Effect of Cr on the Mineral Structure and Composition of Cement Clinker and Its Solidification Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparation
2.3. Characterization and Analysis
3. Results
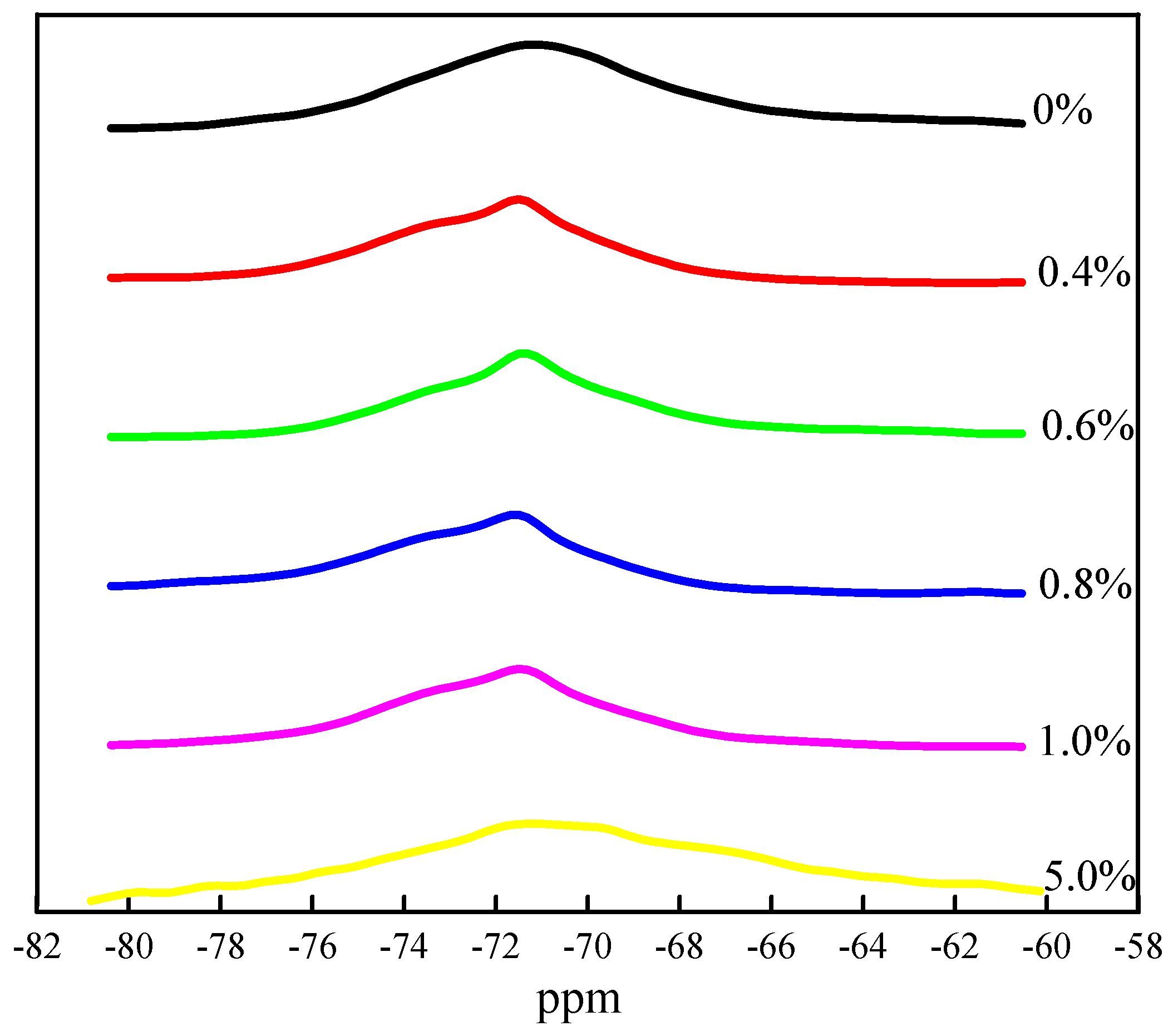
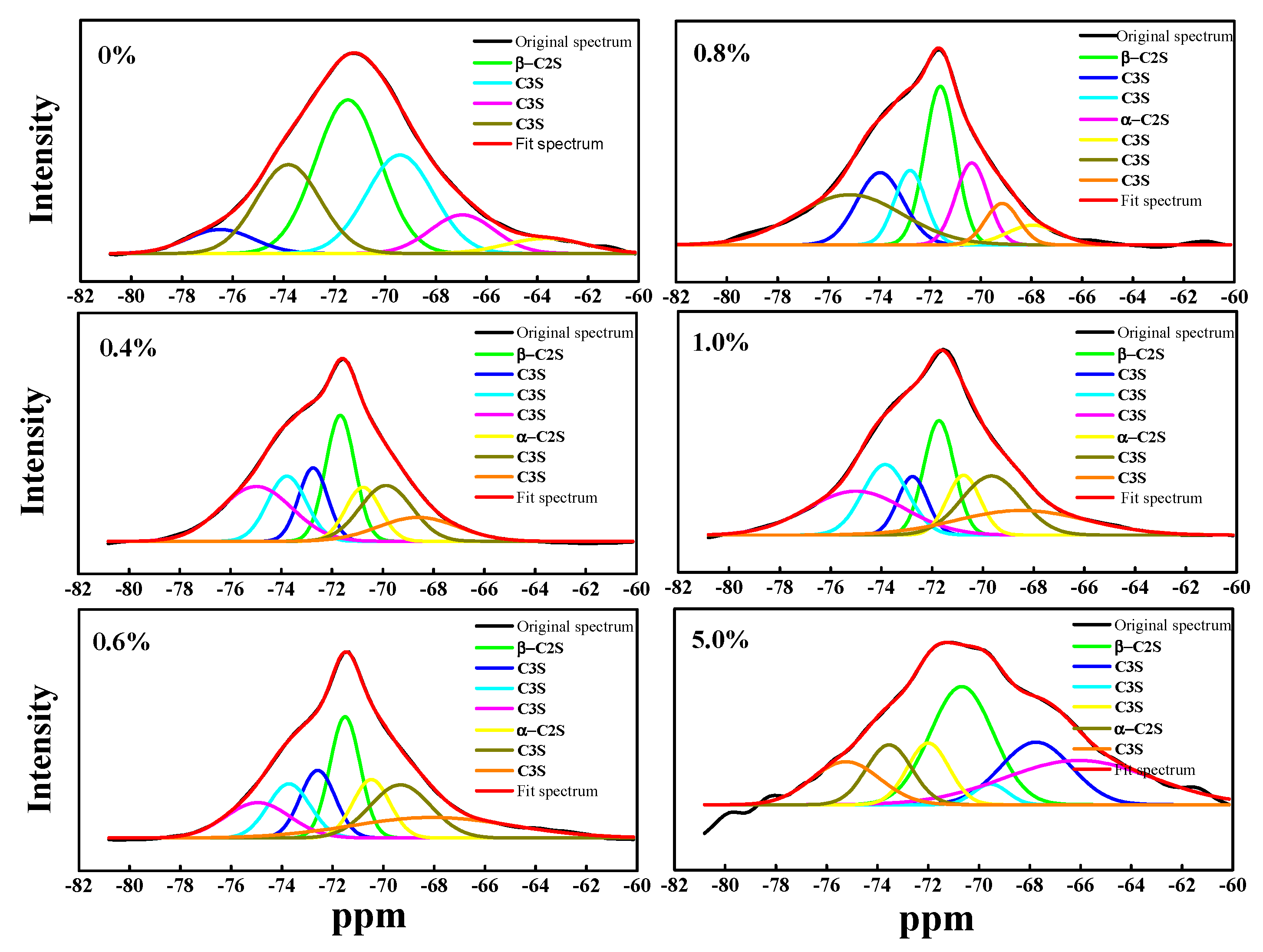
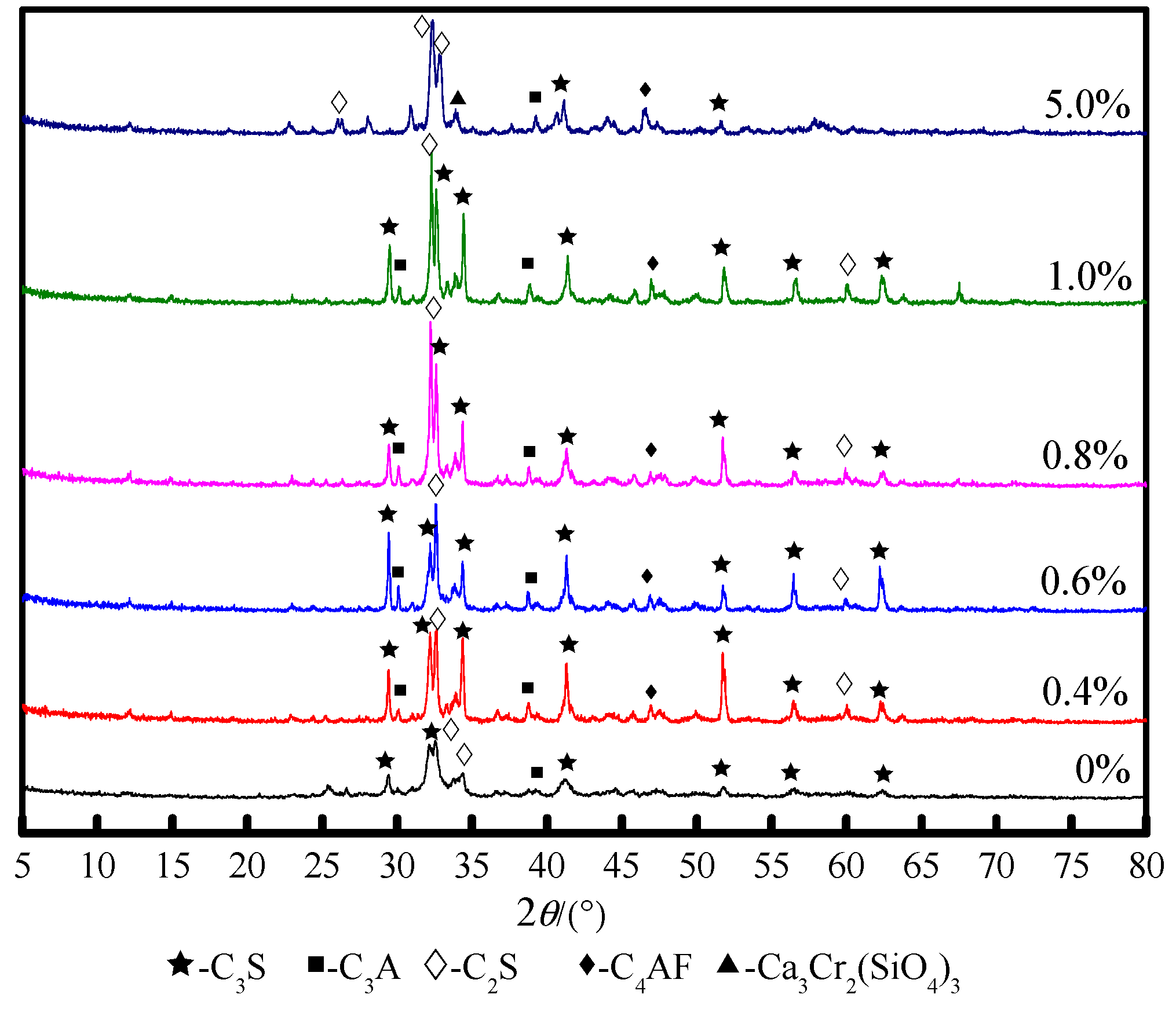
3.1. Effect of Cr on the Structure and Content of Mineral Phase
3.2. Effect of Cr on Mineral Phase Formation
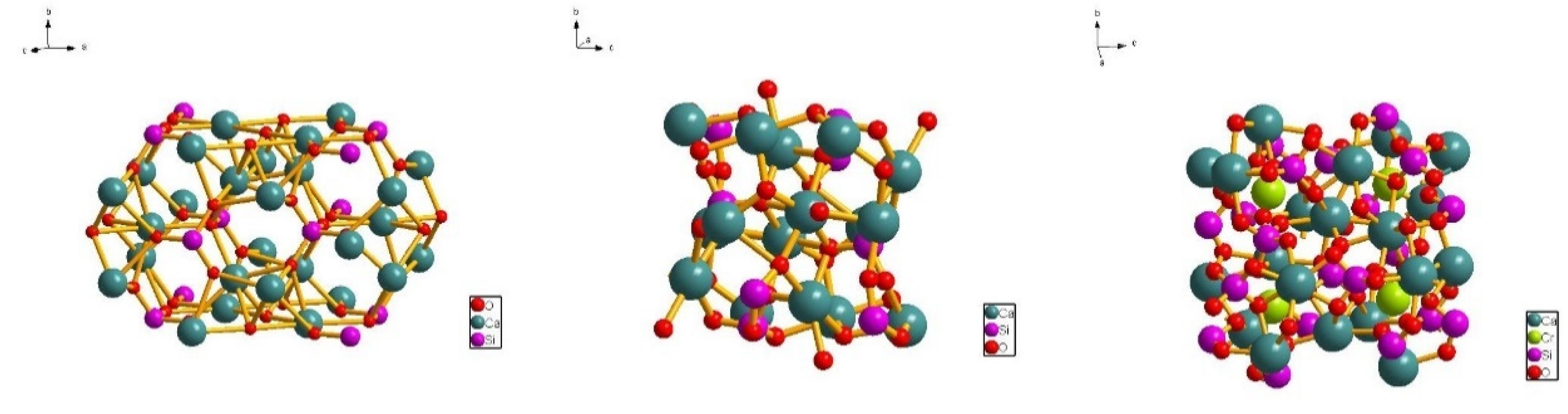
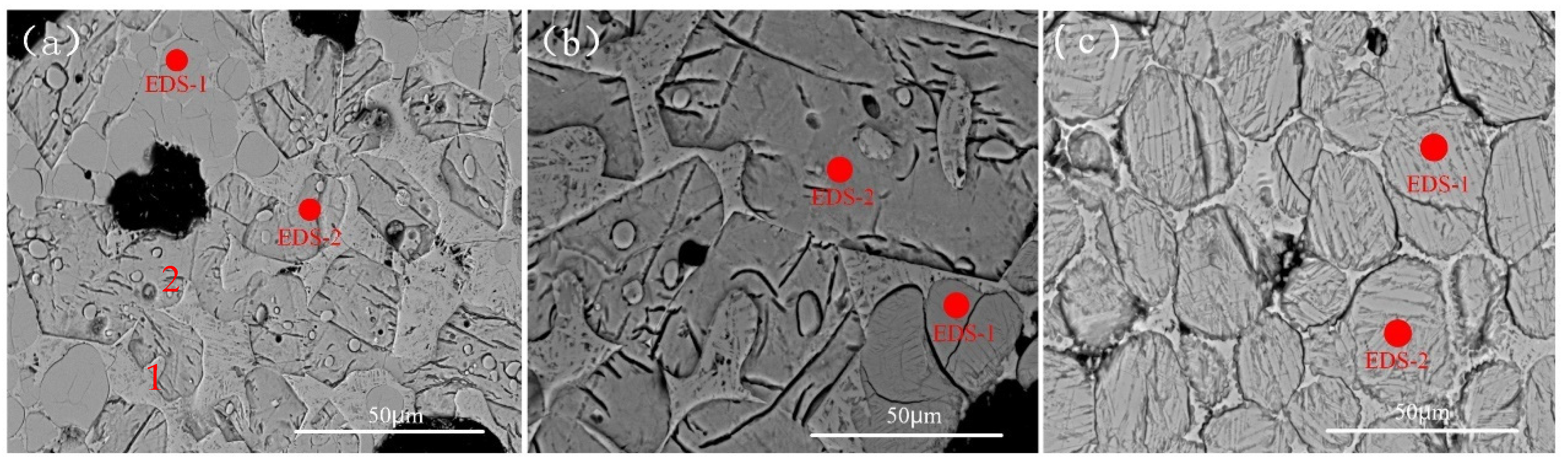
3.3. Effect of Cr on Morphology of the Mineral Phase
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huntzinger, D.N.; Eatmon, T.D. A life-cycle assessment of Portland cement manufacturing: Comparing the traditional process with alternative technologies. J. Clean. Prod. 2009, 17, 668–675. [Google Scholar] [CrossRef]
- Finnveden, G.; Johansson, J.; Lind, P. Life cycle assessment of energy from solid waste—Part 1: General methodology and results. J. Clean. Prod. 2005, 13, 213–229. [Google Scholar] [CrossRef]
- Yang, Q.; Lv, Z.F. Technical Analysis of Cooperative Disposal of Municipal Solid Waste by New Dry-process Cement Kiln. Chem. Eng. Equip. 2012, 195–197. [Google Scholar]
- Xiang, C.Y. Curing Behavior of Heavy Metal Ions in Portland Cement Hydration Products. Ph.D. Thesis, Wuhan University of Technology, Wuhan, China, 2015. [Google Scholar]
