g-C3N4/CeO2 Binary Composite Prepared and Its Application in Automobile Exhaust Degradation
Abstract
1. Introduction
2. Experiment and Methods
2.1. Materials and Equipment
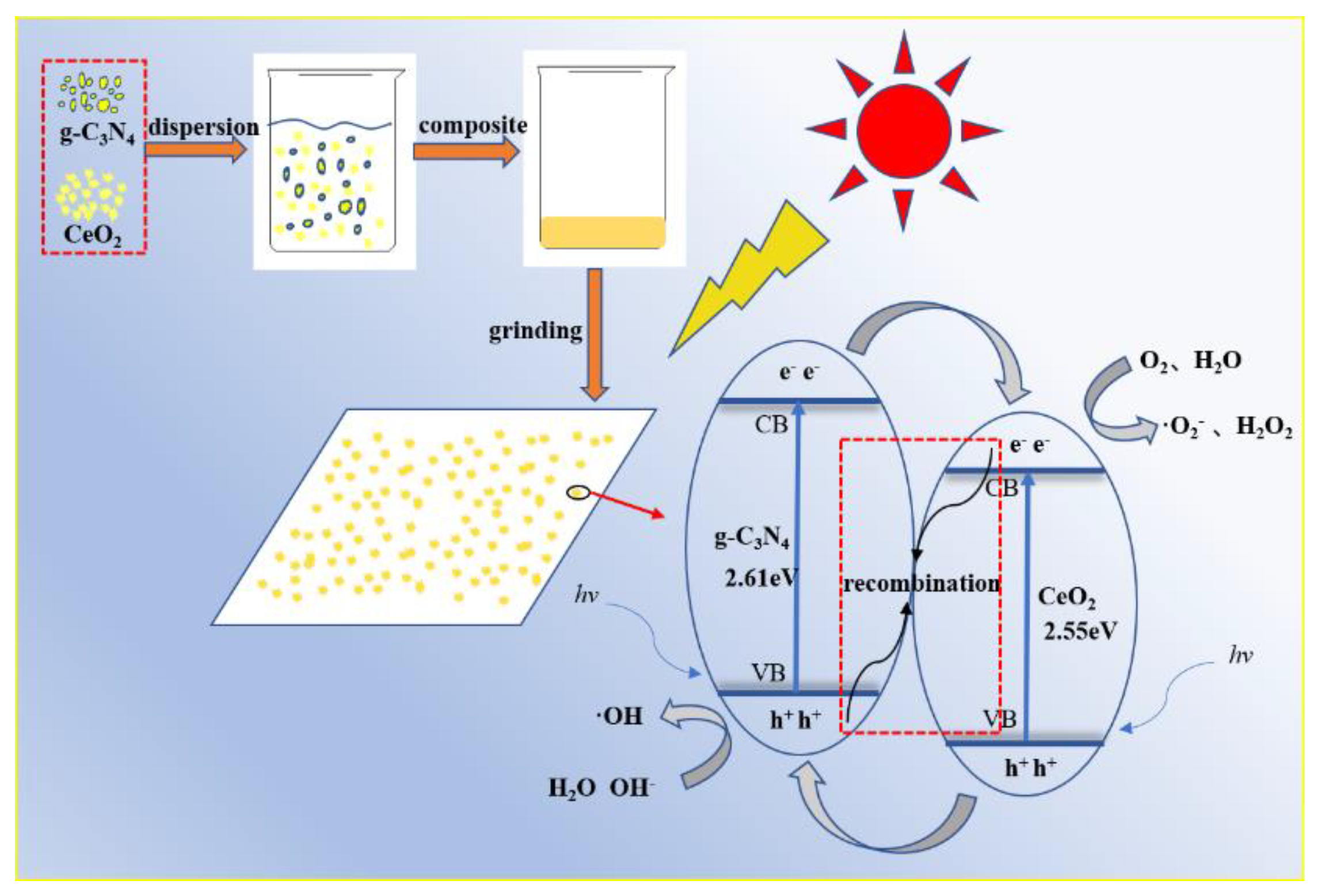
2.2. Preparation
2.3. Indoor Exhaust Gas Purification Step and Evaluation Method
- (1)
- The g-C3N4/CeO2 photocatalytic material was prepared by following the above steps, and 3 g of g-C3N4/CeO2 were weighed and then spread evenly on clean A4 paper;
- (2)
- The exhaust purification system was connected in sequence. The exhaust purification system in this work is shown in Figure 1. Firstly, the DTN220B-NO2 portable nitrogen dioxide detector was put into the reaction box, and a small fan was turned on to keep gas flowing. Then, the photocatalytic material was put into the reaction box, which was closed tightly. Secondly, the reaction chamber was covered with a curtain to prevent light from entering. Then, the air inlet valve was opened, and the air outlet valve was closed. The engine was connected to the air inlet and injected exhaust gas into the reaction box.
- (3)
- The NHA-506 (5G) car exhaust gas analyzer (Nanhua Instrument Co., Ltd., Foshan, China) was turned on. After injecting for a period of time, the automobile exhaust gas analyzer was used to test the concentration of various components in the exhaust gas. When a certain concentration was reached, the intake valve was closed and the gasoline engine was turned off. The UV or incandescent lamp was turned on and the test was started.
- (4)
- The concentrations of NO, NO2, CO, CO2 and HC were recorded every 10 min. A total of 60 min and 7 groups of data were recorded.
