The Impact of Implant–Abutment Connection on Clinical Outcomes and Microbial Colonization: A Narrative Review
Abstract
1. Introduction
- The absence of clinical mobility of the implants;
- The absence of subjective sensitivity, pain or discomfort;
- The absence of peri-implantitis;
- The absence of persistent radiolucency around the implants;
- Bone loss lower than 0.2 mm annually after the implant’s first year of service;
- According to these criteria, the minimum levels for success are a success rate of 85% at the end of a five-year observation period and 80% at the end of a 10-year period.
Objectives
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search
2.3. Study Selection and Data Collection Process
2.4. Risk of Bias
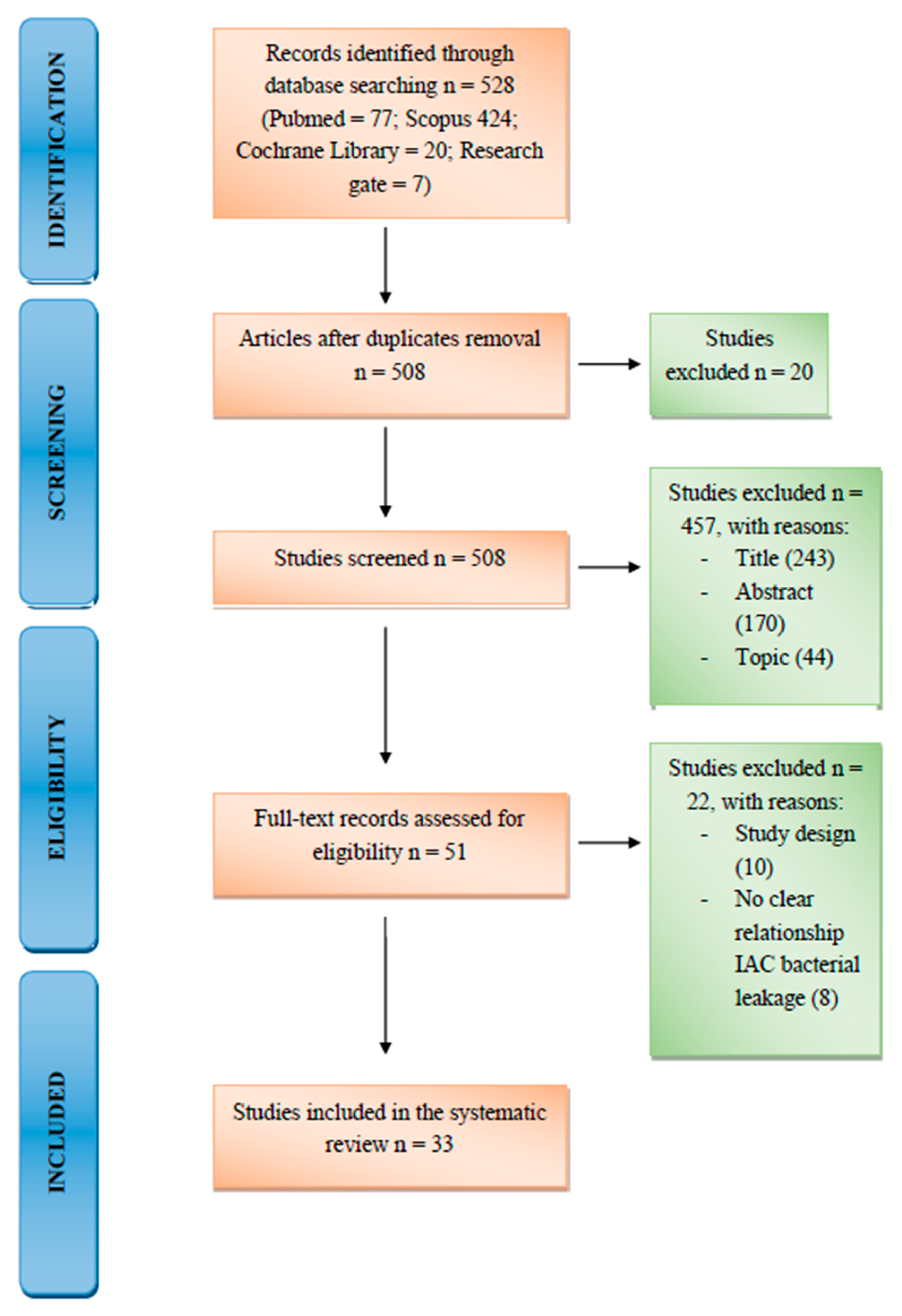
3. Results
4. Discussion
4.1. Internal Hexagonal Connection
4.2. Conical
4.3. Mixed
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ogle, O.E. Implant surface material, design, and osseointegration. Dent. Clin. N. Am. 2015, 592, 505–520. [Google Scholar] [CrossRef]
- Ottria, L.; Lauritano, D.; AndreasiBassi, M.; Palmieri, A.; Candotto, V.; Tagliabue, A.; Tettamanti, L. Mechanical, chemical and biological aspects of titanium and titanium alloys in implant dentistry. J. Biol. Regul. Homeost. Agents 2018, 32 (Suppl. 1), 81–90. [Google Scholar] [PubMed]
- De Oliveira, D.P.; Ottria, L.; Gargari, M.; Candotto, V.; Silvestre, F.J.; Lauritano, D. Surface modification of titanium alloys for biomedical application: From macro to nano scale. J. Biol. Regul. Homeost. Agents 2017, 31 (Suppl. 1), 221–232. [Google Scholar]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.L. Modifications of Dental Implant Surfaces at the Micro- and Nano-Level for Enhanced Osseointegration. Materials (Basel) 2019, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implants 1986, 1, 11–25. [Google Scholar]
- Canullo, L.; Penarrocha-Oltra, D.; Soldini, C.; Mazzocco, F.; Penarrocha, M.; Covani, U. Microbiological assessment of the implant-abutment interface in different connections: Cross-sectional study after 5 years of functional loading. Clin. Oral Implant. Res. 2015, 26, 426–434. [Google Scholar] [CrossRef]
- Sasada, Y.A.B.; Cochran, D.L. Implant-abutment connections: A review of biologic consequences and peri implantitis implications. Int. J. Oral Maxillofac. Implants 2017, 32, 1296–1307. [Google Scholar] [CrossRef]
- Carinci, F.; Lauritano, D.; Cura, F.; Lopez, M.A.; Bassi, M.A.; Confalone, L.; Pezzetti, F. Prevention of bacterial leakage at implant-Abutment connection level: An in vitro study of the efficacy of three different implant systems. J. Biol. Regul. Homeost. Agents 2016, 30 (Suppl. 1), 69–73. [Google Scholar]
- Lopez, M.A.; Bassi, M.A.; Confalone, L.; Gaudio, R.M.; Lombardo, L.; Lauritano, D. The influence of “conical plus octagonal” internal connection on implant survival and success rate: A retrospective study of 66 fixtures. J. Biol. Regul. Homeost. Agents 2016, 30 (Suppl. 1), 49–54. [Google Scholar]
- Bassi, M.A.; Lopez, M.A.; Confalone, L.; Gaudio, R.M.; Lombardo, L.; Lauritano, D. A prospective evaluation of outcomes of two tapered implant systems. J. Biol. Regul. Homeost. Agents 2016, 30 (Suppl. 1), 1–6. [Google Scholar]
