRef]
- Duchanois, T.; Etienne, T.; Cebrián, C.; Liu, L.; Monari, A.; Beley, M.; Assfeld, X.; Haacke, S.; Gros, P.C. An Iron-Based Photosensitizer with Extended Excited-State Lifetime: Photophysical and Photovoltaic Properties. Eur. J. Inorg. Chem. 2015, 2015, 2469–2477. [Google Scholar] [CrossRef]
- Pastore, M.; Duchanois, T.; Liu, L.; Monari, A.; Assfeld, X.; Haacke, S.; Gros, P.C. Interfacial charge separation and photovoltaic efficiency in Fe(ii)-carbene sensitized solar cells. Phys. Chem. Chem. Phys. 2016, 18, 28069–28081. [Google Scholar] [CrossRef] [PubMed]
- Hauch, A.; Georg, A. Diffusion in the electrolyte and charge-transfer reaction at the platinum electrode in dye-sensitized solar cells. Electrochim. Acta 2001, 46, 3457–3466. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Grätzel, M. Molecular Photovoltaics. Acc. Chem. Res. 2000, 33, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Fukui, A.; Komiya, R.; Yamanaka, R.; Islam, A.; Han, L. Effect of a redox electrolyte in mixed solvents on the photovoltaic performance of a dye-sensitized solar cell. Solar Energy Mater. Solar Cells 2006, 90, 649–658. [Google Scholar] [CrossRef]
