Effect of Material and Process Variables on Characteristics of Nitridation-Induced Self-Formed Aluminum Matrix Composites—Part 1: Effect of Reinforcement Volume Fraction, Size, and Processing Temperatures
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
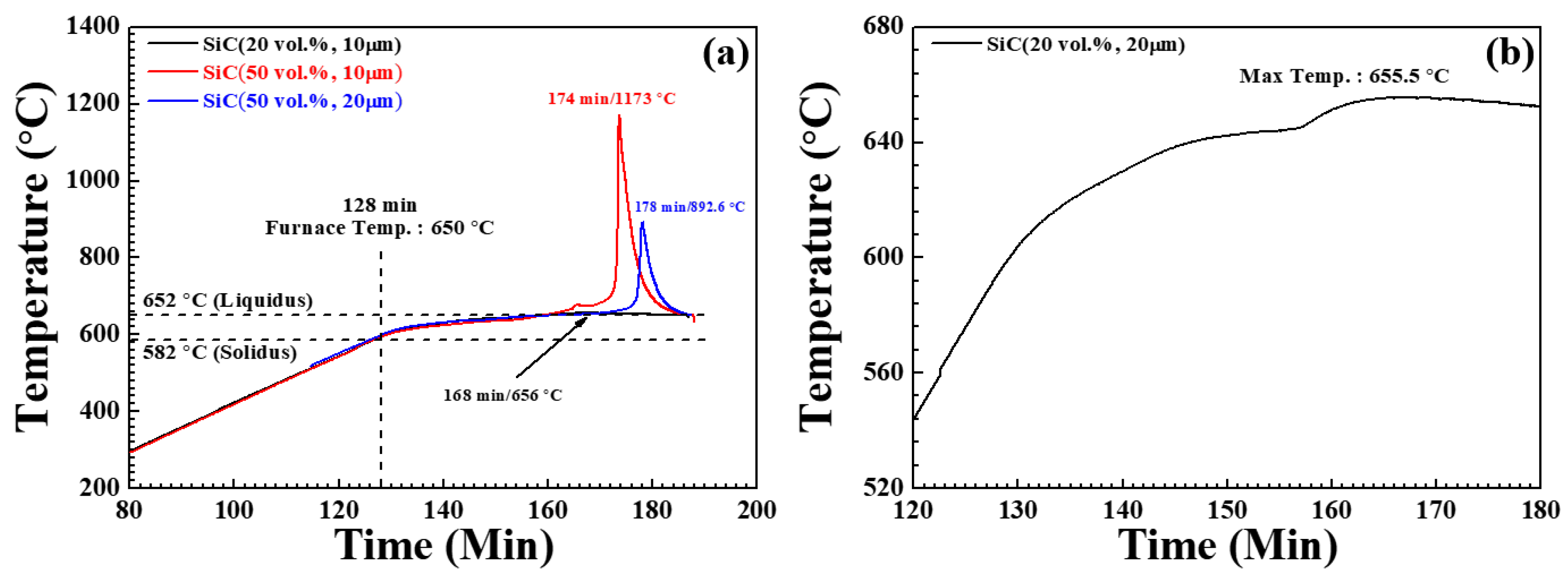
3.1. Temperature Change of Powder Bed by Nitridation
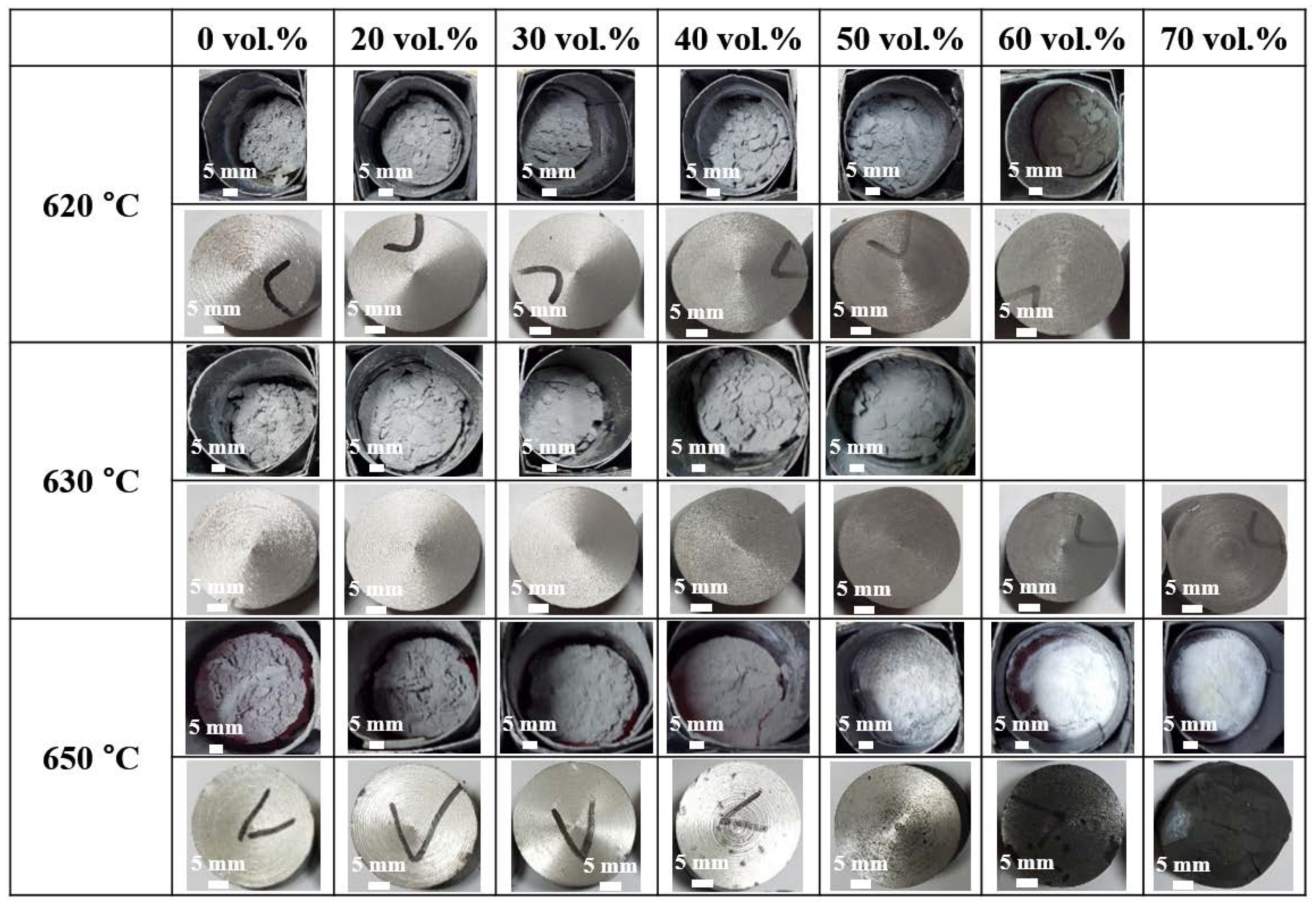
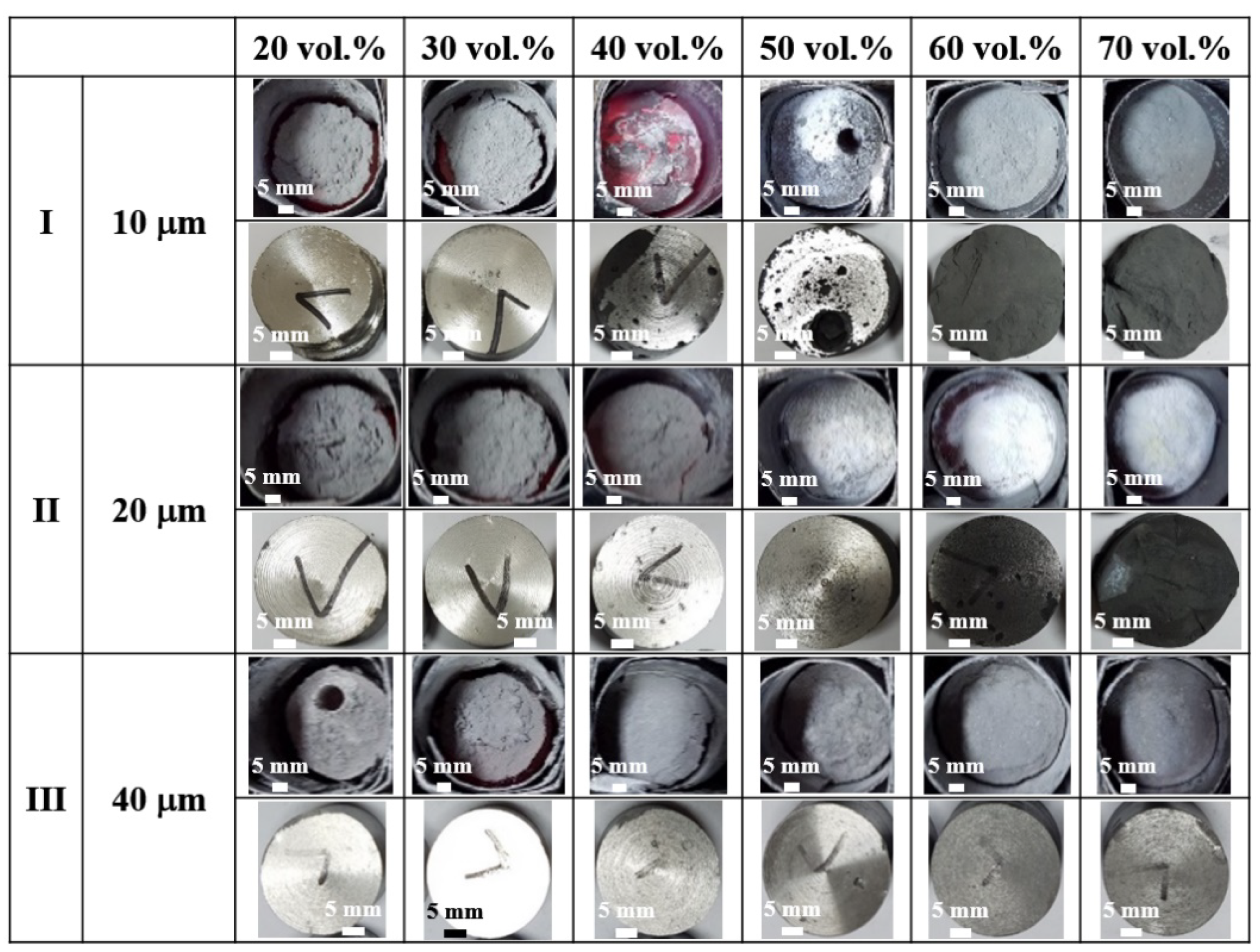
3.2. The Effect of Processing Temperature and Volume Fraction of SiC
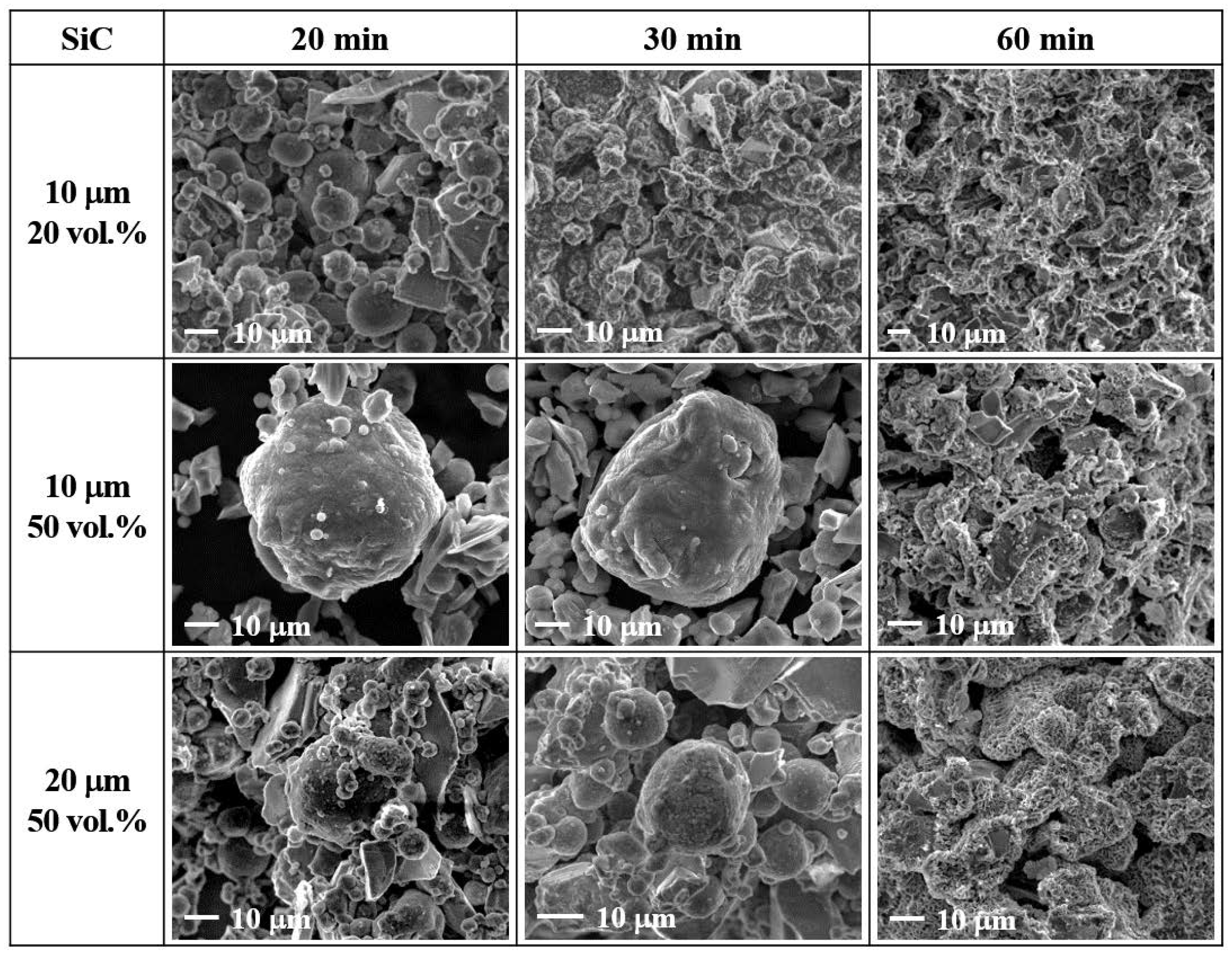
