Electronic Structure of Nitrogen- and Phosphorus-Doped Graphenes Grown by Chemical Vapor Deposition Method
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhong, Y.; Zhen, Z.; Zhu, H. Graphene: Fundamental research and potential applications. FlatChem 2017, 4, 20–32. [Google Scholar] [CrossRef]
- Brownson, D.A.C.; Banks, C.E. The electrochemistry of CVD graphene: Progress and prospects. Phys. Chem. Chem. Phys. 2012, 14, 8264–8281. [Google Scholar] [CrossRef] [PubMed]
- Kraus, J.; Böcklein, S.; Reichelt, R.; Günther, S.; Santos, B.; Menteş, T.O.; Locatelli, A. Towards the perfect graphene membrane?—Improvement and limits during formation of high quality graphene grown on Cu-foils. Carbon N. Y. 2013, 64, 377–390. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y. Controlled Chemical Synthesis in CVD Graphene. Phys. Sci. Rev. 2017, 2. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Chen, S. Large area CVD growth of graphene. Synth. Met. 2015, 210, 95–108. [Google Scholar] [CrossRef]
- Xue, R.; Abidi, I.H.; Luo, Z. Domain size, layer number and morphology control for graphene grown by chemical vapor deposition. Funct. Mater. Lett. 2017, 10, 1730003. [Google Scholar] [CrossRef]
- Jin, Y.; Hu, B.; Wei, Z.; Luo, Z.; Wei, D.; Xi, Y.; Zhang, Y.; Liu, Y. Roles of H2 in annealing and growth times of graphene CVD synthesis over copper foil. J. Mater. Chem. A 2014, 2, 16208–16216. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Xin, J.; Yakobson, B.I.; Ding, F. Role of hydrogen in graphene chemical vapor deposition growth on a copper surface. J. Am. Chem. Soc. 2014, 136, 3040–3047. [Google Scholar] [CrossRef]
- Seah, C.M.; Chai, S.P.; Mohamed, A.R. Mechanisms of graphene growth by chemical vapour deposition on transition metals. Carbon N. Y. 2014, 70, 1–21. [Google Scholar] [CrossRef]
- Plutnar, J.; Pumera, M.; Sofer, Z. The chemistry of CVD graphene. J. Mater. Chem. C 2018, 6, 6082–6101. [Google Scholar] [CrossRef]
- Xu, H.; Ma, L.; Jin, Z. Nitrogen-doped graphene: Synthesis, characterizations and energy applications. J. Energy Chem. 2018, 27, 146–160. [Google Scholar] [CrossRef]
- Chen, N.; Huang, X.; Qu, L. Heteroatom substituted and decorated graphene: Preparation and applications. Phys. Chem. Chem. Phys. 2015, 17, 32077–32098. [Google Scholar] [CrossRef] [PubMed]