- Kusama, H.; Orita, H.; Sugihara, H. TiO2 band shift by nitrogen-containing heterocycles in dye-sensitized solar cells: A periodic density functional theory study. Langmuir 2008, 24, 4411–4419. [Google Scholar] [CrossRef]
- Shi, J.; Peng, B.; Pei, J.; Peng, S.; Chen, J. An Inexpensive and Efficient Pyridine-Based Additive for the Electrolyte of Dye-Sensitized Solar Cells. J. Power Sources 2009, 193, 878–884. [Google Scholar] [CrossRef]
- Zhang, Z.; Zakeeruddin, S.M.; O’Regan, B.C.; Humphry-Baker, R.; Grätzel, M. Influence of 4-Guanidinobutyric Acid as Coadsorbent in Reducing Recombination in Dye-Sensitized Solar Cells. J. Phys. Chem. B 2005, 109, 21818–21824. [Google Scholar] [CrossRef]
- Jennings, J.R.; Wang, Q. Influence of Lithium Ion Concentration on Electron Injection, Transport, and Recombination in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2010, 114, 1715–1724. [Google Scholar] [CrossRef]
- Fürer, S.O.; Luu, L.Y.N.; Bozic-Weber, B.; Constable, E.C.; Housecroft, C.E. Improving performance of copper(I)-based dye sensitized solar cells through I3−/I− electrolyte manipulation. Dyes Pigm. 2016, 132, 72–78. [Google Scholar] [CrossRef]
