Failure Analysis of Retrieved Osteosynthesis Implants
Abstract
1. Introduction
2. Materials and Methods
3. Results
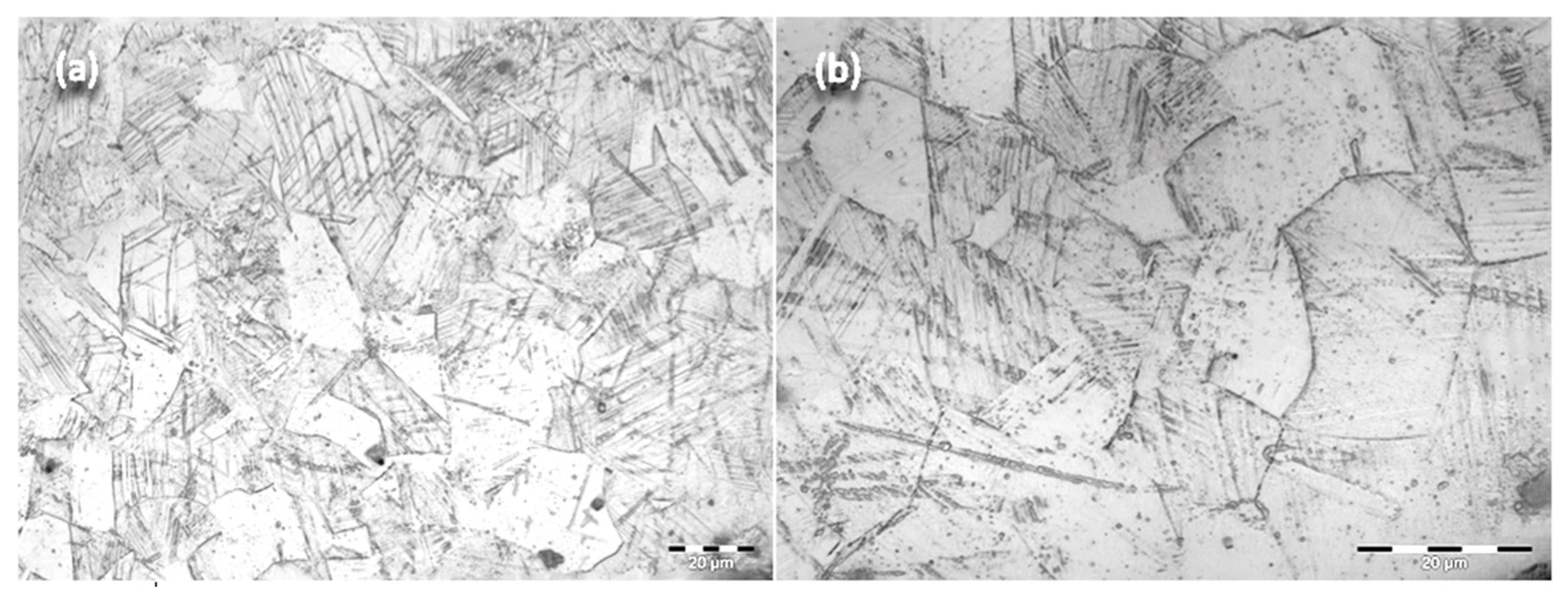
3.1. Metallographic Analysis
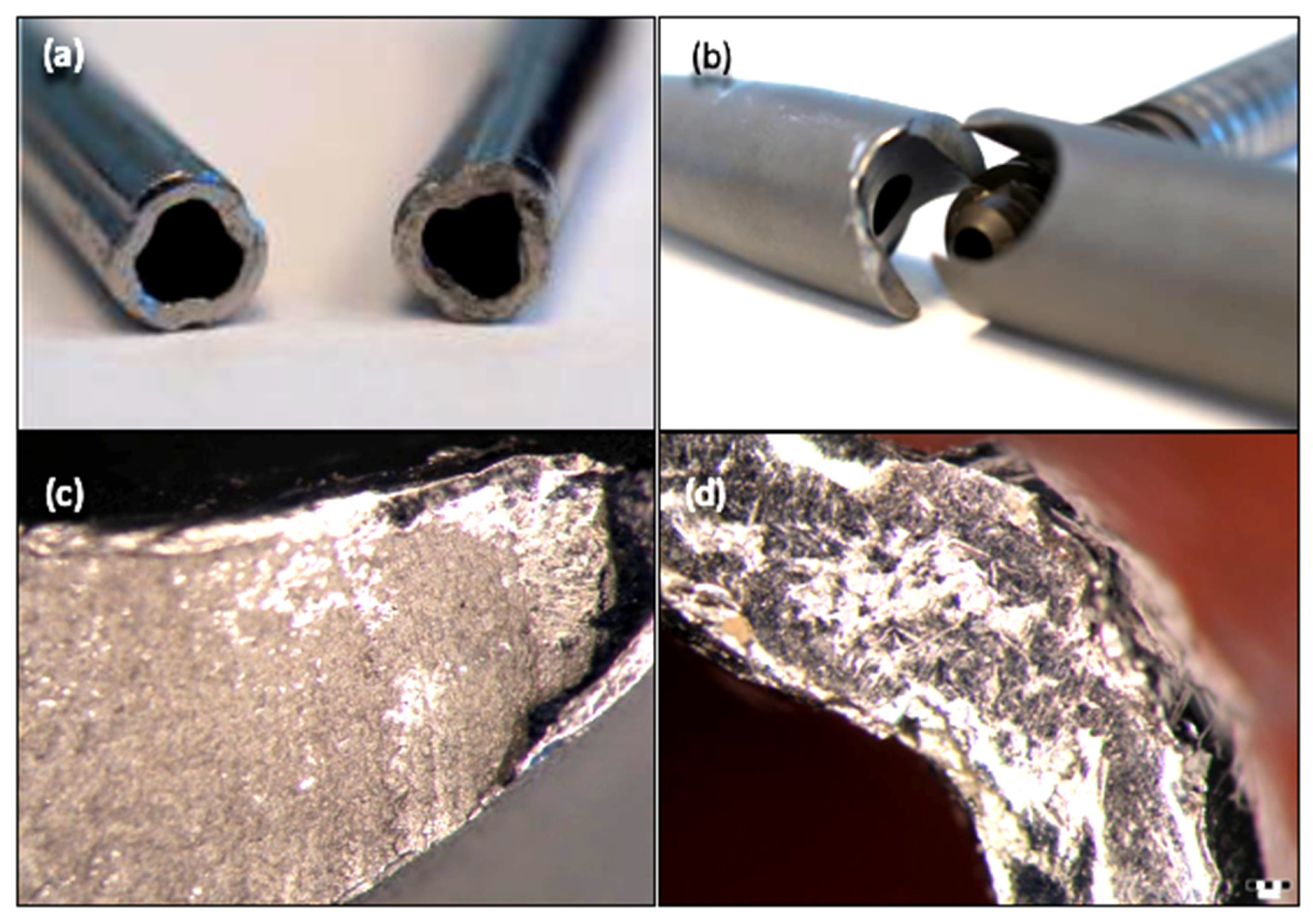
3.2. Stereomicroscopy
3.3. Scanning Electron Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marinescu, R.; Antoniac, V.I.; Stoia, D.I.; Lăptoiu, D.C. Clavicle anatomical osteosynthesis plate breakage-failure analysis report based on patient morphological parameters. Romanian J. Morphol Embryol 2017, 58, 593–598. [Google Scholar]
- Atasiei, T.; Antoniac, I.; Laptoiu, D. Failure causes in hip resurfacing arthroplasty–retrieval analysis. Int. J. Nano Biomater. 2011, 3, 367–381. [Google Scholar] [CrossRef]
- Bane, M.; Miculescu, F.; Blajan, A.I.; Dinu, M.; Antoniac, I. Failure analysis of some retrieved orthopedic implants based on materials characterization. Solid State Phenom. 2012, 188, 114–117. [Google Scholar] [CrossRef]
- Grecu, D.; Antoniac, I.; Trante, O.; Niculescu, N.; Lupescu, O. Failure analysis of retrieved polyethylene insert in total knee replacement. Mat. Plast. 2016, 53, 776–780. [Google Scholar]
- Antoniac, I.V.; Stoia, D.I.; Ghiban, B.; Tecu, C.; Miculescu, F.; Vigaru, C.; Saceleanu, V. Failure analysis of a humeral shaft locking compression plate—Surface investigation and simulation by finite element method. Materials 2019, 12, 1128. [Google Scholar] [CrossRef]
- Mavrodin, C.I.; Pariza, G.; Ion, V.; Antoniac, V.I. Abdominal compartment syndrome—a major complication of large incisional hernia surgery. Chirurgia 2013, 108, 414–417. [Google Scholar]
- Gradinaru, S.; Tabaras, D.; Gheorghe, D.; Gheorghita, D.; Zamfir, R.; Vasilescu, M.; Dobrescu, M.; Grigorescu, G.; Cristescu, I. Analysis of the Anisotropy for 3D Printed PLA Parts Usable in Medicine. U.P.B. Sci. Bull. Series B Chem. Mater. Sci. 2019, 81, 313–324. [Google Scholar]
- Dumitrescu, D.; Savlovschi, C.; Borcan, R.; Pantu, H.; Serban, D.; Gradinaru, S.; Smarandache, G.; Trotea, T.; Branescu, C.; Musat, L.; et al. Clinical case-voluminous diaphragmatic hernia-surgically acute abdomen: Diagnostic and therapeutical challenges. Chirurgia 2011, 106, 657–660. [Google Scholar]