2.4. Error Correction of Exhaust Gas Purification Test
2.5. Analysis of Exhaust Gas Degradation Efficiency
2.6. Microscopic Test
3. Results and Discussion
3.1. Degradation Principle of g-C3N4/CeO2
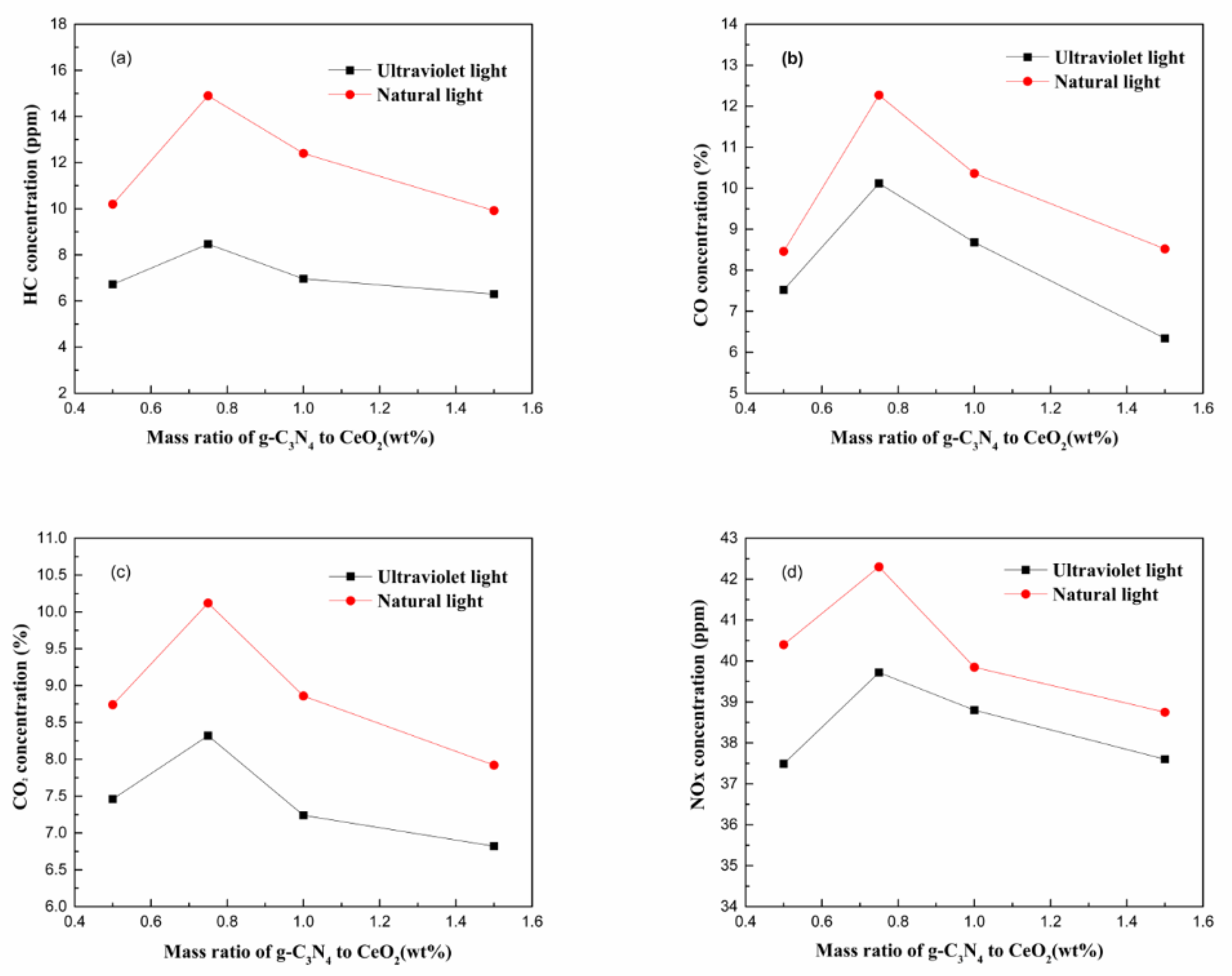
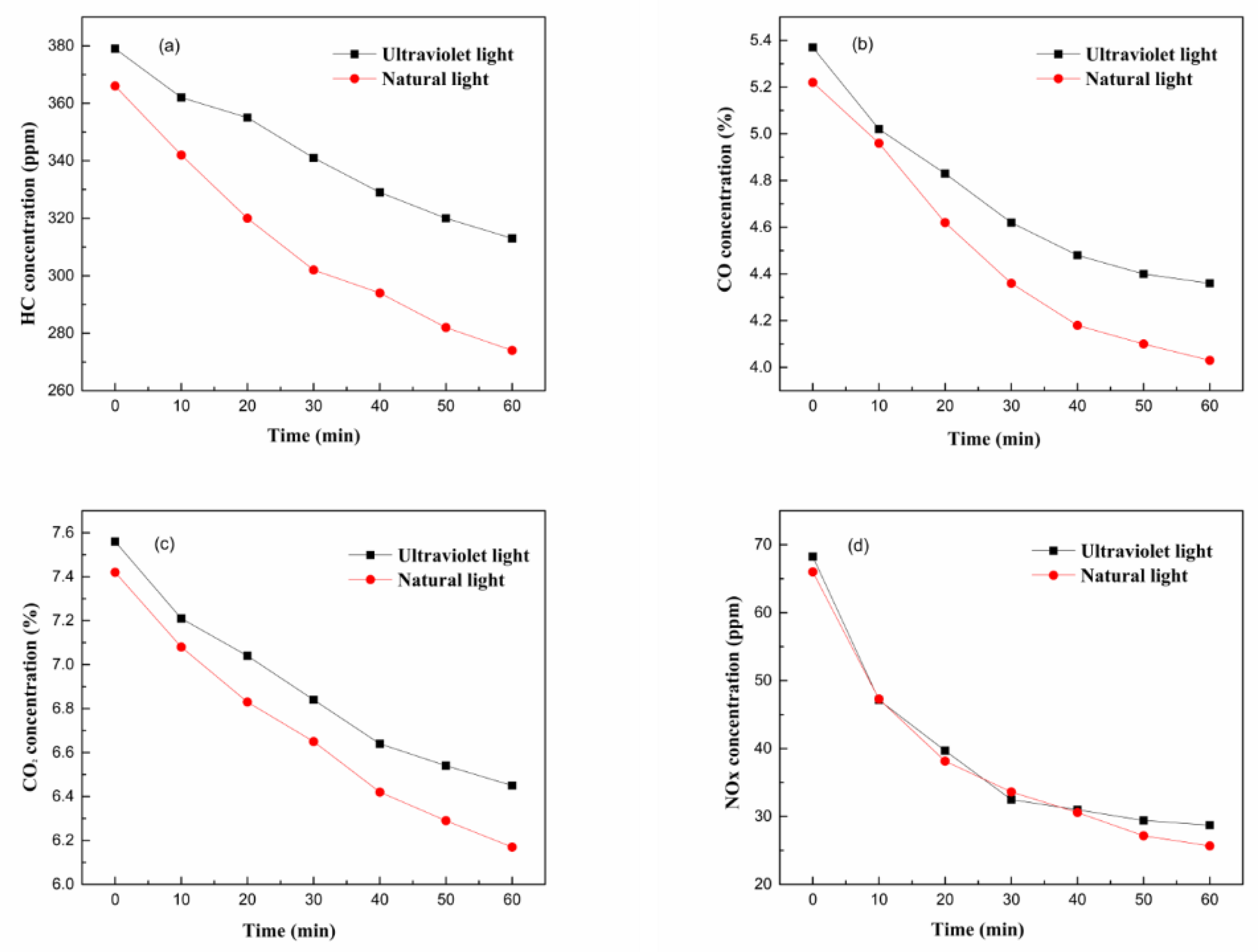
3.2. Degradation Efficiency of g-C3N4/CeO2
3.3. Micro Analysis
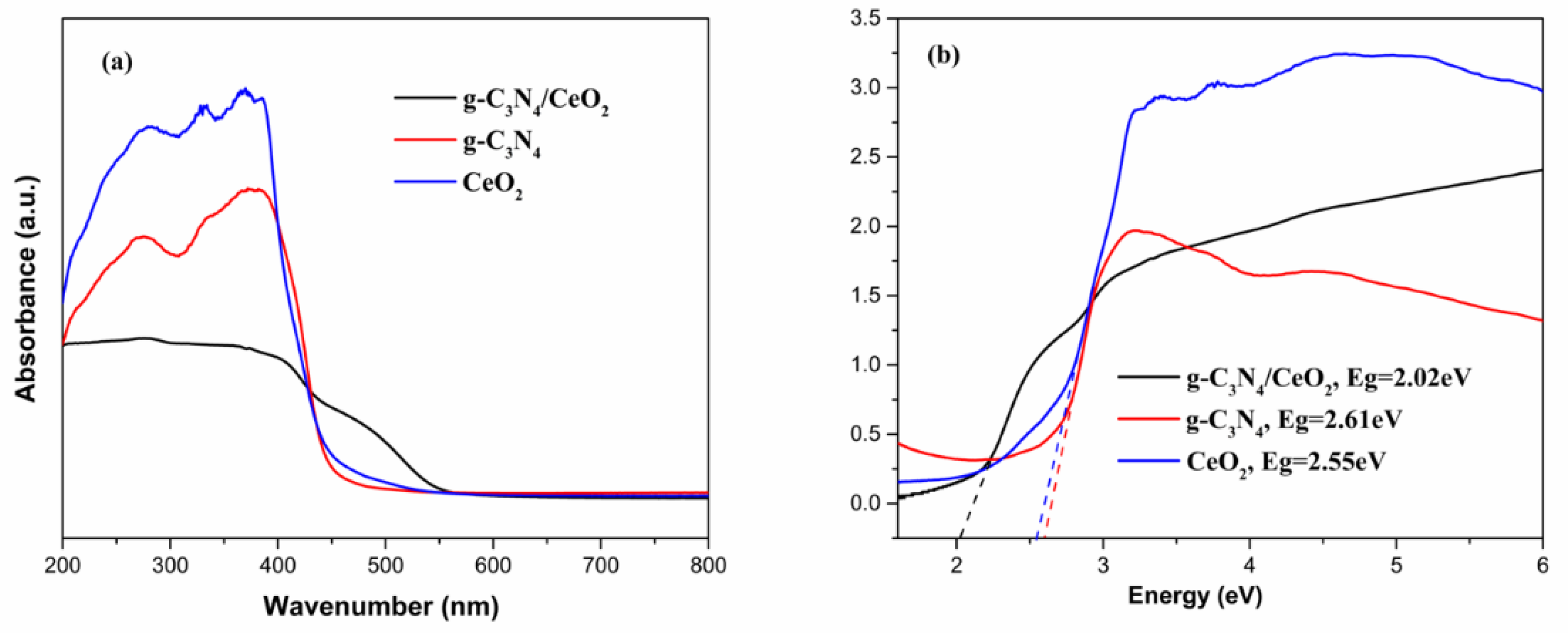
