Model-Based Roentgen Stereophotogrammetric Analysis to Monitor the Head–Taper Junction in Total Hip Arthroplasty in Vivo—And They Do Move
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Cohort—Inclusion Criteria
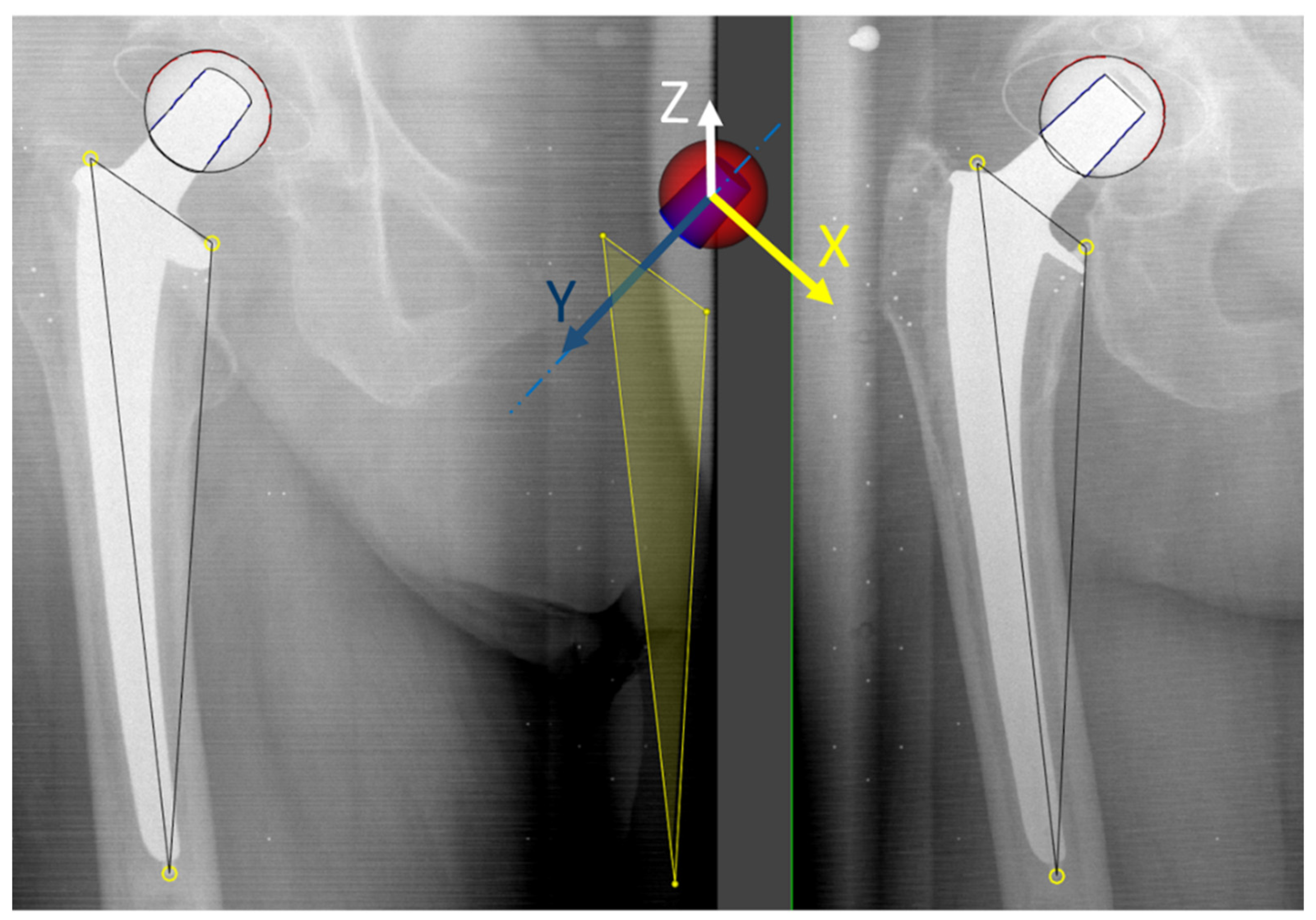
2.2. Image Acqusition and Analysis
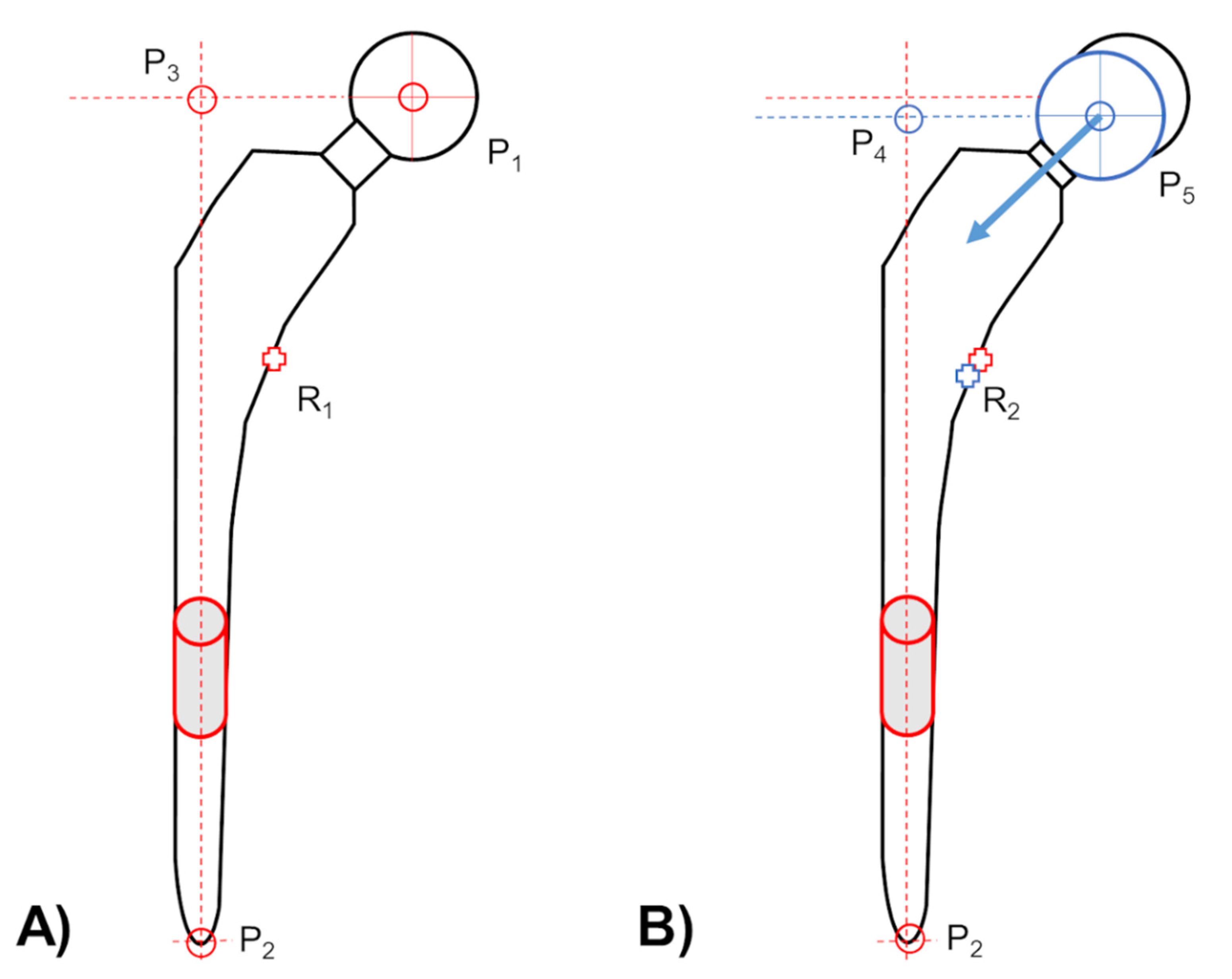
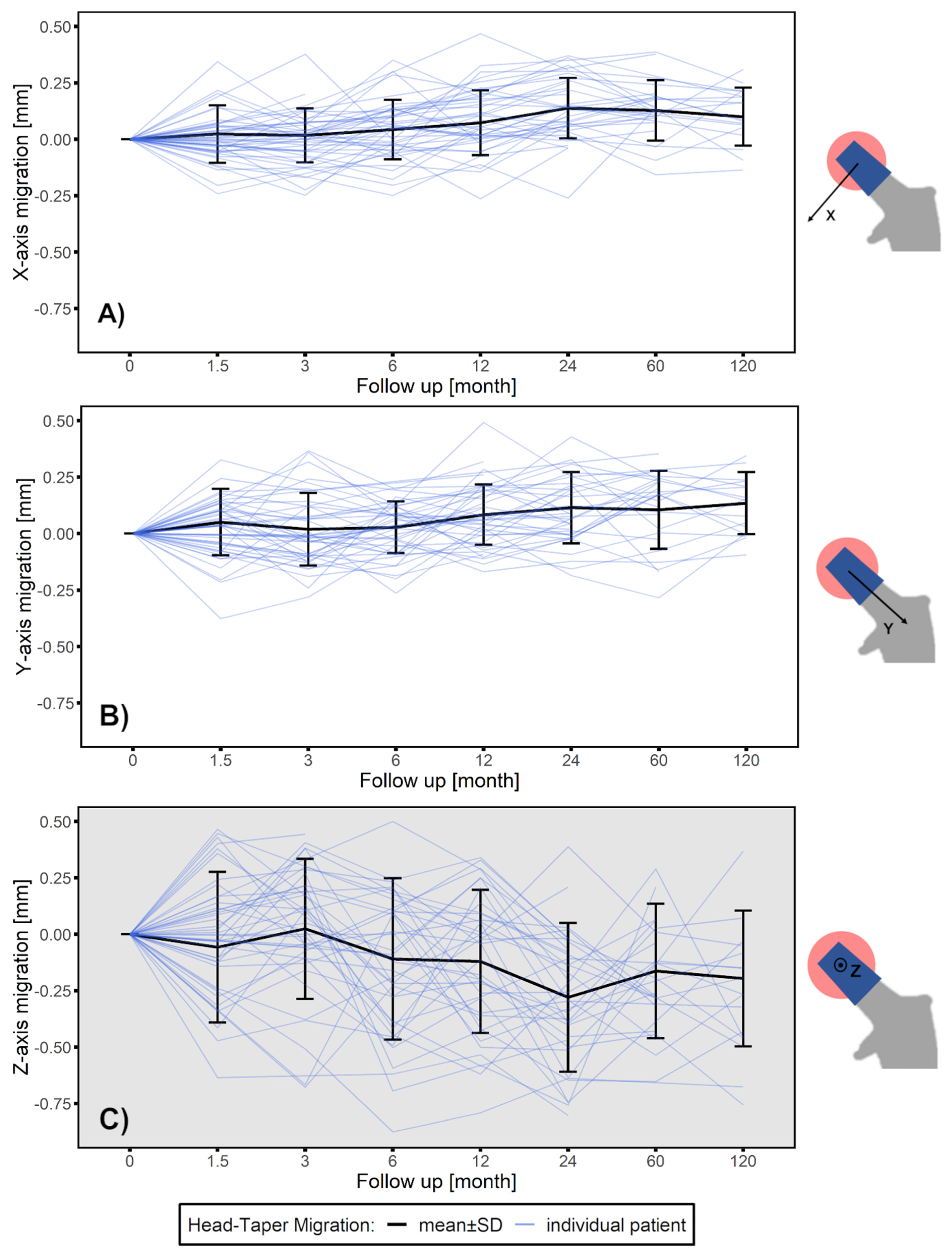
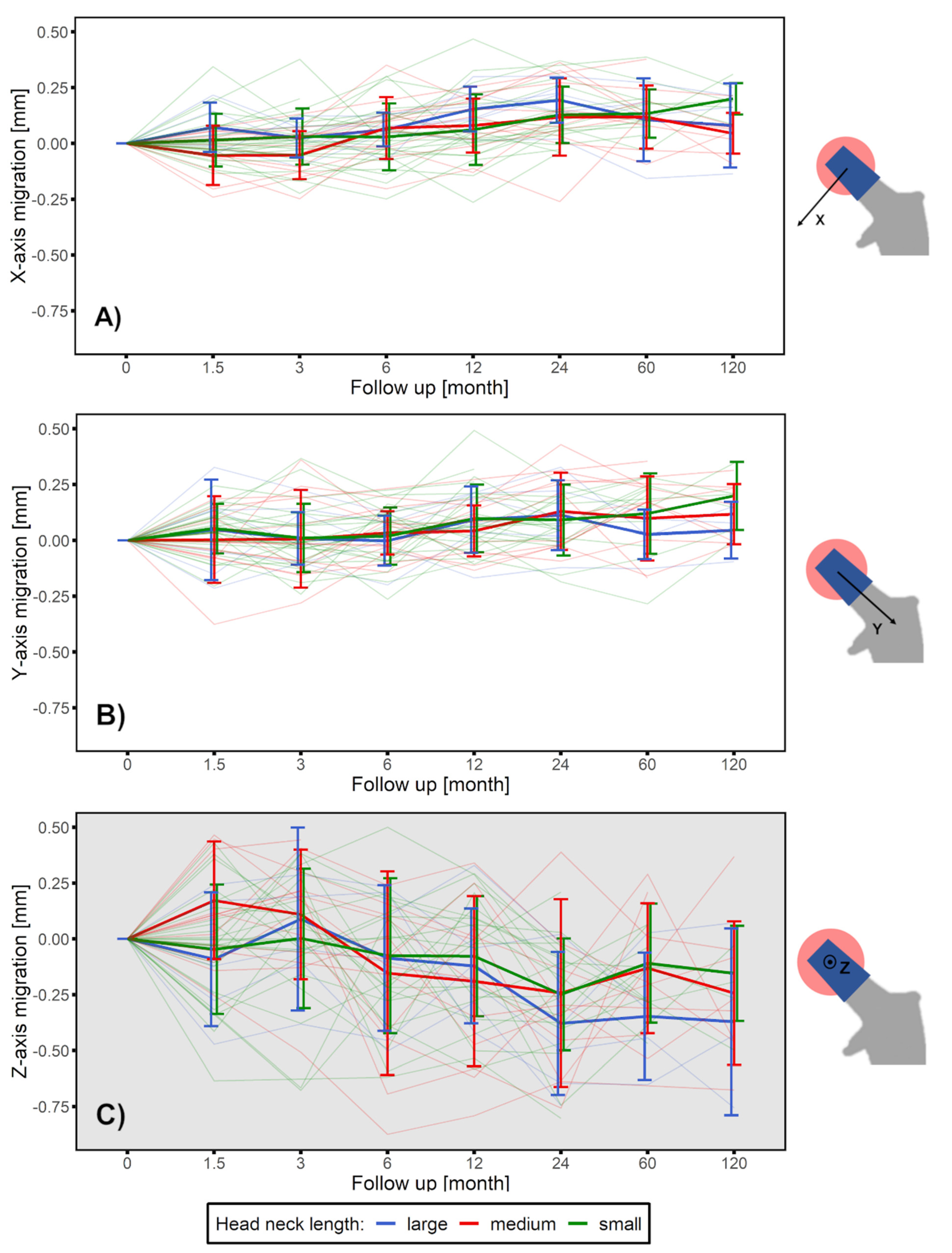
2.3. Head–Taper Migration Measurements
2.4. Experimental Verification of Head–Taper Migration
2.5. Clinical Verification of Potential in Vivo Head–Taper Migrations—Proving Interchangeable Applicability of Marker and Model-Based RSA EGS Method
2.6. Statistics
3. Results
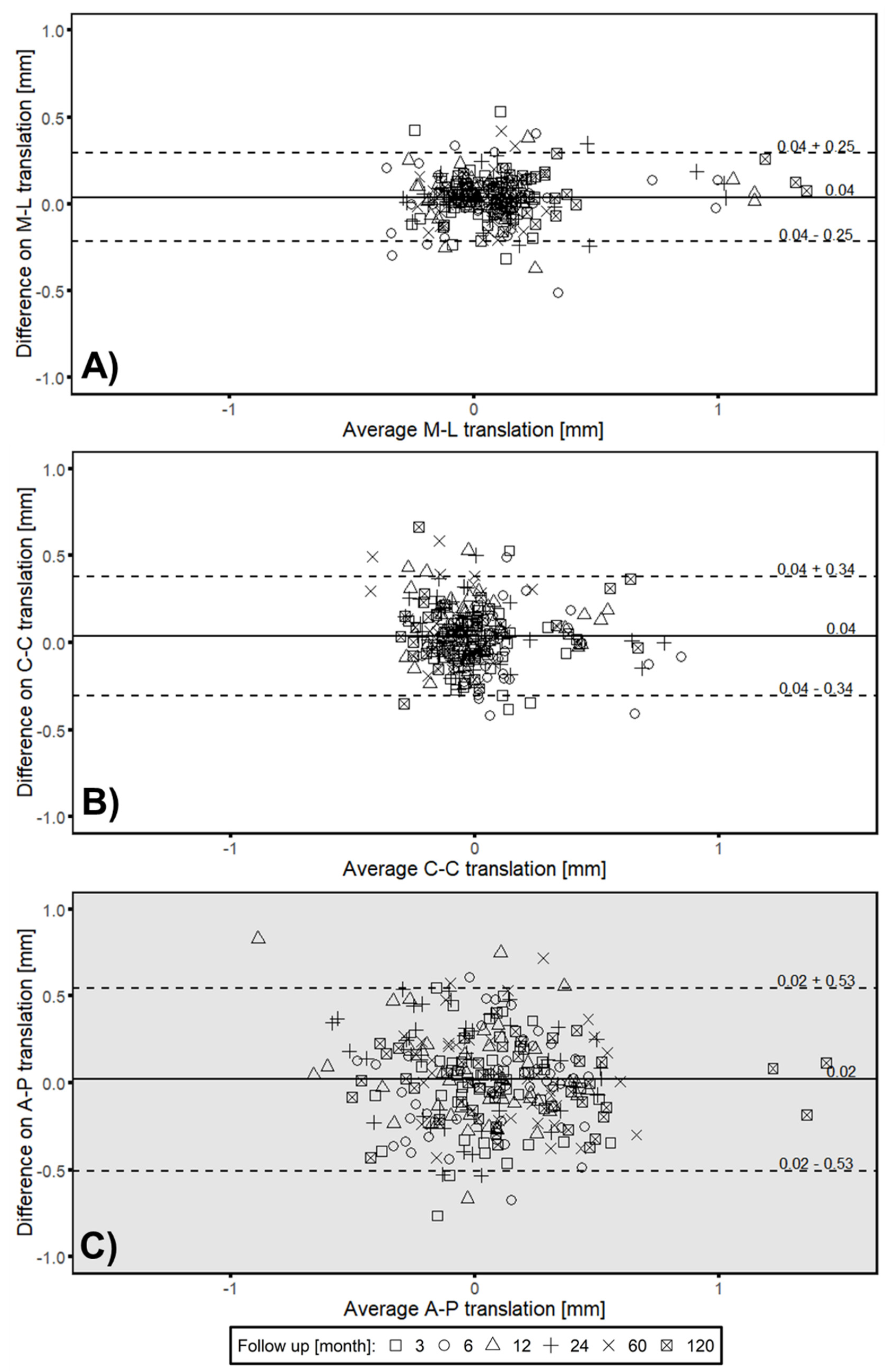
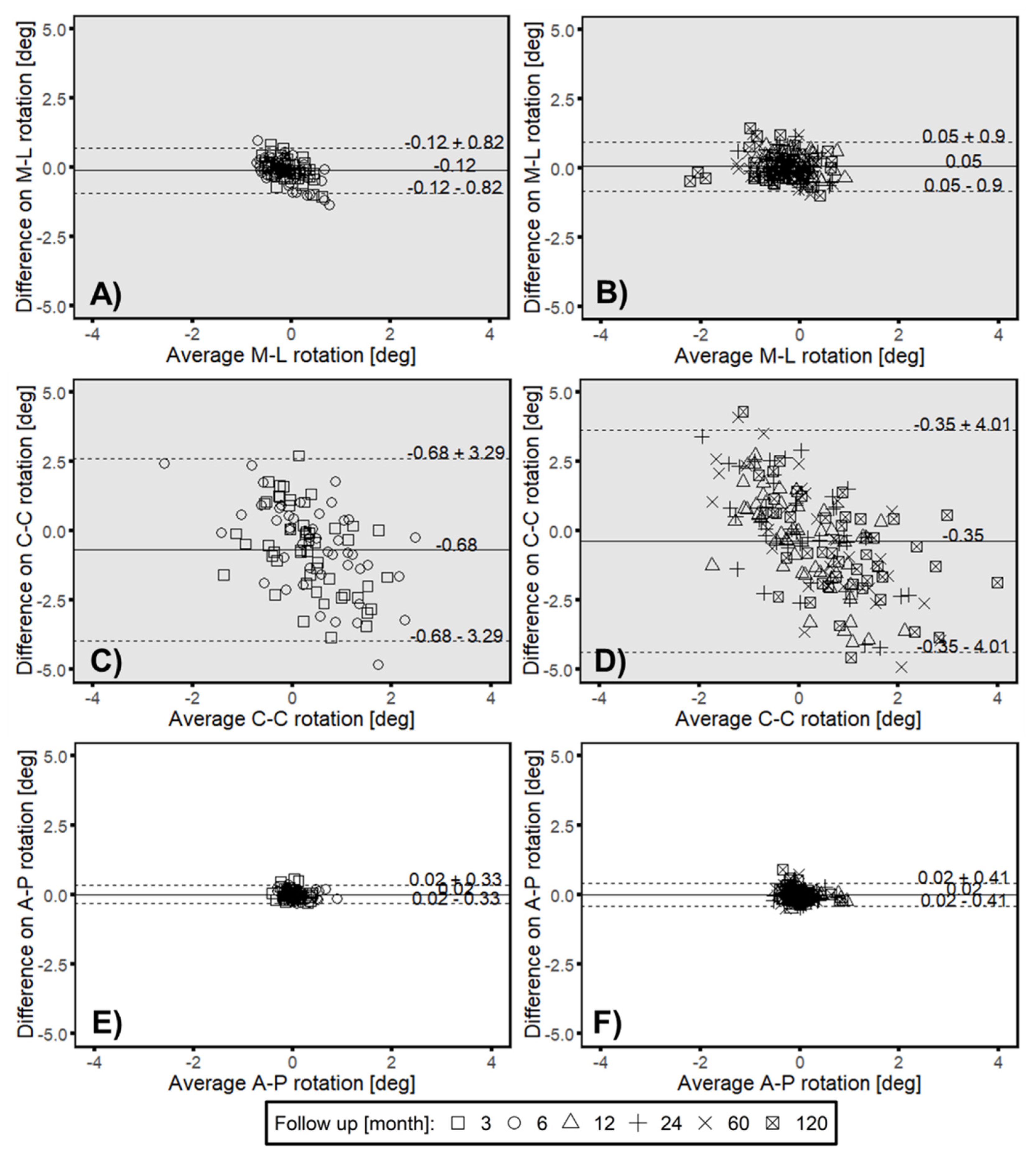
3.1. Head–Taper Migration
3.2. Experimental Verification of the Measured in Vivo Head–taper Movement
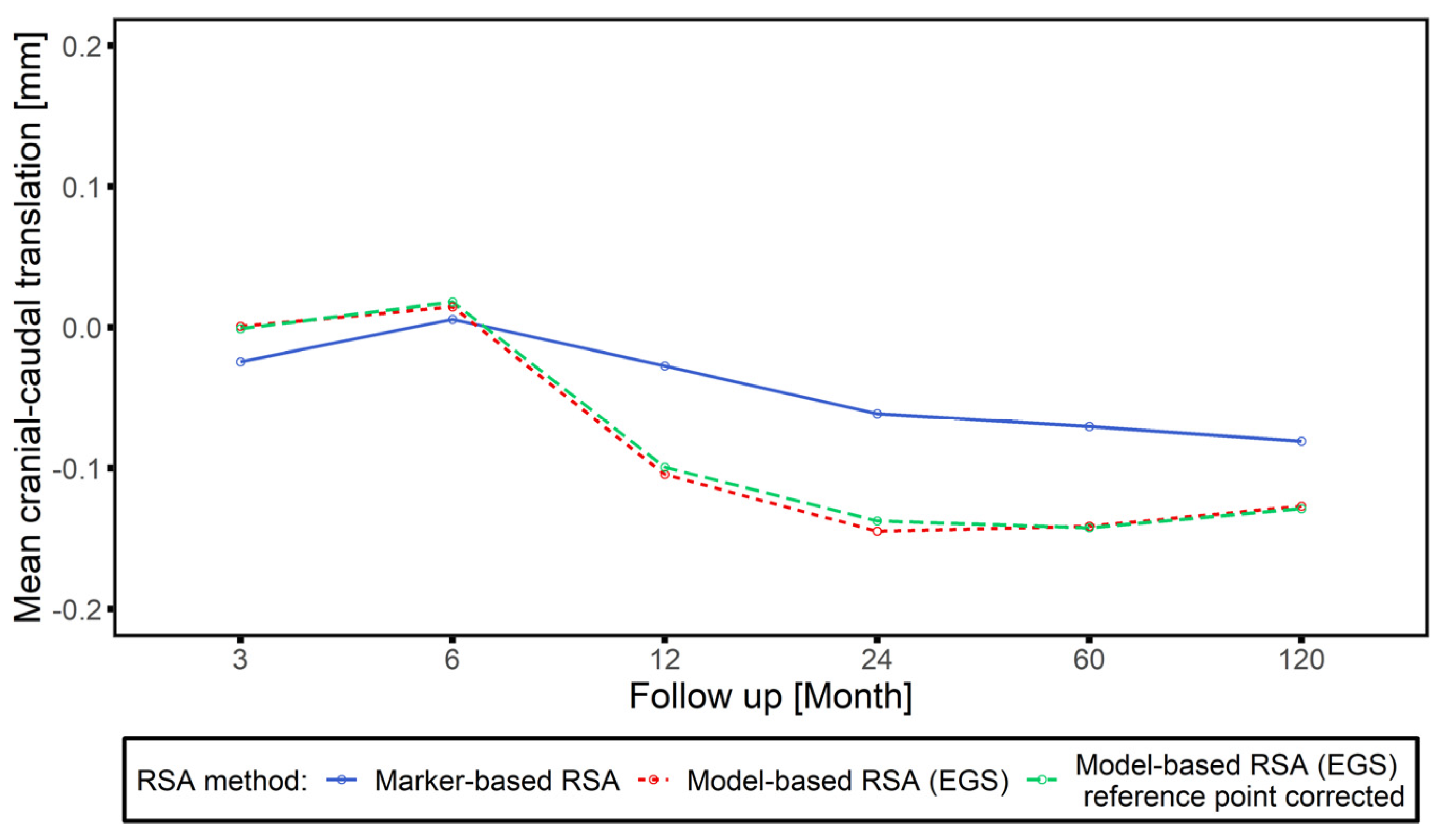
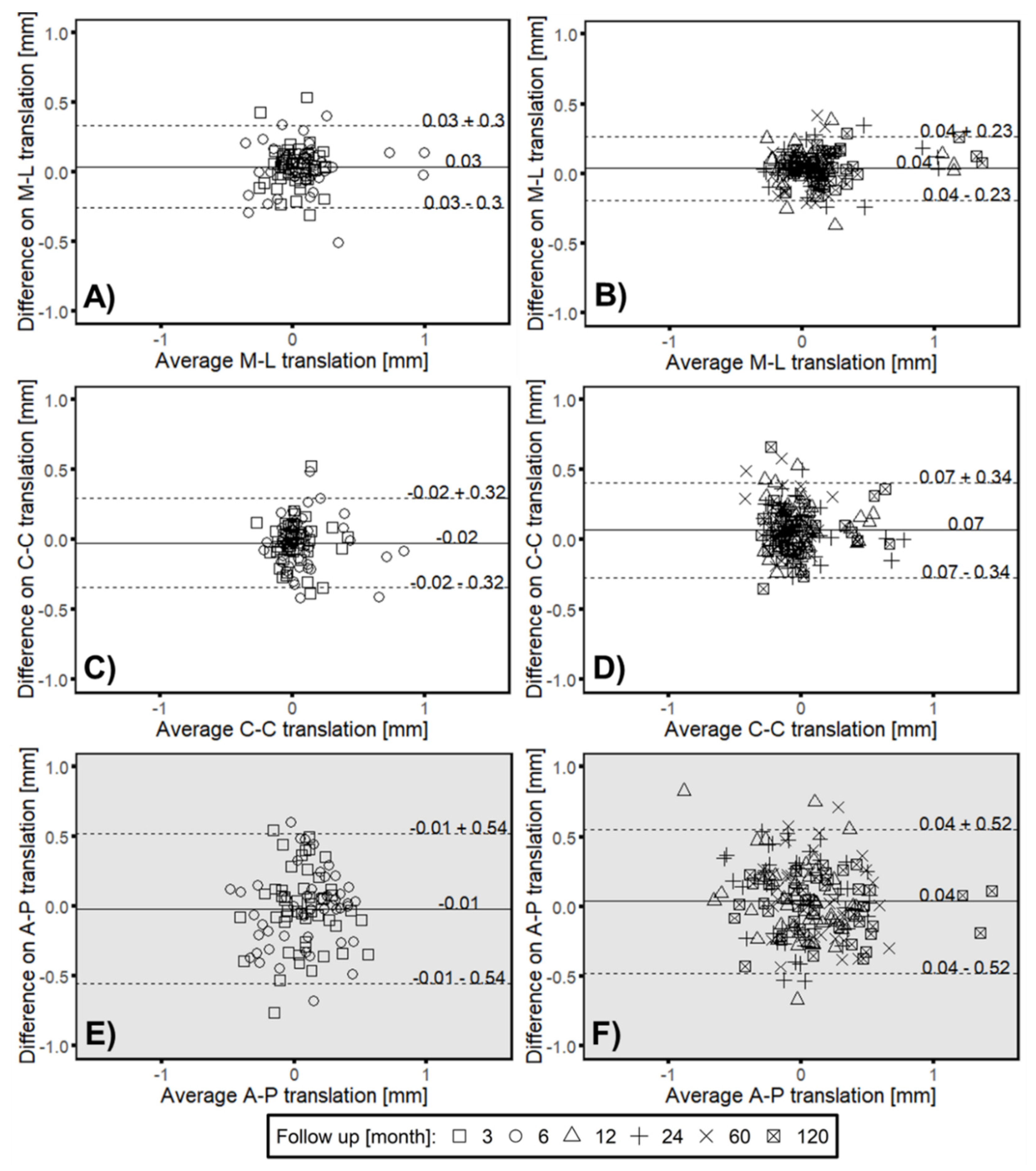
3.3. Clincial Verification—Proof Interchangeability between Marker-Based RSA and Model-based RSA (EGS) Migration Results
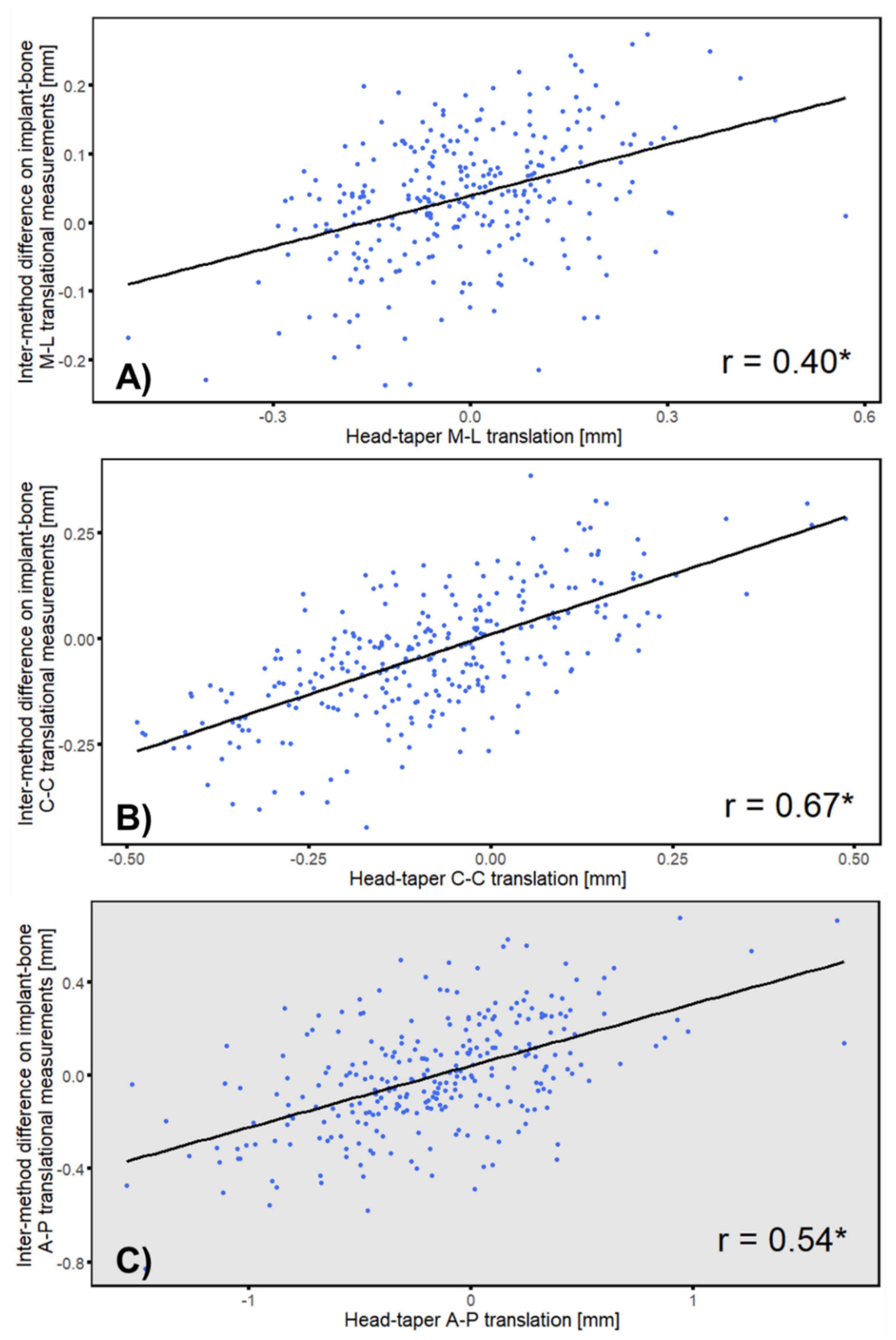
3.4. Correlation between Intermethod Difference and Head–Taper Migration
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Group S | Group M | Group L | ||
|---|---|---|---|---|
| Head Material | Ceramic | Ceramic | Ceramic | |
| Taper Material | CoCrMo | CoCrMo | CoCrMo | |
| Head Diameter | 28 mm | 28 mm | 28 mm | |
| Head Neck Length | S | M | L | |
| Taper | 12/14 | 12/14 | 12/14 | |
| CCD Angle | 126 deg | 126 deg | 126 deg | |
| Numbers | 26 | 13 | 6 | Sum: 45 |
| Follow Up | Group | Sum | ||
|---|---|---|---|---|
| S | M | L | ||
| 1.5 months | 18 | 7 | 5 | 30 |