- Wang, M.G.; Wang, F.Z.; Shang, D.C.; Hu, S.G. Effect of CdO on the formation of tricalcium silicate and its solid solution effect. J. Mater. Sci. Eng. 2016, 34, 208–212. [Google Scholar]
- Hu, S.G.; Nie, S.; Zhu, M.; Li, Y.Z.; Zhan, J.; Wang, F.Z. Performance Analysis of Derived Fuels for Cement Kilns Prepared by Municipal Solid Waste. J. Saf. Environ. 2014, 176–180. [Google Scholar]
- Hou, X.L.; Ma, X.Q. Research Progress on Heavy Metal Pollution and Treatment of Municipal Refuse. Environ. Prot. Sci. 2006, 32, 30–31. [Google Scholar]
- Xiang, C.Y.; He, Y.J.; Lu, L.N.; Wang, F.Z.; Li, Y.Q. Research on the performance of clinker kiln co-processing hazardous waste production clinker. Environ. Sci. Manag. 2013, 38, 81–86. [Google Scholar]
- Katyal, N.K.; Ahluwalia, S.C.; Parkash, R. Effect of Cr2O3 on the formation of C3S in 3CaO:1SiO2:xCr2O3system. Cem. Concr. Res. 2000, 30, 1361–1365. [Google Scholar] [CrossRef]
- Enculescu, M. Influence of oxides of transitional elements on the properties of mineralogical components of clinker. In Proceedings of the 6th International Congress on the Chemistry of Cement, Moscow, Russia, 21–28 September 1974; Volume 19, pp. 351–355. [Google Scholar]
