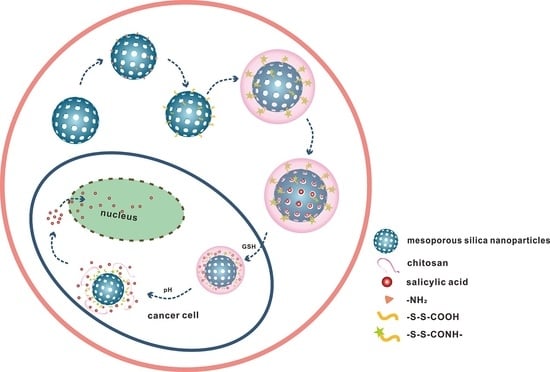
pH and Redox Dual-Responsive MSN-S-S-CS as a Drug Delivery System in Cancer Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MSN and MSN-S-S-CS
2.3. Characterization
2.4. Determination of Stability and Responsiveness
2.5. Drug Loading and Body Fluid Simulation
- (1)
- Simulated gastric juice (pH 1.2): 2.0 g NaCl, 3.2 g pepsin and 7 mL hydrochloric acid, distilled water added to dissolve to a volume of 1000 mL;
- (2)
- Simulated intestinal fluid (pH 7.4): 0.3 g KH2PO4, 2.88 g Na2HPO4•7H2O, 0.20 g KCl, 8 g NaCl, dissolved in distilled water, and diluted to 1000 mL;
- (3)
- Simulated large intestinal fluid (pH 8.4): 3.5 g Na2HPO4•7H2O, 3.5 g KH2PO4, diluted to 1000 mL in distilled water, and adjusted to pH to 8.3 with dilute sodium hydroxide solution;
- (4)
- PBS buffer solution (pH 5.8): 8.3 g KH2PO4 and 0.9 g K2HPO4, dissolved in distilled water, diluted to 1000 mL;
- (5)
- 2 mM GSH solution: 0.6 g GSH dissolved in distilled water, and volume adjusted to a 1000 mL volumetric flask and shaken prior to use;
- (6)
- 10 mM GSH solution: dissolved 3.0 g GSH in distilled water in a 1000 mL volumetric flask, and shaken while standing;
- (7)
- pH 1.2 + 10 mM GSH, pH 5.8 + 10 mM GSH, pH 7.4 + 10 mM GSH: three aliquots of 3.0 g GSH were dissolved in the above 1000 mL pH solutions.
2.6. In Vitro Release
3. Results and Discussion
3.1. Synthesis and SEM Characterization of MSN and MSN-S-S-CS
3.2. Brunauer–Emmett–Teller Surface Area (BET) Analysis
3.3. XRD Analysis
3.4. FTIR Analysis
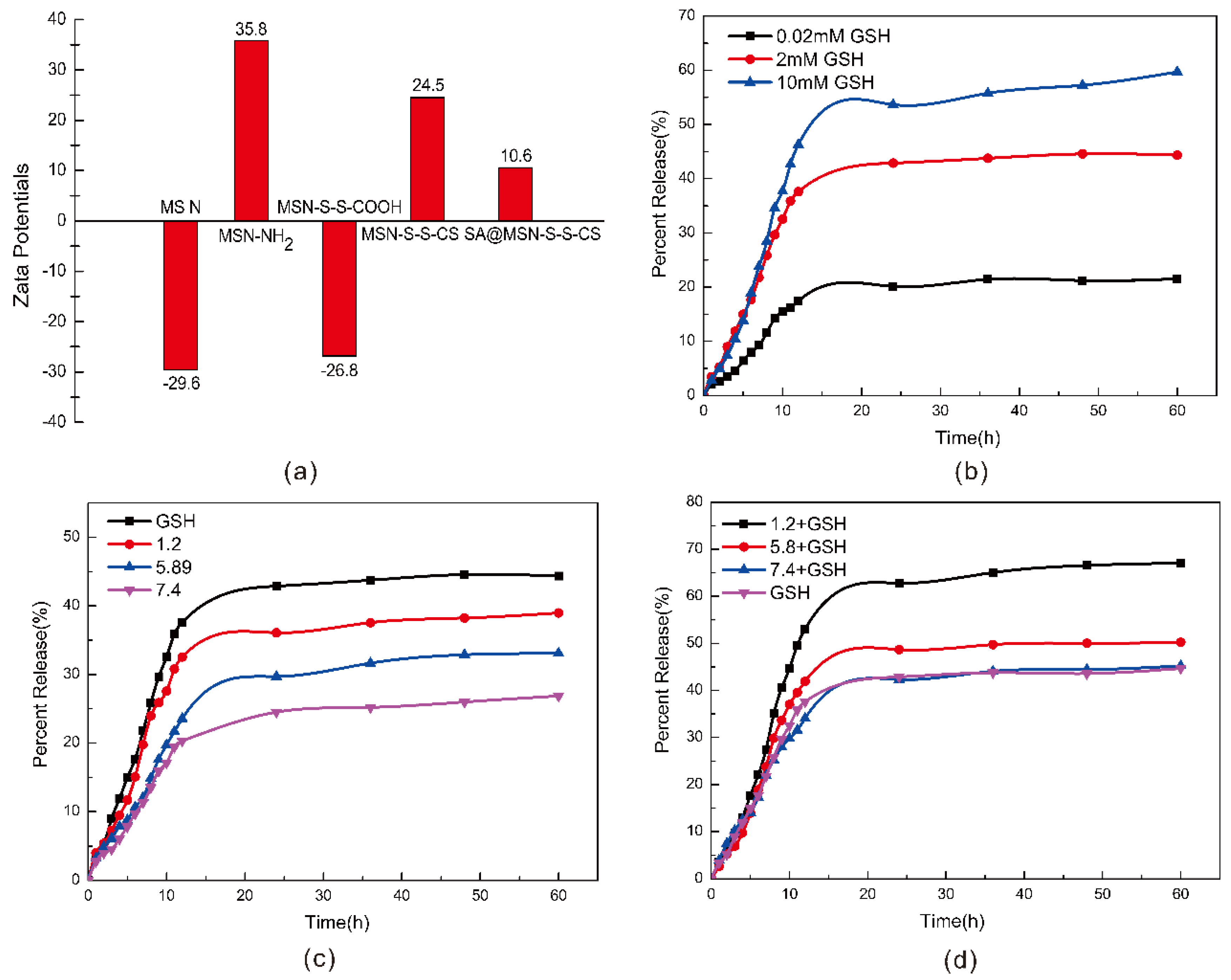
3.5. Size and Zeta Potential
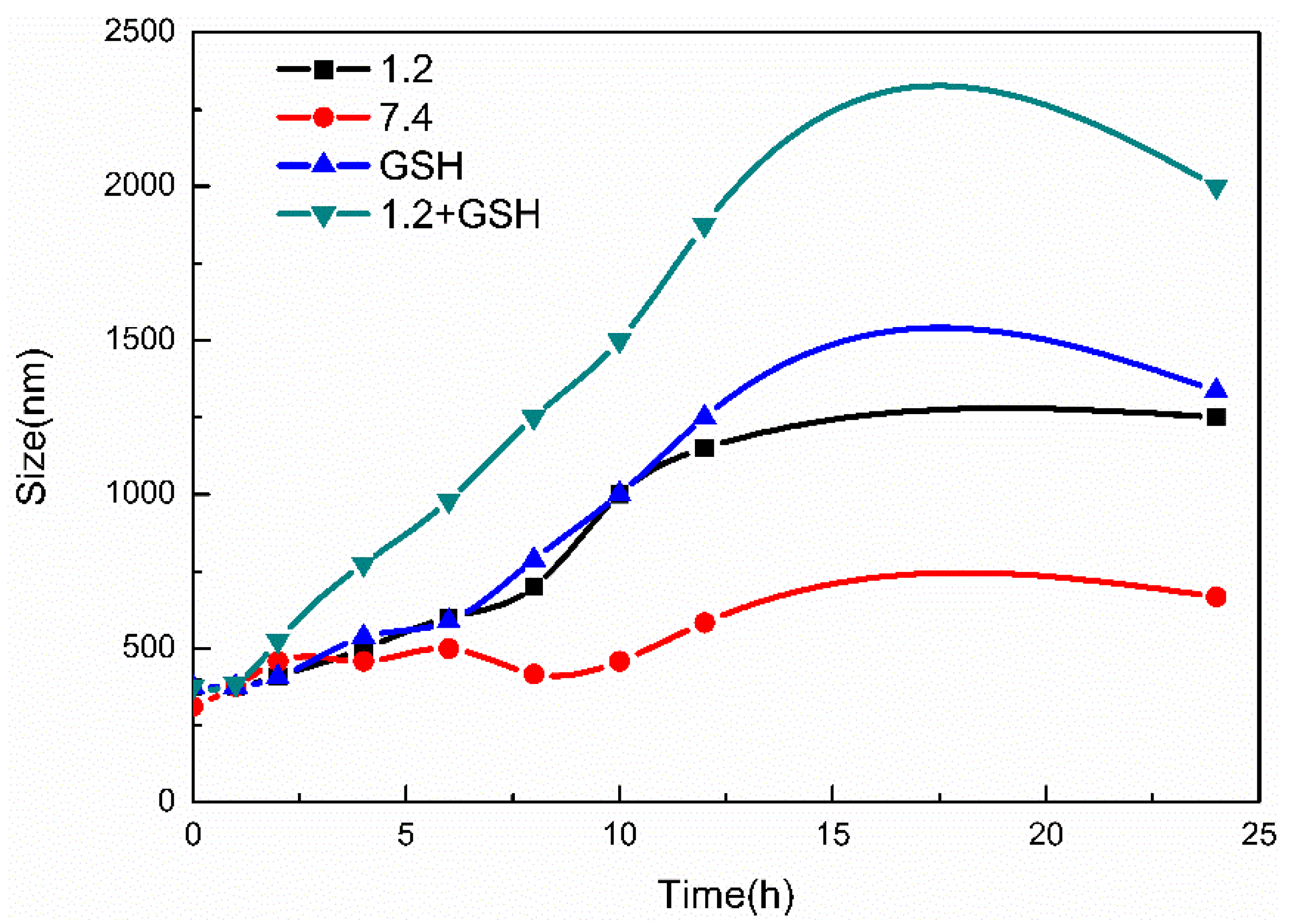
3.6. Stability and Responsiveness Analysis of MSN-S-S-CS
3.7. Simulation Experiment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- He, Q.J.; Shi, J.L. Msn anti-cancer nanomedicines: Chemotherapy enhancement, overcoming of drug resistance, and metastasis inhibition. Adv. Mater. 2014, 26, 391–411. [Google Scholar] [CrossRef]