- Cosmin, B.; Iulian, A.; Florin, M.; Marius, D.; Ionel, D. Investigation of a mechanical valve impairment after eight years of implantation. Key Eng. Mater. 2014, 583, 137–144. [Google Scholar]
- Cirstoiu, M.M.; Antoniac, I.; Ples, L.; Bratila, E.; Munteanu, O. Adverse reactions due to use of two intrauterine devices with different action mechanism in a rare clinical case. Mater. Plast. 2016, 53, 666–669. [Google Scholar]
- Cirstoiu, M.; Cirstoiu, C.; Antoniac, I.; Munteanu, O. Levonorgestrel-releasing intrauterine systems: Device design, biomaterials, mechanism of action and surgical technique. Mater. Plast. 2016, 52, 258–262. [Google Scholar]
- Brătilă, E.; Comandasu, D.; Milea, C.; Berceanu, C.; Vasile, E.; Antoniac, I.; Mehedintu, C. Effect of the surface modification of the synthetic meshes used in the surgical treatment of pelvic organ prolapse on the tissue adhesion and clinical functionality. J. Adhes. Sci. Technol. 2017, 31, 2028–2043. [Google Scholar] [CrossRef]
- Cavalu, S.; Antoniac, I.V.; Fritea, L.; Mates, I.M.; Milea, C.; Laslo, V.; Vicas, S.; Mohan, A. Surface modifications of the titanium mesh for cranioplasty using selenium nanoparticles coating. J. Adhes. Sci. Technol. 2018, 32, 2509–2522. [Google Scholar] [CrossRef]
- Rivis, M.; Pricop, M.; Talpos, S.; Ciocoiu, R.; Antoniac, I.; Gheorghita, D.; Trante, O.; Moldovan, H.; Grigorescu, G.; Seceleanu, V.; et al. Influence of the bone cements processing on the mechanical properties in cranioplasty. Rev. Chim. 2018, 69, 990–993. [Google Scholar] [CrossRef]
- Antoniac, I.V.; Burcea, M.; Ionescu, R.D.; Balta, F. IOL’s opacificatiofn: A complex analysis based on the clinical aspects, biomaterials used and surface characterization of explanted IOL’s. Mater. Plast. 2015, 52, 109–112. [Google Scholar]
- Gabor, A.; Zaharia, C.; Todericiu, V.; Szuhanek, C.; Cojocariu, A.C.; Duma, V.F.; Sticlaru, C.; Negrutiu, M.L.; Antoniav, I.V.; Sinescu, C. Adhesion of scaffolds with implants to the mandibular bone with a defect. Mater. Plast. 2018, 55, 393–397. [Google Scholar] [CrossRef]