3.3.1. UV-Vis Analysis
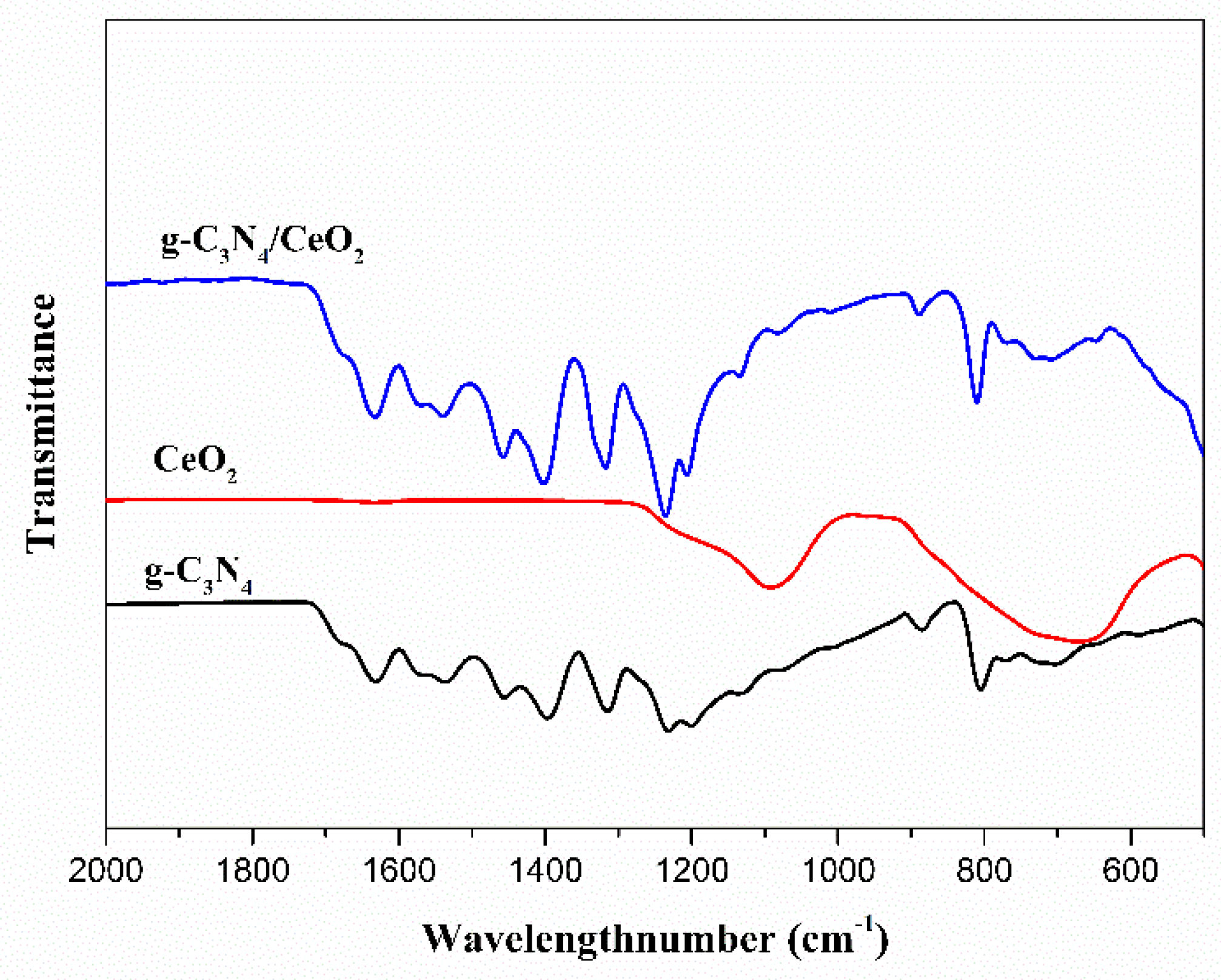
3.3.2. FI-TR Analysis
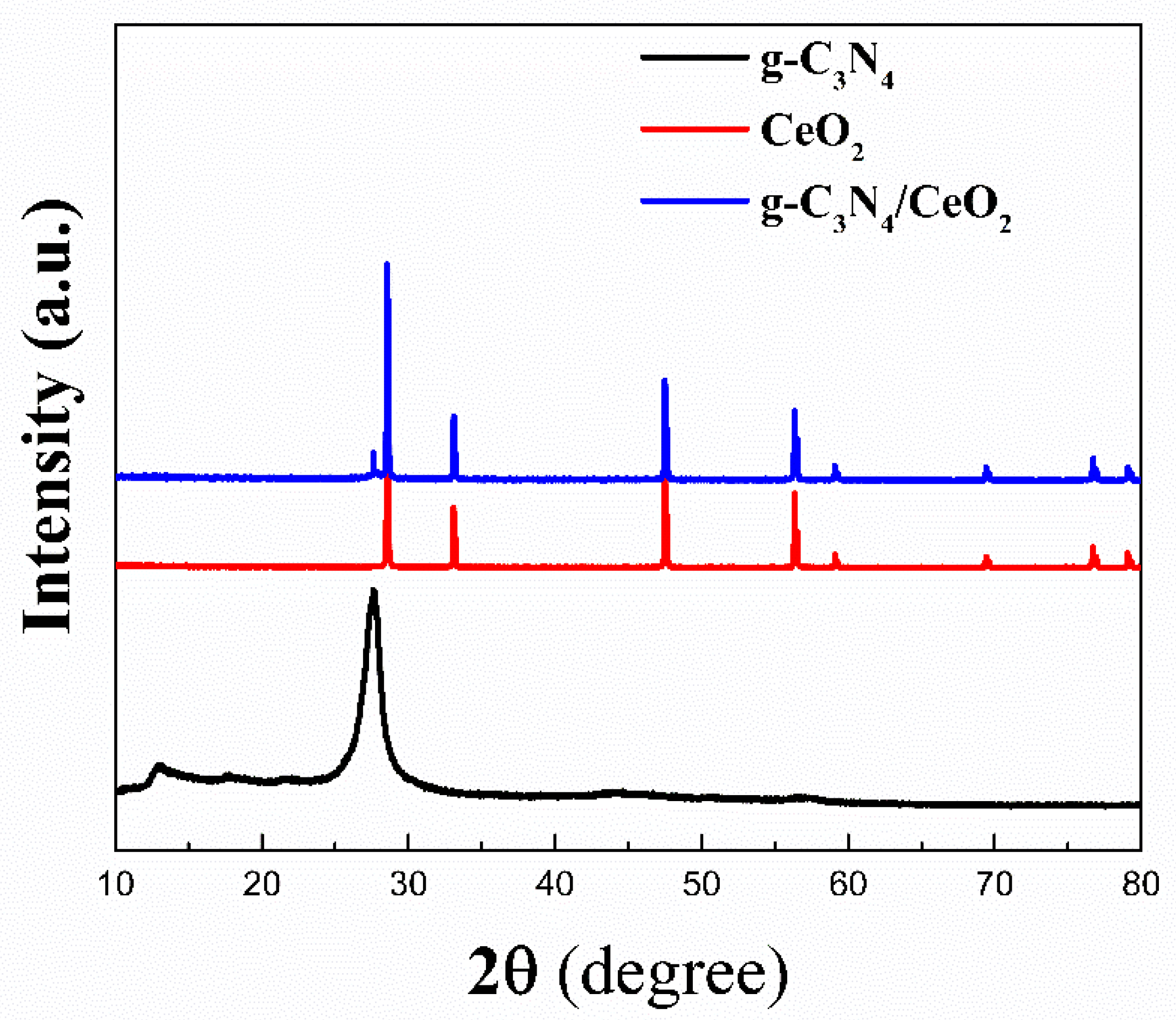
3.3.3. XRD
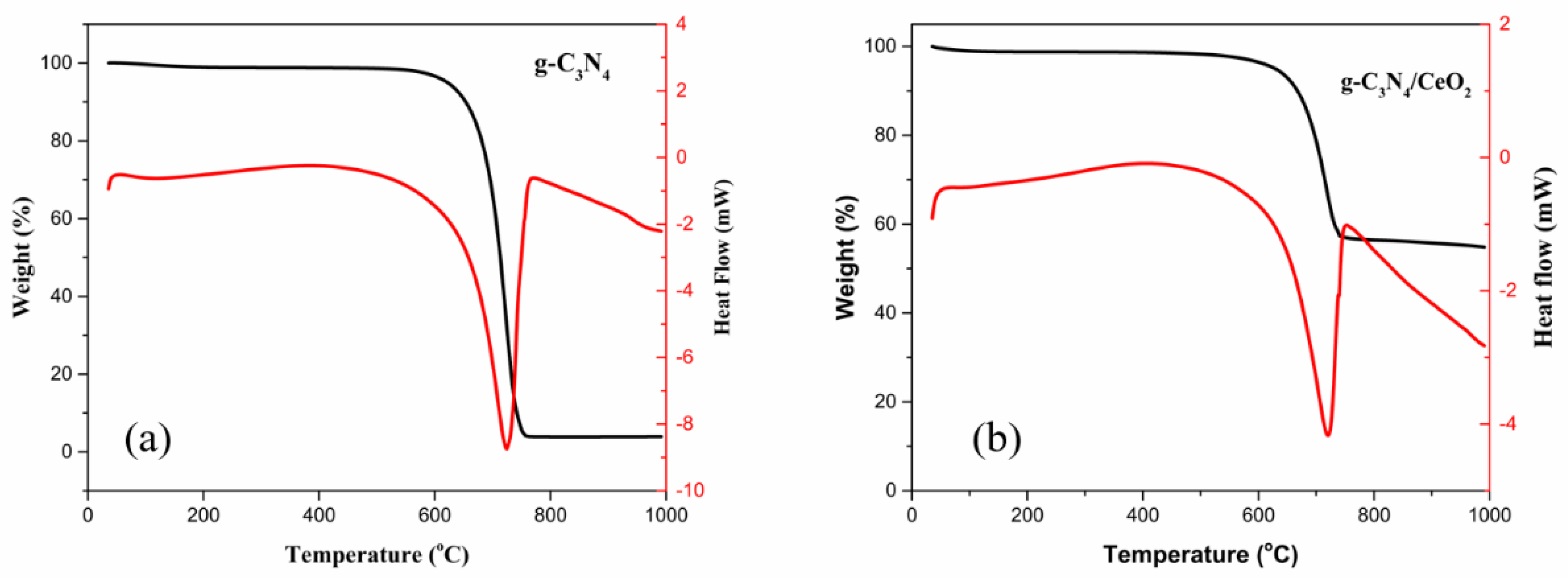