- Xi, Y.Z. Curing mechanism of chromium in Portland cement. J. China Acad. Build. Mater. Sci. 1990, 2, 15–21. [Google Scholar]
- Kolovos, K.; Tsivilis, S.; Kakali, G. SEM examination of clinkers containing foreign elements. Cem. Concr. Compos. 2005, 27, 163–170. [Google Scholar] [CrossRef]
- Tong, D.Z.; Yu, Q.J.; Wang, S.B. XPS and ESR study of chromium ions in cement clinker. J. Chin. Ceram. Soc. 1988, 16, 370–376. [Google Scholar]
- Barros, A.M.; Espinosa, D.C.R.; Tenório, J.A.S. Effect of Cr2O3 and NiO additions on the phase transformations at high temperature in Portland cement. Cem. Concr. Res. 2004, 34, 1795–1801. [Google Scholar] [CrossRef]
- Fierens, P.; Verhaegen, J.P. Structure and reactivity of chromium doped tricalcium silicate. J. Am. Ceram. Soc. 1972, 55, 309–312. [Google Scholar] [CrossRef]
- Stephan, D.; Mallmann, R.; Knöfel, D.; Härdtl, R. High intakes of Cr, Ni, and Zn in clinker: Part I. Influence on burning process and formation of phases. Cem. Concr. Res. 1999, 29, 1949–1957. [Google Scholar] [CrossRef]
- Ste, D.; Maleki, H. Influence of Cr, Ni and Zn on the properties of puree linker phases: Part I: C3S. Cem. Concr. Res. 1999, 29, 545–552. [Google Scholar]
- Y, W.; L, W.; X, W.; Liu, Y.J.; Wei, L.Y. Solid solution behavior of Cr3+ ion in tricalcium silicate. Bull. Chin. Ceram. Soc. 2013, 32, 1253–1257. [Google Scholar]