- Corobea, M.S.; Albu, M.G.; Ion, R.; Cimpean, A.; Miculescu, F.; Antoniac, I.V.; Raditoiu, V.; Sirbu, I.; Stoenescu, M.; Voicu, S.; et al. Modification of titanium surface with collagen and doxycycline as a new approach in dental implants. J. Adhes. Sci. Technol. 2015, 29, 2537–2550. [Google Scholar] [CrossRef]
- Ionescu, R.; Mardare, M.; Dorobantu, A.; Vermesan, S.; Marinescu, E.; Saban, R.; Antoniac, L.; Ciocan, D.N.; Ceausu, M. Correlation between materials, design and clinical issues in the case of associated use of different stainless steels as implant materials. Key Eng. Mater. 2014, 583, 41–44. [Google Scholar] [CrossRef]
- Perren, S.M. Physical and biological aspects of fracture healing with special reference to internal fixation. Clin. Orthop. Relat. Res. 1979, 138, 175–196. [Google Scholar]
- Panti, Z.; Cretu, B.; Panaitescu, C.; Nica, M.; Tecu, C.; Semenescu, A.; Ene, D.; Ene, R. Implant associated local recurrence in primary bone sarcoma. Rev. Chim. 2020, 71, 172–175. [Google Scholar] [CrossRef]
- Iulian, A.; Dan, L.; Camelia, T.; Claudia, M.; Sebastian, G. Synthetic materials for osteochondral tissue engineering. Osteochondral Tissue Eng. 2018, 1058, 31–52. [Google Scholar]
- Marinescu, R.; Antoniac, I.; Laptoiu, D.; Antoniac, A.; Grecu, D. Complications Related to Biocomposite Screw Fixation in ACL Reconstruction Based on Clinical Experience and Retrieval Analysis. Mater. Plast. 2015, 52, 340–344. [Google Scholar]
- Stefanescu, T.; Antoniac, I.V.; Popovici, R.A.; Galuscan, A.; Tirca, T. Ni-Ti rotary instrument fracture analysis after clinical use. Structure changes in used instruments. Environ. Eng. Manag. J. 2016, 15, 981–988. [Google Scholar]
- Wadood, A. Brief overview on nitinol as biomaterial. Adv. Mater. Sci. Eng. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Cirstoiu, C.; Ene, R.; Panti, Z.; Ene, P.; Cîrstoiu, M.-M. Particularities of shoulder recovery after arthroscopic bankart repair with bioabsorbable and metallic suture anchors. Mater. Plast. 2015, 52, 361–363. [Google Scholar]