- Jia, X.; Tian, K.; Zhao, X.; Zhou, T.; Pei, M.; Liu, P. Fluorescent amphiphilic copolymer-based tumor theranostics for facile dox-loading and tumor microenvironment-triggered release. Mater. Des. 2016, 105, 333–340. [Google Scholar] [CrossRef]
- Liang, C.Y.; Wang, H.P.; Zhang, M.; Cheng, W.; Li, Z.H.; Nie, J.P.; Liu, G.; Lian, D.Z.; Xie, Z.H.; Huang, L.Q.; et al. Self-controlled release of oxaliplatin prodrug from d-alpha-tocopheryl polyethylene glycol 1000 succinate (tpgs) functionalized mesoporous silica nanoparticles for cancer therapy. J. Colloid. Interf. Sci. 2018, 525, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Nie, J.P.; Gao, N.S.; Liu, G.; Tao, W.; Xiao, X.J.; Jiang, L.J.; Liu, Z.G.; Zeng, X.W.; Mei, L.A. Multifunctional nanoplatform against multidrug resistant cancer: Merging the best of targeted chemo/gene/photothermal therapy. Adv. Funct. Mater. 2017, 27, 1704135. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regi, M. New developments in ordered mesoporous materials for drug delivery. J. Mater. Chem. 2010, 20, 5593–5604. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Edit. 2007, 46, 7548–7558. [Google Scholar] [CrossRef]
- Wang, S.B. Ordered mesoporous materials for drug delivery. Microporous Mesoporous Mater. 2009, 117, 1–9. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.Y.; Yang, B.X.; Xu, L.; Zheng, N.; Chen, H.T.; Li, S.M. Control-release microcapsule of famotidine loaded biomimetic synthesized mesoporous silica nanoparticles: Controlled release effect and enhanced stomach adhesion in vitro. Mat. Sci. Eng. C Mater. 2016, 58, 273–277. [Google Scholar] [CrossRef]