| 3 months | 21 | 9 | 5 | 35 |
| 6 months | 22 | 11 | 6 | 39 |
| 12 months | 23 | 12 | 6 | 41 |
| 24 months | 21 | 11 | 6 | 38 |
| 60 months | 11 | 8 | 4 | 23 |
| 120 months | 7 | 8 | 3 | 18 |
| Medial-Lateral | Cranial–Caudal | Anterior–Posterior | ||
|---|---|---|---|---|
| Follow up [months] | 3 | 0.42 * | 0.66 * | 0.58 * |
| 6 | 0.49 * | 0.74 * | 0.52 * | |
| 12 | 0.39 * | 0.68 * | 0.60 * | |
| 24 | 0.27 | 0.55 * | 0.45 * | |
| 60 | 0.44 * | 0.65 * | 0.58 * | |
| 120 | 0.28 | 0.61 * | 0.44 * |

References
- Pivec, R.; Johnson, A.J.; Mears, S.C.; Mont, M.A. Hip arthroplasty. Lancet 2012, 380, 1768–1777. [Google Scholar] [CrossRef]
- Sundfeldt, M.; V Carlsson, L.; B Johansson, C.; Thomsen, P.; Gretzer, C. Aseptic loosening, not only a question of wear: A review of different theories. Acta Orthop. 2006, 77, 177–197. [Google Scholar] [CrossRef] [PubMed]
- MacInnes, S.J.; Gordon, A.; Wilkinson, J.M. Risk factors for aseptic loosening following total hip arthroplasty. In Recent Advances in Arthroplasty, 1st ed.; Fokter, S., Ed.; InTech: Rijeka, Croatia, 2012; pp. 275–294. [Google Scholar] [CrossRef] [Green Version]
- Valstar, E.R.; Gill, R.H. Radiostereometric analysis in orthopaedic surgery: Editorial comment. Clin. Orthop. Relat. Res. 2006, 448, 2. [Google Scholar] [CrossRef] [PubMed]
- Kaptein, B.; Valstar, E.; Stoel, B.; Rozing, P.; Reiber, J. A new model-based RSA method validated using CAD models and models from reversed engineering. J. Biomech. 2003, 36, 873–882. [Google Scholar] [CrossRef]
