Fluorescence Microscopic Investigations of the Retarding Effect of Superplasticizers in Cementitious Systems of UHPC
Abstract
1. Introduction
2. Materials and Methods
2.1. Solids
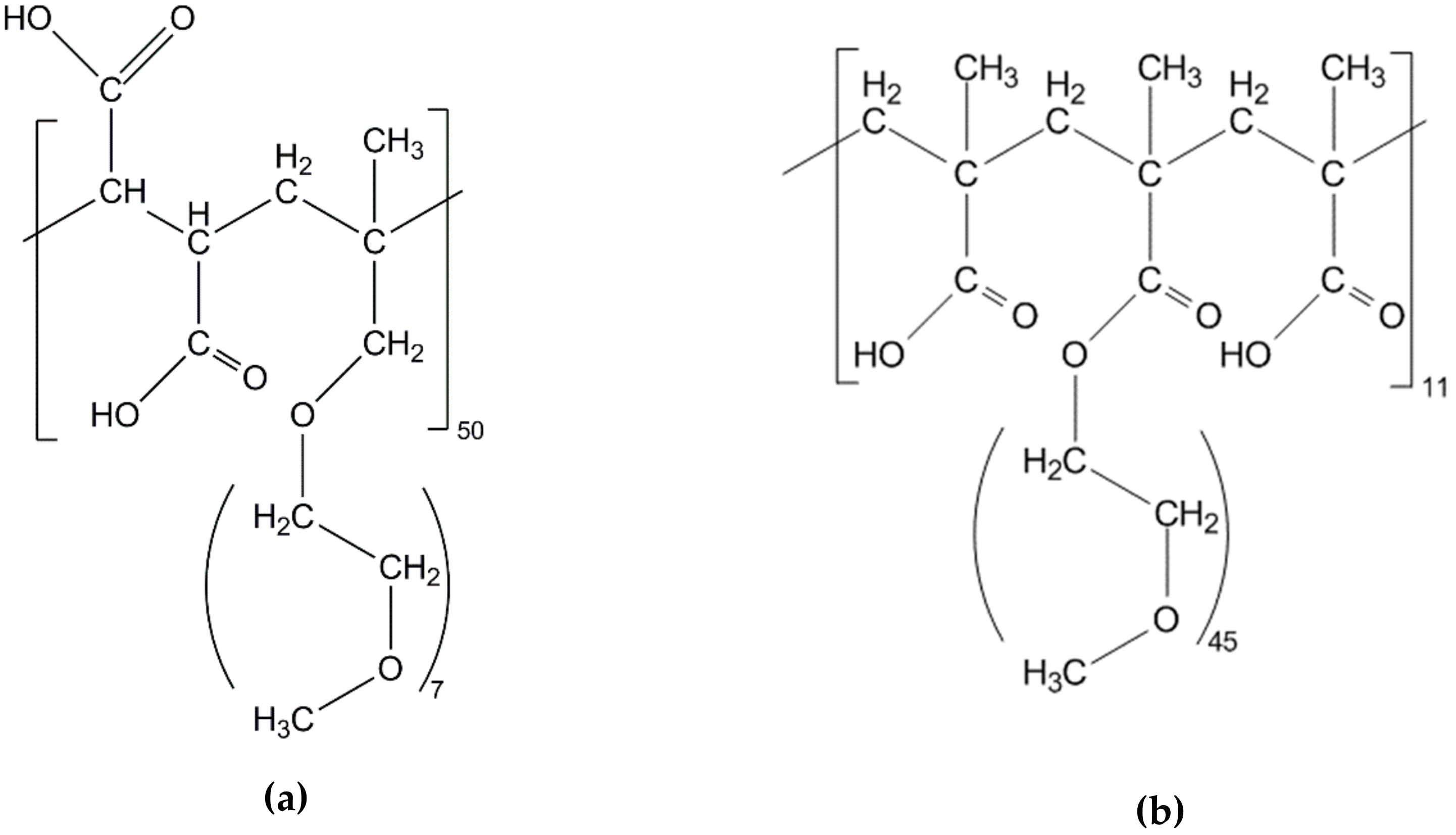
2.2. Superplasticizers
2.3. Calorimetry
2.4. Staining
2.5. Fluorescence Microscopy
3. Results and Discussion
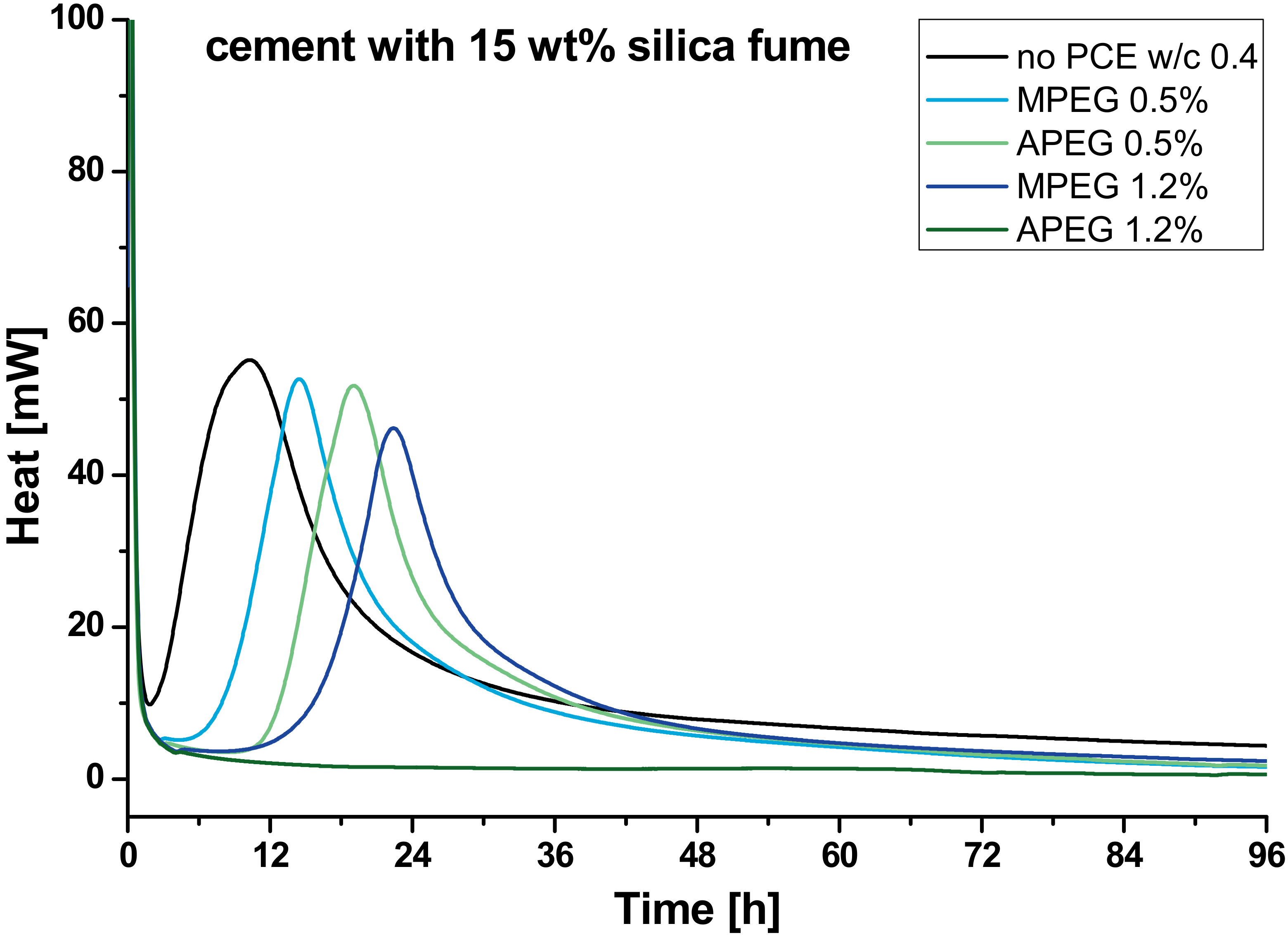
3.1. Calorimetry
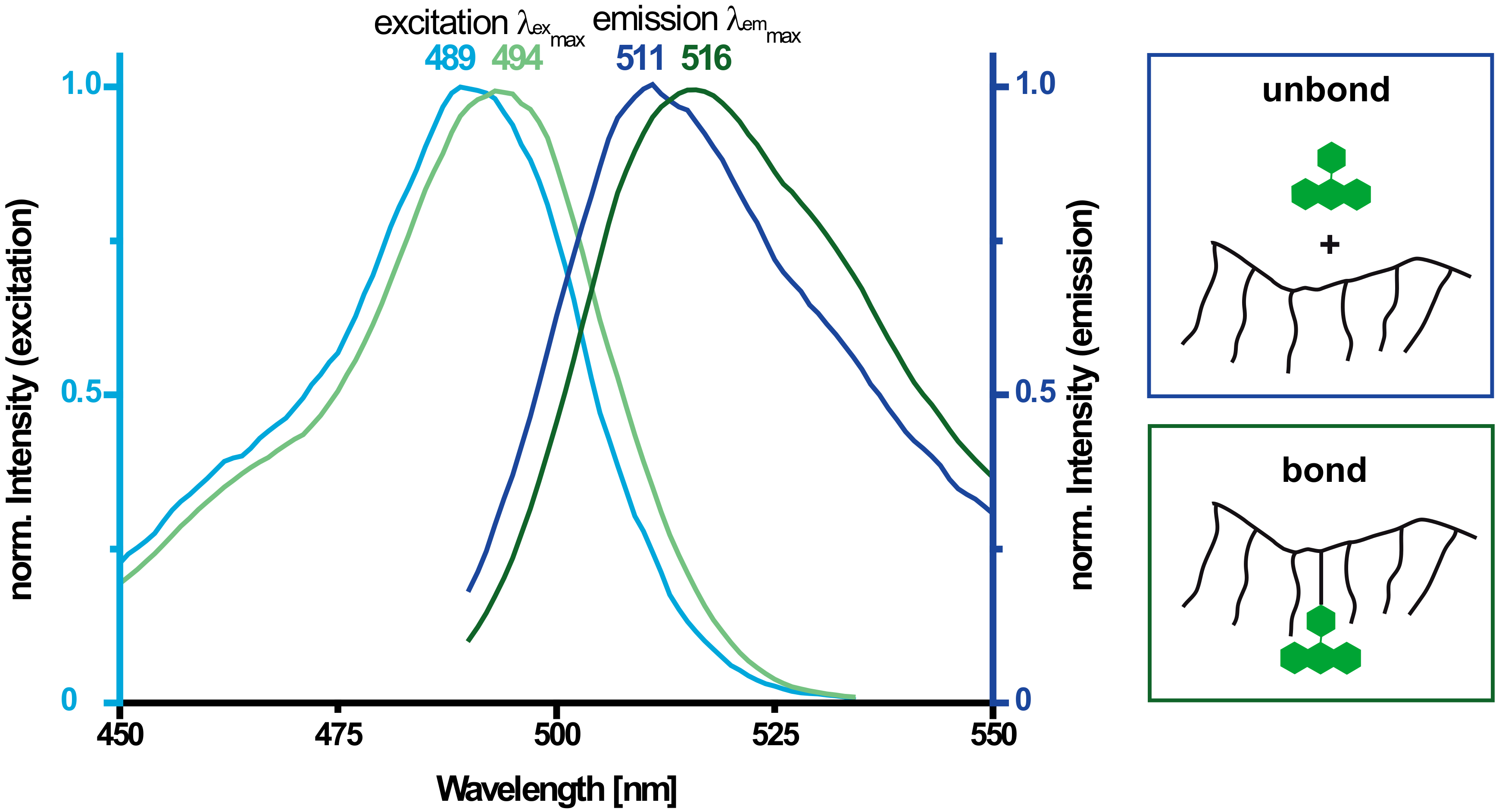
3.2. Fluorescence Spectroscopy
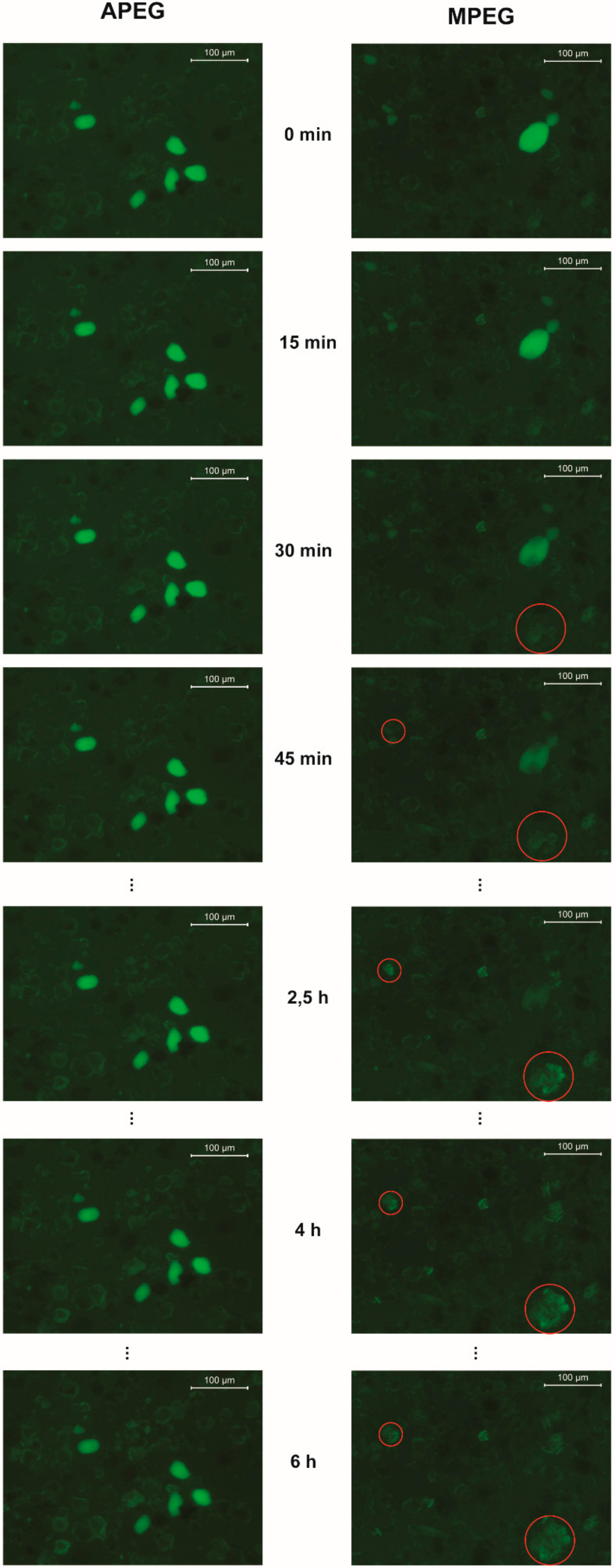
3.3. Fluorescence Microscopy
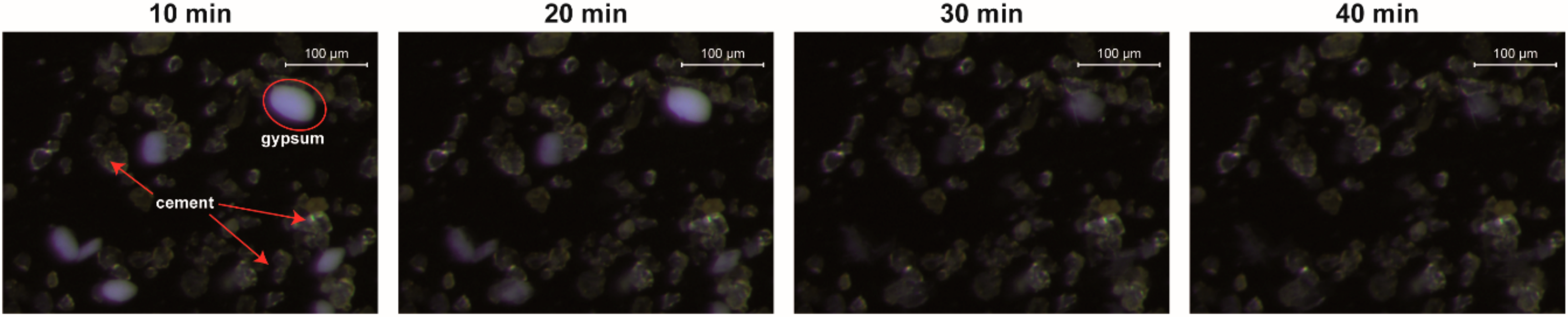
3.3.1. Pure Cement
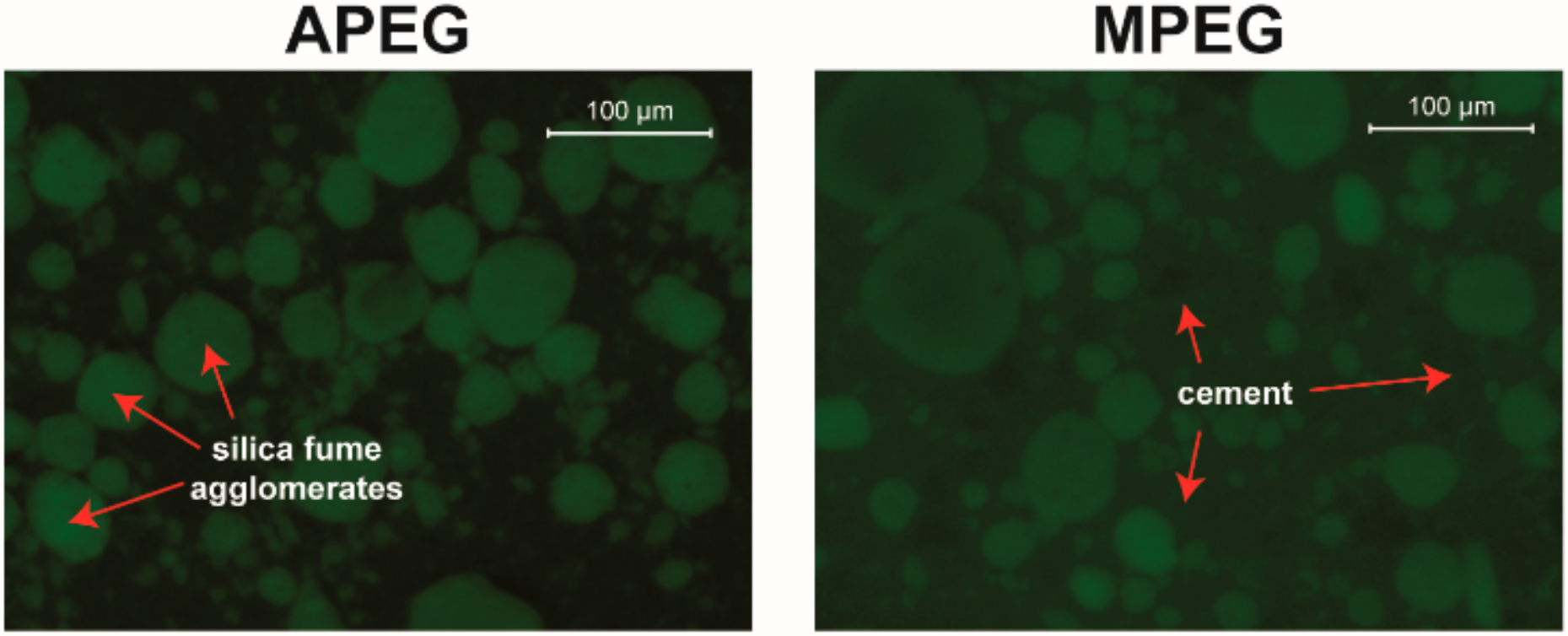
3.3.2. Cement with Silica Fume
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, D.; Shi, C.; Wu, Z.; Xiao, J.; Huang, Z.; Fang, Z. A review on ultra high performance concrete: Part II. Hydration, microstructure and properties. Constr. Build. Mater. 2015, 96, 368–377. [Google Scholar] [CrossRef]