- Saini, K.; Prabhuraj, R.S.; Bandyopadhyaya, R. Development of mesoporous silica nanoparticles of tunable pore diameter for superior gemcitabine drug delivery in pancreatic cancer cells. J. Nanosci. Nanotechnol. 2020, 20, 3084–3096. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Sekhar, V.C.; Nair, S.S. Polyelectrolyte complexes of carboxymethyl chitosan/alginate based drug carrier for targeted and controlled release of dual drug. J. Drug. Deliv. Sci. Technol. 2019, 51, 569–582. [Google Scholar] [CrossRef]
- Lu, Y.; Ballauff, M. Thermosensitive core-shell microgels: From colloidal model systems to nanoreactors. Prog. Polym. Sci. 2011, 36, 767–792. [Google Scholar] [CrossRef]
- Chakraborty, I.; Mascharak, P.K. Mesoporous silica materials and nanoparticles as carriers for controlled and site-specific delivery of gaseous signaling molecules. Microporous Mesoporous Mater. 2016, 234, 409–419. [Google Scholar] [CrossRef]
- Hu, J.J.; Xiao, D.; Zhang, X.Z. Advances in peptide functionalization on mesoporous silica nanoparticles for controlled drug release. Small 2016, 12, 3344–3359. [Google Scholar] [CrossRef] [PubMed]
- Aquib, M.; Farooq, M.A.; Banerjee, P.; Akhtar, F.; Filli, M.S.; Boakye-Yiadom, K.O.; Kesse, S.; Raza, F.; Maviah, M.B.J.; Mavlyanova, R.; et al. Targeted and stimuli-responsive mesoporous silica nanoparticles for drug delivery and theranostic use. J. Biomed. Mater. Res. A 2019, 107, 2643–2666. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.W.; Liu, G.; Tao, W.; Ma, Y.; Zhang, X.D.; He, F.; Pan, J.M.; Mei, L.; Pan, G.Q. A drug-self-gated mesoporous antitumor nanoplatform based on ph-sensitive dynamic covalent bond. Adv. Funct. Mater. 2017, 27, 1605985. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, D.; Li, M.F.; Feng, J. Multifunctional mesoporous silica nanoparticles based on charge-reversal plug-gate nanovalves and acid-decomposable zno quantum dots for intracellular drug delivery. Acs. Appl. Mater. Inter. 2015, 7, 26666–26673. [Google Scholar] [CrossRef]