- Hurschler, C.; Seehaus, F.; Emmerich, J.; Kaptein, B.L.; Windhagen, H. Comparison of the model-based and marker-based roentgen stereophotogrammetry methods in a typical clinical setting. J. Arthroplast. 2009, 24, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Seehaus, F.; Emmerich, J.; Kaptein, B.L.; Windhagen, H.; Hurschler, C. Experimental analysis of Model-Based Roentgen Stereophotogrammetric Analysis (MBRSA) on four typical prosthesis components. J. Biomech. Eng. 2009, 131, 041004. [Google Scholar] [CrossRef]
- Valstar, E.R.; de Jong, F.W.; Vrooman, H.A.; Rozing, P.M.; Reiber, J.H. Model-based Roentgen stereophotogrammetry of orthopaedic implants. J. Biomech. 2001, 34, 715–722. [Google Scholar] [CrossRef]
- Collier, J.P.; Surprenant, V.A.; Jensen, R.E.; Mayor, M.B.; Surprenant, H.P. Corrosion between the components of modular femoral hip prostheses. J. Bone Jt. Surg. Br. 1992, 74, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Koper, M.; Mathijssen, N.; Witt, F.; Morlock, M.; Vehmeijer, S. Severe Wear and Pseudotumor Formation Due to Taper Mismatch in a Total Hip Arthroplasty: A Case Report. JBJS Case Connect. 2015, 5, e29. [Google Scholar] [CrossRef]
- Morlock, M.; Bünte, D.; Gührs, J.; Bishop, N. Corrosion of the head-stem taper junction—Are we on the verge of an epidemic? HSS J. 2017, 13, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Jennings, J.M.; Dennis, D.A.; Yang, C.C. Corrosion of the head-neck junction after total hip arthroplasty. J. Am. Acad. Orthop. Surg. 2016, 24, 349–356. [Google Scholar] [CrossRef]
- Pastides, P.S.; Dodd, M.; Sarraf, K.M.; Willis-Owen, C.A. Trunnionosis: A pain in the neck. World J. Orthop. 2013, 4, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.A.; Cantrell, W.A.; Khlopas, A.; Berger, R.J.; Sodhi, N.; Molloy, R.M.; Krebs, V.E.; Mont, M.A. Evidence-based management of trunnionosis in metal-on-polyethylene total hip arthroplasty: A systematic review. J. Arthroplast. 2018, 33, 3343–3353. [Google Scholar] [CrossRef] [PubMed]