- Granzier-Nakajima, T.; Fujisawa, K.; Anil, V.; Terrones, M.; Yeh, Y.T. Controlling nitrogen doping in graphene with atomic precision: Synthesis and characterization. Nanomaterials 2019, 9, 425. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Wu, B.; Jiang, L.; Guo, Y.; Huang, L.; Chen, J.; Tan, J.; Geng, D.; Luo, B.; Hu, W.; et al. Low temperature growth of highly nitrogen-doped single crystal graphene arrays by chemical vapor deposition. J. Am. Chem. Soc. 2012, 134, 11060–11063. [Google Scholar] [CrossRef]
- Susi, T.; Hardcastle, T.P.; Hofsäss, H.; Mittelberger, A.; Pennycook, T.J.; Mangler, C.; Drummond-Brydson, R.; Scott, A.J.; Meyer, J.C.; Kotakoski, J. Single-atom spectroscopy of phosphorus dopants implanted into graphene. 2D Mater. 2017, 4, 021013. [Google Scholar] [CrossRef]
- Some, S.; Kim, J.; Lee, K.; Kulkarni, A.; Yoon, Y.; Lee, S.; Kim, T.; Lee, H. Highly air-stable phosphorus-doped n-type graphene field-effect transistors. Adv. Mater. 2012, 24, 5481–5486. [Google Scholar] [CrossRef]
- Larrude, D.G.; Garcia-Basabe, Y.; Freire, F.L.; Rocco, M.L.M. Electronic structure and ultrafast charge transfer dynamics of phosphorous doped graphene layers on a copper substrate: A combined spectroscopic study. RSC Adv. 2015, 5, 74189–74197. [Google Scholar] [CrossRef]
- Ovezmyradov, M.; Magedov, I.V.; Frolova, L.V.; Chandler, G.; Garcia, J.; Bethke, D.; Shaner, E.A.; Kalugin, N.G. Chemical vapor deposition of phosphorous and boron-doped graphene using phenyl-containing molecules. J. Nanosci. Nanotechnol. 2015, 15, 4883–4886. [Google Scholar] [CrossRef]
- Schiros, T.; Nordlund, D.; Pálová, L.; Prezzi, D.; Zhao, L.; Kim, K.S.; Wurstbauer, U.; Gutiérrez, C.; Delongchamp, D.; Jaye, C.; et al. Connecting dopant bond type with electronic structure in n-doped graphene. Nano Lett. 2012, 12, 4025–4031. [Google Scholar] [CrossRef]
- Sedelnikova, O.V.; Bulusheva, L.G.; Okotrub, A.V. Graphitic and pyridinic nitrogen in carbon nanotubes: Energetic and polarization aspects. J. Nanophotonics 2015, 10, 012510. [Google Scholar] [CrossRef]
- Gaillard, P.; Schoenhalz, A.L.; Moskovkin, P.; Lucas, S.; Henrard, L. Growth of nitrogen-doped graphene on copper: Multiscale simulations. Surf. Sci. 2016, 644, 102–108. [Google Scholar] [CrossRef]
- Sui, Y.; Zhu, B.; Zhang, H.; Shu, H.; Chen, Z.; Zhang, Y.; Zhang, Y.; Wang, B.; Tang, C.; Xie, X.; et al. Temperature-dependent nitrogen configuration of N-doped graphene by chemical vapor deposition. Carbon N. Y. 2015, 81, 814–820. [Google Scholar] [CrossRef]