- Chen, Y.J.; Khan, A.R.; Yu, D.X.; Zhai, Y.J.; Ji, J.B.; Shi, Y.K.; Zhai, G.X. Pluronic f127-functionalized molybdenum oxide nanosheets with ph-dependent degradability for chemo-photothermal cancer therapy. J. Colloid Interface Sci. 2019, 553, 567–580. [Google Scholar] [CrossRef]
- Banyai, I.; Keri, M.; Nagy, Z.; Berka, M.; Balogh, L.P. Self-diffusion of water and poly(amidoamine) dendrimers in dilute aqueous solutions. Soft Matter 2013, 9, 1645–1655. [Google Scholar] [CrossRef]
- Shen, Y.Q.; Zhou, Z.X.; Sui, M.H.; Tang, J.B.; Xu, P.S.; Van Kirk, E.A.; Murdoch, W.J.; Fan, M.H.; Radosz, M. Charge-reversal polyamidoamine dendrimer for cascade nuclear drug delivery. Nanomedicine. 2010, 5, 1205–1217. [Google Scholar] [CrossRef]
- Martinez-Cuezva, A.; Valero-Moya, S.; Alajarin, M.; Berna, J. Light-responsive peptide [2] rotaxanes as gatekeepers of mechanised nanocontainers. Chem. Commun. 2015, 51, 14501–14504. [Google Scholar] [CrossRef]
- Ganguly, A.; Das, S. Expulsion of a potent cancer-cell photosensitizer from its micelle-bound state using beta-cyclodextrin: A tenable model for efficient drug release. Spectrochim. Acta A 2020, 224, 117411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.H.; Cheng, F.F.; Zhou, R.; Cao, J.T.; Li, J.J.; Burda, C.; Min, Q.H.; Zhu, J.J. DNA-hybrid-gated multifunctional mesoporous silica nanocarriers for dual-targeted and microrna-responsive controlled drug delivery**. Angew Chem. Int. Edit. 2014, 53, 2371–2375. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Q.; Zhao, X.Y.; Cheng, M.; Wang, Y.; Li, C.L.; Hu, S.Q. Facile preparation of redox-responsive hollow mesoporous silica spheres for the encapsulation and controlled release of corrosion inhibitors. Prog. Org. Coat. 2019, 136, 105302. [Google Scholar] [CrossRef]