3.3.4. TG-DSC
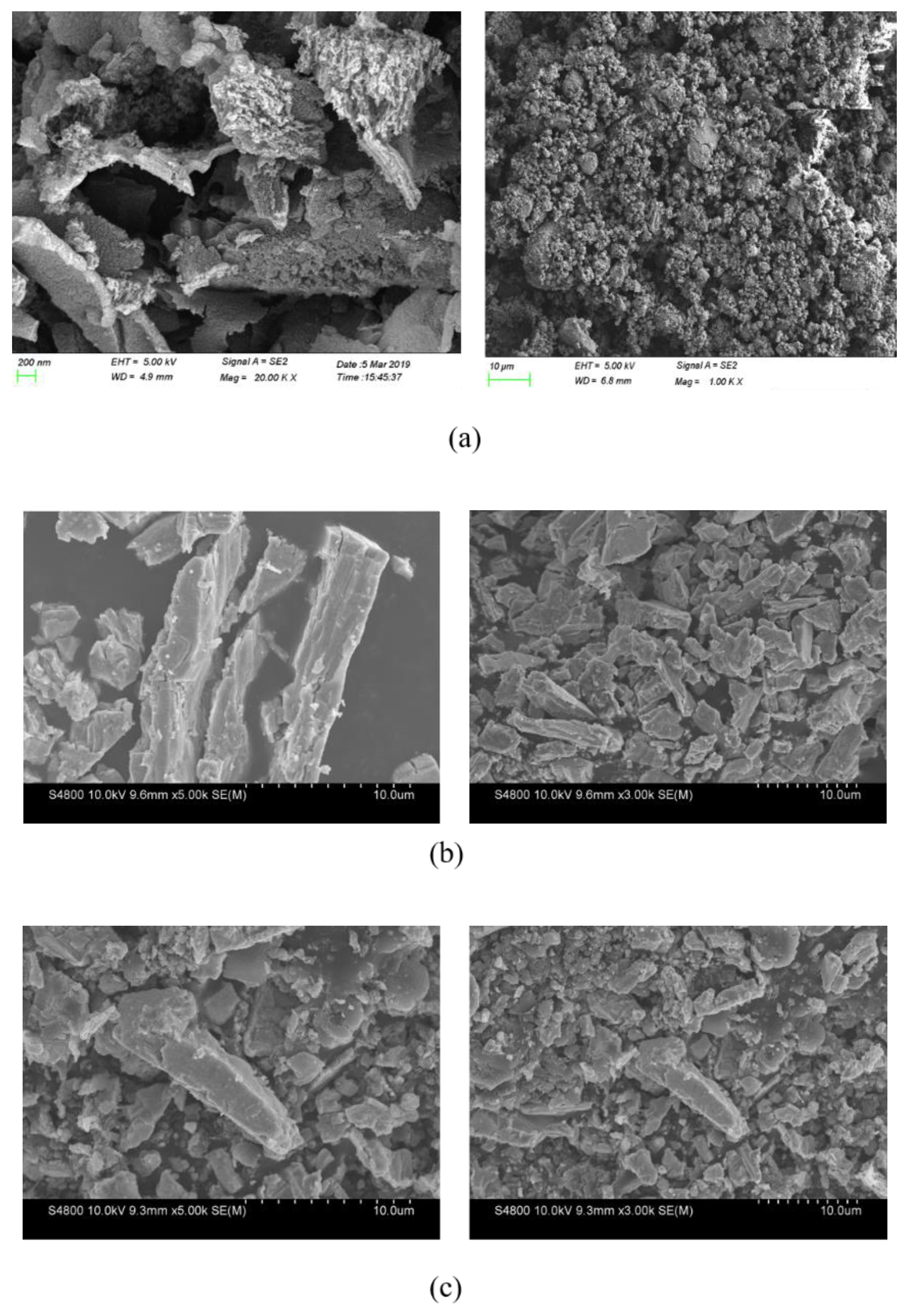
3.3.5. SEM
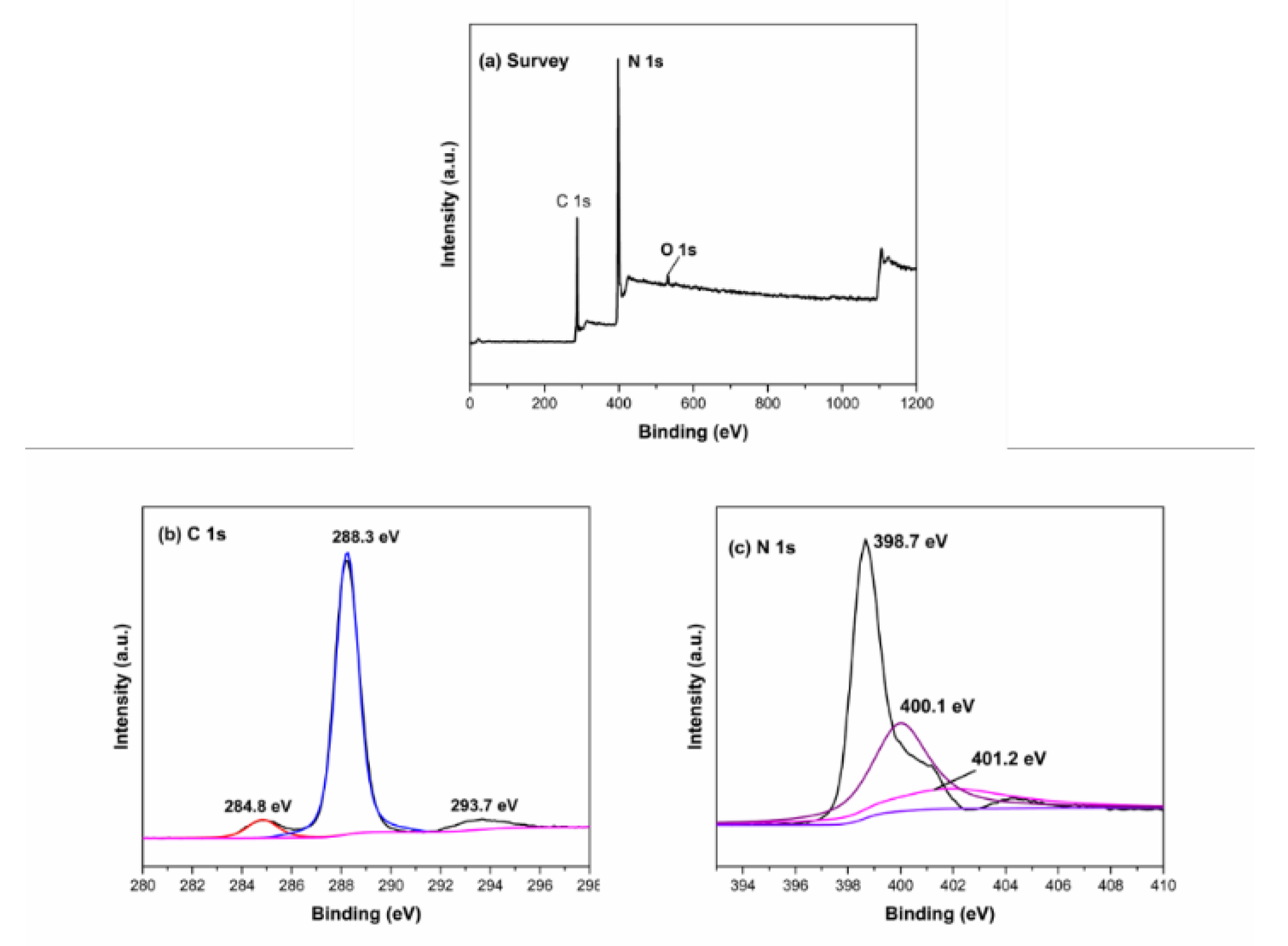
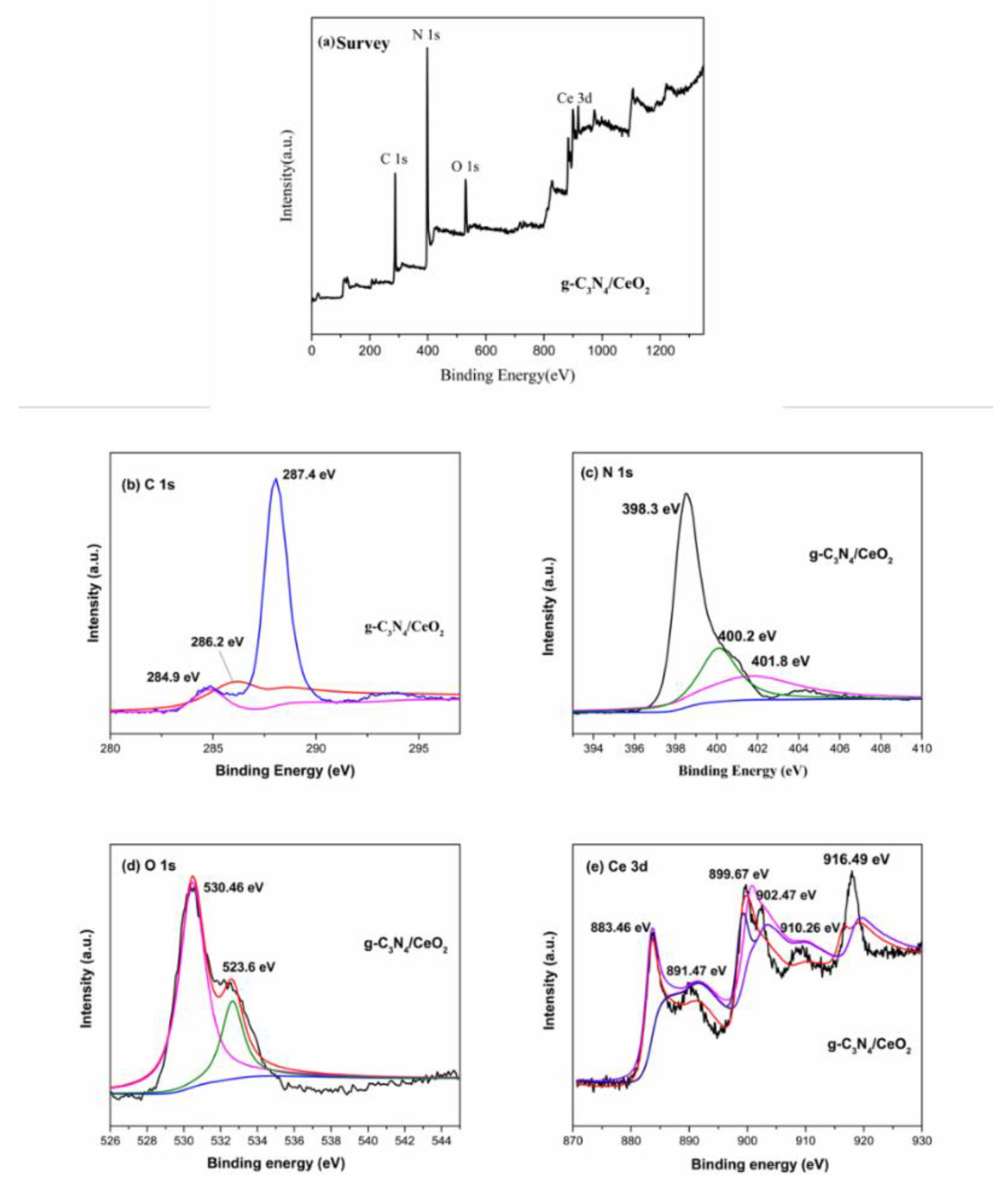
3.3.6. XPS
4. Conclusions
- (a)
- The exhaust gas purification efficiency of the g-C3N4/CeO2 composite under natural light irradiation was higher than that under ultraviolet light. The best mass ratio preparation of g-C3N4/CeO2 was 0.75.