- Guazzo, R.; Gardin, C.; Bellin, G.; Sbricoli, L.; Ferroni, L.; Ludovichetti, F.S.; Piattelli, A.; Antoniac, I.; Bressan, E.; Zavan, B. Graphene-Based Nanomaterials for Tissue Engineering in the Dental Field. Nanomaterials. 2018, 8, 349. [Google Scholar] [CrossRef] [PubMed]
- Pascoletti, G.; Cianetti, F.; Putame, G.; Terzini, M.; Zanetti, E.M. Numerical simulation of an intramedullary elastic nail: Expansion phase and load-bearing behavior. Front. Bioeng. Biotechnol. 2018, 6, 174. [Google Scholar] [CrossRef]
- Putame, G.; Pascoletti, G.; Franceschini, G.; Dichio, G.; Terzini, M. Prosthetic hip ROM from multibody software simulation. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 5386–5389. [Google Scholar]
- Gheorghe, D.; Pencea, I.; Antoniac, I.V.; Turcu, R.-N. Investigation of the Microstructure, Hardness and Corrosion Resistance of a New 58Ag24Pd11Cu2Au2Zn1.5In1.5Sn Dental Alloy. Materials 2019, 12, 4199. [Google Scholar] [CrossRef]
- Antoniac, I.; Negrusoiu, M.; Mardare, M.; Socoliuc, C.; Zazgyva, A.; Niculescu, M. Adverse local tissue reaction after 2 revision hip replacements for ceramic liner fracture: A case report. Medicine 2017, 96, e6687. [Google Scholar] [CrossRef]
- Buzatu, M.; Geanta, V.; Stefanoiu, R.; Buţu, M.; Petrescu, M.-I.; Buzatu, M.; Ghica, S.-I.; Antoniac, I.; Iacob, G.; Niculescu, F.; et al. Mathematical Modeling for Correlation of the Resistance to Compression with the Parameters Md, Bo and E/A, for the Design of Titanium Beta Alloys Developed for Medical Applications. U.P.B. Sci. Bull. Series B Chem. Mater. Sci. 2019, 81, 183–192. [Google Scholar]
- Aksakal, B.; Yildirim, Ö.S.; Gul, H. Metallurgical failure analysis of various implant materials used in orthopedic applications. J. Fail. Anal. Prev. 2004, 4, 17–23. [Google Scholar] [CrossRef]
- Ene, R.; Panti, Z.; Nica, M.; Pleniceanu, M.; Ene, P.; Cirstoiu, M.; Antoniac, V.I.; Cirstoiu, C. Mechanical failure of angle locking plates in distal comminuted tibial fractures. Key Eng. Mater. 2016, 695, 118–122. [Google Scholar] [CrossRef]