- Wang, P.; Zakeeruddin, S.M.; Moser, J.E.; Humphry-Baker, R.; Grätzel, M. A Solvent-Free, SeCN–/(SeCN)3− Based Ionic Liquid Electrolyte for High-Efficiency Dye-Sensitized Nanocrystalline Solar Cells. J. Am. Chem. Soc. 2004, 126, 7164–7165. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Cao, Y.; Zhang, J.; Wang, M.; Li, R.; Wang, P.; Zakeeruddin, S.M.; Grätzel, M. High-performance dye-sensitized solar cells based on solvent-free electrolytes produced from eutectic melts. Nat. Mater. 2008, 7, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.; Bobbink, F.D.; Păunescu, E.; Scopelliti, R.; Dyson, P.J. Influence of Elemental Iodine on Imidazolium-Based Ionic Liquids: Solution and Solid-State Effects. Inorg. Chem. 2015, 54, 10504–10512. [Google Scholar] [CrossRef] [PubMed]
- Karpacheva, M.; Housecroft, C.E.; Constable, E.C. Electrolyte tuning in dye-sensitized solar cells with N-heterocyclic carbene (NHC) iron(II) sensitizers. Beilstein J. Nanotechnol. 2018, 9, 3069–3078. [Google Scholar] [CrossRef]
- Karpacheva, M.; Malzner, F.J.; Wobill, C.; Büttner, A.; Constable, E.C.; Housecroft, C.E. Cuprophilia: Dye-sensitized solar cells with copper(I) dyes and copper(I)/(II) redox shuttles. Dyes Pigm. 2018, 156, 410–416. [Google Scholar] [CrossRef]