- Van Damme, H. Concrete material science: Past, present, and future innovations. Cem. Concr. Res. 2018. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Z.; Xiao, J.; Wang, D.; Huang, Z.; Fang, Z. A review on ultra high performance concrete: Part I. Raw materials and mixture design. Constr. Build. Mater. 2015, 101, 741–751. [Google Scholar] [CrossRef]
- Hirata, T.; Tsubakimoto, T.; Hosoido, M.; Tawara, H. Cement Dispersant 84 2022. JP Patent S59-018338, 26 April 1984. [Google Scholar]
- Qian, Y.; de Schutter, G. Different Effects of NSF and PCE Superplasticizer on Adsorption, Dynamic Yield Stress and Thixotropy of Cement Pastes. Materials 2018, 11, 695. [Google Scholar] [CrossRef] [PubMed]
- Uchikawa, H.; Hanehara, S.; Sawaki, D. The role of steric repulsive force in the dispersion of cement particles in fresh paste prepared with organic admixture. Cem. Concr. Res. 1997, 27, 37–50. [Google Scholar] [CrossRef]
- Lowke, D.; Gehlen, C. The zeta potential of cement and additions in cementitious suspensions with high solid fraction. Cem. Concr. Res. 2017, 95, 195–204. [Google Scholar] [CrossRef]