- Popescu, D.; Ene, R.; Popescu, A.; Cirstoiu, M.; Sinescu, R.; Cirstoiu, C. Total hip joint replacement in young male patient with osteoporosis, secondary to hypogonadotropic hypogonadism. Acta Endocrinol. Buchar. 2015, 11, 109–113. [Google Scholar] [CrossRef]
- Popa, M.; Ene, R.; Streinu-Cercel, A.; Popa, V.; Pleniceanu, M.; Nica, M.; Panti, Z.; Cirstoiu, M.; Cirstoiu, C. Algic syndrome in osteoarticular infectious pathology; detection and rapid treatment of the causative agent using microcalorimetry. In Proceedings of the 14th National Congress of Urogynecology and the National Conference of the Romanian Association for the Study of Pain, Eforie, Romania, 7–9 September 2017; pp. 589–594. [Google Scholar]
- Ene, R.; Panti, Z.A.; Nica, M.; Popa, M.G.; Cîrstoiu, M.M.; Munteanu, O.; Vasilescu, S.L.; Simion, G.; Vasilescu, A.; Daviţoiu, D.V.; et al. Chondrosarcoma of the pelvis–case report. Rom. J. Morphol. Embryol. 2018, 59, 927–931. [Google Scholar]
- Álvarez, D.B.; Aparicio, J.P.; Fernandez, E.L.; Mugica, I.G.; Batalla, D.N.; Jiménez, J.P. Implant breakage, a rare complication with the Gamma nail. A review of 843 fractures of the proximal femur treated with a Gamma nail. Acta Orthop. Belg. 2004, 70, 435–443. [Google Scholar]
- Shahgaldi, B.F.; Compson, J. Wear and corrosion of sliding counterparts of stainless-steel hip screw-plates. Injury 2000, 31, 85–92. [Google Scholar] [CrossRef]
- Proverbio, E.; Bonaccorsi, L.M. Microstructural analysis of failure of a stainless steel bone plate implant. Pract. Fail. Anal. 2001, 1, 33–38. [Google Scholar] [CrossRef]
- Walczak, J.; Shahgaldi, F.; Heatley, F. In vivo corrosion of 316L stainless-steel hip implants: Morphology and elemental compositions of corrosion products. Biomaterials 1998, 19, 229–237. [Google Scholar] [CrossRef]
- ISO 12891–1:1999(E)—Retrieval and analysis of surgical implants—Part 1: Retrieval and handling; International Organization for Standardization: Geneva, Switzerland, 1999.