- Ferber, J.; Stangl, R.; Luther, J. An Electrical Model for the Dye-Sensitized Solar Cell. Solar Energy Mater. Solar Cells 1998, 53, 29–54. [Google Scholar] [CrossRef]
- Malzner, F.J.; Brauchli, S.Y.; Schönhofer, E.; Constable, E.C.; Housecroft, C.E. To deprotect or not to deprotect: Phosphonate ester versus phosphonic acid anchor ligands in copper(I)-based dye-sensitized solar cells. Polyhedron 2014, 82, 116–121. [Google Scholar] [CrossRef]
- Halme, J.; Vahermaa, P.; Miettunen, K.; Lund, P. Device physics of dye solar cells. Adv. Mater. 2010, 22, E210–E234. [Google Scholar] [CrossRef]
- Ho, P.; Bao, L.Q.; Ahn, K.S.; Cheruku, R.; Kim, J.H. P-Type dye-sensitized solar cells: Enhanced performance with a NiO compact blocking layer. Synth. Met. 2016, 217, 314–321. [Google Scholar] [CrossRef]
- Every, H.A.; Bishop, A.G.; MacFarlane, D.R.; Orädd, G.; Forsyth, M. Transport properties in a family of dialkylimidazolium ionic liquids. Phys. Chem. Chem. Phys. 2004, 6, 1758–1765. [Google Scholar] [CrossRef]
- Wang, P.; Wenger, B.; Humphry-Baker, R.; Moser, J.E.; Teuscher, J.; Kantlehner, W.; Mezger, J.; Stoyanov, E.V.; Zakeeruddin, S.M.; Grätzel, M. Charge separation and efficient light energy conversion in sensitized mesoscopic solar cells based on binary ionic liquids. J. Am. Chem. Soc. 2005, 127, 6850–6856. [Google Scholar] [CrossRef] [PubMed]