- Wang, B.; Tsetseris, L.; Pantelides, S.T. Introduction of nitrogen with controllable configuration into graphene via vacancies and edges. J. Mater. Chem. A 2013, 1, 14927–14934. [Google Scholar] [CrossRef]
- Gao, H.; Song, L.; Guo, W.; Huang, L.; Yang, D.; Wang, F.; Zuo, Y.; Fan, X.; Liu, Z.; Gao, W.; et al. A simple method to synthesize continuous large area nitrogen-doped graphene. Carbon N. Y. 2012, 50, 4476–4482. [Google Scholar] [CrossRef]
- Lv, R.; Li, Q.; Botello-Méndez, A.R.; Hayashi, T.; Wang, B.; Berkdemir, A.; Hao, Q.; Eléas, A.L.; Cruz-Silva, R.; Gutiérrez, H.R.; et al. Nitrogen-doped graphene: Beyond single substitution and enhanced molecular sensing. Sci. Rep. 2012, 2, 586. [Google Scholar] [CrossRef]
- Cui, T.; Lv, R.; Huang, Z.H.; Zhu, H.; Zhang, J.; Li, Z.; Jia, Y.; Kang, F.; Wang, K.; Wu, D. Synthesis of nitrogen-doped carbon thin films and their applications in solar cells. Carbon N. Y. 2011, 49, 5022–5028. [Google Scholar] [CrossRef]
- Garcia, A.G.; Baltazar, S.E.; Castro, A.H.R.; Robles, J.F.P.; Rubio, A. Influence of S and P doping in a graphene sheet. J. Comput. Theor. Nanosci. 2008, 5, 2221–2229. [Google Scholar] [CrossRef]
- He, S.M.; Huang, C.C.; Liou, J.W.; Woon, W.Y.; Su, C.Y. Spectroscopic and electrical characterizations of low-damage phosphorous-doped graphene via ion implantation. ACS Appl. Mater. Interfaces 2019. [Google Scholar] [CrossRef]
- Shin, D.W.; Kim, T.S.; Yoo, J.B. Phosphorus doped graphene by inductively coupled plasma and triphenylphosphine treatments. Mater. Res. Bull. 2016, 82, 71–75. [Google Scholar] [CrossRef]
- Arkhipov, V.E.; Gusel′nikov, A.V.; Popov, K.M.; Gevko, P.N.; Fedoseeva, Y.V.; Smirnov, D.A.; Bulusheva, L.G.; Okotrub, A.V. Optimization of parameters of graphene synthesis on copper foil at low methan pressure. J. Struct. Chem. 2018, 59, 759–765. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Kanygin, M.A.; Arkhipov, V.E.; Popov, K.M.; Fedoseeva, Y.V.; Smirnov, D.A.; Okotrub, A.V. In situ X-ray photoelectron spectroscopy study of lithium nnteraction with graphene and nitrogen-doped graphene films produced by chemical vapor deposition. J. Phys. Chem. C 2017, 121, 5108–5114. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Sedelnikova, O.V.; Okotrub, A.V. Many-body effects in optical response of graphene-based structures. Int. J. Quantum Chem. 2016, 116, 270–281. [Google Scholar] [CrossRef]