- (b)
- The degradation efficiencies of the g-C3N4/CeO2 composite for HC, CO, CO2 and NOX in 60 min were 7.59%, 12.10%, 8.25% and 36.82%, respectively. Under natural light irradiation, the degradation efficiencies for HC, CO, CO2 and NOX in the 60 min were 15.88%, 16.22%, 10.45% and 40.58%, respectively.
- (c)
- The microstructure characterized the crystal structure and micro-morphology of g-C3N4/CeO2 composite. This indicated that g-C3N4/CeO2 heterostructure nanocomposite was successfully prepared.
Author Contributions
Funding
Conflicts of Interest
References
- Dong, F.; Sun, Y.J.; Zhang, Y.X. Micro-Nano Structure Regulation of Graphite Phase C3N4 and Application of Photocatalytic Environmental Purification, 1st ed.; Science Press: Beijing, China, 2018. [Google Scholar]
- Smith, T.W.; Axon, C.J.; Darton, R.C. The impact on human health of car-related air pollution in the UK, 1995–2005. Atmos. Environ. 2013, 77, 260–266. [Google Scholar] [CrossRef]
- Dong, P.Y.; Xi, X.G. Visible Light-Responsive Photocatalytic Material, 2nd ed.; Science Press: Beijing, China, 2017. [Google Scholar]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.S.; Zhao, X.J. Heterogeneous Photocatalysis in Semiconductors: Research and Experimental Demonstration of Thermodynamic Mechanism, 1st ed.; Science Press: Beijing, China, 2013. [Google Scholar]
- Cui, S.C.; Li, R.; Ma, W.S.; Li, M.M.; Cui, J.X.; Pei, J.Z. Preparation, photocatalytic properties investigation and degradation rate study on nano-TiO2 aerogels doped with Fe3+ for automobile emission purification. Mater. Res. Express 2018, 5, 115513. [Google Scholar] [CrossRef]
- Chen, Y.F.; Tang, X.N.; Zhang, B.; Luo, Y.; Li, Y. TiO2@SiO2 Composites: Preparation and Photocatalytic Antimicrobial Performance. J. Inorg. Mater. 2019, 34, 1325–1333. [Google Scholar] [CrossRef]
- Ton, N.Q.T.; Le, T.N.T.; Kim, S.; Dao, V.A.; Yi, J.; Vu, T.H.T. High-Efficiency Photo-Generated Charges of ZnO/TiO2 Heterojunction Thin Films for Photocatalytic and Antibacterial Performance. J. Nanosci. Nanotechnol. 2020, 20, 2214–2222. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.M.; Wang, G.L.; Li, L.J.; Wang, C.; Zhang, X.F. Fabrication of black TiO2/TiO2 homojunction for enhanced photocatalytic degradation. J. Mater. Sci. 2019, 54, 14320–14329. [Google Scholar] [CrossRef]
- Tao, W.; Wang, M.K.; Ali, R. Multi-layered porous hierarchical TiO2/g-C3N4 hybrid coating for enhanced visible light photocatalysis. Appl. Surf. Sci. 2019, 495, 143435. [Google Scholar] [CrossRef]
- Haga, Y.; An, H.; Yosomiya, R. Photoconductive properties of TiO2 films prepared by the sol–gel method and its application. J. Mater. Sci. 1997, 32, 3183–3188. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Gong, M.D.; Liu, X.; Ji, L.J.; Yang, Z.L.; Zhu, X.S. Preparation of activated carbon nanotube foams loaded with Ag-doped TiO2 for highly efficient photocatalytic degradation under UV and visible light. J. Mater. Sci. 2019, 54, 975–2989. [Google Scholar] [CrossRef]
- Ma, L.P.; Gu, Y.S.; Duan, Z.J.; Yuan, L.; Pang, S.J. Scanning tunneling microscopy investigation of carbon nitride thin films grown by microwave plasma chemical vapor deposition. Thin Solid Films 1999, 349, 10–13. [Google Scholar] [CrossRef]
- Montigaud, H.; Tanguy, B.; Demazeau, G.; Alves, I.; Courjault, S. C3N4: Dream or reality? Solvothermal synthesis as macroscopic samples of the C3N4 graphitic form. J. Mater. Sci. 2000, 35, 2547–2552. [Google Scholar] [CrossRef]
- Guo, Q.X.; Xie, Y.; Wang, X.J.; Lv, S.C.; Hou, T.; Liu, X.M. Characterization of well-crystallized graphitic carbon nitride nanocrystallites via a benzene-thermal route at low temperatures. Chem. Phys. Lett. 2003, 308, 84–87. [Google Scholar] [CrossRef]
- Wang, X.C.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Naseri, A.; Samadi, M.; Pourjavadi, A.; Moshfegh, A.Z.; Ramakrishna, S. Graphitic carbon nitride (g-C3N4)-based photocatalysts for solar hydrogen generation: Recent advances and future development directions. J. Mater. Chem. A 2017, 5, 23406–23433. [Google Scholar] [CrossRef]
- Sun, S.D.; Gou, X.F.; Tao, S.S.; Cui, J.; Li, J.; Yang, Q.; Liang, S.H.; Yang, Z.M. Mesoporous graphitic carbon nitride (g-C3N4) nanosheets synthesized from carbonated beverage-reformed commercial melamine for enhanced photocatalytic hydrogen evolution. Mater. Chem. Front. 2019, 3, 597–605. [Google Scholar] [CrossRef]
- Jourshabani, M.; Shariatinia, Z.; Badiei, A. In situ fabrication of SnO2/S-doped g-C3N4 nanocomposites and improved visible light driven photodegradation of methylene blue. J. Mol. Liq. 2017, 248, 688–702. [Google Scholar] [CrossRef]
- Jia, J.K.; Jiang, C.Y.; Zhang, X.R.; Li, P.J.; Xiong, J.X.; Zhang, Z.; Wu, T.; Wang, Y.P. Urea-modified carbon quantum dots as electron mediator decorated g-C3N4/WO3 with enhanced visible-light photocatalytic activity and mechanism insight. Appl. Surf. Sci. 2019, 498, 143524. [Google Scholar] [CrossRef]