- ISO 12891–2:2000(E)—Retrieval and analysis of surgical implants—Part 2: Analysis of retrieved metallic surgical implants; International Organization for Standardization: Geneva, Switzerland, 2000.
- Azevedo, C.R.F.; Hippert Jr, E.D.U.A.R.D.O. Failure analysis of surgical implants in Brazil. Eng. Fail. Anal. 2002, 9, 621–633. [Google Scholar] [CrossRef]
- Buzatu, M.; Geanta, V.; Stefanoiu, R.; Butu, M.; Petrescu, M.I.; Buzatu, M.; Antoniac, I.; Iacob, G.; Niculescu, F.; Ghica, S.I.; et al. Investigations into Ti-15Mo-W alloys developed for medical applications. Materials 2019, 12, 147. [Google Scholar] [CrossRef]

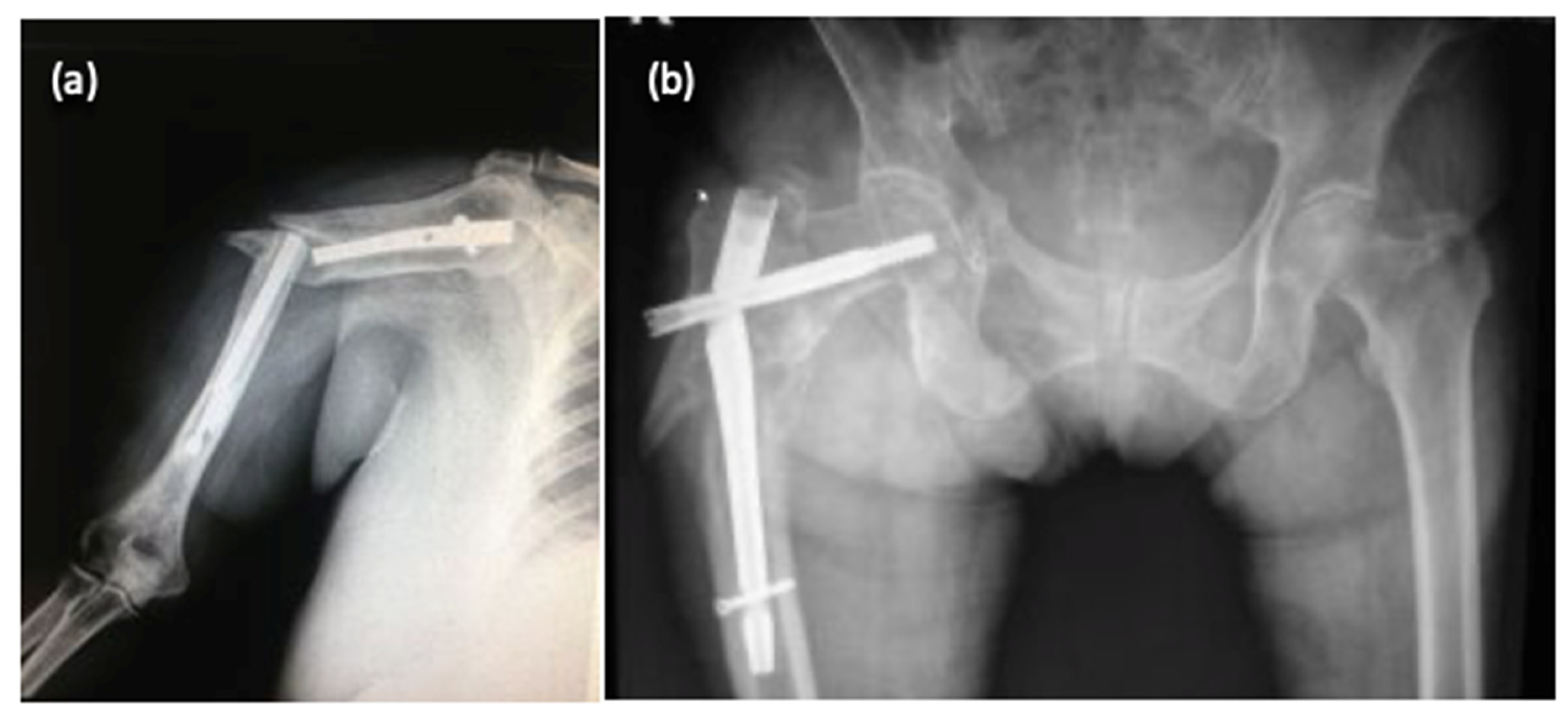

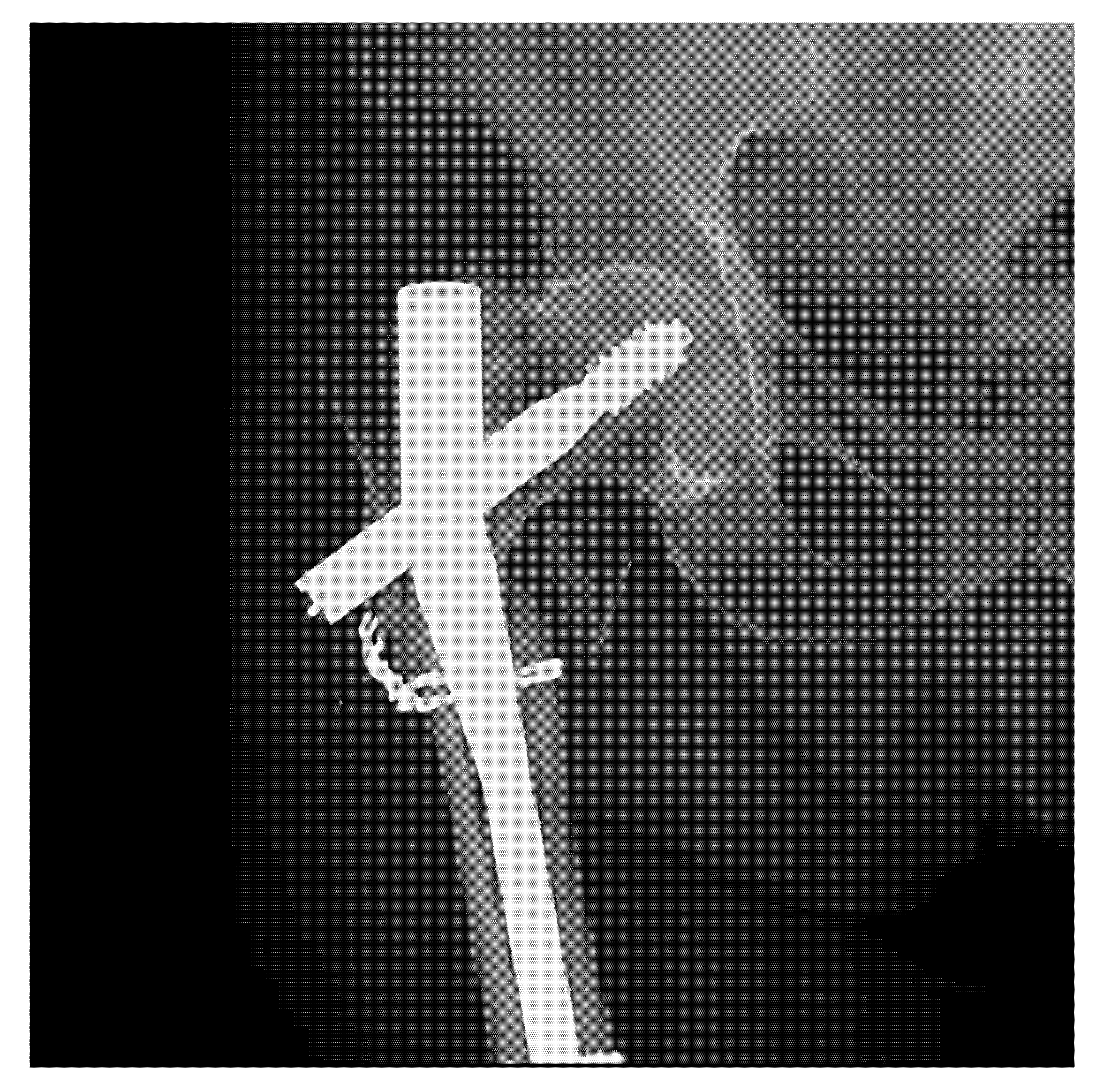
| Radiographic Images (Multiple Views) | Implant Length | Implant Diameter |
|---|---|---|
| Bone morphology | Contralateral bone x-rays (magnified) | IM canal isthmus (the narrowest portion of the canal) |
| Shape of the intramedullary (IM) canal | Traction radiographs | 1.0 to 1.5 mm greater than anticipated IM nail diameter |