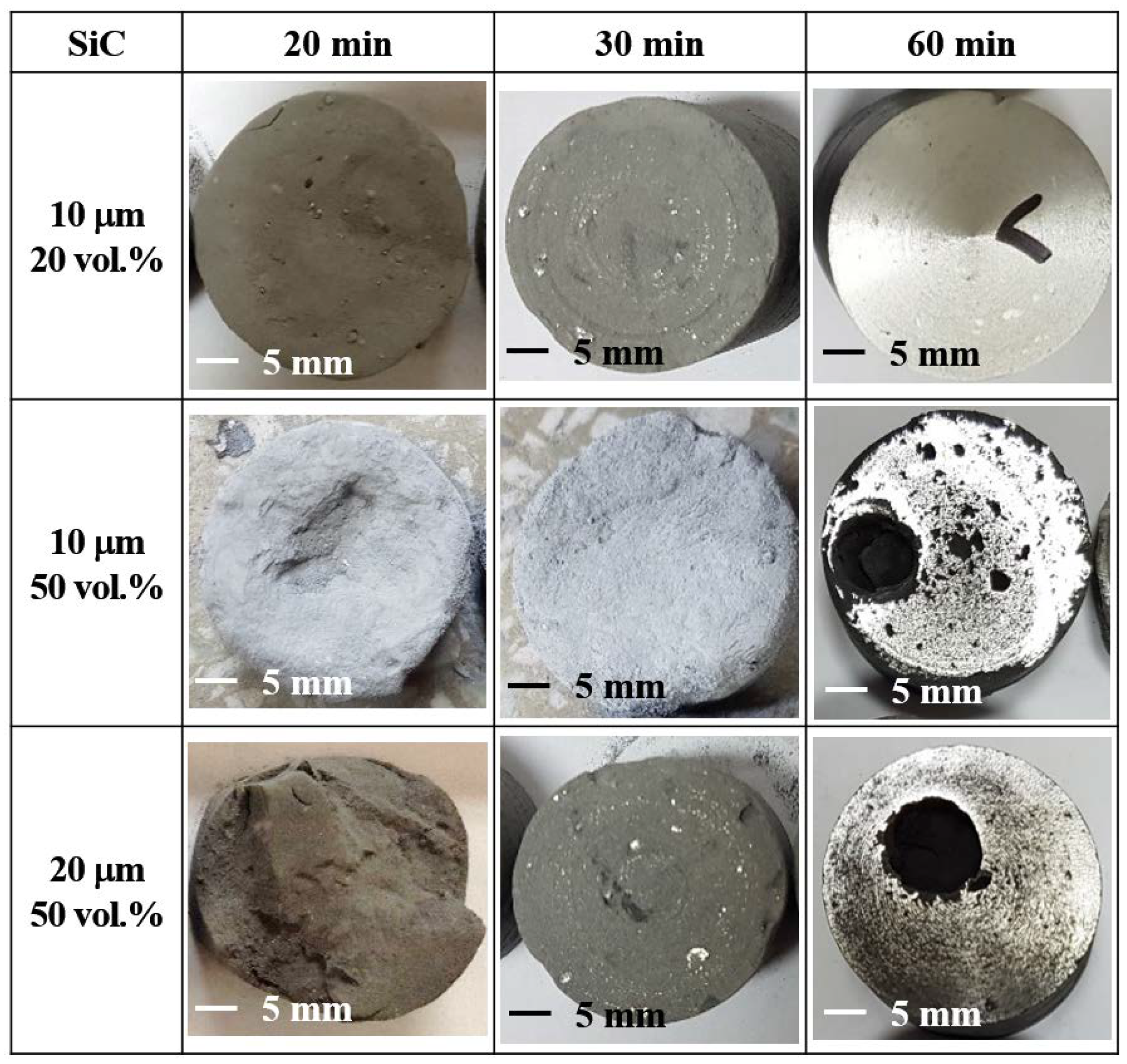
3.3. Effect of SiC Particle Size
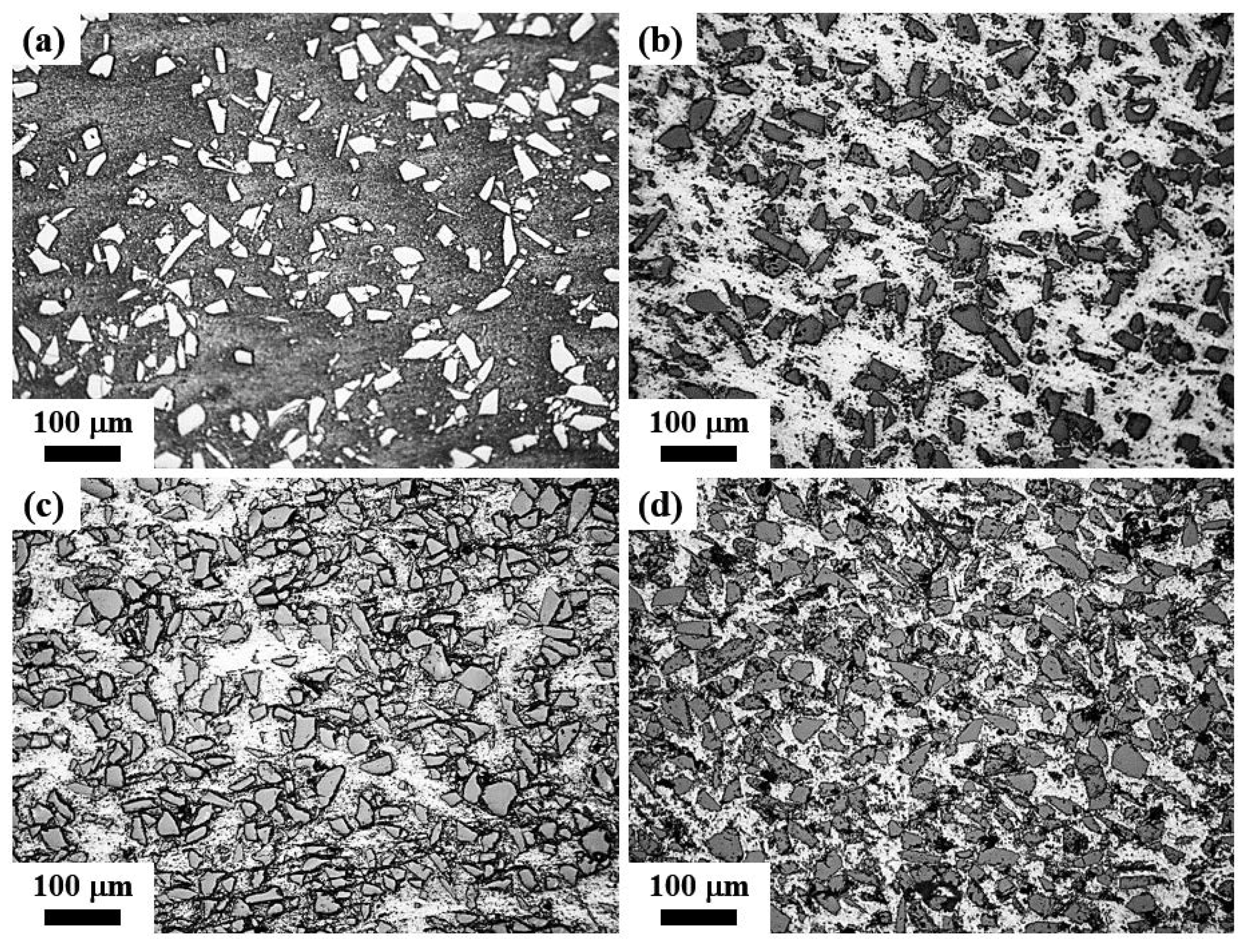
3.4. Microstructures of the Obtained Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miracle, D.B. Metal matrix composites-from science to technological significance. Compos. Sci. Technol. 2005, 65, 2526–2540. [Google Scholar] [CrossRef]
- Mortensen, A.; Llorca, J. Metal Matrix Composites. Ann. Rev. Mater. Res. 2010, 40, 243–270. [Google Scholar] [CrossRef]