- Mencio, F.; De Angelis, F.; Papi, P.; Rosella, D.; Pompa, G.; Di Carlo, S. A randomized clinical trial about presence of pathogenic microflora and risk of peri-implantitis: comparison of two different types of implant-abutment connections. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1443–1451. [Google Scholar] [PubMed]
- Mencio, F.; Papi, P.; di Carlo, S.; Pompa, G. Salivary bacterial leakage into implant-abutment connections: Preliminary results of an in vitro study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2476–2483. [Google Scholar] [PubMed]
- Mishra, S.K.; Chowdhary, R.; Kumari, S. Microleakage at the Different Implant Abutment Interface: A Systematic Review. J. Clin. Diagn. Res. 2017, 11, ZE10–ZE15. [Google Scholar] [CrossRef] [PubMed]
- Garrana, R.; Mohangi, G.; Malo, P.; Nobre, M. Leakage of Microbial Endotoxin through the Implant-Abutment Interface in Oral Implants: An In Vitro Study. BioMed Res. Int. 2016, 2016, 9219071. [Google Scholar] [CrossRef] [PubMed]
- Tawse-Smith, A.; Ma, S.; Duncan, W.J.; Gray, A.; Reid, M.R.; Rich, A.M. Implications of wear at the titanium-zirconia implant-abutment interface on the health of peri-implant tissues. Int. J. Oral Maxillofac. Implants 2017, 32, 599–609. [Google Scholar] [CrossRef]
- Scarano, A.; Murmura, G.; Carinci, F.; Lauritano, D. Immediately loaded small-diameter dental implants: Evaluation of retention, stability and comfort for the edentulous patient. Eur. J. Inflamm. 2012, 10, 19–23. [Google Scholar]
- Bassi, M.A.; Lopez, M.A.; Confalone, L.; Gaudio, R.M.; Lombardo, L.; Lauritano, D. Clinical outcome of a two-piece implant system with an internal hexagonal connection: A prospective study. J. Biol. Regul. Homeost. Agents 2016, 30 (Suppl. 1), 7–12. [Google Scholar]
- Ugurel, C.S.; Steiner, M.; Isik-Ozkol, G.; Kutay, O.; Kern, M. Mechanical resistance of screwless morse taper and screw-retained implant-abutment connections. Clin. Oral Implants Res. 2015, 26, 137–142. [Google Scholar] [CrossRef]
- Hajaj, T.; Talpos, S.; Stoian, C.; Negrutiu, M.L.; Szuhanek, C.; Popa, M.; Stan, A.T.; Zaharia, C.; Hajaj, K.R.; Licker, M.; et al. Determining the biological sealing quality of the implant-abutment interface using streptococcus mutans in both, conical and internal hex connections. Rev. de Chim. 2018, 69, 1429–1430. [Google Scholar] [CrossRef]
- Costa, G.N.; Martinez, E.F.; Ruellas, A.M.D.O.; Peruzzo, D.C.; Joly, J.C.; Napimoga, M.H. Microbiological Sealing Analysis of a Tapered Connection and External Hexagon System. Int. J. Dent. 2017, 2017, 3849085. [Google Scholar] [CrossRef]
- Ceruso, F.M.; Barnaba, P.; Mazzoleni, S.; Ottria, L.; Gargari, M.; Zucconi, A.; Bruno, G.; di Fiore, A. Implant-abutment connections on single crowns: A systematic review. Oral Implantol. (Rome) 2017, 10, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, K.; Ayukawa, Y.; Matsuzaki, T.; Kihara, M.; Koyano, K. The influence of implant-abutment connection on the screw loosening and microleakage. Int. J. Implant Dent. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- He, Y.; Fok, A.; Aparicio, C.; Teng, W. Contact analysis of gap formation at dental implant-abutment interface under oblique loading: A numerical-experimental study. Clin. Implant Dent. Relat. Res. 2019, 21, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Pardal-Peláez, B.; Montero, J. Preload loss of abutment screws after dynamic fatigue in single implant-supported restorations. A systematic review. J. Clin. Exp. Dent. 2017, 9, e1355–e1361. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; De Carvalho Serra, R. Load fatigue performance of conical implant-abutment connection: Effect of torque level and interface junction. Minerva Stomatol. 2015, 64, 1–7. [Google Scholar] [PubMed]