- Tu, X.M.; Zhou, R.T.; Guo, H.L.; Tao, W.Y. Heterojunction semiconductor g-C3N4/BiVO4 with an enhanced photocatalytic activity based on the effective chemical bonding. J. Mater. Technol. 2019, 34, 827–837. [Google Scholar] [CrossRef]
- Yan, H.; Zhu, Z.W.; Long, Y.M.; Li, W.F. Single-source-precursor-assisted synthesis of porous WO3/g-C3N4 with enhanced photocatalytic property. J. Colloid Surf. A 2019, 582, 123857. [Google Scholar] [CrossRef]
- Li, T. Preparation and Electrochemical Performance of Cerium Oxide-Based Composites. Master’s Thesis, Shaanxi University of Science and Technology, Xi’an, China, 30 May 2018. [Google Scholar]
- He, L.Y.; Su, Y.M.; Jiang, L.H.; Shi, S.K. Recent advances of cerium oxide nanoparticles in synthesis. luminescence and biomedical studies: A review. J. Rare Earth 2015, 33, 791–799. [Google Scholar] [CrossRef]
- Xie, X.W.; Li, Y.; Liu, Z.Q.; Haruta, M.; Shen, W.J. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 2009, 7239, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, W.; Feng, X.L.; Liu, D.P.; Zhang, Y. Decoration of Pt on Cu/Co double-doped CeO2 nanospheres and their greatly enhanced catalytic activity. Chem. Sci. 2016, 7, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Dhal, G.C.; Mohan, D.; Prasad, R. Application of hopcalite catalyst for controlling carbon monoxide emission at cold-start emission conditions. J. Traffic Transp. Eng. 2019, 6, 419–440. [Google Scholar] [CrossRef]
- Zhang, J.S.; Wang, B.; Wang, X.C. Carbon nitride polymeric semiconductor for photocatalysis. Prog. Chem. 2014, 26, 19–29. [Google Scholar]
- Cui, S.C.; Li, R.; Zhu, C.D.; Pei, J.Z.; Wen, Y. Cerium-bismuth solid solution material prepared and application in automobile exhaust purification. Sep. Purif. Technol. 2020, 239, 116520. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Zhou, C.; Jin, Y.R.; Jing, Q.Y.; Yang, Y.L.; Shen, X.; Tang, Q.; Mu, Y.H.; Du, A.K. Facile synthesis of 3D porous thermally exfoliated g-C3N4 nanosheet with enhanced photocatalytic degradation of organic dye. J. Colloid Interface Sci. 2016, 468, 211–219. [Google Scholar] [CrossRef]
- Makula, P.; Pacia, M.; Macyk, W. How to Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Cui, P.P.; Hu, Y.; Zheng, M.M.; Wei, C.H. Enhancement of visible-light photocatalytic activities of BiVO4 coupled with g-C3N4 prepared using different precursors. Environ. Sci. Pollut. Res. 2018, 25, 32466–32477. [Google Scholar] [CrossRef]
- Cui, S.C.; Li, R.; Pei, J.Z.; Wen, Y.; Li, Y.; Xing, X.Y. Automobile exhaust purification over g-C3N4 catalyst material. Mater. Chem. Phys. 2020, 112867. [Google Scholar]

| Device Name | Model | Factory |
|---|---|---|
| Alumina crucible | Φ100 mL | Shanghai Kesheng Ceramics Co., Ltd. (Shanghai, China) |
| Electronic balance | FA2004B | Shanghai Jingke Tianmei Scientific Instrument Co., Ltd. (Shanghai, China) |
| Muffle furnace | 100 mL | Shanghai Pudong Physical Optical Instrument Factory (Shanghai, China) |
| Planetary ball mill | 450 rpm | Changsha Miqi Equipment Co., Ltd. (Changsha, China) |
| Magnetic stirrer | 0–2400 r/min | Changzhou Deke Instrument Manufacturing Co., Ltd. (Changzhou, China) |
| Electric centrifuge | 0–4000 rpm | Jintan District Xicheng Xinrui Instrument Factory (Changzhou, China) |
| Reagent Name | Model | Factory |
|---|---|---|
| Dicyandiamide | AR | Tianjin Fuchen Chemical Reagent Factory (Tianjin, China) |
| Cerium Oxide | AR | Tianjin Kemiou Chemical Reagent Co., Ltd. (Tianjin, China) |
| Exhaust Gas Composition | 0–10 min | 10–20 min | 20–30 min | 30–40 min | 40–50 min | 50–60 min |
|---|---|---|---|---|---|---|
| HC (ppm) | 10 | 6 | 8 | 6 | 6 | 5 |
| CO (%) | 0.1 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
| CO2 (%) | 0.1 | 0.1 | 0.1 | 0.1 | 0.08 | 0.08 |
| NOX (ppm) | 8 | 5 | 6 | 2 | 1 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, S.; Xie, B.; Li, R.; Pei, J.; Tian, Y.; Zhang, J.; Xing, X. g-C3N4/CeO2 Binary Composite Prepared and Its Application in Automobile Exhaust Degradation. Materials 2020, 13, 1274. https://doi.org/10.3390/ma13061274
Cui S, Xie B, Li R, Pei J, Tian Y, Zhang J, Xing X. g-C3N4/CeO2 Binary Composite Prepared and Its Application in Automobile Exhaust Degradation. Materials. 2020; 13(6):1274. https://doi.org/10.3390/ma13061274
Chicago/Turabian StyleCui, Shengchao, Baowen Xie, Rui Li, Jianzhong Pei, Yefei Tian, Jiupeng Zhang, and Xiangyang Xing. 2020. "g-C3N4/CeO2 Binary Composite Prepared and Its Application in Automobile Exhaust Degradation" Materials 13, no. 6: 1274. https://doi.org/10.3390/ma13061274
APA StyleCui, S., Xie, B., Li, R., Pei, J., Tian, Y., Zhang, J., & Xing, X. (2020). g-C3N4/CeO2 Binary Composite Prepared and Its Application in Automobile Exhaust Degradation. Materials, 13(6), 1274. https://doi.org/10.3390/ma13061274





