The key to determining the success of the NISFAC process is the formation of sufficient molten Al to fill the voids inside the powder bed. In particular, when the production temperature is lower than the melting point (or liquidus) of Al (or alloy), the Al powder can only melt when the temperature is locally increased due to the exothermic nitridation reaction. Hence, the amount of molten Al is determined by the degree of nitridation, which can be controlled by the various process parameters.
3.1. Effect of Nitrogen and Argon Gas
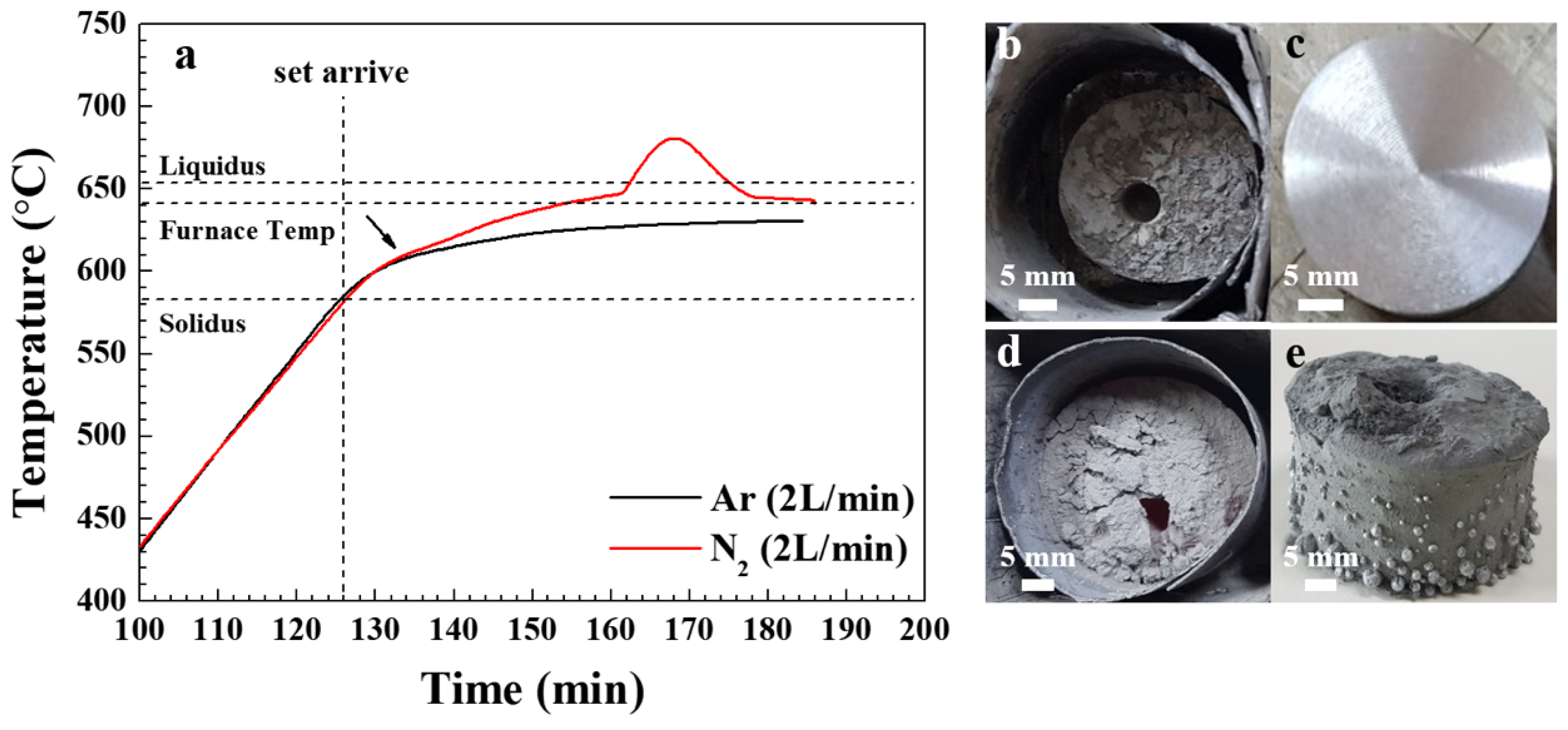
Figure 1a shows the internal temperature of the powder bed when heating a mixture of Al 6061 alloy and SiC (40 μm, 20 vol.%) from room temperature (RT) to 640 °C for 1 h under an atmosphere of argon or nitrogen (2 L/min). For both types of atmosphere, the temperature of the powder bed increased at approximately the same rate during the heating from RT to 610 °C. However, when the temperature of the powder bed exceeded 610 °C, a more rapid temperature increase was observed under the nitrogen atmosphere than the argon atmosphere. This is due to the exothermic nature of the nitridation reaction. Furthermore, under the nitrogen atmosphere, the temperature rise of the powder bed became rapid after reaching approximately 645 °C, and subsequently became more gradual as the temperature approached the maximum value of about 680 °C.