- Gherlone, E.F.; Capparé, P.; Pasciuta, R.; Grusovin, M.G.; Mancini, N.; Burioni, R. Evaluation of resistance against bacterial microleakage of a new conical implant-abutment connection versus conventional connections: An in vitro study. New Microbiol. 2016, 39, 49–56. [Google Scholar]
- Gehrke, S.A.; Shibli, J.A.; Aramburu Junior, J.S.; Sánchez de VAL, J.E.M.; Calvo-Girardo, J.L.; Dedavid, B.A. Effects of different torque levels on the implant-abutment interface in a conical internal connection. Braz. Oral Res. 2016, 30, pii: S1806-83242016000100233. [Google Scholar] [CrossRef]
- Cassetta, M.; Di Mambro, A.; Giansanti, M.; Brandetti, G.; Calasso, S. A 36-month follow-up prospective cohort study on peri-implant bone loss of Morse Taper connection implants with platform switching. J. Oral Sci. 2016, 58, 49–57. [Google Scholar] [CrossRef][Green Version]
- Lauritano, D.; Bignozzi, C.A.; Pazzi, D.; Cura, F.; Carinci, F. Efficacy of a new coating of implant-abutment connection in reducing microbial contamination: An in vitro study. Oral Implantol. (Rome) 2017, 10, 1–10. [Google Scholar] [CrossRef]
- Schmitt, C.M.; Nogueira-Filho, G.; Tenenbaum, H.C.; Lai, J.Y.; Brito, C.; Doering, H.; Nonhoff, J. Performance of conical abutment (Morse Taper) connection implants: A systematic review. J. Biomed. Mater. Res. A 2014, 102, 552–574. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.G.; Fernandes, A.; Kulkarni, R.; Ajantha, G.S.; Lekha, K.; Nadiger, R. Efficacy of Antibacterial Sealing Gel and O-Ring to Prevent Microleakage at the Implant Abutment Interface: An In Vitro Study. J. Oral Implantol. 2014, 40, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Shirani, G.; Refoua, S.; Rahimi Yeganeh, M. Comparative study of abutment screw loosening with or without adhesive material. J. Adv. Prosthodont. 2017, 9, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Siadat, H.; Arshad, M.; Mahgoli, H.A.; Fallahi, B. Microleakage Evaluation at Implant-Abutment Interface Using Radiotracer Technique. J. Dent. (Tehran) 2016, 13, 176–183. [Google Scholar]
- Romanos, G.E.; Biltucci, M.T.; Kokaras, A.; Paster, B.J. Bacterial Composition at the Implant-Abutment Connection under Loading in vivo. Clin. Implant Dent. Relat. Res. 2016, 18, 138–145. [Google Scholar] [CrossRef]
- Enkling, N.; Jöhren, P.; Katsoulis, J.; Bayer, S.; Jervøe-Storm, P.-M.; Mericske-Stern, R.; Jepsen, S. Influence of Platform Switching on Bone-level Alterations: A Three-year Randomized Clinical Trial. J. Dent. Res. 2013, 92 (Suppl. 12), 139S–145S. [Google Scholar] [CrossRef]
- Sesma, N.; Garaicoa-Pazmino, C.; Zanardi, P.R.; Chun, E.P.; Laganá, D.C. Assessment of Marginal Bone Loss around Platform- Matched and Platform-Switched Implants—A Prospective Study. Braz. Dent. J. 2016, 27, 712–716. [Google Scholar] [CrossRef]