- Fang, Y.H. Application of solid high resolution nuclear magnetic resonance in cement chemistry research. J. Build. Mater. 2003, 54–60. [Google Scholar]
- Feng, C.H.; Wang, X.J.; Li, D.X. Application progress of 29Si and 27Al solid nuclear magnetic resonance in cement-based materials. Nucl. Technol. 2014, 37, 48–53. [Google Scholar]
- Xiao, J.M.; Fan, H.H.; Wu, Y.L.; Ma, Z. 29Si solid high resolution nuclear magnetic resonance analysis of sludge ash replacing clay calcined cement clinker. J. Mater. Sci. Eng. 2016, 34, 460–464. [Google Scholar]
- He, Y.J.; Hu, S.G. Application of 29Si solid nuclear magnetic resonance technology in cement chemistry research. J. Mater. Sci. Eng. 2007, 105, 148–157. [Google Scholar]
- Trapote-Barreira, A.; Porcar, L.; Cama, J.; Soler, J.M.; Allen, A.J. Structural changes in C–S–H gel during dissolution: Small-angle neutron scattering and Si-NMR characterization. Cem. Concr. Res. 2015, 72, 76–89. [Google Scholar] [CrossRef]
- Yang, N.R.; Yue, W.H. Manual of Atlas of Inorganic Nonmetallic Materials; Wuhan University of Technology Press: Wuhan, China, 2000; pp. 571–628. [Google Scholar]
- Lu, P. Introduction to Cement Materials Science; Tongji University Press: Shanghai, China, 1991; pp. 18–55. [Google Scholar]