- Yoshioka, K.; Tazawa, E.-I.; Kawai, K.; Enohata, T. Adsorption characteristics of superplasticizers on cement component minerals. Cem. Concr. Res. 2002, 32, 1507–1513. [Google Scholar] [CrossRef]
- Plank, J.; Sachsenhauser, B. Experimental determination of the effective anionic charge density of polycarboxylate superplasticizers in cement pore solution. Cem. Concr. Res. 2009, 39, 1–5. [Google Scholar] [CrossRef]
- Keppert, M.; Scheinherrová, L.; Jerman, M.; Doušová, B.; Kobera, L.; Brus, J.; Černý, R. Hydration of Ordinary Portland Cement in Presence of Lead Sorbed on Ceramic Sorbent. Materials 2018, 12. [Google Scholar] [CrossRef]
- Weeks, C.; Hand, R.J.; Sharp, J.H. Retardation of cement hydration caused by heavy metals present in ISF slag used as aggregate. Cem. Concr. Compos. 2008, 30, 970–978. [Google Scholar] [CrossRef]
- Abile, R.; Russo, A.; Limone, C.; Montagnaro, F. Impact of the charge density on the behaviour of polycarboxylate ethers as cement dispersants. Constr. Build. Mater. 2018, 180, 477–490. [Google Scholar] [CrossRef]
- Caruso, F.; Mantellato, S.; Palacios, M.; Flatt, R.J. ICP-OES method for the characterization of cement pore solutions and their modification by polycarboxylate-based superplasticizers. Cem. Concr. Res. 2017, 91, 52–60. [Google Scholar] [CrossRef]
- Marchon, D.; Juilland, P.; Gallucci, E.; Frunz, L.; Flatt, R.J. Molecular and submolecular scale effects of comb-copolymers on tri-calcium silicate reactivity: Toward molecular design. J. Am. Ceram. Soc. 2017, 817–841. [Google Scholar] [CrossRef]
- Marchon, D.; Boscaro, F.; Flatt, R.J. First steps to the molecular structure optimization of polycarboxylate ether superplasticizers: Mastering fluidity and retardation. Cem. Concr. Res. 2019, 116–123. [Google Scholar] [CrossRef]