- Chen, J.J.; Liu, J.X.; Hu, Y.P.; Tian, Z.F.; Zhu, Y.F. Metal-organic framework-coated magnetite nanoparticles for synergistic magnetic hyperthermia and chemotherapy with ph-triggered drug release. Sci. Technol. Adv. Mat. 2019, 20, 1043–1054. [Google Scholar] [CrossRef]
- Hei, M.Y.; Wang, J.; Wang, K.; Zhu, W.P.; Ma, P.X. Dually responsive mesoporous silica nanoparticles regulated by upper critical solution temperature polymers for intracellular drug delivery. J. Mater. Chem. B 2017, 5, 9497–9501. [Google Scholar] [CrossRef]
- Croissant, J.G.; Qi, C.; Mongin, O.; Hugues, V.; Blanchard-Desce, M.; Raehm, L.; Cattoen, X.; Man, M.W.C.; Maynadier, M.; Gary-Bobo, M.; et al. Disulfide-gated mesoporous silica nanoparticles designed for two-photon-triggered drug release and imaging. J. Mater. Chem. B. 2015, 3, 6456–6461. [Google Scholar] [CrossRef]
- Shi, H.; Gao, T.; Shi, L.; Chen, T.S.; Xiang, Y.; Li, Y.Y.; Li, G.X. Molecular imaging of telomerase and the enzyme activity-triggered drug release by using a conformation-switchable nanoprobe in cancerous cells. Sci Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.T.; Feng, N.P. Mesoporous silica nanoparticles: Synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery. Expert. Opin. Drug. Del. 2019, 16, 219–237. [Google Scholar] [CrossRef]
- Wu, J.; Sailor, M. Chitosan hydrogel-capped porous sio2 as a ph responsive nano-valve for triggered release of insulin. Adv. Funct. Mater. 2009, 19, 733–741. [Google Scholar] [CrossRef]
- Bazban-Shotorbani, S.; Hasani-Sadrabadi, M.M.; Karkhaneh, A.; Serpooshan, V.; Jacob, K.I.; Moshaverinia, A.; Mahmoudi, M. Revisiting structure-property relationship of ph-responsive polymers for drug delivery applications. J. Control Release 2017, 253, 46–63. [Google Scholar] [CrossRef]