- Mueller, U.; Braun, S.; Schroeder, S.; Sonntag, R.; Kretzer, J.P. Same Same but Different? 12/14 Stem and Head Tapers in Total Hip Arthroplasty. J. Arthroplast. 2017, 32, 3191–3199. [Google Scholar] [CrossRef]
- Plummer, D.R.; Berger, R.A.; Paprosky, W.G.; Sporer, S.M.; Jacobs, J.J.; Della Valle, C.J. Diagnosis and management of adverse local tissue reactions secondary to corrosion at the head-neck junction in patients with metal on polyethylene bearings. J. Arthroplast. 2016, 31, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Lash, N.; Whitehouse, M.; Greidanus, N.; Garbuz, D.; Masri, B.; Duncan, C. Delayed dislocation following metal-on-polyethylene arthroplasty of the hip due to ‘silent’trunnion corrosion. Bone Jt. J. 2016, 98, 187–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrory, B.J.; MacKenzie, J.; Babikian, G. A high prevalence of corrosion at the head–neck taper with contemporary Zimmer non-cemented femoral hip components. J. Arthroplast. 2015, 30, 1265–1268. [Google Scholar] [CrossRef]
- Hussey, D.K.; McGrory, B.J. Ten-year cross-sectional study of mechanically assisted crevice corrosion in 1352 consecutive patients with metal-on-polyethylene total hip arthroplasty. J. Arthroplast. 2017, 32, 2546–2551. [Google Scholar] [CrossRef]
- Nawabi, D.H.; Gold, S.; Lyman, S.; Fields, K.; Padgett, D.E.; Potter, H.G. MRI predicts ALVAL and tissue damage in metal-on-metal hip arthroplasty. Clin. Orthop. Relat. Res. 2014, 472, 471–481. [Google Scholar] [CrossRef] [Green Version]