| Fracture pattern and comminution | Distance between palpable bony landmarks |
| No. | Patient Age | Fracture Location (Bone Type) | Type of Implant | Failure Causes | ||||
|---|---|---|---|---|---|---|---|---|
| External Factors (Traumatic Event) | Surgical Causes | Implant Defects | ||||||
| Inadequate Implant Size | Deficient Fracture Reduction | Materials Defects | Surface Defects | |||||
| 1 | 36 | Tibia | IMN | (+) | ||||
| 2 | 65 | Femur | IMN | (+) | (+) | |||
| 3 | 72 | Femur | GN | (+) | ||||
| 4 | 26 | Humerus | PS | (+) | ||||
| 5 | 44 | Tibia | IMN | (+) | (+) | (+) | (+) | |
| 6 | 31 | Tibia | PS | (+) | (+) | (+) | ||
| 7 | 71 | Femur | GN | (+) | ||||
| 8 | 67 | Femur | DHS | (+) | (+) | |||
| 9 | 39 | Humerus | IMN | (+) | (+) | |||
| 10 | 78 | Femur | DHS | (+) | ||||
| 11 | 46 | Tibia | IMN | (+) | (+) | (+) | ||
| 12 | 42 | Humerus | PS | (+) | (+) | |||
| 13 | 69 | Femur | DHS | (+) | ||||
| 14 | 18 | Humerus | IMN | (+) | ||||
| 15 | 68 | Femur | DHS | |||||
| 16 | 32 | Humerus | PS | (+) | (+) | |||
| 17 | 74 | Femur | GN | (+) | ||||
| 18 | 59 | Femur | DHS | (+) | ||||