- Kim, H.; Mattevi, C.; Calvo, M.R.; Oberg, J.C.; Artiglia, L.; Agnoli, S.; Hirjibehedin, C.F.; Chhowalla, M.; Saiz, E. Activation energy paths for graphene nucleation and growth on Cu. ACS Nano 2012, 6, 3614–3623. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, S.; Rhee, K.Y.; Park, S.Y. A catalytic, catalyst-free, and roll-to-roll production of graphene via chemical vapor deposition: Low temperature growth. Carbon 2018, 127, 1–12. [Google Scholar] [CrossRef]
- Choi, J.-H.; Li, Z.; Cui, P.; Fan, X.; Zhang, H.; Zeng, C.; Zhang, Z. Drastic reduction in the growth temperature of graphene on copper via enhanced London dispersion force. Sci. Rep. 2013, 3, 1925. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studing the properties of graphene. Nat. Nanotech. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Fates, R.; Bouridah, H.; Raskin, J.-P. Probing carrier concentration in gated single, bi- and tri-layer CVD graphene using Raman spectroscopy. Carbon 2019, 149, 390–399. [Google Scholar] [CrossRef]
- Das, A.; Pisana, S.; Chakraborty, B.; Piscanec, S.; Saha, S.K.; Waghmare, U.V.; Novoselov, K.S.; Krishnamurthy, H.R.; Geim, A.K.; Ferrari, A.C.; et al. Monitoring dopants by Raman scattering in an electrochemically top-gated graphene transistor. Nat. Nanotech. 2006, 3, 210–215. [Google Scholar] [CrossRef]
- Zhao, L.; He, R.; Zabet-Khosousi, A.; Kim, K.S.; Schiros, T.; Roth, M.; Kim, P.; Flynn, G.W.; Pinczuk, A.; Pasupathy, A.N. Dopant segregation in polycrystalline monolayer graphene. Nano Lett. 2015, 15, 1428–1436. [Google Scholar] [CrossRef]
- Blume, R.; Rosenthal, D.; Tessonnier, J.-P.; Li, H.; Knop-Gericke, A.; Schlögl, R. Characterizing graphitic carbon with X-ray photoelectron spectroscopy: A step-by-step approach. ChemCatChem 2015, 7, 2871–2881. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Okotrub, A.V.; Asanov, I.P.; Fonseca, A.; Nagy, J.B. Comparative study on the electronic structure of arc-discharge and catalytic carbon nanotubes. J. Phys. Chem. B 2001, 105, 4853–4859. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Stolyarova, S.G.; Chuvilin, A.L.; Shubin, Y.V.; Asanov, I.P.; Sorokin, A.M.; Mel’Gunov, M.S.; Zhang, S.; Dong, Y.; Chen, X.; et al. Creation of nanosized holes in graphene planes for improvement of rate capability of lithium-ion batteries. Nanotechnology 2018, 29, 134001. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qiu, Y.; Hu, P.A. Three dimensional P-doped graphene synthesized by eco-fiendly chamical vapor deposition for oxygen reduction reactions. J. Nanosci. Nanotech. 2016, 16, 6216–6222. [Google Scholar] [CrossRef]
