Compressive Strength, Chloride Ion Penetrability, and Carbonation Characteristic of Concrete with Mixed Slag Aggregate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mixing Proportions and Specimen Preparation
3. Results and Discussion
3.1. Slump and Air Content
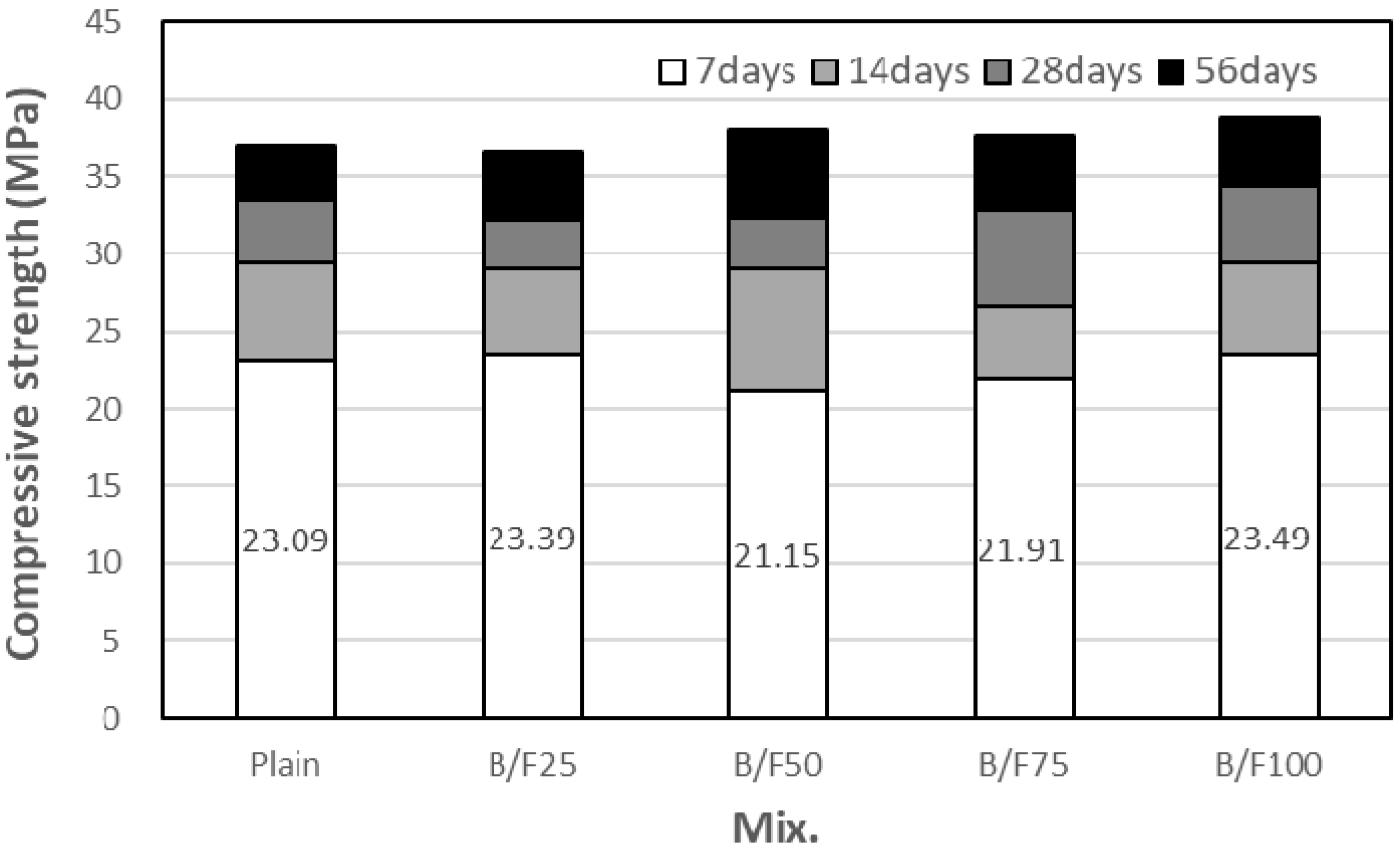
3.2. Compressive Strength
3.3. Chloride Ion Penetrability
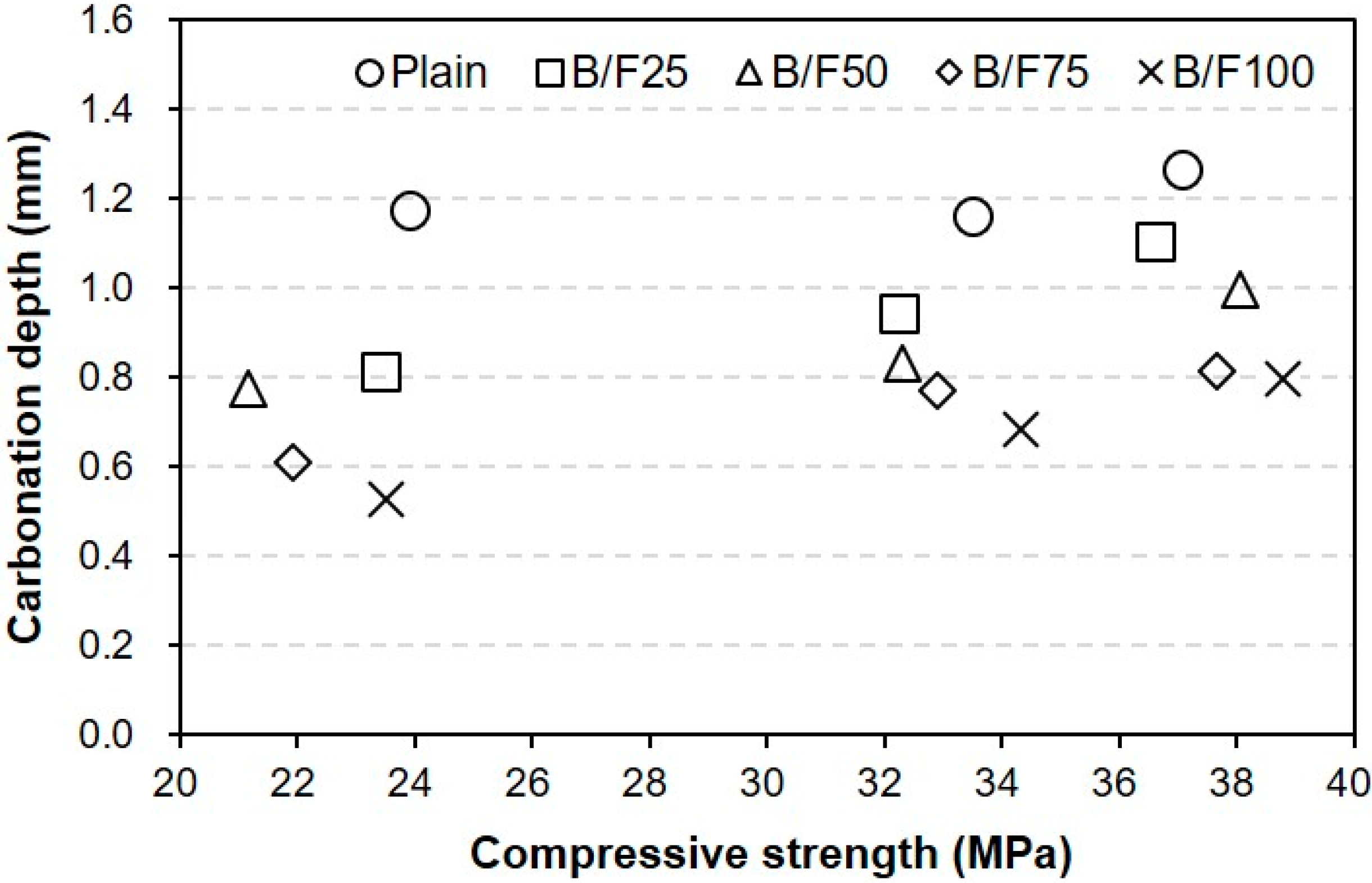
3.4. Carbonation Depth
4. Conclusions
- (1)
- The slumps of all mixtures were similar (200 to 210 mm), regardless of the replacement ratio of MSFA. The WRA dosage decreased with the increase in replacement ratio of MSFA.
- (2)
- The compressive strength of the plain sample was approximately 23.9 MPa, while those of the samples with the MSFAs were in the range of 21.2 to 23.5 MPa after seven days. After 56 days, the highest compressive strength (approximately 38.8 MPa) was observed for the B/F100 sample. The increase in compressive strength could be explained as the particle size distribution of the MSFA was similar to that of the NS and the formation of the secondary CSH gel was initiated.