- Schwarz, F.; Hegewald, A.; Becker, J. Impact of implant–abutment connection and positioning of the machined collar/microgap on crestal bone level changes: A systematic review. Clin. Oral Implants Res. 2014, 25, 417–425. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| In vivo and in vitro studies | No clear reference to the relationship between implant-abutment-connection (IAC) and bacterial leakage |
| Prospective and retrospective studies | Case report because of limited clinical relevance |
| Systematic and narrative reviews | Studied with less than fifteen implants as sample size |
| Less than 48 h of follow-up for in vitro studies | |
| Less than 14 days of follow-up for in vivo studies |
| Authors, Year | Type of Study | N° Implant Included | Type of Implant | Type of Connection | Follow Up |
|---|---|---|---|---|---|
| Gherlone, 2015 | In vitro | 80 | Sweden and Martina | DAT connection—double action tight (internal connection) | 96 h |
| Enkling, 2013 | Rand. clinical trial | 25 | SICace, SIC-Invent AG | Internal hexagonical | 3 years |
| Cassetta, 2015 | Prospect. cohort study | 748 | Impladent | Platform-switching | 3 years |
| Bassi, 2016 | Prospect. clinical study | 52 | Elisir cylindrical, Elisir EVO conical | Internal hexagonical | 4 years |
| Lopez, 2016 | Retrospect. study | 66 | I-Fix | External Hex, Deep Conical, Internal Octagon, Internal Hex, Conical Connection | 40 m |
| Garrana, 2016 | In vitro | 27 | Southern Implants, Neodent, Straumann, Dentsply-Ankylos | External Hex, Deep Conical, Internal Octagon, Internal Hex, Conical Connection | 7 days |
| Canullo, 2015 | Cross-sectional study | 40 | BIOMET 3i, Sweden and Martina, ASTRA TECH Implant System | Double internal hexagon, internal hexagon, conical connection | 5 years |
| Costa, 2017 | In vitro case-control | 24 | Intraoss | External hexagonal | 14 days |
| Arshad, 2017 | In vitro case-control | 20 | Dentium | NR | 1 m |
| Siadat, 2016 | In vitro case-control | 17 | Nobel Biocare | NR | 12 days |
| Romanos, 2014 | Case-control | 240 | Dentsply Implants, Biomet 3i | Platform-switching, Morse tapered, internal polygonal butt-joint | 2 years |
| Scarano, 2016 | Clinical trial | 146 | Dental Tech | NR | NR |
| Ugurel, 2013 | Case-control | 64 | Tasarimmed, Straumann, Biohorizons, Dentsply friadent | Screwless Morse taper | NR |
| Lauritano, 2017 | In vitro case-control | 40 | Edierre Implants System | NR | 96 h |
| Andreasi Bassi, 2016 | Prospect. clinical study | 133 | EVO | Tapered connection | NR |
| Lopez, 2016 | Retrospect. clinical study | 215 | Falappa Medical Devices | Conical connection | 5 years |
| Carinci, 2016 | In vitro case-control | 17 | Implant System FMD | FN, NQ, Eisir by FMD, Rome, Italy | 48 h |
| Mencio, 2017 | Rand. clinical trial | 20 | NR | Cemented implant-abutment and screwed implant-abutment connection | 1 year |
| Gehrke, 2016 | Case-control | 40 | NR | Conical internal connection | NR |
| Sesma, 2016 | Case-control | 36 | Conexão (Conect AR) | Platform-switched and platform matched | 15 m |
| Nayak, 2016 | In vitro case-control | 45 | ADIN DentalImplant System | NR | 48 h |
| Mencio, 2016 | In vitro case-control | 15 | Winsix, BioSAF IN | NR | 14 days |