- Lange, A.; Plank, J. Optimization of comb-shaped polycarboxylate cement dispersants to achieve fast-flowing mortar and concrete. J. Appl. Polym. Sci. 2015, 132, 42529. [Google Scholar] [CrossRef]
- Lange, A.; Hirata, T.; Plank, J. Influence of the HLB value of polycarboxylate superplasticizers on the flow behavior of mortar and concrete. Cem. Concr. Res. 2014, 60, 45–50. [Google Scholar] [CrossRef]
- Ferrari, L.; Kaufmann, J.; Winnefeld, F.; Plank, J. Interaction of cement model systems with superplasticizers investigated by atomic force microscopy, zeta potential, and adsorption measurements. J. Colloid Interface Sci. 2010, 347, 15–24. [Google Scholar] [CrossRef]
- Matsuzawa, K.; Atarashi, D.; Miyauchi, M.; Sakai, E. Interactions between fluoride ions and cement paste containing superplasticizer. Cem. Concr. Res. 2017, 91, 33–38. [Google Scholar] [CrossRef]
- Meier, M.R.; Napharatsamee, T.; Plank, J. Dispersing performance of superplasticizers admixed to aged cement. Constr. Build. Mater. 2017, 139, 232–240. [Google Scholar] [CrossRef]
- Plank, J.; Schröfl, C.; Gruber, M.; Lesti, M.; Sieber, R. Effectiveness of Polycarboxylate Superplasticizers in Ultra-High Strength Concrete: The Importance of PCE Compatibility with Silica Fume. J. Adv. Concr. Technol. 2009, 5–12. [Google Scholar] [CrossRef]
- Winnefeld, F.; Becker, S.; Pakusch, J.; Götz, T. Effects of the molecular architecture of comb-shaped superplasticizers on their performance in cementitious systems. Cem. Concr. Compos. 2007, 29, 251–262. [Google Scholar] [CrossRef]
- Zingg, A.; Winnefeld, F.; Holzer, L.; Pakusch, J.; Becker, S.; Gauckler, L. Adsorption of polyelectrolytes and its influence on the rheology, zeta potential, and microstructure of various cement and hydrate phases. J. Colloid Interface Sci. 2008, 323, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Arend, J.; Wetzel, A.; Middendorf, B. In-situ investigation of superplasticizers: From fluorescence microscopy to concrete rheology. Cem. Concr. Res. 2018, 113, 178–185. [Google Scholar] [CrossRef]
- Sustainable Building with Ultra-High Performance Concrete, Results of the German Priority Programme 1182 Funded by Deutsche Forschungsgemeinschaft (DFG); Schmidt, M., Fehling, E., Fröhlich, S., Eds.; Kassel Univ. Press: Kassel, Germany, 2014; ISBN 9783862194810. [Google Scholar]
- Conte, T.; Plank, J. Impact of molecular structure and composition of polycarboxylate comb polymers on the flow properties of alkali-activated slag. Cem. Concr. Res. 2019, 116, 95–101. [Google Scholar] [CrossRef]
- Habbaba, A.; Plank, J. Interaction Between Polycarboxylate Superplasticizers and Amorphous Ground Granulated Blast Furnace Slag. J. Am. Ceram. Soc. 2010, 93, 2857–2863. [Google Scholar] [CrossRef]
- Thermo Scientific. Crosslinking Technical Handbook. Reactivity Chemistries, Applications and Structure References. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/Handbooks/crosslinking-technical-handbook.pdf (accessed on 28 February 2012).