Photographic images of the air-cooled crucibles after heating for 1 h under a nitrogen or argon atmosphere, respectively, are presented in
Figure 1b,d, while images of the respective samples after machine working are presented in
Figure 1c. For the sample heated in nitrogen, the significant amount of shrinkage observed after cooling (
Figure 1b) suggests that sufficient molten Al was produced during heating. Moreover, a bright metallic luster was obtained for this sample after lathe working (
Figure 1c), demonstrating the production of a sound composite. When heated in argon, the maximum temperature inside the bed was 631 °C, which is lower than the programmed furnace target temperature of 640 °C but higher than the solidus of the Al 6061 alloy (582 °C), hence melting still occurred. However, unlike the powder heated in nitrogen, little shrinkage was observed after solidification (
Figure 1d) and molten Al alloy leaked out of the bed surface (
Figure 1e) due to the poor wettability between the molten Al and SiC [
2,
3] as in the previous studies. Hence, high-quality composites were not produced in this case.
Figure 2 indicates the effect of varying the amount of nitrogen during the production of the same composites by heating the mixtures for 1 h at a set temperature of 640 °C. The change in temperature inside the powder bed is seen to vary according to the nitrogen flow rate (1 to 4 L/min) and, hence, the amount of nitrogen supplied. At nitrogen flow rates of 1–3 L/min, the temperature increase was first gradual and became rapid at around 645 °C due to the exothermic nitridation reaction. Furthermore, as the amount of nitrogen increased, the rapid temperature rise occurred sooner, but the maximum temperature was significantly decreased. We reported in the previous study that the temperature at the center of the bed was very high compared to that at the bottom or top during the nitridation of Al powders [
25]. This indicates that nitridation begins at the center of the powder bed. Nitrogen gas supplied into the chamber passes through the surface of the powder bed and moves inwards through the pore network. Nitrogen reaching the top of the bed is soon discharged through the exhaust pipe. Hence, contact between nitrogen and the Al particle surface is maintained by the continuous supply of fresh nitrogen gas into the chamber. Therefore, nitridation does not occur at the top of the powder bed because the contact time between the nitrogen gas and the Al powder is very short there. By contrast, since the nitrogen gas reaching the center of the bed via the pore network remains in a relatively closed area, nitridation can be initiated during a longer contact time relative to the upper and lower portions of the bed for a given flow rate. Thus, increasing the amount of nitrogen allows a high nitrogen concentration to be maintained in a relatively wider volume in the center of the bed (
Figure 2b). Hence, as the quantity of Al particles experiencing nitridation increases, the heat dissipated by the exotherm increases, and the temperature of 645 °C is reached sooner, at which stage a rapid temperature rise occurs. This can be seen from the fact that the bed temperature before the onset of rapid temperature rise is relatively higher as the amount of nitrogen increases, as shown in
Figure 2a. As will be explained later, however, no rapid temperature rise was observed when the nitrogen flow rate was increased to 4 L/min.
The degrees of nitridation and the maximum temperatures of the powder bed under various nitrogen flow rates are indicated in
Supplementary Figure S1 along with photographic images of the composites in their crucibles and after lathe working. As the inlet flow rate and, hence, the amount of nitrogen supplied increases, the degree of nitridation and maximum temperature both decrease. Since the amount of AlN formed by the nitridation reaction affects the properties of the composite, the amount of AlN should be controlled according to the requirement of the end user.