- Susi, T.; Pichler, T.; Ayala, P. X-ray photoelectron spectroscopy of graphitic carbon nanomaterials doped with heteroatoms. Beilstein J. Nanotechnol. 2015, 6, 177–192. [Google Scholar] [CrossRef]
- Li, J.; Ren, Z.; Zhou, Y.; Wu, X.; Xu, X.; Qi, M.; Li, W.; Bai, J.; Wang, L. Scalable synthesis of pyrrolic N-doped graphene by atmospheric pressure chemical vapor deposition and its terahertz response. Carbon N. Y. 2013, 62, 330–336. [Google Scholar] [CrossRef]
- Wang, Z.; Li, P.; Chen, Y.; Liu, J.; Tian, H.; Zhou, J.; Zhang, W.; Li, Y. Synthesis of nitrogen-doped graphene by chemical vapour deposition using melamine as the sole solid source of carbon and nitrogen. J. Mater. Chem. C 2014, 2, 7396–7401. [Google Scholar] [CrossRef]
- Capasso, A.; Dikonimos, T.; Sarto, F.; Tamburrano, A.; De Bellis, G.; Sarto, M.S.; Faggio, G.; Malara, A.; Messina, G.; Lisi, N. Nitrogen-doped graphene films from chemical vapor deposition of pyridine: Influence of process parameters on the electrical and optical properties. Beilstein J. Nanotechnol. 2015, 6, 2028–2038. [Google Scholar] [CrossRef]
- Scardamaglia, M.; Struzzi, C.; Aparicio Rebollo, F.J.; De Marco, P.; Mudimela, P.R.; Colomer, J.F.; Amati, M.; Gregoratti, L.; Petaccia, L.; Snyders, R.; et al. Tuning electronic properties of carbon nanotubes by nitrogen grafting: Chemistry and chemical stability. Carbon N. Y. 2015, 83, 118–127. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Li, R.; Sun, X.; Abou-Rachid, H. Thermal and chemical durability of nitrogen-doped carbon nanotubes. J. Nanoparticle Res. 2012, 14, 1016. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Okotrub, A.V.; Fedoseeva, Y.V.; Kurenya, A.G.; Asanov, I.P.; Vilkov, O.Y.; Koós, A.A.; Grobert, N. Controlling pyridinic, pyrrolic, graphitic, and molecular nitrogen in multi-wall carbon nanotubes using precursors with different N/C ratios in aerosol assisted chemical vapor deposition. Phys. Chem. Chem. Phys. 2015, 17, 23741–23747. [Google Scholar] [CrossRef] [PubMed]
- Okotrub, A.V.; Fedorovskaya, E.O.; Senkovskiy, B.V.; Bulusheva, L.G. Nitrogen species in few-layer graphene produced by thermal exfoliation of fluorinated graphite intercalation compounds. Phys. Status Solidi Basic Res. 2015, 252, 2444–2450. [Google Scholar] [CrossRef]
