Influence of Mg, Cu, and Ni Dopants on Amorphous TiO2 Thin Films Photocatalytic Activity
Abstract
1. Introduction
2. Experimental Method
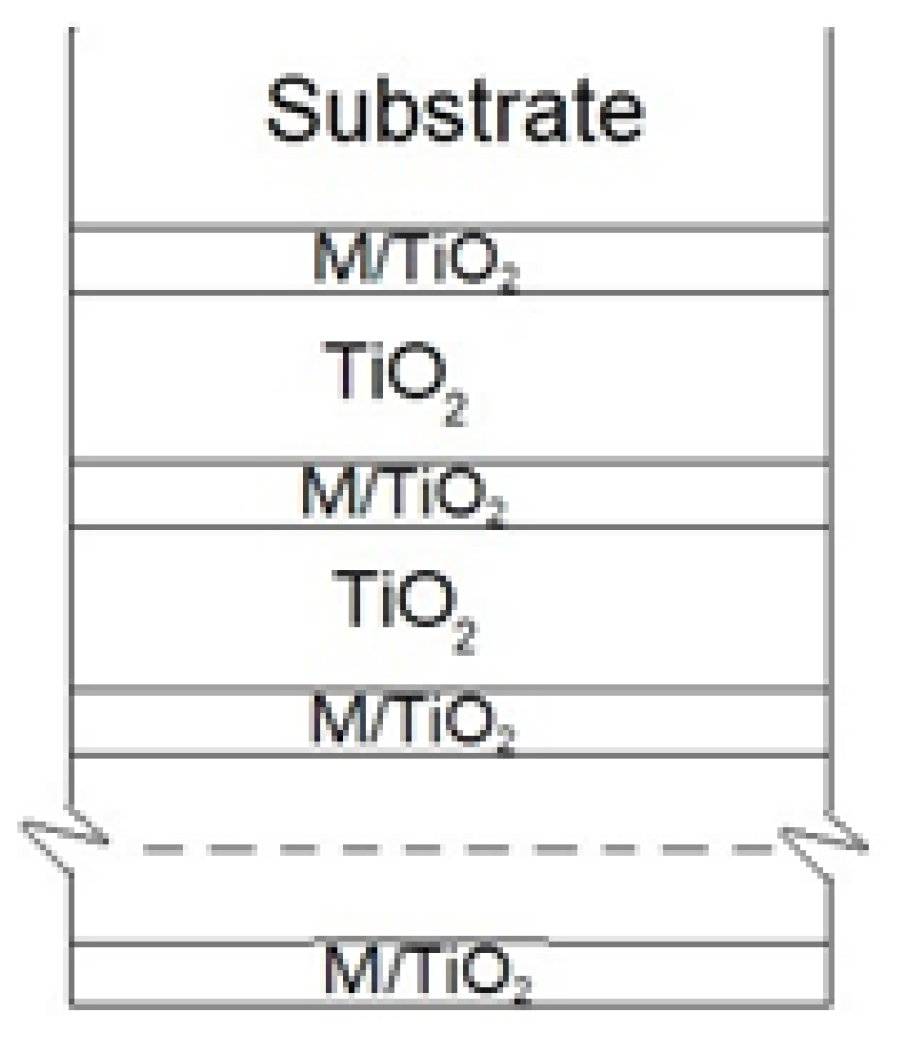
2.1. Films Preparation
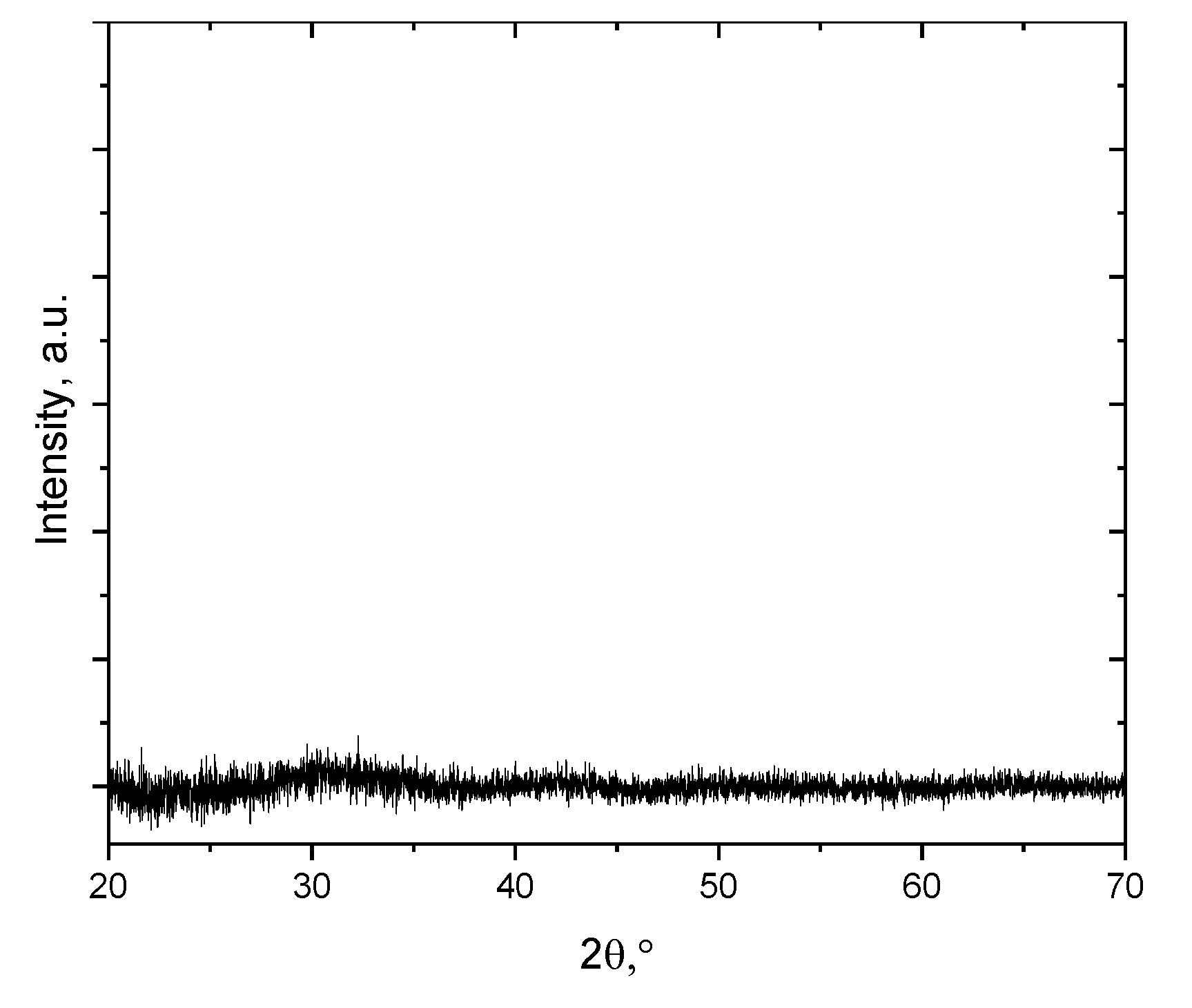