- Nishii, T.; Sakai, T.; Takao, M.; Yoshikawa, H.; Sugano, N. Is ultrasound screening reliable for adverse local tissue reaction after hip arthroplasty? J. Arthroplast. 2014, 29, 2239–2244. [Google Scholar] [CrossRef]
- Sesselmann, S.; Hong, Y.; Schlemmer, F.; Wiendieck, K.; Söder, S.; Hussnaetter, I.; Müller, L.A.; Forst, R.; Wierer, T. Migration measurement of the cemented Lubinus SP II hip stem–a 10-year follow-up using radiostereometric analysis. Biomed. Tech. 2017, 62, 271–278. [Google Scholar] [CrossRef] [PubMed]
- RSAcore. RSAcore User Manual Model-Based RSA 4.1; RSAcore: Leiden, The Netherlands, 2015; Volume Version 1.1. [Google Scholar]
- Valstar, E.R.; Gill, R.; Ryd, L.; Flivik, G.; Borlin, N.; Karrholm, J. Guidelines for standardization of radiostereometry (RSA) of implants. Acta Orthop. 2005, 76, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Karrholm, J.; Borssen, B.; Lowenhielm, G.; Snorrason, F. Does early micromotion of femoral stem prostheses matter? 4-7-year stereoradiographic follow-up of 84 cemented prostheses. J. Bone Jt. Surg. Br. 1994, 76, 912–917. [Google Scholar] [CrossRef]
- Ryd, L.; Albrektsson, B.; Carlsson, L.; Dansgard, F.; Herberts, P.; Lindstrand, A.; Regner, L.; Toksvig-Larsen, S. Roentgen stereophotogrammetric analysis as a predictor of mechanical loosening of knee prostheses. J. Bone Jt. Surg. Br. 1995, 77, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Pearson, K. Note on regression and inheritance in the case of two parents. Proc. R. Soc. Lond. 1895, 58, 240–242. [Google Scholar]
- Fisher, R.A. Frequency distribution of the values of the correlation coefficient in samples from an indefinitely large population. Biometrika 1915, 10, 507–521. [Google Scholar] [CrossRef]