- Scardamaglia, M.; Aleman, B.; Amati, M.; Ewels, C.; Pochet, P.; Reckinger, N.; Colomer, J.F.; Skaltsas, T.; Tagmatarchis, N.; Snyders, R.; et al. Nitrogen implantation of suspended graphene flakes: Annealing effects and selectivity of sp2 nitrogen species. Carbon N. Y. 2014, 73, 371–381. [Google Scholar] [CrossRef]
- Okotrub, A.V.; Kanygin, M.A.; Koroteev, V.O.; Stolyarova, S.G.; Gorodetskiy, D.V.; Fedoseeva, Y.V.; Asanov, I.P.; Bulusheva, L.G.; Vyalikh, A. Phosphorus incorporation into graphitic material via hot pressing of graphite oxide and triphenylphosphine. Synth. Met. 2019, 248, 53–58. [Google Scholar] [CrossRef]
- Hasegawa, G.; Deguchi, T.; Kanamori, K.; Kobayashi, Y.; Kageyama, H.; Abe, T.; Nakanishi, K. High-level doping of nitrogen, phosphorus, and sulfur into activated carbon monoliths and their electrochemical capacitances. Chem. Mater. 2015, 27, 4703–4712. [Google Scholar] [CrossRef]
- Fedoseeva, Y.; Lapteva, L.L.; Makarova, A.A.; Bulusheva, L.G.; Okotrub, A.V. Charge polarization in partially lithiated single-walled carbon nanotubes. Phys. Chem. Chem. Phys. 2018, 20, 22592–22599. [Google Scholar] [CrossRef]
- Zatsepin, D.A.; MacK, P.; Wright, A.E.; Schmidt, B.; Fitting, H.J. XPS analysis and valence band structure of a low-dimensional SiO2/Si system after Si+ ion implantation. Phys. Status Solidi Appl. Mater. Sci. 2011, 208, 1658–1661. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Okotrub, A.V.; Kuznetsov, V.L.; Vyalikh, D.V. Soft X-ray spectroscopy and quantum chemistry characterization of defects in onion-like carbon produced by nanodiamond annealing. Diam. Relat. Mater. 2007, 16, 1222–1226. [Google Scholar] [CrossRef]
- Fedoseeva, Y.V.; Okotrub, A.V.; Koroteev, V.O.; Borzdov, Y.M.; Palyanov, Y.N.; Shubin, Y.V.; Maksimovskiy, E.A.; Makarova, A.A.; Münchgesang, W.; Bulusheva, L.G.; et al. Graphitization of 13C enriched fine-grained graphitic material under high-pressure annealing. Carbon N. Y. 2019, 141, 323–330. [Google Scholar] [CrossRef]
- Pacilé, D.; Meyer, J.C.; Fraile Rodríguez, A.; Papagno, M.; Gómez-Navarro, C.; Sundaram, R.S.; Burghard, M.; Kern, K.; Carbone, C.; Kaiser, U. Electronic properties and atomic structure of graphene oxide membranes. Carbon N. Y. 2011, 49, 966–972. [Google Scholar] [CrossRef]
- Iyer, G.R.S.; Wang, J.; Wells, G.; Bradley, M.P.; Borondics, F. Nanoscale imaging of freestanding nitrogen doped single layer graphene. Nanoscale 2015, 7, 2289–2294. [Google Scholar] [CrossRef] [PubMed]