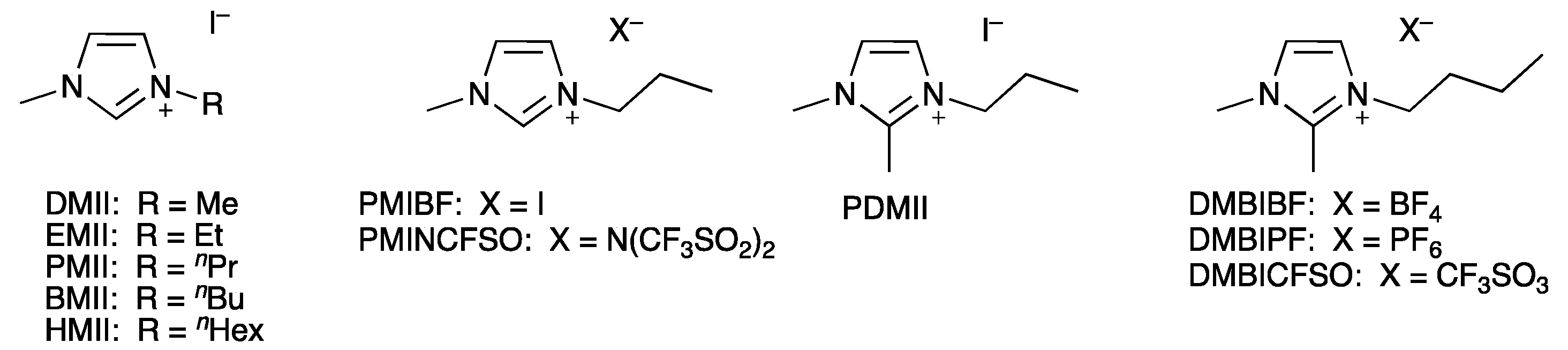


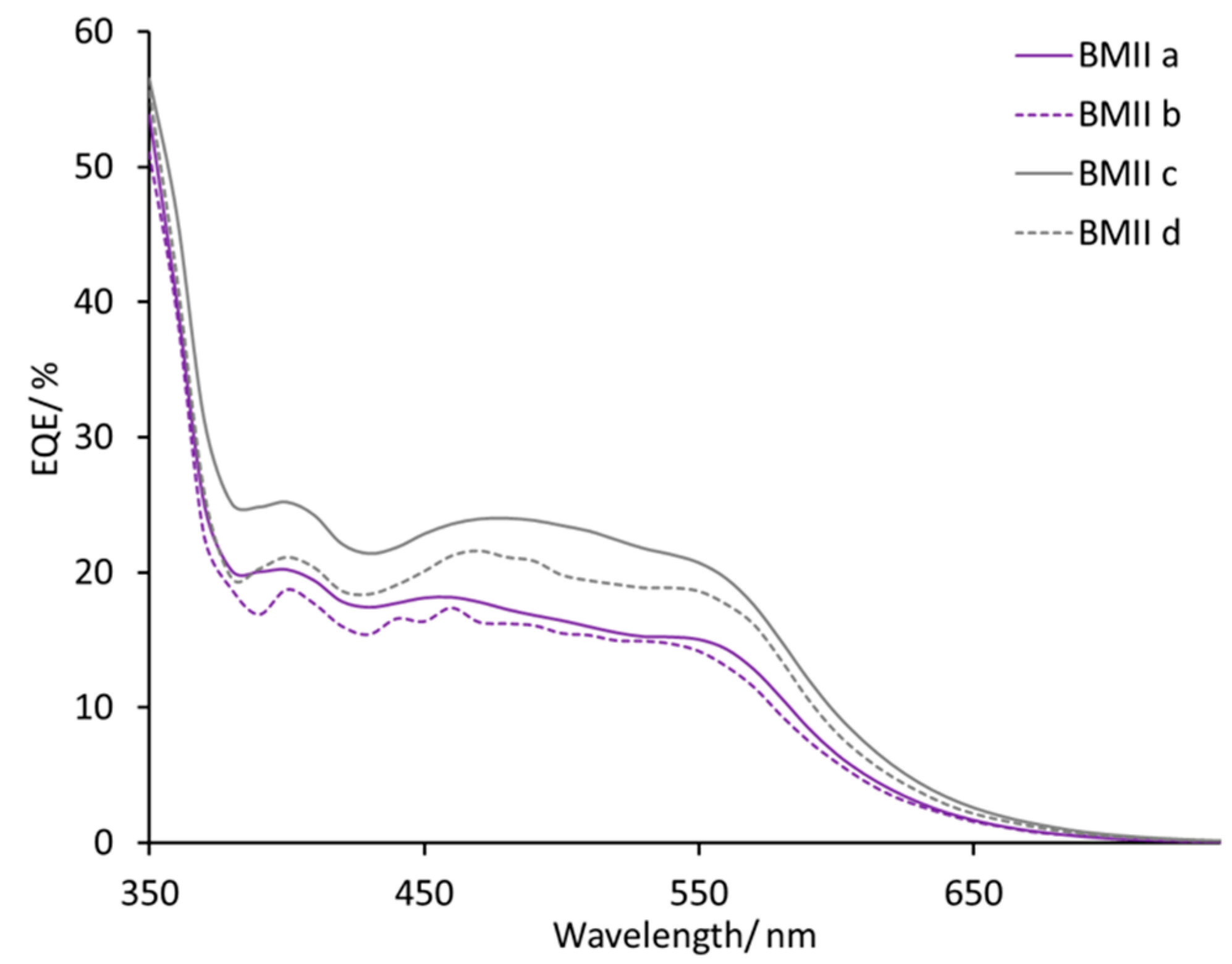
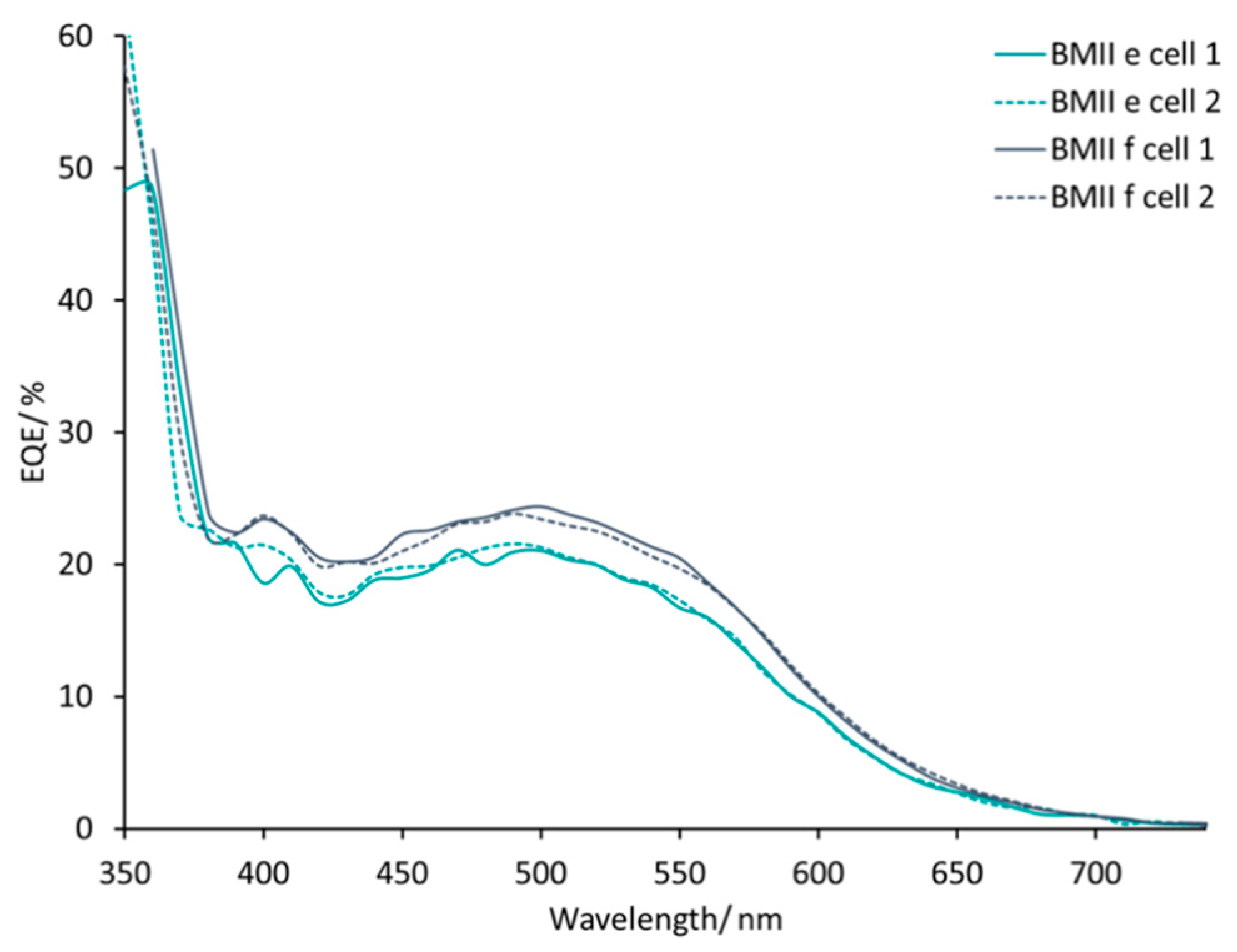
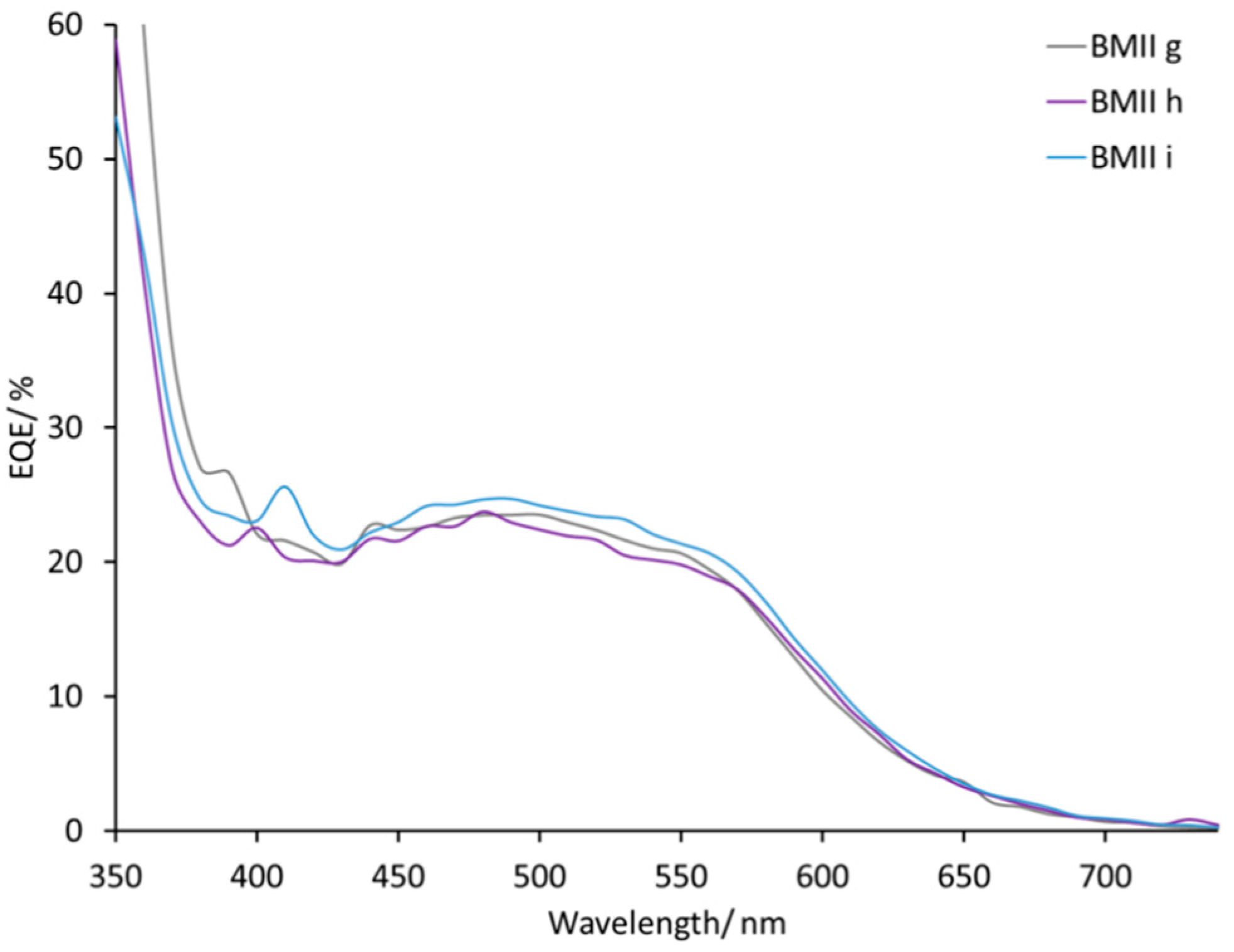

| Electrolyte 1 | LiI/M | LiPF6/M | I2/M | IL/M |
|---|---|---|---|---|
| PMIIa | 0.10 | – | 0.05 | PMII/0.60 |
| PMIIb | – | 0.10 | 0.05 | PMII/0.60 |
| PMIIc | 0.18 | – | 0.05 | PMII/0.60 |
| PMIId | – | 0.18 | 0.05 | PMII/0.60 |
| BMIIa | 0.10 | – | 0.05 | BMII/0.60 |
| BMIIb | – | 0.10 | 0.05 | BMII/0.60 |
| BMIIc | 0.18 | – | 0.05 | BMII/0.60 |
| BMIId | – | 0.18 | 0.05 | BMII/0.60 |
| BMIIe | 0.26 | – | 0.05 | BMII/0.60 |
| BMIIf | 0.34 | – | 0.05 | BMII/0.60 |
| BMIIg | 0.18 | – | 0.05 | BMII/0.52 |
| BMIIh | 0.26 | – | 0.05 | BMII/0.44 |
| BMIIi | 0.34 | – | 0.05 | BMII/0.36 |
| PDMIIa | 0.18 | – | 0.05 | PDMII/0.60 |
| PDMIIb | 0.18 | – | 0.05 | PDMII/0.52 |
| PMIIe | 0.18 | – | 0.05 | PMII/0.52 |
| Electrolyte 1 | JSC/mA cm−2 | VOC/mV | ff/% | η/% | Rel. η/% 2 |
|---|---|---|---|---|---|
| PMIIa | 2.34 | 371 | 66 | 0.57 | 10.2 |
| PMIIb | 2.71 | 264 | 57 | 0.41 | 7.3 |
| PMIIc | 3.01 | 315 | 62 | 0.59 | 10.5 |