- Liebau, F. Structural Chemistry of Silicate; China Construction Industry Press: Beijing, China, 1989. [Google Scholar]
- Zhang, Q.T. Science Foundation of Inorganic Materials; East China University of Science and Technology Press: Shanghai, China, 2007. [Google Scholar]
| Composition | CaO | SiO2 | Al2O3 | Fe2O3 |
|---|---|---|---|---|
| Limestone | 56.80 | 0.28 | 0.12 | 0.05 |
| Clay | 14.52 | 48.25 | 10.28 | 4.60 |
| Copper slag | 6.55 | 30.17 | 8.93 | 48.04 |
| Raw Material | C0 | C0.4 | C0.6 | C0.8 | C1.0 | C5.0 |
|---|---|---|---|---|---|---|
| Cr | 0 | 0.4 | 0.6 | 0.8 | 1.0 | 5.0 |
| Limestone | 68.73 | 68.73 | 68.73 | 68.73 | 68.73 | 68.73 |
| Clay | 28.92 | 28.92 | 28.92 | 28.92 | 28.92 | 28.92 |
| Copper slag | 2.35 | 2.35 | 2.35 | 2.35 | 2.35 | 2.35 |
| Content | 0% | 0.4% | 0.6% | 0.8% | 1.0% | 5.0% |
|---|---|---|---|---|---|---|
| β-C2S | 36.2 | 19.43 | 19.03 | 23.51 | 23.12 | 27.44 |
| C2S | 36.2 | 29.36 | 30.21 | 36.13 | 36.58 | 37.44 |
| C3S | 63.8 | 70.64 | 70.72 | 63.87 | 63.42 | 62.56 |
| Ion | Ionic Radius/pm | Electronegativity | Ligancy |
|---|---|---|---|
| Ca2+ | 100 | 1.0 | 6 |
| Si4+ | 40 | 1.9 | 4 |
| Cr3+ | 69 | 1.6 | 6 |
| Number | Mass Fraction/% | |||||
|---|---|---|---|---|---|---|
| O | Al | Si | Ca | Cr | ||