- Lapteva, L.L.; Fedoseeva, Y.V.; Shlyakhova, E.V.; Makarova, A.A.; Bulusheva, L.G.; Okotrub, A.V. NEXAFS spectroscopy study of lithium interaction with nitrogen incorporated in porous graphitic material. J. Mater. Sci. 2019, 54, 11168–11178. [Google Scholar] [CrossRef]
- Hua, W.; Gao, B.; Li, S.; Ågren, H.; Luo, Y. X-ray absorption spectra of graphene from first-principles simulations. Phys. Rev. B. 2010, 82, 1–7. [Google Scholar] [CrossRef]
- Piazza, A.; Giannazzo, F.; Buscarino, G.; Fisichella, G.; La Magna, A.; Roccaforte, F.; Cannas, M.; Gelardi, F.M.; Agnello, S. Effect of air on oxygen p-doped graphene on SiO2. Phys. Status Solidi Appl. Mater. Sci. 2016, 213, 2341–2344. [Google Scholar] [CrossRef]
- Fedoseeva, Y.V.; Gorodetskiy, D.V.; Baskakova, K.I.; Asanov, I.P.; Bulusheva, L.G.; Makarova, A.A.; Yudin, I.B.; Plotnikov, M.Y.; Emelyanov, A.A.; Rebrov, A.K.; et al. Structure of diamond films grown using high-speed flow of a thermally activated CH4-H2 gas mixture. Materials 2020, 13, 219. [Google Scholar] [CrossRef]
- Matsoso, B.J.; Ranganathan, K.; Mutuma, B.K.; Lerotholi, T.; Jones, G.; Coville, N.J. Time-dependent evolution of the nitrogen configurations in N-doped graphene films. RSC Adv. 2016, 6, 106914–106920. [Google Scholar] [CrossRef]
- Kim, K.J.; Yang, S.; Park, Y.; Lee, M.; Kim, B.; Lee, H. Annealing effects after nitrogen ion casting on monolayer and multilayer graphene. J. Phys. Chem. C 2013, 117, 2129–2134. [Google Scholar] [CrossRef]
- Usachov, D.; Fedorov, A.; Vilkov, O.; Senkovskiy, B.; Adamchuk, V.K.; Yashina, L.V.; Volykhov, A.A.; Farjam, M.; Verbitskiy, N.I.; Grüneis, A.; et al. The chemistry of imperfections in N-graphene. Nano Lett. 2014, 14, 4982–4988. [Google Scholar] [CrossRef]
- Isacsson, A.; Cummings, A.W.; Colombo, L.; Colombo, L.; Kinaret, J.M.; Roche, S. Scaling properties of polycrystalline graphene: A review. 2D Mater. 2017, 4, 012002. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Jaroniec, M.; Qiao, S.Z. Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes. ACS Catal. 2015, 5, 5207–5234. [Google Scholar] [CrossRef]
- Pham, T.V.; Kim, J.-G.; Jung, J.Y.; Kim, J.H.; Cho, H.; Seo, T.H.; Lee, H.; Kim, N.D.; Kim, M.J. High areal capacitance of N-doped graphene synthesized by arc discharge. Adv. Funct. Mater. 2019, 29, 1905511. [Google Scholar] [CrossRef]