| PMIId | 3.91 | 281 | 53 | 0.59 | 10.5 |
| BMIIa | 2.42 | 374 | 61 | 0.55 | 9.8 |
| BMIIb | 1.77 | 359 | 62 | 0.39 | 6.9 |
| BMIIc | 3.40 | 301 | 59 | 0.61 | 10.9 |
| BMIId | 3.13 | 315 | 59 | 0.58 | 10.4 |
| BMIIe | 3.02 | 299 | 62 | 0.56 | 10.0 |
| BMIIf | 3.45 | 307 | 60 | 0.64 | 11.4 |
| BMIIg | 3.33 | 298 | 62 | 0.62 | 11.1 |
| BMIIh | 3.46 | 301 | 60 | 0.63 | 11.3 |
| BMIIi | 3.61 | 264 | 62 | 0.59 | 10.5 |
| PDMIIa | 3.27 | 348 | 58 | 0.66 | 11.8 |
| PDMIIb | 3.21 | 337 | 57 | 0.62 | 11.1 |
| PMIIe | 2.80 | 368 | 62 | 0.64 | 11.4 |
| Reference cell with dye N719 | 12.53 | 654 | 68 | 5.60 | 100 |
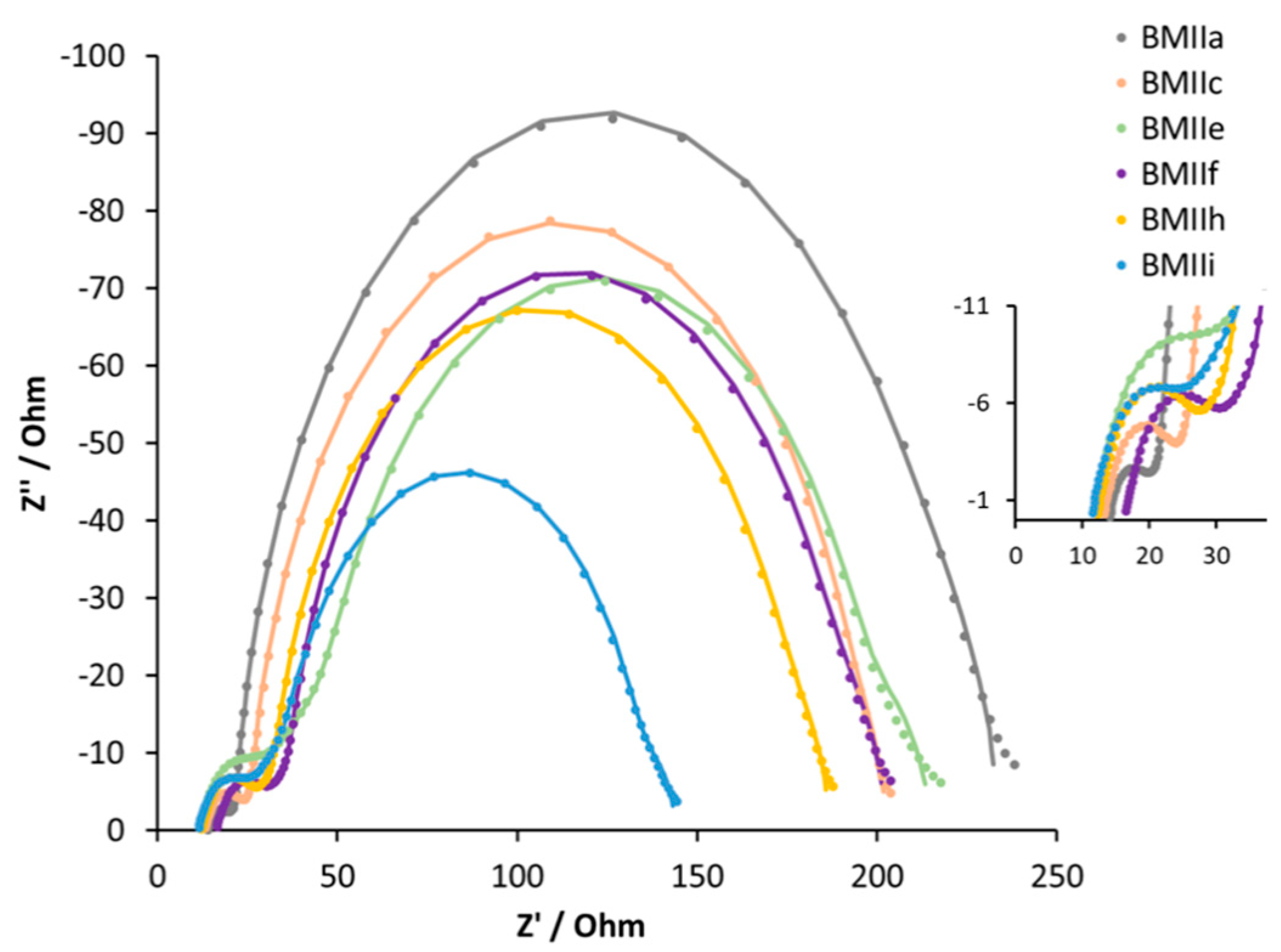
| Electrolyte 1 | Rrec/Ω | Cμ/μF | Rtr/Ω | τ/ms | τt/ms | Ld/μm | Rs/Ω | RPt/Ω | CPt/μF |
|---|---|---|---|---|---|---|---|---|---|
| BMIIa | 192 | 384 | 9 | 74 | 3 | 56 | 14 | 4 | 6 |
| BMIIc | 169 | 307 | 13 | 52 | 4 | 43 | 13 | 8 | 6 |
| BMIIe | 150 | 258 | 69 | 39 | 18 | 18 | 11 | 11 | 7 |
| BMIIf | 152 | 374 | 26 | 57 | 10 | 29 | 16 | 11 | 5 |
| BMIIh | 144 | 378 | 23 | 54 | 9 | 30 | 12 | 12 | 5 |
| BMIIi | 99 | 302 | 40 | 30 | 12 | 19 | 11 | 10 | 6 |
| Electrolyte 1,2 | JSC/mA cm−2 | VOC/mV | ff/% | η/% | Rel. η/% 3 |
|---|---|---|---|---|---|