Figure 3 presents optical micrographs of the composites prepared under different nitrogen flow rates. Uniform distribution of SiC is observed for all samples and no significant microstructural differences were detected. In addition, the XRD patterns in
Figure 4 indicate that AlN was formed in all the composites regardless of nitrogen flow rate and that other reaction products such as Al
4C
3 were not detected. In particular, Al
4C
3 was not detected at a nitrogen flow rate of 1 L/min, even though the maximum temperature was approximately that at which this carbide can form (742 °C). As indicated in the above discussion, the temperature rise was localized and the holding time for this temperature range was evidently too short for carbides to form. In this study, composites were prepared at 640 °C, which is below the liquidus (652 °C) of the Al 6061 alloy. Hence, it is possible to produce the composite even at a temperature below the liquidus line, which is one of the advantages of the NISFAC process. Unlike conventional processes involving liquid phases, composites can be produced even below the melting point (or liquidus) of Al and its alloys, which not only reduces the formation of undesirable reactants (e.g. Al
4C
3 when SiC is used), but also reduces the energy-consumption during the manufacturing processes.
To examine in detail the effect of nitrogen concentration upon the composite production, the change in the microstructure of the powder bed was tracked during heating under nitrogen flow rates of 1 and 4 L/min at a set temperature of 640 °C. The degrees of nitridation and powder bed temperatures of the composites produced with various nitrogen flow rates and heating times are presented in
Table 1, while
Supplementary Figure S2 presents photographic images of the respective powders in their crucibles (rows I and V) and in the powder beds (rows II and VI), along with images of the samples after cutting (rows III and VII) and after lathe working (rows IV and VIII). In addition, SEM images of the powder bed microstructures observed at various heating times are presented in
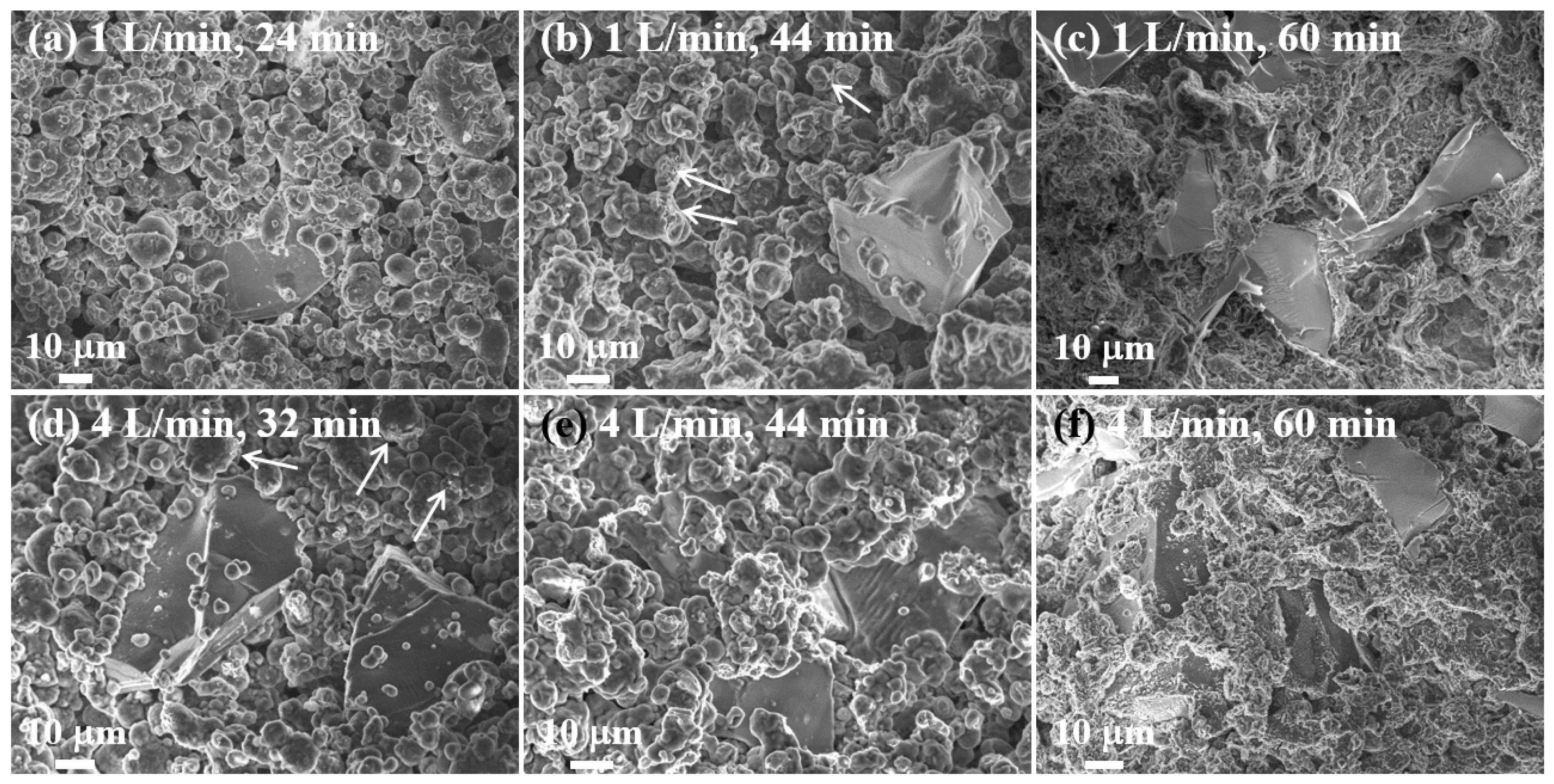
Figure 5. It can be seen that a nitrogen flow rate of 1 L/min resulted in a very small degree of nitridation (0.1%) after heating for 15 mins with a set temperature of 640 °C, so that the sample remained in the powder form. This is because the temperature inside the powder bed remained relatively low (~ 622 °C) and, although this was above the Al 6061 alloy solidus (582 °C), almost no molten Al was formed (