- (3)
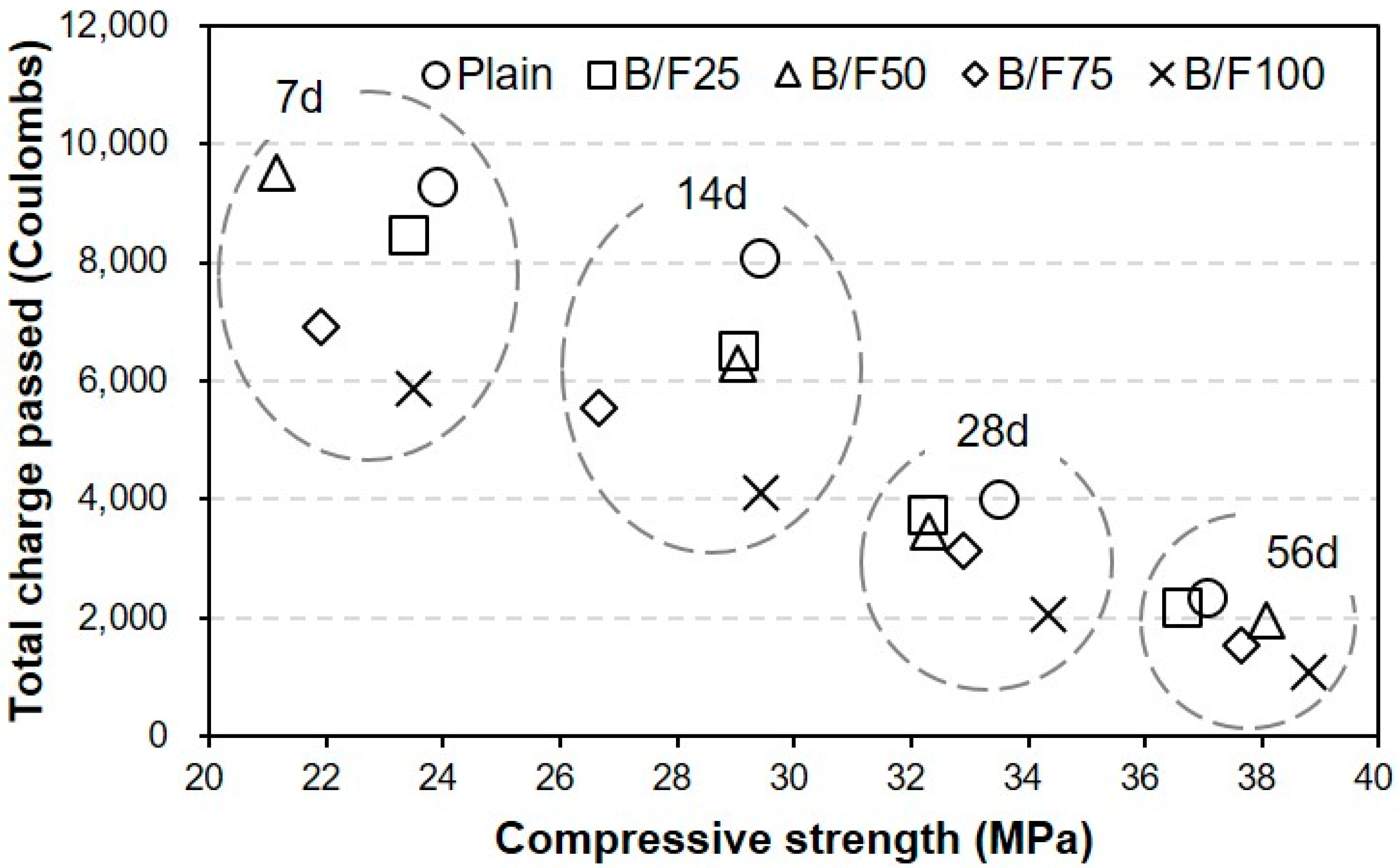
- After seven days, the charge passed through B/F100 was the smallest, approximately 37% smaller than that through the plain sample. After 28 days of curing, the chloride ion penetrabilities of all samples were moderate level according to ASTM C 1202. After 56 days, the chloride ion penetrabilities of B/F50, B/F75, and B/F100 were low level, approximately 17%, 34%, and 54% lower than that of the plain sample, respectively.