- Campbell, F.C. Introduction to composite materials. In Structural Composite Materials; Campbell, F.C., Ed.; ASM International: Novelty, OH, USA, 2010. [Google Scholar]
- Surappa, M.K. Aluminium matrix composites: Challenges and opportunities. Sadhana 2003, 28, 319–334. [Google Scholar] [CrossRef]
- Kainer, K.U. Metal Matrix Composites: Custom-made Materials for Automotive and Aerospace Engineering; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Chawla, N.; Chawla, K.K. Metal Matrix Composites; Springer: New York, NY, USA, 2006. [Google Scholar]
- Mortensen, A.; Jin, I. Solidification processing of metal matrix composites. Int. Mater. Rev. 1992, 37, 101–128. [Google Scholar] [CrossRef]
- Ibrahim, I.A.; Mohamed, F.A.; Lavernia, E.J. Particulate reinforced metal matrix composites—A review. J. Mater. Sci. 1991, 26, 1137–1156. [Google Scholar] [CrossRef]
- Rohatgi, P.K.; Asthana, R.; Das, S. Solidification, structures, and properties of cast metal-ceramic particle composites. Int. Met. Rev. 1986, 31, 115–139. [Google Scholar] [CrossRef]
- Rana, R.S.; Purohit, R.; Das, S. Review of recent Studies in Al matrix composites. Int. J. Sci. Eng. Res. 2012, 3, 1–16. [Google Scholar]
- Rittner, M. Metal Matrix Composites in the 21st Century: Markets and Opportunities; BCC, Inc.: Norwalk, CT, USA, 2000. [Google Scholar]
- Evans, A.; Marchi, C.S.; Mortensen, A. Metal Matrix Composites in Industry: An Introduction and a Survey; Springer Science & Business Media: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Degischer, H.P.; Prader, P.; Marchi, C.S. “Assessment of Metal Matrix Composites for Innovations”—Intermediate report of a European Thematic Network. Compos. Pt. A-Appl. Sci. Manuf. 2001, 32, 1161–1166. [Google Scholar] [CrossRef]
- Qu, X.H.; Zhang, L.; Wu, M.; Ren, S.B. Review of metal matrix composites with high thermal conductivity for thermal management applications. Porg. Nat. Sci.: Mater. Int. 2011, 21, 189–197. [Google Scholar] [CrossRef]
- The Materials Society (TMS), Metal-Matrix Composites in Industry: A Database of Companies, Materials, and Products. Available online: http://members.tms.org/MMCDatabase/MMC.asp (accessed on 15 November 2019).