- Morlock, M.M.; Dickinson, E.C.; Gunther, K.P.; Bunte, D.; Polster, V. Head Taper Corrosion Causing Head Bottoming Out and Consecutive Gross Stem Taper Failure in Total Hip Arthroplasty. J. Arthroplast. 2018, 33, 3581–3590. [Google Scholar] [CrossRef] [Green Version]
- Pennock, A.T.; Schmidt, A.H.; Bourgeault, C.A. Morse-type tapers: Factors that may influence taper strength during total hip arthroplasty. J. Arthroplast. 2002, 17, 773–778. [Google Scholar] [CrossRef]
- Witt, F.; Gührs, J.; Morlock, M.M.; Bishop, N.E. Quantification of the contact area at the head-stem taper interface of modular hip prostheses. PLoS ONE 2015, 10, e0135517. [Google Scholar] [CrossRef] [Green Version]
- Osman, K.; Panagiotidou, A.; Khan, M.; Blunn, G.; Haddad, F. Corrosion at the head-neck interface of current designs of modular femoral components: Essential questions and answers relating to corrosion in modular head–neck junctions. Bone Jt. J. 2016, 98, 579–584. [Google Scholar] [CrossRef] [PubMed]
- McTighe, T.; Brazil, D.; Keppler, L.; Keggi, J.; McPherson, E. Metallic Modular Taper Junctions in Total Hip Arthroplasty. Reconstr. Rev. 2015, 5, 29–42. [Google Scholar] [CrossRef]
- Panagiotidou, A.; Meswania, J.; Osman, K.; Bolland, B.; Latham, J.; Skinner, J.; Haddad, F.; Hart, A.; Blunn, G. The effect of frictional torque and bending moment on corrosion at the taper interface: An in vitro study. Bone Jt. J. 2015, 97, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Natu, S.; Sidaginamale, R.P.; Gandhi, J.; Langton, D.J.; Nargol, A.V. Adverse reactions to metal debris: Histopathological features of periprosthetic soft tissue reactions seen in association with failed metal on metal hip arthroplasties. J. Clin. Pathol. 