- Shah, P.V.; Rajput, S.J. Facile synthesis of chitosan capped mesoporous silica nanoparticles: A ph responsive smart delivery platform for raloxifene hydrochloride. Aaps. Pharmscitech. 2018, 19, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Bhaysar, D.; Gajjar, J.; Sawant, K. Formulation and development of smart ph responsive mesoporous silica nanoparticles for breast cancer targeted delivery of anastrozole: In Vitro and In Vivo characterizations. Microporous Mesoporous Mater. 2019, 279, 107–116. [Google Scholar] [CrossRef]
- Wang, S.Q.; Yang, L.T.; Cho, H.Y.; Chueng, S.T.D.; Zhang, H.P.; Zhang, Q.Y.; Lee, K.B. Programmed degradation of a hierarchical nanoparticle with redox and light responsivity for self-activated photo-chemical enhanced chemodynamic therapy. Biomaterials 2019, 224, 119498. [Google Scholar] [CrossRef] [PubMed]
- Rianna, C.; Radmacher, M. Influence of microenvironment topography and stiffness on the mechanics and motility of normal and cancer renal cells. Nanoscale 2017, 9, 11222–11230. [Google Scholar] [CrossRef]
- Giovaninni, G.; Moore, C.J.; Hall, A.J.; Byrne, H.J.; Gubala, V. Ph-dependent silica nanoparticle dissolution and cargo release. Colloid Surf. B 2018, 169, 242–248. [Google Scholar] [CrossRef]
- Akrami, S.; Karami, B.; Farahi, M. Preparation and characterization of novel phthalhydrazide-functionalized mcm-41 and its application in the one-pot synthesis of coumarinfused triazolopyrimidines. Rsc. Adv. 2017, 7, 34315–34320. [Google Scholar] [CrossRef]
- Yokozaki, Y.; Shimoyama, Y. Loading of vitamin e into silicone hydrogel by supercritical carbon dioxide impregnation toward controlled release of timolol maleate. J. Supercrit. Fluid 2018, 131, 11–18. [Google Scholar] [CrossRef]
- Zhai, Y.L.; Zhou, X.; Zhang, Z.Q.; Zhang, L.; Wang, D.Y.; Wang, X.H.; Sun, W. Design, synthesis, and characterization of schiff base bond-linked ph-responsive doxorubicin prodrug based on functionalized mpeg-pcl for targeted cancer therapy. Polymers. 2018, 10, 1127. [Google Scholar] [CrossRef]