- Lee, K.B.; Kim, S.H.; Kim, D.Y.; Cha, P.R.; Kim, H.S.; Choi, H.J.; Ahn, J.P. Aluminum Matrix Composites Manufactured using Nitridation-Induced Self-Forming Process. Sci. Rep. 2019, 9, 20389. [Google Scholar] [CrossRef]
- Lee, K.B.; Ahn, J.P.; Kim, H.S.; Choi, H.J. Method of Fabricating an Aluminum Matrix Composite and an Aluminum Matrix Composite Fabricated by the same. U.S. Patent 10094006, 9 October 2018. [Google Scholar]
- Kim, S.H.; Noh, J.H.; Ahn, J.P.; Lee, J.C.; Kwon, H.; Lee, J.G.; Yang, H.R.; Lee, K.B. Effects of Surface Oxide on the Nitridation Behavior of Aluminum Particles. Metall. Mater. Trans. A 2015, 46, 496–504. [Google Scholar] [CrossRef]
- Lee, K.B.; Kim, Y.H.; Choi, H.J.; Ahn, J.P. Effect of carbon on the nitridation behavior of aluminum powder. J. Alloys Comp. 2016, 689, 218–224. [Google Scholar] [CrossRef]
- Lee, K.B.; Yoo, S.H.; Kim, H.S.; Won, S.O.; Yang, B.J.; Ahn, J.P.; Choi, H.J. Nitridation-assisted Al infiltration for fabricating Al composites. J. Mater. Sci. 2017, 52, 4333–4344. [Google Scholar] [CrossRef]
- Lee, K.B.; Yoo, S.H.; Kim, Y.H.; Han, C.W.; Won, S.O.; Ahn, J.P.; Choi, H.J. A cost-effective route to produce Al/AlN composites with low coefficient of thermal expansion. J. Compo. Mater. 2017, 51, 2845–2851. [Google Scholar] [CrossRef]
- Haibo, J.; Chen, K.; Heping, Z.; Agathopoulos, S.; Fabrichnaya, O.; Ferreira, J.M.F. Direct nitridation of molten Al (Mg, Si) alloy to AlN. J. Cryst. Growth 2005, 281, 639–645. [Google Scholar] [CrossRef]
- Okada, T.; Toriyama, M.; Kanzaki, S. Direct nitridation of aluminum compacts at low temperature. J. Mater. Sci. 2000, 35, 3105–3111. [Google Scholar] [CrossRef]
- Chang, A.J.; Rhee, S.W.; Baik, S.G. Kinetics and Mechanisms for Nitridation of Floating Aluminum Powder. J. Am. Ceram. Soc. 1995, 78, 33–40. [Google Scholar] [CrossRef]
- NIST-JANAF Thermochemical Tables. Available online: http://kinetics.nist.gov/janaf/ (accessed on 20 November 2019).
- Zheng, Q.; Wu, B.; Reddy, R.G. In-Situ Processing of Al Alloy Composites. Adv. Eng. Mater. 2003, 5, 167–172. [Google Scholar] [CrossRef]
- Scholz, H.; Greil, P. Nitridation reactions of molten Al-(Mg,Si) alloys. J. Mater. Sci. 1991, 26, 669–677. [Google Scholar] [CrossRef]
- Zheng, Q.; Reddy, R.G. Mechanism of in situ formation of AlN in Al melt using nitrogen gas. J. Mater. Sci. 2004, 39, 141–149. [Google Scholar] [CrossRef]
- Kumari, S.S.; Pillai, U.T.S.; Pai, B.C. Synthesis and characterization of in situ Al-AlN composite by nitrogen gas bubbling method. J. Alloy. Compd. 2011, 509, 2503–2509. [Google Scholar] [CrossRef]
- Radwan, M.; Bahgat, M. A modified direct nitridation method for formation of nano-AlN whiskers. J. Mater. Proc. Tech. 2007, 181, 99–105. [Google Scholar] [CrossRef]
- Jia, L.; Kondoh, K.; Imai, H.; Onishi, M.; Chen, B.; Li, S.F. Nano-Scale AlN powders and AlN/Al composites by full and partial direct nitridation of aluminum in solid-state. J. Alloy. Compd. 2015, 629, 184–187. [Google Scholar] [CrossRef]
- Aluminum 6061-T6; 7429-90-ss5 [Online]. ASM Aerospace Specification Metals Inc.: Pompano Beach, FL, USA, 1978. Available online: http://asm.matweb.com// (accessed on 20 November 2019).