- (4)
- The resistances to penetration of chloride ions of the samples with the MSFAs were better than that of the plain sample. The tendency that the concrete with BFS has a good resistance to chloride ions is similar to those in previous reports.
- (5)
- The chloride ion penetrability decreased with the increase in compressive strength. In addition, the chloride ion penetrabilities of the samples with the MSFAs were lower than that of the plain sample at the same compressive strength.
- (6)
- The higher replacement ratio of MSFA led to a smaller carbonation depth. The carbonation depth (0.79 mm) of B/F100 was the smallest after 56 days. The results show that the use of the MSFA in the mortar or concrete can be effective for the improvement in carbonation resistance.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khatib, J.M. Properties of concrete incorporating fine recycled aggregate. Cem. Concr. Res. 2005, 35, 763–769. [Google Scholar] [CrossRef]
- Cho, B.S.; Kim, Y.U.; Kim, D.B.; Choi, S.J. Effect of ferronickel slag powder on microhydration heat, flow, compressive strength, and drying shrinkage of mortar. Adv. Civ. Eng. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Maslehuddin, M.; Sharif, A.M.; Shameem, M.; Ibrahim, M.; Barry, M.S. Comparison of properties of steel slag and crushed limestone aggregate concretes. Constr. Build. Mater. 2003, 17, 105–112. [Google Scholar] [CrossRef]
- Arellano Aguilar, R.; Burciaga Díaz, O.; Escalante García, J.I. Lightweight concretes of activated metakaolin-fly ash binders, with blast furnace slag aggregates. Constr. Build. Mater. 2010, 24, 1166–1175. [Google Scholar] [CrossRef]
- Saha, A.K.; Sarker, P.K. Durability of Mortar Incorporating Ferronickel Slag Aggregate and Supplementary Cementitious Materials Subjected to Wet-Dry Cycles. Int. J. Concr. Struct. Mater. 2018, 12, 29. [Google Scholar] [CrossRef]
- Choi, Y.C.; Choi, S. Alkali-silica reactivity of cementitious materials using ferro-nickel slag fine aggregates produced in different cooling conditions. Constr. Build. Mater. 2015, 99, 279–287. [Google Scholar] [CrossRef]
- Lee, H.G.; Bae, S.H.; Lee, H.J.; Choi, Y.W.; Cho, B.S. Mechanical Properties and Resistance to Freezing and Thawing of Concrete Using Air-Cooled Ferronickel Slag Fine Aggregate. J. Rec. Const. Resour. 2018, 6, 319–323. [Google Scholar]
- Ulubeyli, G.C.; Artir, R. Sustainability for Blast Furnace Slag: Use of Some Construction Wastes. Procedia Soc. Behav. Sci. 2015, 195, 2191–2198. [Google Scholar] [CrossRef]