Figure 5a and
Supplementary Figure S2, row II).
After 32 minutes of heating, the degree of nitridation increased to 2.9% and the powder bed temperature increased to 639 °C. However, the amount of molten Al phase was not enough to form highly dense composites and the final composites were easily broken after solidification (
Supplementary Figure S2, row II). In addition, the arrows in
Figure 5b,c indicate that molten Al was released from the powder and covered the surfaces of adjacent Al and SiC particles. Molten Al was thus in intimate contact with a very large surface area of the SiC particles. The melting of the Al powder was caused by the localized increase in temperature above the Al melting temperature due to the exothermic nitridation reaction.
After heating for 44 mins, the top of the powder bed remained in powder form (
Figure S2, row I), whereas sufficient shrinkage occurred at the bottom of the powder bed to allow complete solidification (
Supplementary Figure S2, row II). This was because the powder bed temperature reached ~ 649 °C or more, which is close to the liquidus temperature of the Al 6061 alloy (652 °C), so enough molten Al was formed to fill the voids in the powder bed (
Figure 5e,f). However, as can be seen from the cross-section image in
Supplementary Figure S2 (row III) and the SEM image in
Figure 5d, some un-melted areas were present near the surface of the powder bed.
With a nitrogen flow rate of 1 L/min, the temperature inside the bed continued to rise after 60 mins of heating (
Figure 2), hence the nitridation reaction continued. This can also be seen in the cross-section image in
Supplementary Figure S2 (row III) and the SEM images in
Figure 5g,h. This indicates that much more melt was formed in the center than at the top of the bed. In addition, the amount of molten Al was significantly increased relative to that observed after 50 mins of heating (
Figure 5f,h). As shown in the crucible image, some powder remained on the top even after heating for more than 1 h, but the bed temperature decreased after reaching the maximum temperature of 742 °C while the amount of molten Al increased (
Figure 5i).