- Rafi, A.A.; Mahkam, M.; Davaran, S.; Hamishehkar, H.A. Smart ph-responsive nano-carrier as a drug delivery system: A hybrid system comprised of mesoporous nanosilica mcm-41 (as a nano-container) & a ph-sensitive polymer (as smart reversible gatekeepers): Preparation, characterization and in vitro release studies of an anti-cancer drug. Eur. J. Pharm. Sci. 2016, 93, 64–73. [Google Scholar]
- Verlooy, P.; Aerts, A.; Lebedev, O.I.; Van Tendeloo, G.; Kirschhock, C.; Martens, J.A. Synthesis of highly stable pure-silica thin-walled hexagonally ordered mesoporous material. Chem. Commun. 2009, 28, 4287–4289. [Google Scholar] [CrossRef]
- Appaturi, J.N.; Ramalingam, R.J.; Al-Lohedan, H.A. Synthesis, characterization and catalytic activity of melamine immobilized mcm-41 for condensation reactions. J. Porous Mater. 2018, 25, 629–641. [Google Scholar] [CrossRef]
- Liu, R.; Cai, Z.W.; Xu, B.J. Characterization and quantification of flavonoids and saponins in adzuki bean (vigna angularis L.) by hplc-dad-esi-msn analysis. Chem. Cent. J. 2017, 11, 93:1–93:17. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi-Alibeik, M.; Rezaeipoor-Anari, A. Mcm-41 functionalized with b-f bond: Preparation, characterization and catalytic application in the synthesis of polyhydroquinolines. Lett. Org. Chem. 2015, 12, 651–658. [Google Scholar] [CrossRef]
- Ahern, R.J.; Hanrahan, J.P.; Tobin, J.M.; Ryan, K.B.; Crean, A.M. Comparison of fenofibrate-mesoporous silica drug-loading processes for enhanced drug delivery. Eur. J. Pharm. Sci. 2013, 50, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.P.; Ding, C.Q.; Liu, X.L.; Gao, J.; Wu, D.T.; Qin, Y.; Kong, Y. A redox and ph dual-triggered drug delivery platform based on chitosan grafted tubular mesoporous silica. Ceram. Int. 2019, 45, 22603–22609. [Google Scholar] [CrossRef]
- Albayati, S.H.M.; Deveci, P. Ph and gsh dual responsive smart silica nanocarrier for doxorubicin delivery. Mater. Res. Express 2019, 6, 065705. [Google Scholar] [CrossRef]