- Li, G.; Zhao, X. Properties of concrete incorporating fly ash and ground granulated blast-furnace slag. Cem. Concr. Compos. 2003, 25, 293–299. [Google Scholar] [CrossRef]
- Sanjuán, M.Á.; Estévez, E.; Argiz, C.; del Barrio, D. Effect of curing time on granulated blast-furnace slag cement mortars carbonation. Cem. Concr. Compos. 2018, 90, 257–265. [Google Scholar] [CrossRef]
- Yazici, H. The effect of curing conditions on compressive strength of ultra high strength concrete with high volume mineral admixtures. Build. Environ. 2007, 42, 2083–2089. [Google Scholar] [CrossRef]
- Nataraja, M.C.; Dileep Kumar, P.G.; Manu, A.S.; Sanjay, M.C. Use of granulated blast furnace slag as fine aggregate in cement mortar. Int. J. Struct. Civ. Eng. Res. 2013, 2, 1–10. [Google Scholar]
- Patra, R.K.; Mukharjee, B.B. Influence of incorporation of granulated blast furnace slag as replacement of fine aggregate on properties of concrete. J. Clean. Prod. 2017, 165, 468–476. [Google Scholar] [CrossRef]
- KS F 2402. Standard Test Method for Concrete Slump; Korea Standards Association: Seoul, Korea, 2017. [Google Scholar]
- KS F 2421. Standard Test Method for Air Content of Fresh Concrete by the Pressure Method (Air Receiver Method); Korea Standards Association: Seoul, Korea, 2016. [Google Scholar]
- KS F 2405. Standard Test Method for Compressive Strength of Concrete; Korea Standards Association: Seoul, Korea, 2017. [Google Scholar]
- ASTM C1202. Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration; ASTM International: Philadelphia, PA, USA, 2000. [Google Scholar]
- KS L 2584. Standard Test Method for Accelerated Carbonation of Concrete; Korea Standards Association: Seoul, Korea, 2015. [Google Scholar]
- Valcuende, M.; Benito, F.; Parra, C.; Miñano, I. Shrinkage of self-compacting concrete made with blast furnace slag as fine aggregate. Constr. Build. Mater. 2015, 76, 1–9. [Google Scholar] [CrossRef]
- Subathra Devi, V.; Gnanavel, B.K. Properties of concrete manufactured using steel slag. Procedia Eng. 2014, 97, 95–104. [Google Scholar] [CrossRef]
- Leng, F.; Feng, N.; Lu, X. Experimental study on the properties of resistance to diffusion of chloride ions of fly ash and blast furnace slag concrete. Cem. Concr. Res. 2000, 30, 989–992. [Google Scholar] [CrossRef]
- Yeau, K.Y.; Kim, E.K. An experimental study on corrosion resistance of concrete with ground granulate blast-furnace slag. Cem. Concr. Res. 2005, 35, 1391–1399. [Google Scholar] [CrossRef]