| SiC | 20 min | 30 min | 60 min | Max Temp. (°C) /Time (min) | |||
|---|---|---|---|---|---|---|---|
| Degree of Nitridation | Temperature of Bed (°C) | Degree of Nitridation | Temperature of Bed (°C) | Degree of Nitridation | Temperature of Bed (°C) | ||
| 20 vol.% 10 μm | 2.3 | 640 | 4.0 | 645 | 5.4 | 650 | 656/40 |
| 50 vol.% 10 μm | 2.1 | 633 | 3.8 | 646 | 49.8 | 650 | 1173/46 |
| 50 vol.% 20 μm | 2.7 | 638 | 4.2 | 648 | 31.3 | 650 | 893/50 |
| 0 vol.% | 20 vol.% | 30 vol.% | 40 vol.% | 50 vol.% | 60 vol.% | 70 vol.% | |
|---|---|---|---|---|---|---|---|
| 620 °C | 2.3 | 3.8 | 4.3 | 7.1 | 10.2 | 9.4 | |
| 630 °C | 2.1 | 3.3 | 5.0 | 5.6 | 6.2 | 8.3 | 12.3 |
| 650 °C | 4.6 | 4.4 | 5.2 | 11.5 | 33.6 | 66.7 | 94.2 |
| 20 vol.% | 30 vol.% | 40 vol.% | 50 vol.% | 60 vol.% | 70 vol.% | |
|---|---|---|---|---|---|---|
| 10 μm | 5.7 | 6.9 | 54.0 | 49.8 | 7.7 | 12.1 |
| 20 μm | 4.4 | 5.2 | 11.5 | 33.6 | 66.7 | 94.2 |
| 40 μm | 4.7 | 5.6 | 6.3 | 7.7 | 9.0 | 16.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-Y.; Cha, P.-R.; Nam, H.-S.; Choi, H.-J.; Lee, K.-B. Effect of Material and Process Variables on Characteristics of Nitridation-Induced Self-Formed Aluminum Matrix Composites—Part 1: Effect of Reinforcement Volume Fraction, Size, and Processing Temperatures. Materials 2020, 13, 1309. https://doi.org/10.3390/ma13061309
Kim D-Y, Cha P-R, Nam H-S, Choi H-J, Lee K-B. Effect of Material and Process Variables on Characteristics of Nitridation-Induced Self-Formed Aluminum Matrix Composites—Part 1: Effect of Reinforcement Volume Fraction, Size, and Processing Temperatures. Materials. 2020; 13(6):1309. https://doi.org/10.3390/ma13061309
Chicago/Turabian StyleKim, Dae-Young, Pil-Ryung Cha, Ho-Seok Nam, Hyun-Joo Choi, and Kon-Bae Lee. 2020. "Effect of Material and Process Variables on Characteristics of Nitridation-Induced Self-Formed Aluminum Matrix Composites—Part 1: Effect of Reinforcement Volume Fraction, Size, and Processing Temperatures" Materials 13, no. 6: 1309. https://doi.org/10.3390/ma13061309
APA StyleKim, D.-Y., Cha, P.-R., Nam, H.-S., Choi, H.-J., & Lee, K.-B. (2020). Effect of Material and Process Variables on Characteristics of Nitridation-Induced Self-Formed Aluminum Matrix Composites—Part 1: Effect of Reinforcement Volume Fraction, Size, and Processing Temperatures. Materials, 13(6), 1309. https://doi.org/10.3390/ma13061309