| a | EDS-1 | 50.65 | 1.51 | 14.48 | 32.02 | 1.35 |
| EDS-2 | 53.90 | 1.30 | 11.63 | 32.07 | —— | |
| b | EDS-1 | 53.51 | 1.29 | 14.28 | 29.57 | 1.34 |
| EDS-2 | 53.22 | 1.02 | 12.11 | 32.19 | —— | |
| c | EDS-1 | 54.33 | 1.51 | 14.15 | 29.03 | 3.98 |
| EDS-2 | 53.98 | 1.77 | 14.02 | 29.13 | 4.10 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, H.; Lv, M.; Wang, X.; Xiao, J.; Mi, X.; Jia, L. Effect of Cr on the Mineral Structure and Composition of Cement Clinker and Its Solidification Behavior. Materials 2020, 13, 1529. https://doi.org/10.3390/ma13071529
Fan H, Lv M, Wang X, Xiao J, Mi X, Jia L. Effect of Cr on the Mineral Structure and Composition of Cement Clinker and Its Solidification Behavior. Materials. 2020; 13(7):1529. https://doi.org/10.3390/ma13071529
Chicago/Turabian StyleFan, Haihong, Mengqi Lv, Xiaosha Wang, Jianmin Xiao, Xiaofan Mi, and Luwei Jia. 2020. "Effect of Cr on the Mineral Structure and Composition of Cement Clinker and Its Solidification Behavior" Materials 13, no. 7: 1529. https://doi.org/10.3390/ma13071529
APA StyleFan, H., Lv, M., Wang, X., Xiao, J., Mi, X., & Jia, L. (2020). Effect of Cr on the Mineral Structure and Composition of Cement Clinker and Its Solidification Behavior. Materials, 13(7), 1529. https://doi.org/10.3390/ma13071529