2012, 65, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Morlock, M.M. The taper disaster-how could it happen? Hip. Int. 2015, 25, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Kocagöz, S.B.; Hanzlik, J.A.; Underwood, R.J.; Gilbert, J.L.; MacDonald, D.W.; Lee, G.-C.; Mont, M.A.; Kraay, M.J.; Klein, G.R. Do ceramic femoral heads reduce taper fretting corrosion in hip arthroplasty? A retrieval study. Clin. Orthop. Relat. Res. 2013, 471, 3270–3282. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.C.; Lau, A.C.; Del Balso, C.; Howard, J.L.; Lanting, B.A.; Teeter, M.G. Tribocorrosion: Ceramic and oxidized zirconium vs cobalt-chromium heads in total hip arthroplasty. J. Arthroplast. 2016, 31, 2064–2071. [Google Scholar] [CrossRef]
- Khatod, M.; Cafri, G.; Inacio, M.C.; Schepps, A.L.; Paxton, E.W.; Bini, S.A. Revision total hip arthoplasty: Factors associated with re-revision surgery. J. Bone Jt. Surg. Am. 2015, 97, 359–366. [Google Scholar] [CrossRef]
- Kocagöz, S.B.; Underwood, R.J.; Sivan, S.; Gilbert, J.L.; MacDonald, D.W.; Day, J.S.; Kurtz, S.M. Does taper angle clearance influence fretting and corrosion damage at the head–stem interface? A matched cohort retrieval study. Semin. Arthroplast. 2013, 24, 246–254. [Google Scholar] [CrossRef] [Green Version]
- Del Balso, C.; Teeter, M.; Tan, S.; Lanting, B.; Howard, J. Taperosis: Does head length affect fretting and corrosion in total hip arthroplasty? Bone Jt. J. 2015, 97, 911–916. [Google Scholar] [CrossRef]
- Panagiotidou, A.; Meswania, J.; Hua, J.; Muirhead-Allwood, S.; Hart, A.; Blunn, G. Enhanced Wear and Corrosion in Modular Tapers in Total Hip Replacement Is Associated With the Contact Area and Surface Topography. J. Orthop. Res. 2013, 31, 2032–2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Parameter | Value |