| Summary of Exclusion Criteria | Summary of Confounding Factors | Summary of Limitation of the Studies | Type of Analysis (In Vivo Studies) | Type of Analysis (In Vitro Studies) |
|---|---|---|---|---|
| Alcohol Drug Smoking General health condition (liver, blood or kidney disease; immune-suppressed patients, corticosteroids therapy) Local tumors and ulcers Bruxism Pregnant women History of bisphosphonate Active periodontal or peri-implant pathology | Not clear best torque value Not all patients fulfill follow-up periods | Short follow-up Used periapical radiographs with no visibility of lingual and buccal bone Finding an appropriate adhesive material Sample size Lack of cyclic loading | Bone loss valued with periapical radiograph Sampling with paper point and following microbiological analysis (culture exam or PCR) * | Microbiological exam: PCR, culture exam, limulus, amoebocyte lysate * Cyclic loading and valuation of detorque, screw or implant fracture |
| Study | Implant Connection | Outcome Measure | Results |
|---|---|---|---|
| Gherlone, 2015 | Internal conical connection (ICC) | % of contaminated implants | ICC = 30% Controls = 100% |
| Sesma, 2016 | Platform switching connection (PSC) | Vertical bone change one year after functional loading | 0.40 ± 0.19 |
| Cassetta, 2016 | Platform switching connection (PSC) | Mean marginal bone remodeling | −0.56 mm |
| Enkling, 2013 | Platform switching connection (PSC) | Mean radiographic peri-implant bone loss | PSC = 0.69 ± 0.43 mm Controls = 0.74 ± 0.57 mm |
| Cannullo, 2015 | Conical connection (CC) vs. double internal hexagon connections (DIHC) | Positivity to red complex bacteria | CC = 10% DIHC = 68% |
| Lauritano, 2017 | Antimicrobial polysiloxane coating on the implant-abutment junction (PCJ) vs cemented implant-abutment connection (CC) | Total bacterial count on average | PCJ = 3.7E + 08 CC = 2.1E + 08 |
| Carinci, 2016 | Internal conical connection design: nano fix vs. uNiQo vs. Elisir implant systems by FMD, Rome, Italy | Median percentage of bacteria living in the inner side of the implants | uNiQo = 1.4% nano fix = 1.9% Elisir = 2.6% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauritano, D.; Moreo, G.; Lucchese, A.; Viganoni, C.; Limongelli, L.; Carinci, F. The Impact of Implant–Abutment Connection on Clinical Outcomes and Microbial Colonization: A Narrative Review. Materials 2020, 13, 1131. https://doi.org/10.3390/ma13051131
Lauritano D, Moreo G, Lucchese A, Viganoni C, Limongelli L, Carinci F. The Impact of Implant–Abutment Connection on Clinical Outcomes and Microbial Colonization: A Narrative Review. Materials. 2020; 13(5):1131. https://doi.org/10.3390/ma13051131
Chicago/Turabian StyleLauritano, Dorina, Giulia Moreo, Alberta Lucchese, Chiara Viganoni, Luisa Limongelli, and Francesco Carinci. 2020. "The Impact of Implant–Abutment Connection on Clinical Outcomes and Microbial Colonization: A Narrative Review" Materials 13, no. 5: 1131. https://doi.org/10.3390/ma13051131
APA StyleLauritano, D., Moreo, G., Lucchese, A., Viganoni, C., Limongelli, L., & Carinci, F. (2020). The Impact of Implant–Abutment Connection on Clinical Outcomes and Microbial Colonization: A Narrative Review. Materials, 13(5), 1131. https://doi.org/10.3390/ma13051131