- Uryga-Polowy, V.; Kosslick, D.; Freund, C.; Rademann, J. Resin-bound aminofluorescein for C-terminal labeling of peptides: High-affinity polarization probes binding to polyproline-specific GYF domains. Chembiochem 2008, 9, 2452–2462. [Google Scholar] [CrossRef]
- Baueregger, S.; Perello, M.; Plank, J. Role of PVOH and kaolin on colloidal stability of liquid and powder EVA and SB latexes in cement pore solution. Colloids Surf. A Physicochem. Eng. Asp. 2013, 434, 145–153. [Google Scholar] [CrossRef]
- Zingg, A.; Winnefeld, F.; Holzer, L.; Pakusch, J.; Becker, S.; Figi, R.; Gauckler, L. Interaction of polycarboxylate-based superplasticizers with cements containing different C3A amounts. Cem. Concr. Compos. 2009, 31, 153–162. [Google Scholar] [CrossRef]
- Stroh, J.; Schlegel, M.-C.; Schmidt, W.; Thi, Y.N.; Meng, B.; Emmerling, F. Time-resolved in situ investigation of Portland cement hydration influenced by chemical admixtures. Constr. Build. Mater. 2016, 106, 18–26. [Google Scholar] [CrossRef]
- Plank, J.; Hirsch, C. Impact of zeta potential of early cement hydration phases on superplasticizer adsorption. Cem. Concr. Res. 2007, 37, 537–542. [Google Scholar] [CrossRef]
- Schröfl, C.; Gruber, M.; Plank, J. Preferential adsorption of polycarboxylate superplasticizers on cement and silica fume in ultra-high performance concrete (UHPC). Cem. Concr. Res. 2012, 42, 1401–1408. [Google Scholar] [CrossRef]
- Hommer, H. Interaction of polycarboxylate ether with silica fume. J. Eur. Ceram. Soc. 2009, 29, 1847–1853. [Google Scholar] [CrossRef]

| Phase | C3S | C2S | C3A | C2AF | Gypsum and Hemihydrate |
|---|---|---|---|---|---|
| Content [%] | 61.3 | 15.9 | 3.9 | 13.9 | 3.3 |
| Oxide Content [wt%] | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | P2O5 | Others | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| Silica fume | 95.6 | 0.1 | 0.1 | 0.5 | 0.3 | 0.7 | 0.2 | 0.1 | 1.0 | 1.4 |
| OPC | 21.5 | 3.7 | 4.3 | 64.3 | 0.9 | 0.4 | 0.3 | 0.2 | 2.7 | 1.7 |
| SP | %bwob | SP Conc. | SP Total Mass [g] | Cement [g] | Silica Fume [g] | Water [g] | Total [g] |
|---|---|---|---|---|---|---|---|
| - | - | - | - | 15 | 0 | 6 | 21 |
| APEG | 0.5 | 35 wt% | 0.21 | 15 | 0 | 3.61 | 18.82 |
| MPEG | 0.5 | 40 wt% | 0.19 | 15 | 0 | 3.63 | 18.82 |
| APEG | 1.2 | 35 wt% | 0.51 | 15 | 0 | 3.42 | 18.93 |
| MPEG | 1.2 | 40 wt% | 0.46 | 15 | 0 | 3.47 | 18.93 |
| - | - | - | - | 12.75 | 2.25 | 6 | 21 |
| APEG | 0.5 | 35 wt% | 0.21 | 12.75 | 2.25 | 3.61 | 18.82 |
| MPEG | 0.5 | 40 wt% | 0.19 | 12.75 | 2.25 | 3.63 | 18.82 |
| APEG | 1.2 | 35 wt% | 0.51 | 12.75 | 2.25 | 3.42 | 18.93 |
| MPEG | 1.2 | 40 wt% | 0.46 | 12.75 | 2.25 | 3.47 | 18.93 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arend, J.; Wetzel, A.; Middendorf, B. Fluorescence Microscopic Investigations of the Retarding Effect of Superplasticizers in Cementitious Systems of UHPC. Materials 2020, 13, 1057. https://doi.org/10.3390/ma13051057
Arend J, Wetzel A, Middendorf B. Fluorescence Microscopic Investigations of the Retarding Effect of Superplasticizers in Cementitious Systems of UHPC. Materials. 2020; 13(5):1057. https://doi.org/10.3390/ma13051057
Chicago/Turabian StyleArend, Johannes, Alexander Wetzel, and Bernhard Middendorf. 2020. "Fluorescence Microscopic Investigations of the Retarding Effect of Superplasticizers in Cementitious Systems of UHPC" Materials 13, no. 5: 1057. https://doi.org/10.3390/ma13051057
APA StyleArend, J., Wetzel, A., & Middendorf, B. (2020). Fluorescence Microscopic Investigations of the Retarding Effect of Superplasticizers in Cementitious Systems of UHPC. Materials, 13(5), 1057. https://doi.org/10.3390/ma13051057