| 19 | 46 | Humerus | IMN | (+) | (+) | |||
| 20 | 55 | Tibia | IMN | (+) | ||||
| 21 | 69 | Femur | DHS | (+) | ||||
| IMN=Intramedullary Nail; GN=Gamma Nail; DHS=Dynamic Hip Screw; PS=Plate-Screw System | ||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nica, M.; Cretu, B.; Ene, D.; Antoniac, I.; Gheorghita, D.; Ene, R. Failure Analysis of Retrieved Osteosynthesis Implants. Materials 2020, 13, 1201. https://doi.org/10.3390/ma13051201
Nica M, Cretu B, Ene D, Antoniac I, Gheorghita D, Ene R. Failure Analysis of Retrieved Osteosynthesis Implants. Materials. 2020; 13(5):1201. https://doi.org/10.3390/ma13051201
Chicago/Turabian StyleNica, Mihai, Bogdan Cretu, Dragos Ene, Iulian Antoniac, Daniela Gheorghita, and Razvan Ene. 2020. "Failure Analysis of Retrieved Osteosynthesis Implants" Materials 13, no. 5: 1201. https://doi.org/10.3390/ma13051201
APA StyleNica, M., Cretu, B., Ene, D., Antoniac, I., Gheorghita, D., & Ene, R. (2020). Failure Analysis of Retrieved Osteosynthesis Implants. Materials, 13(5), 1201. https://doi.org/10.3390/ma13051201