- Mo, L.; Zhang, F.; Deng, M.; Jin, F.; Al-Tabbaa, A.; Wang, A. Accelerated carbonation and performance of concrete made with steel slag as binding materials and aggregates. Cem. Concr. Compos. 2017, 83, 138–145. [Google Scholar] [CrossRef]







| Type | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Comp. str. (MPa) | |
|---|---|---|---|---|---|---|---|---|
| 7 day | 28 day | |||||||
| Cement (C) | 17.43 | 6.50 | 3.57 | 64.40 | 2.55 | 1.17 | 42.7 | 56.5 |
| Blast furnace slag (BFS) powder | 30.61 | 13.98 | 0.32 | 40.71 | 6.43 | 0.60 | - | - |
| Fly ash (FA) | 64.88 | 20.56 | 6.06 | 2.58 | 0.80 | 1.45 | - | - |
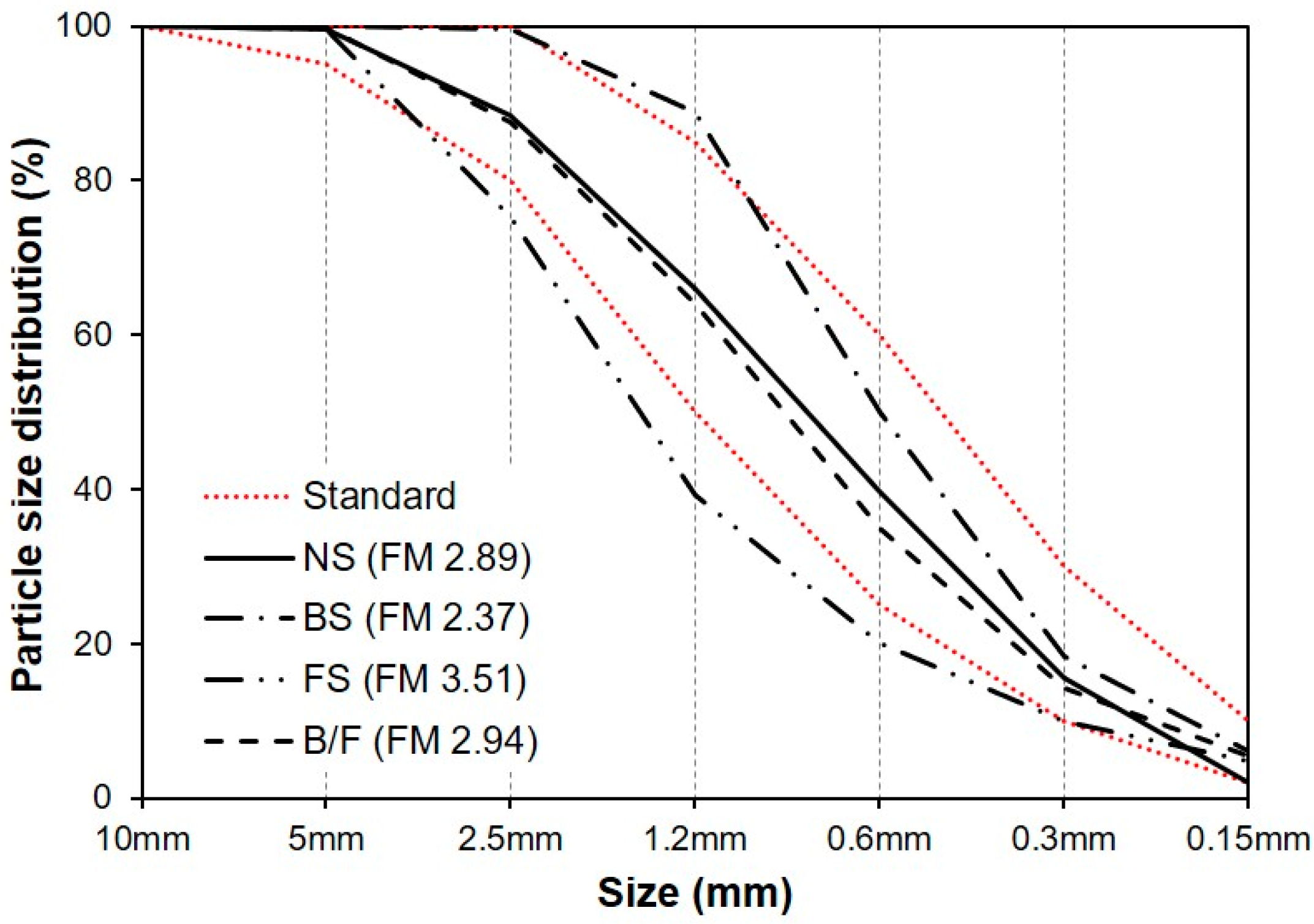
| Type | FM | Density (g/cm3) | Water Absorption (%) | Unit Weight (kg/L) | Ratio of Absolute Volume (%) |
|---|---|---|---|---|---|
| Natural sand (NS) | 2.89 | 2.63 | 1.1 | 1.645 | 62.56 |
| BFS sand (BS) | 2.37 | 2.81 | 2.1 | 1.737 | 61.80 |
| FNS sand (FS) | 3.51 | 3.04 | 0.6 | 1.871 | 61.56 |
| Mix | W/B (%) | S/a (%) | Unit Weight (kg/m3) | WRA (B*%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Water | Cement | BFS Powder | Fly Ash | NS | BS | FS | Gravel | ||||
| Plain | 51.8 | 47 | 176 | 238 | 68 | 34 | 812 | - | - | 916 | 0.9 |
| B/F25 | 176 | 238 | 68 | 34 | 609 | 109 | 117 | 916 | 0.7 | ||
| B/F50 | 176 | 238 | 68 | 34 | 406 | 219 | 234 | 916 | 0.5 | ||
| B/F75 | 176 | 238 | 68 | 34 | 203 | 328 | 351 | 916 | 0.3 | ||
| B/F100 | 176 | 238 | 68 | 34 | - | 437 | 469 | 916 | 0.2 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-J.; Kim, Y.-U.; Oh, T.-G.; Cho, B.-S. Compressive Strength, Chloride Ion Penetrability, and Carbonation Characteristic of Concrete with Mixed Slag Aggregate. Materials 2020, 13, 940. https://doi.org/10.3390/ma13040940
Choi S-J, Kim Y-U, Oh T-G, Cho B-S. Compressive Strength, Chloride Ion Penetrability, and Carbonation Characteristic of Concrete with Mixed Slag Aggregate. Materials. 2020; 13(4):940. https://doi.org/10.3390/ma13040940
Chicago/Turabian StyleChoi, Se-Jin, Young-Uk Kim, Tae-Gue Oh, and Bong-Suk Cho. 2020. "Compressive Strength, Chloride Ion Penetrability, and Carbonation Characteristic of Concrete with Mixed Slag Aggregate" Materials 13, no. 4: 940. https://doi.org/10.3390/ma13040940
APA StyleChoi, S.-J., Kim, Y.-U., Oh, T.-G., & Cho, B.-S. (2020). Compressive Strength, Chloride Ion Penetrability, and Carbonation Characteristic of Concrete with Mixed Slag Aggregate. Materials, 13(4), 940. https://doi.org/10.3390/ma13040940