- Ito, Y.; Christodoulou, C.; Nardi, M.V.; Koch, N.; Sachdev, H.; Müllen, K. Chemical vapor deposition of N-doped graphene and carbon films: The role of precursors ans gas phase. ACS Nano 2014, 8, 3337–3346. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-F.; Lo, S.-T.; Lin, J.-C.; Zhang, W.; Lu, J.-Y.; Liu, F.-H.; Tseng, C.-M.; Lee, Y.-H.; Liang, C.-T.; Li, L.-J. Nitrogen-doped graphene sheets grown by chemical vapor deposition: Synthesis and influence of nitrogen impurities on carrier transport. ACS Nano 2013, 7, 6522–6532. [Google Scholar] [CrossRef] [PubMed]
- Sedelnikova, O.V.; Fedoseeva, Yu.V.; Romanenko, A.I.; Dusel’nikov, A.V.; Vilkov, O.Yu.; Maksimovskiy, E.A.; Bychanok, D.S.; Kuzhir, P.P.; Bulusheva, L.G.; Okotrub, A.V. Effect of boron and nitrogen additives on structure and transport properties of arc-produced carbon. Carbon 2019, 143, 660–668. [Google Scholar] [CrossRef]
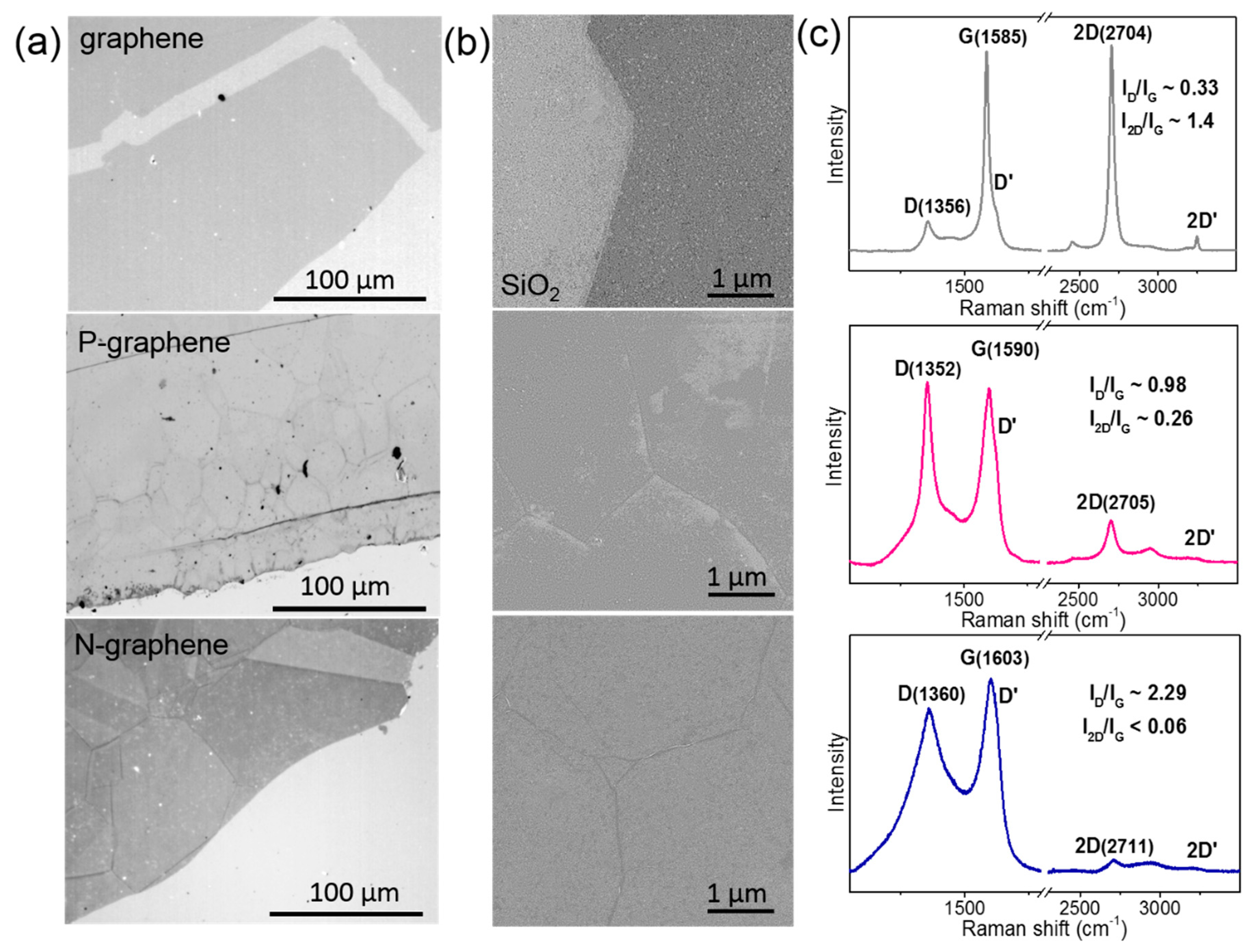
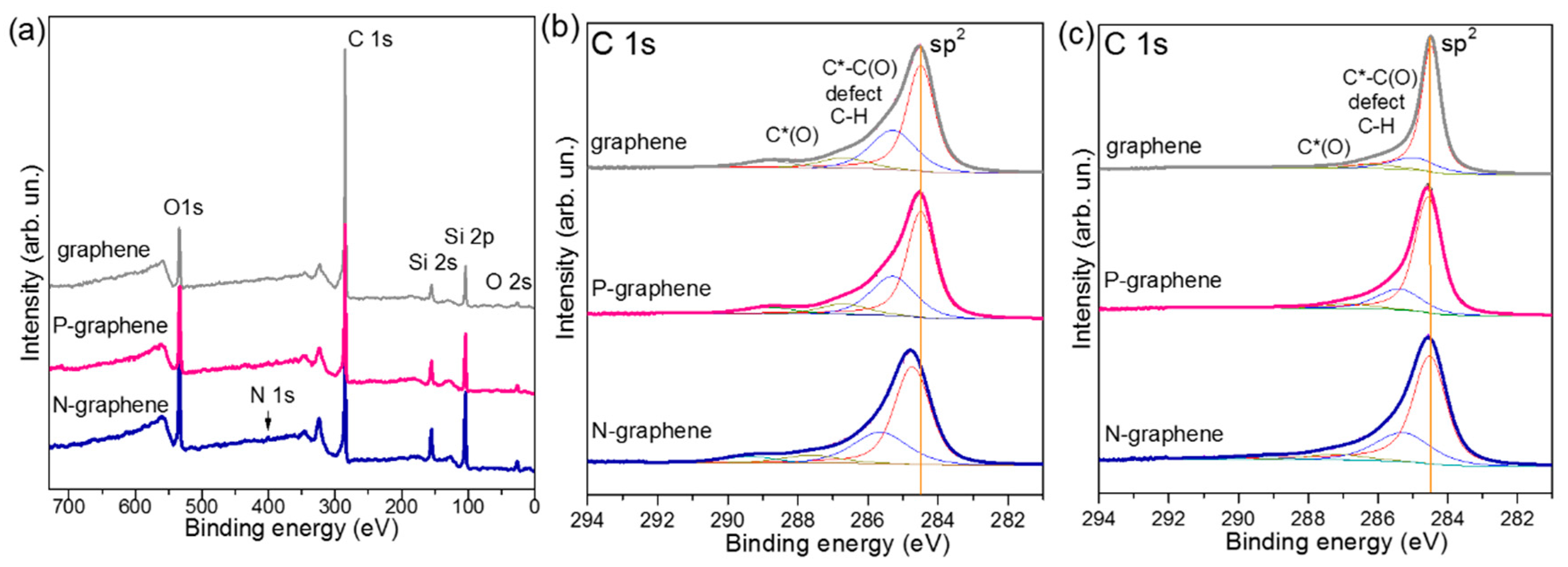

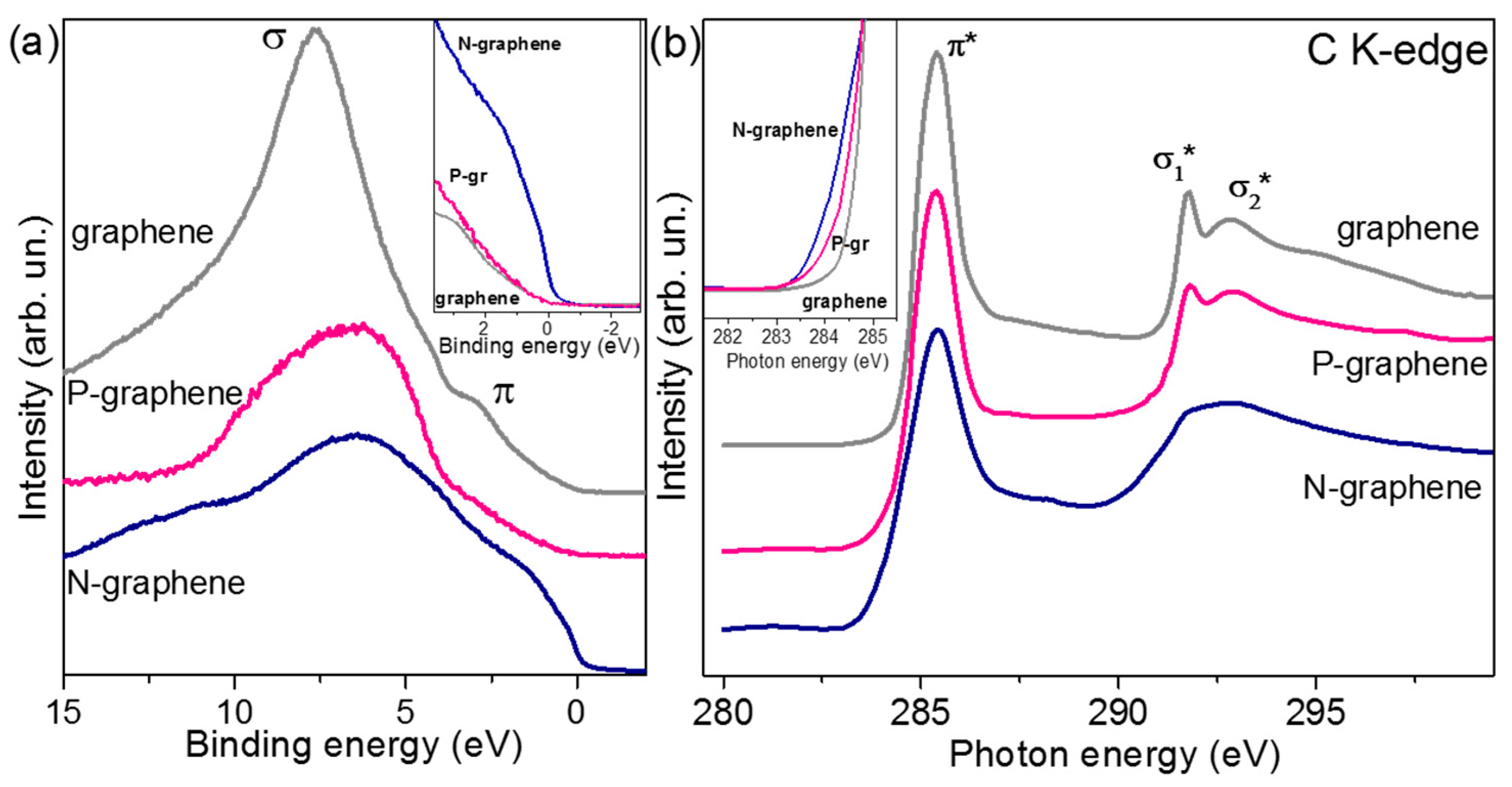
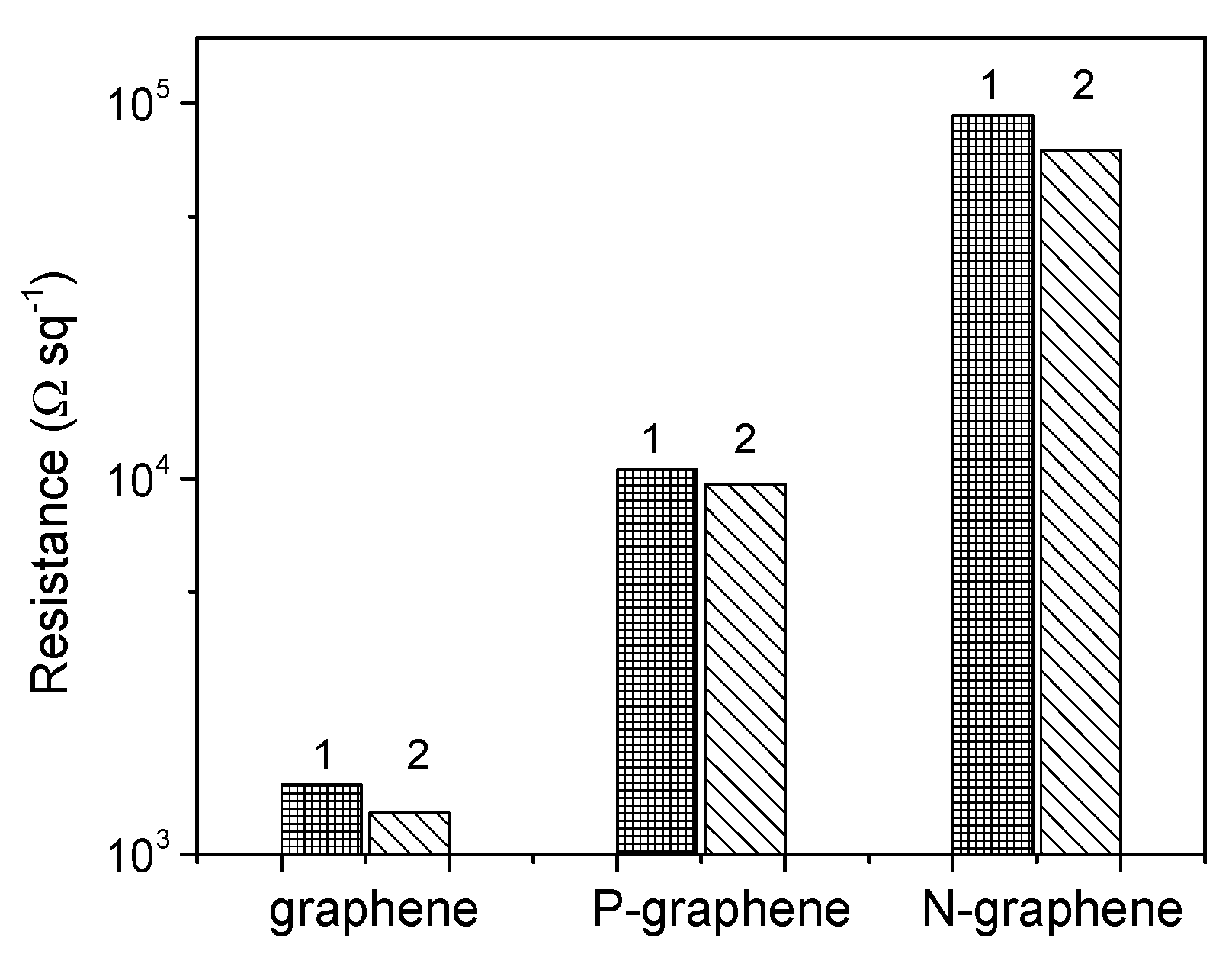
| Sample | TS (K) | Precursor | ID/IG | NL | Heteroatom | BE (eV) | Resistance (kΩ/□) |
|---|---|---|---|---|---|---|---|
| Graphene | 1323 | CH4 | 0.33 | ~5 | - | 284.47 | 1.3 |
| P-graphene | 1123 | 10CH4/PH3 | 0.98 | ~4 | <0.1% P | 284.56 | 9.7 |
| N-graphene | 1173 | CH3CN | 2.29 | ~3 | ~0.7% N | 284.51 | 75.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulusheva, L.G.; Arkhipov, V.E.; Popov, K.M.; Sysoev, V.I.; Makarova, A.A.; Okotrub, A.V. Electronic Structure of Nitrogen- and Phosphorus-Doped Graphenes Grown by Chemical Vapor Deposition Method. Materials 2020, 13, 1173. https://doi.org/10.3390/ma13051173
Bulusheva LG, Arkhipov VE, Popov KM, Sysoev VI, Makarova AA, Okotrub AV. Electronic Structure of Nitrogen- and Phosphorus-Doped Graphenes Grown by Chemical Vapor Deposition Method. Materials. 2020; 13(5):1173. https://doi.org/10.3390/ma13051173
Chicago/Turabian StyleBulusheva, L. G., V. E. Arkhipov, K. M. Popov, V. I. Sysoev, A. A. Makarova, and A. V. Okotrub. 2020. "Electronic Structure of Nitrogen- and Phosphorus-Doped Graphenes Grown by Chemical Vapor Deposition Method" Materials 13, no. 5: 1173. https://doi.org/10.3390/ma13051173
APA StyleBulusheva, L. G., Arkhipov, V. E., Popov, K. M., Sysoev, V. I., Makarova, A. A., & Okotrub, A. V. (2020). Electronic Structure of Nitrogen- and Phosphorus-Doped Graphenes Grown by Chemical Vapor Deposition Method. Materials, 13(5), 1173. https://doi.org/10.3390/ma13051173