The temperature of the bed and the degree of nitridation for the composites after heating for 24 mins under a nitrogen flow rate of 4 L/min were ~ 639 °C and 2.9%, respectively. These values were similar to those obtained after heating for 32 mins under a flow rate of 1 L/min. In addition, the crucible images indicate a larger shrinkage after 24 mins of heating at 4 L/min than after 32 mins of heating at 1 L/min, and that the amount of powder remaining on the bed was also reduced and completely solidified at the higher flow rate (
Supplementary Figure S2, row V). The images obtained after cutting show similar morphologies after 44 mins of heating at both flow rates. In addition, after 44 mins of heating at 4 L/min, the peak temperature of 641 °C has already been passed so that sufficient shrinkage has occurred and the amount of powder remaining on the upper part of the bed has been greatly reduced (
Supplementary Figure S2, rows V-VIII).
As shown in
Figure 6, these macroscopic changes were also reflected in the microstructure. After heating for 44 mins, the amount of molten Al obtained at 4 L/min was greatly increased relative to that obtained at 1 L/min. Since the rate of nitridation at a nitrogen flow rate of 4L/min is greater than that at 1 L/min, the temperature inside the powder bed increases more rapidly and molten Al is formed relatively faster. As a result, the molten Al fills the pore space inside the bed and blocks the nitrogen supply, suppressing further nitridation and preventing a rapid temperature rise. By contrast, at a nitrogen flow rate of 1 L/min, the nitridation rate is slow, hence the formation of the liquid phase is slow, nitrogen is supplied into the bed for a relatively longer time, the nitridation time increases and, hence, the temperature rises rapidly due to the increase in the degree of nitridation.
3.2. Effect of Heating Temperature at a Fixed Nitrogen Flow Rate
Since the rapid temperature rise was not observed when heating at a nitrogen flow rate of 4 L/min, the composite was prepared at a fixed flow rate of 4 L/min while heating for 1 h at set temperatures ranging from 610 to 650 °C in order to analyze the effect of the production temperature.
Figure 7 shows the temperature changes measured inside the powder bed for various heating temperatures. For all production temperatures, each graph displays several changes of slope such that the second change of slope (marked by the arrows in
Figure 7) indicates the temperature rise due to nitridation. The temperature rise stems from two main reasons, namely: i) the exothermic nitridation reaction and ii) the rise in temperature the furnace towards the set temperature. Therefore, the temperature rise due to nitridation may be most clearly distinguished at the lowest set temperature of 610 °C. As shown in
Figure 7, at 610 °C, the temperature gradient changes near the solidus temperature (582 °C) of the Al 6061 alloy, indicating that the nitridation of Al begins at very low temperatures. Thus, the time for initiation of nitridation (as indicated by the second change in slope) decreases with increasing production temperature, but the powder bed temperature then increases due to the relatively increased effect of heating. In contrast to the temperature behavior when heating to set temperatures in the range of 610 to 640 °C, heating to 650 °C generated a sharp internal temperature change at about 645 °C, as was observed in
Figure 2. The peak internal temperatures together with the set temperatures are summarized in
Supplementary Figure S3 along with photographic images of the composites in the crucibles and after machine working. Thus, the peak internal temperature did not vary significantly with set temperatures except for the set temperature of 650 °C, which exhibited the sharp increase in powder bed temperature. The composites prepared at a set temperature of 610 °C (
Supplementary Figure S3, row II) exhibited a reduced metallic luster and a smaller amount of shrinkage compared to those produced at higher set temperatures. Therefore, in order to manufacture a high-quality composite, manufacturing temperatures of 620 °C or higher are preferable. In addition, since the maximum temperature inside the powder bed remains below the liquidus of the Al 6061 alloy (652 °C) at set temperatures in the range of 620 to 640 °C, this can greatly limit the formation of undesirable reaction products. When the heating time was increased to 90 mins for the set temperature of 610 °C, however, the crucible shrinkage and metal gloss increased significantly (
Supplementary Figure S3, row I). As described above, when manufacturing the composite using the NISFAC process, it is possible to control the amount of molten Al by adjusting the process variables, so that the composite may be manufactured under a variety of conditions. This diversity of processing is another advantage of the NISFAC process. That is, even for the same composite system (e.g., the Al/SiC system studied herein), it is possible to tailor the characteristics of the final product by controlling various process parameters such as the composition of the selected Al alloy, the matrix powder size, the SiC particle size, the volume fraction of SiC, the manufacturing time and temperature, the nitrogen concentration, etc.