|---|---|
| Taper angle | 5.611 ± 0.004 deg |
| Ra | 3.58 ± 0.06 µm |
| Rz | 14.26 ± 0.29 µm |
| RSm | 122.93 ± 0.20 µm |
| Rp | 7.50 ± 0.19 µm |
| Rk | 13.90 ± 0.34 µm |
| Head–Taper Junction | 0 > 2 kN | 2 > 4 kN | Total |
|---|---|---|---|
| #1 | 160 µm | 105 µm | 265 µm |
| #2 | 155 µm | 95 µm | 250 µm |
| #3 | 160 µm | 125 µm | 285 µm |
| #4 | 173 µm | 100 µm | 273 µm |
| #5 | 160 µm | 105 µm | 265 µm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Sonntag, R.; Kretzer, J.P.; Taylor, D.; Forst, R.; Seehaus, F. Model-Based Roentgen Stereophotogrammetric Analysis to Monitor the Head–Taper Junction in Total Hip Arthroplasty in Vivo—And They Do Move. Materials 2020, 13, 1543. https://doi.org/10.3390/ma13071543
Xu J, Sonntag R, Kretzer JP, Taylor D, Forst R, Seehaus F. Model-Based Roentgen Stereophotogrammetric Analysis to Monitor the Head–Taper Junction in Total Hip Arthroplasty in Vivo—And They Do Move. Materials. 2020; 13(7):1543. https://doi.org/10.3390/ma13071543
Chicago/Turabian StyleXu, Jing, Robert Sonntag, J. Philippe Kretzer, Dominic Taylor, Raimund Forst, and Frank Seehaus. 2020. "Model-Based Roentgen Stereophotogrammetric Analysis to Monitor the Head–Taper Junction in Total Hip Arthroplasty in Vivo—And They Do Move" Materials 13, no. 7: 1543. https://doi.org/10.3390/ma13071543
APA StyleXu, J., Sonntag, R., Kretzer, J. P., Taylor, D., Forst, R., & Seehaus, F. (2020). Model-Based Roentgen Stereophotogrammetric Analysis to Monitor the Head–Taper Junction in Total Hip Arthroplasty in Vivo—And They Do Move. Materials, 13(7), 1543. https://doi.org/10.3390/ma13071543