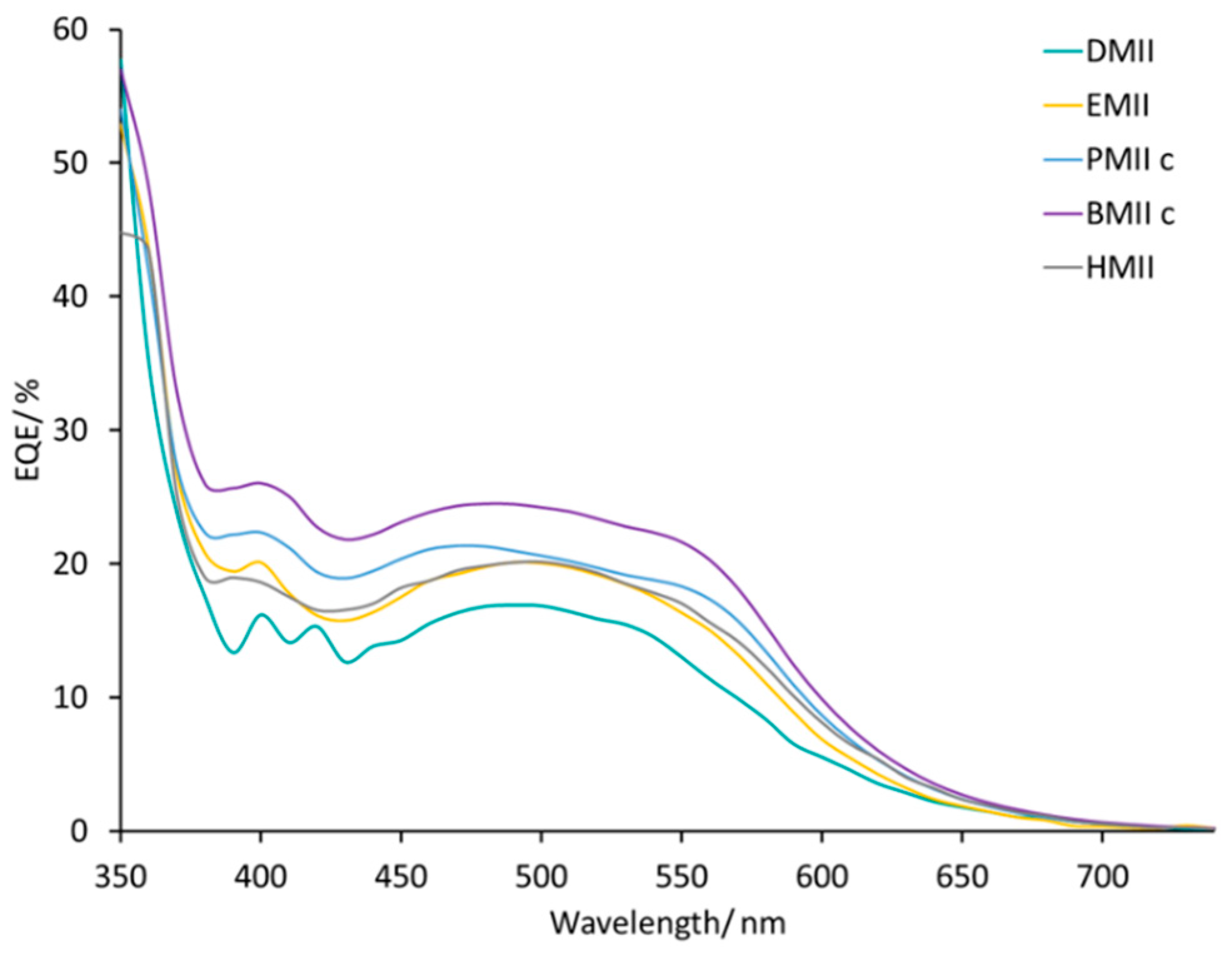
| DMII | 2.31 | 362 | 63 | 0.53 | 9.5 |
| EMII | 2.47 | 344 | 62 | 0.52 | 9.3 |
| PMIIc | 3.01 | 315 | 62 | 0.59 | 10.5 |
| BMIIc | 3.40 | 301 | 59 | 0.61 | 10.9 |
| HMII | 3.14 | 316 | 60 | 0.60 | 10.7 |
| Reference cell with dye N719 | 12.53 | 654 | 68 | 5.60 | 100 |
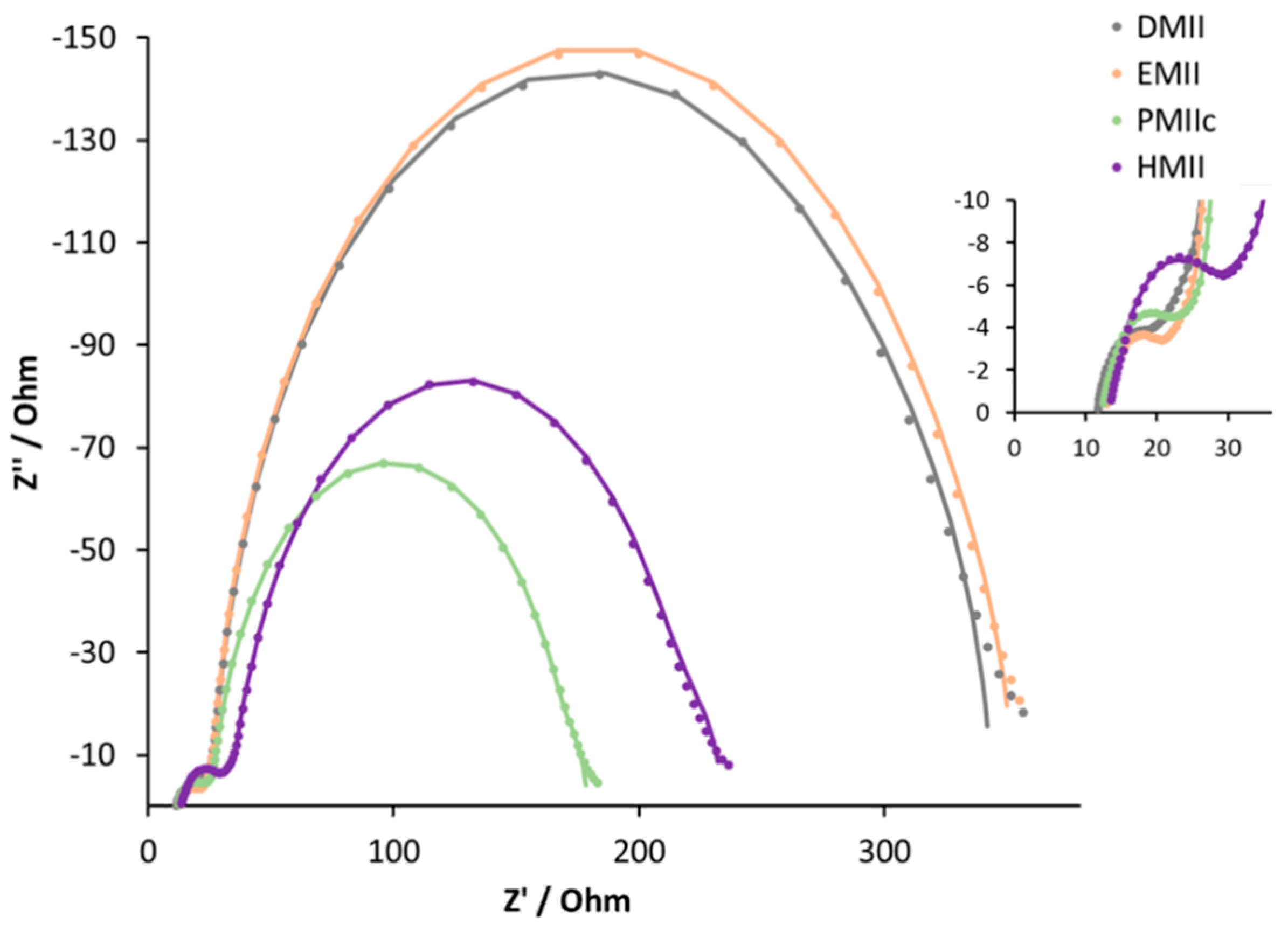
| Electrolyte 1,2 | Rrec/Ω | Cμ/μF | Rtr/Ω | τ/ms | τt/ms | Ld/μm | Rs/Ω | RPt/Ω | CPt/μF |
|---|---|---|---|---|---|---|---|---|---|
| DMII | 269 | 416 | 22 | 112 | 9 | 42 | 12 | 6 | 7 |
| EMII | 299 | 464 | 19 | 139 | 9 | 48 | 13 | 6 | 5 |
| PMIIc | 141 | 297 | 19 | 42 | 6 | 33 | 12 | 8 | 5 |
| BMIIc | 169 | 307 | 13 | 52 | 4 | 43 | 13 | 8 | 6 |
| HMII | 179 | 328 | 30 | 59 | 10 | 29 | 13 | 11 | 5 |
| Electrolyte 1,2 | JSC/mA cm−2 | VOC/mV | ff/% | η/% | Rel. η/% 3 |
|---|---|---|---|---|---|
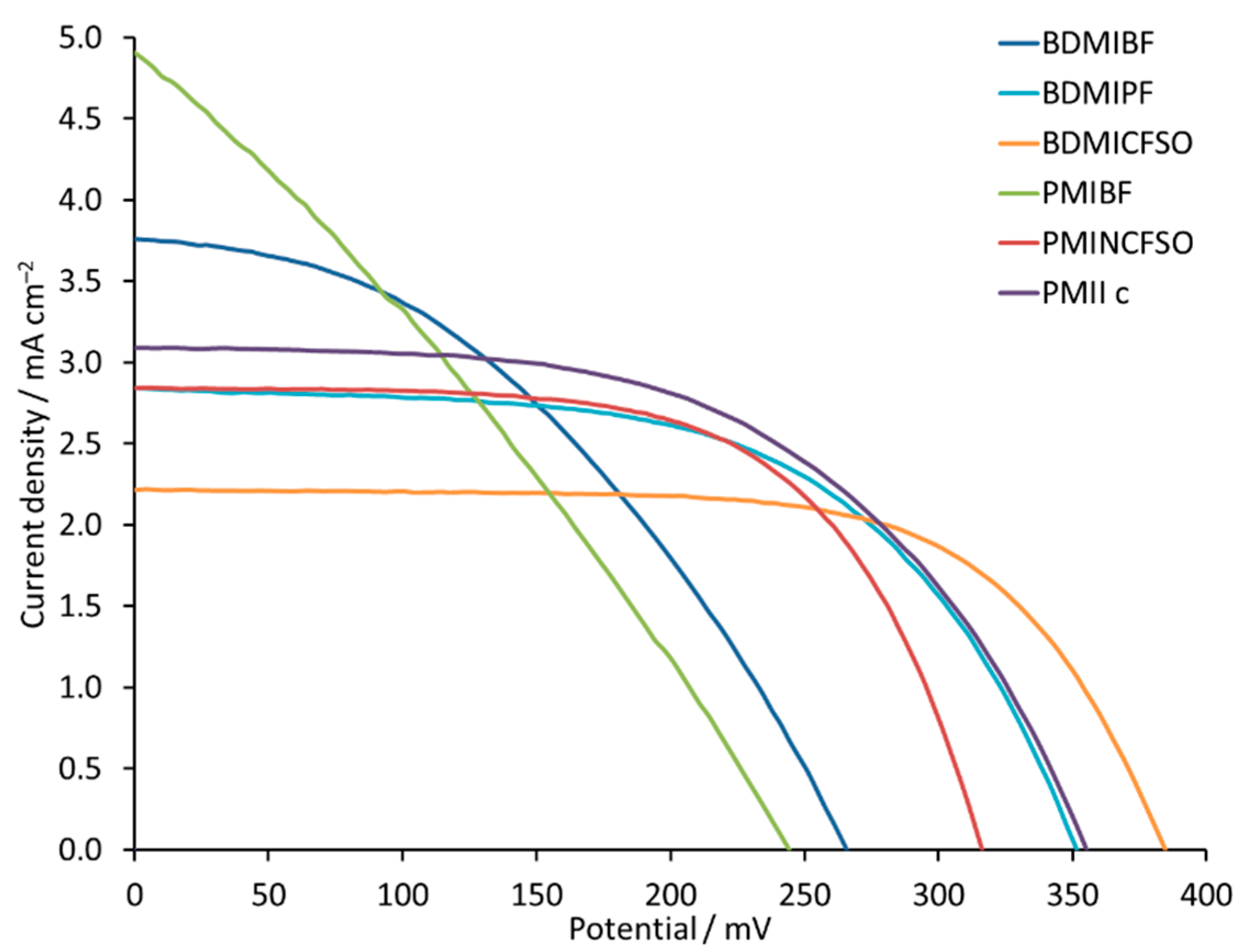
| BDMIBF | 3.80 | 266 | 41 | 0.41 | 7.3 |
| BDMIPF | 2.84 | 351 | 58 | 0.57 | 10.2 |
| BDMICFSO | 2.22 | 385 | 66 | 0.56 | 10.0 |
| PMIBF | 4.90 | 244 | 30 | 0.35 | 6.3 |
| PMINCFSO | 2.84 | 316 | 62 | 0.56 | 10.0 |
| PMII c | 3.01 | 315 | 62 | 0.59 | 10.5 |