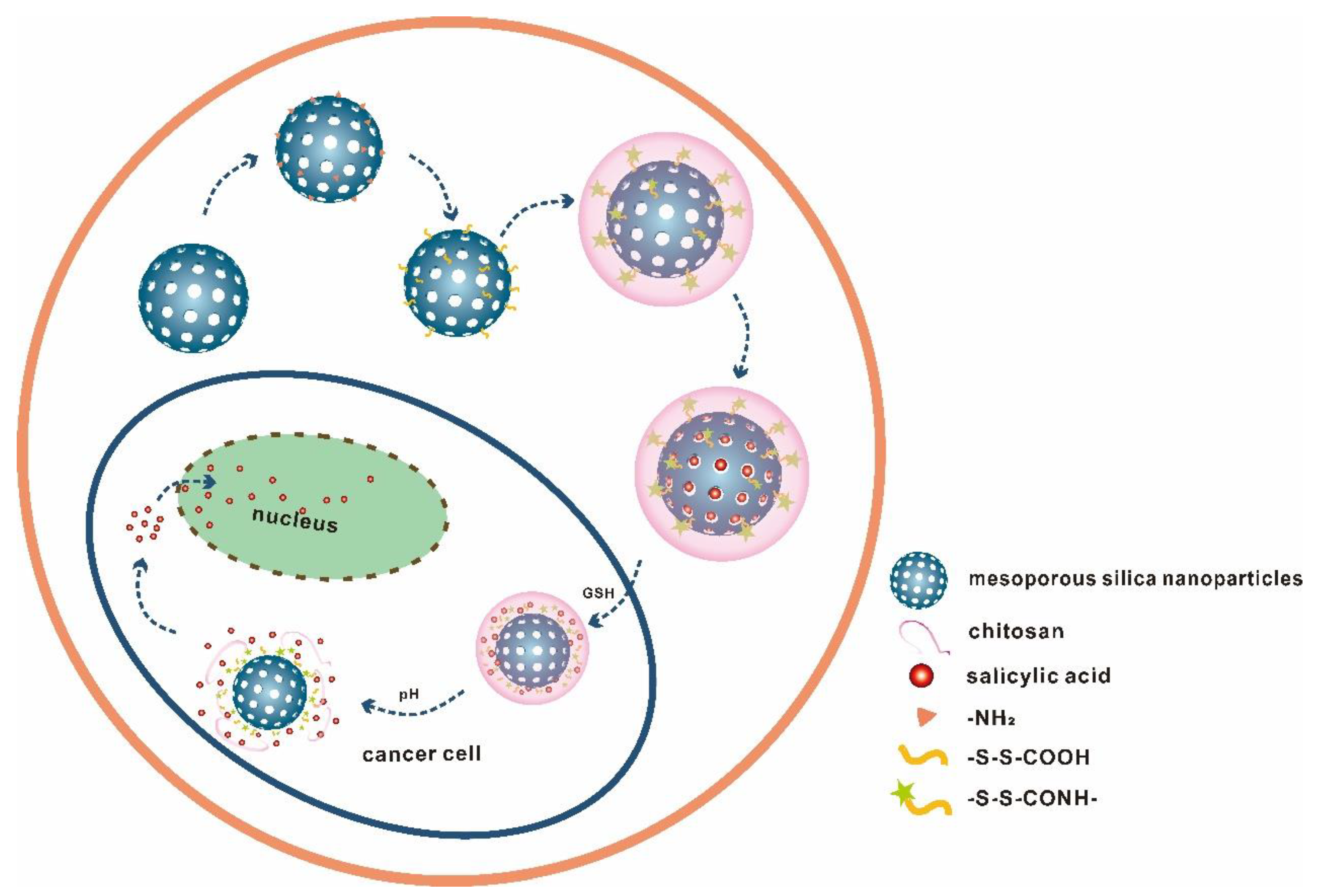
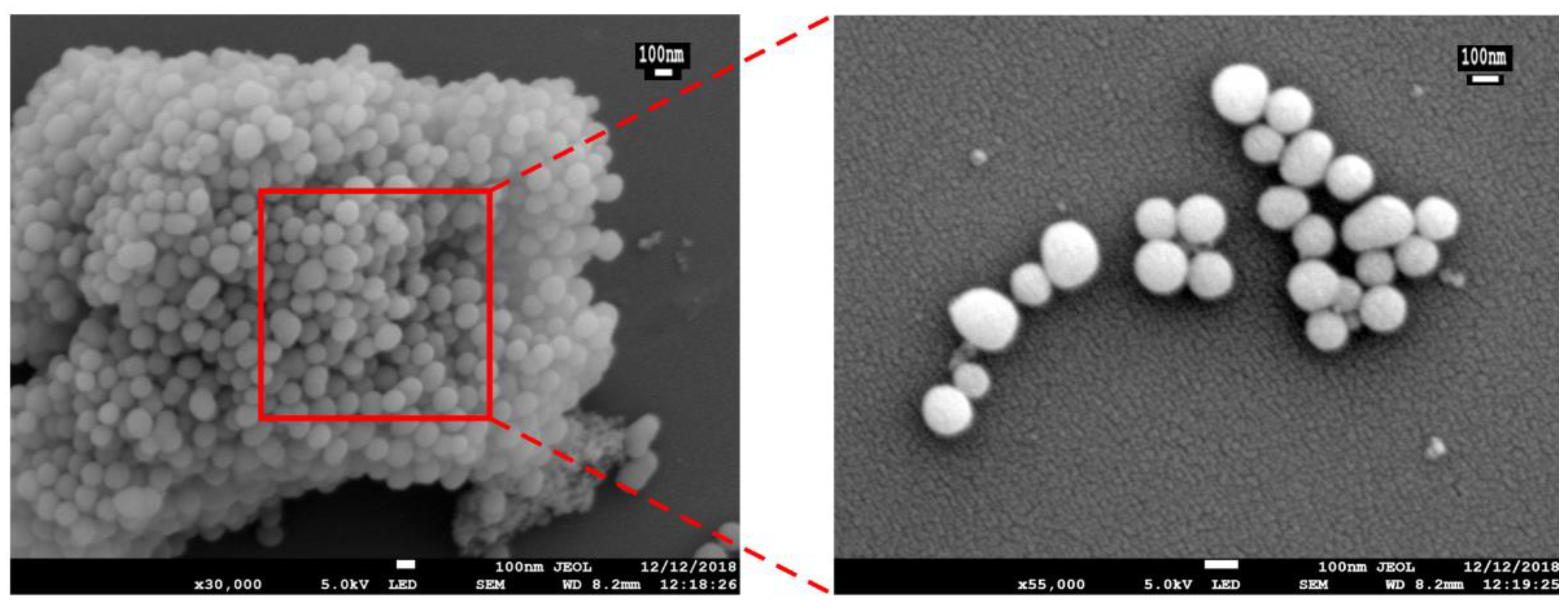

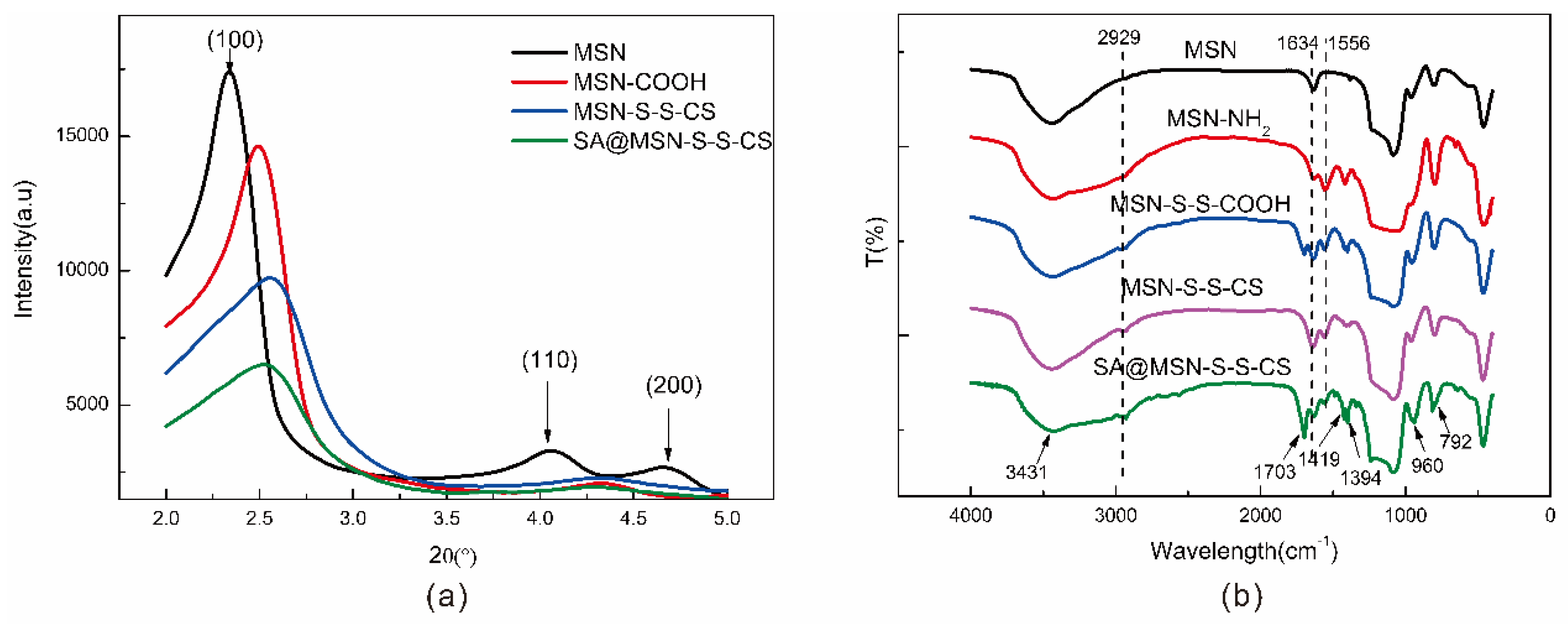
| No. | Specific Surface Area (m2/g) | Aperture (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| MSN | 1080.22 | 3.769 | 0.453 |
| MSN-S-S-CS | 654.01 | 3.746 | 0.416 |
| SA@MSN-S-S-CS | 356.30 | 3.516 | 0.233 |
| No. | 2θ (°) | d100 (nm) | a0 (nm) |
|---|---|---|---|
| MSN | 2.34 | 3.764 | 4.272 |
| MSN-COOH | 2.50 | 3.533 | 4.108 |
| MSN-S-S-CS | 2.56 | 3.449 | 4.010 |
| SA@MSN-S-S-CS | 2.52 | 3.499 | 4.068 |
| Time (h) | SN | MSN-S-S-CS | ||
|---|---|---|---|---|
| PDI | ζ (mV) | PDI | ζ (mV) | |
| 0 | 0.267 | −20.3 | 0.432 | 7.8 |
| 1 | 0.331 | −26.7 | 0.608 | 5.7 |
| 2 | 0.275 | −24.6 | 0.54 | 4.6 |
| 4 | 0.432 | −19.8 | 0.396 | 7.4 |
| 6 | 0.341 | −21.6 | 0.324 | 8.8 |
| 8 | 0.473 | −16.8 | 0.732 | 5.9 |
| 10 | 0.212 | −23.9 | 0.473 | 7.5 |
| 12 | 0.435 | −17.4 | 0.401 | 8.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Xiao, L.; Chang, Y.; Cao, Y.; Chen, C.; Wang, D. pH and Redox Dual-Responsive MSN-S-S-CS as a Drug Delivery System in Cancer Therapy. Materials 2020, 13, 1279. https://doi.org/10.3390/ma13061279
Xu Y, Xiao L, Chang Y, Cao Y, Chen C, Wang D. pH and Redox Dual-Responsive MSN-S-S-CS as a Drug Delivery System in Cancer Therapy. Materials. 2020; 13(6):1279. https://doi.org/10.3390/ma13061279
Chicago/Turabian StyleXu, Yanqin, Liyue Xiao, Yating Chang, Yuan Cao, Changguo Chen, and Dan Wang. 2020. "pH and Redox Dual-Responsive MSN-S-S-CS as a Drug Delivery System in Cancer Therapy" Materials 13, no. 6: 1279. https://doi.org/10.3390/ma13061279
APA StyleXu, Y., Xiao, L., Chang, Y., Cao, Y., Chen, C., & Wang, D. (2020). pH and Redox Dual-Responsive MSN-S-S-CS as a Drug Delivery System in Cancer Therapy. Materials, 13(6), 1279. https://doi.org/10.3390/ma13061279