| Reference cell with dye N719 | 12.53 | 654 | 68 | 5.60 | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpacheva, M.; Wyss, V.; Housecroft, C.E.; Constable, E.C. There Is a Future for N-Heterocyclic Carbene Iron(II) Dyes in Dye-Sensitized Solar Cells: Improving Performance through Changes in the Electrolyte. Materials 2019, 12, 4181. https://doi.org/10.3390/ma12244181
Karpacheva M, Wyss V, Housecroft CE, Constable EC. There Is a Future for N-Heterocyclic Carbene Iron(II) Dyes in Dye-Sensitized Solar Cells: Improving Performance through Changes in the Electrolyte. Materials. 2019; 12(24):4181. https://doi.org/10.3390/ma12244181
Chicago/Turabian StyleKarpacheva, Mariia, Vanessa Wyss, Catherine E. Housecroft, and Edwin C. Constable. 2019. "There Is a Future for N-Heterocyclic Carbene Iron(II) Dyes in Dye-Sensitized Solar Cells: Improving Performance through Changes in the Electrolyte" Materials 12, no. 24: 4181. https://doi.org/10.3390/ma12244181
APA StyleKarpacheva, M., Wyss, V., Housecroft, C. E., & Constable, E. C. (2019). There Is a Future for N-Heterocyclic Carbene Iron(II) Dyes in Dye-Sensitized Solar Cells: Improving Performance through Changes in the Electrolyte. Materials, 12(24), 4181. https://doi.org/10.3390/ma12244181