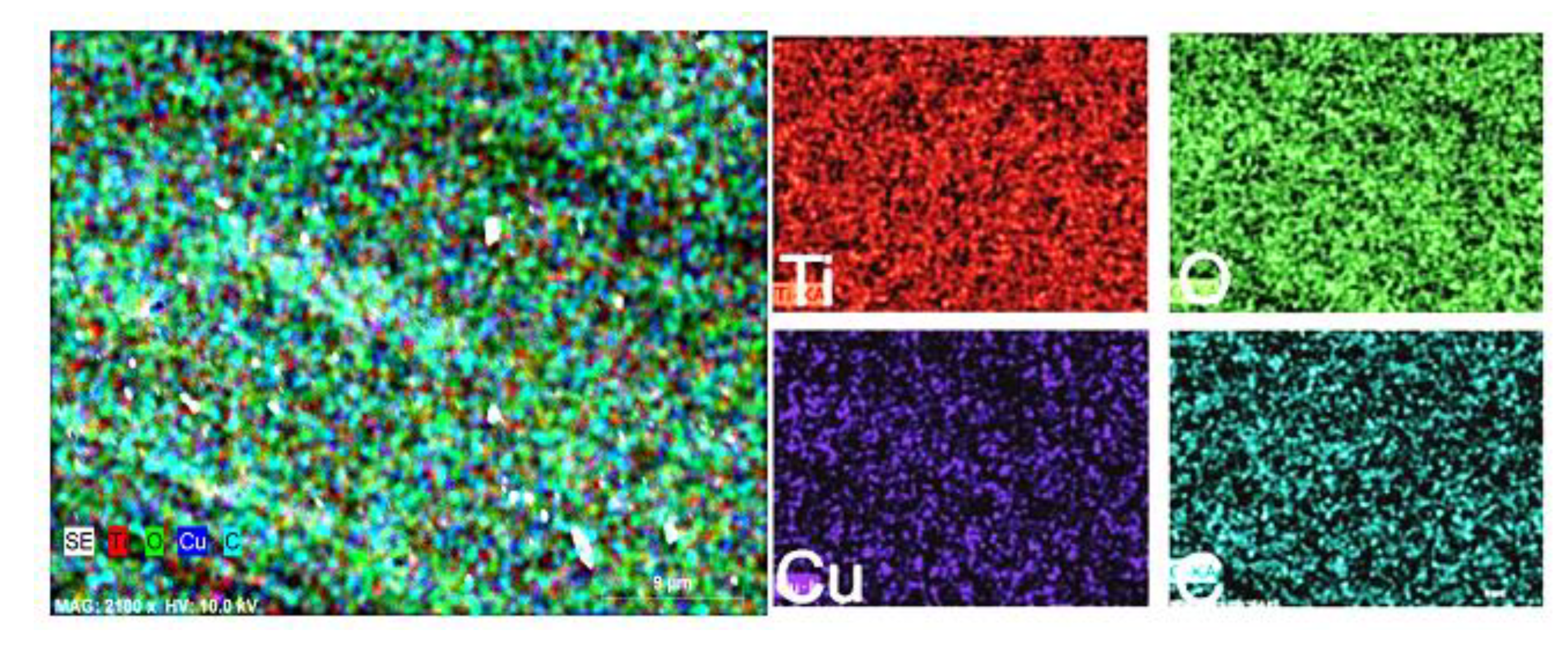
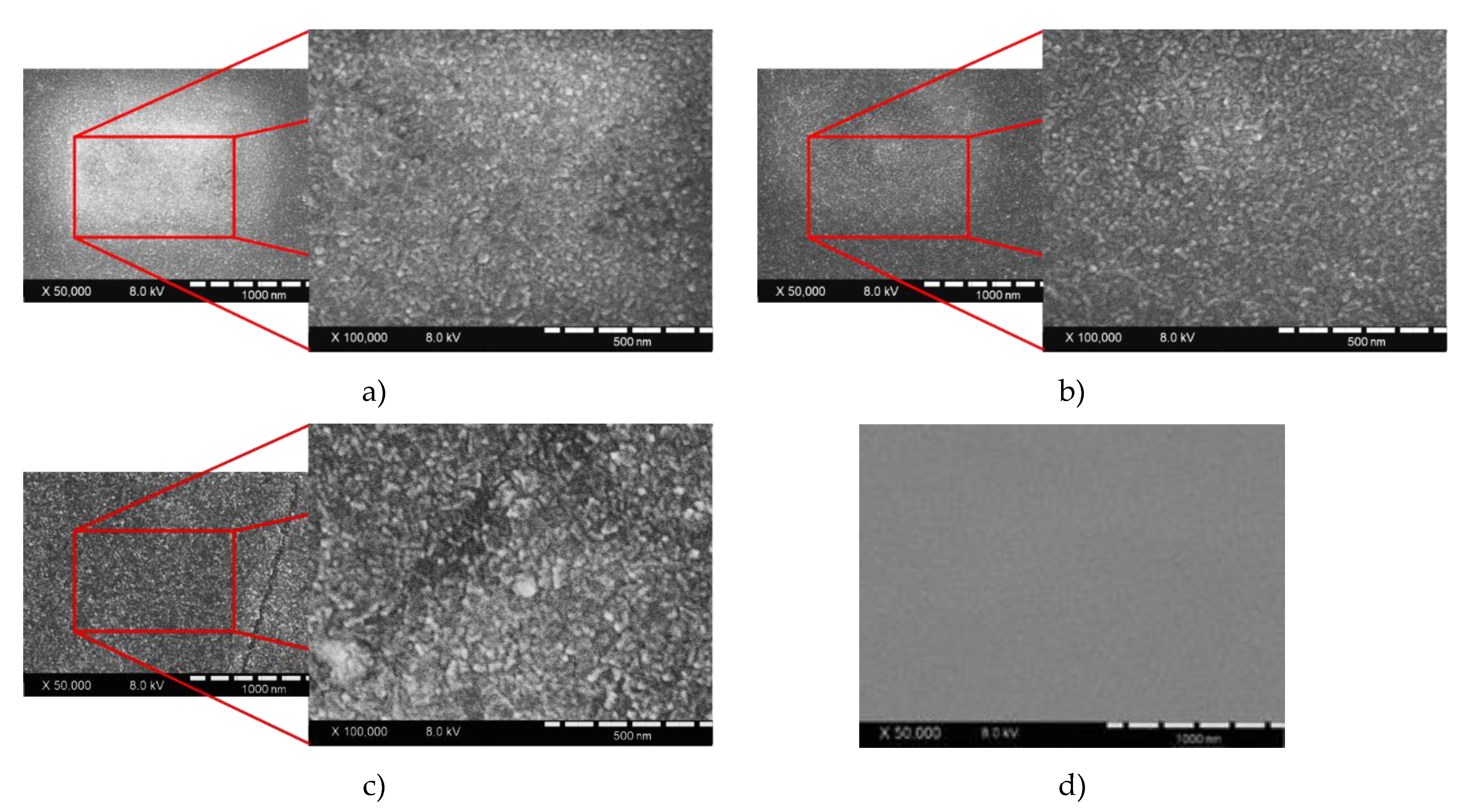
2.2. The Morphology and Structural Analysis
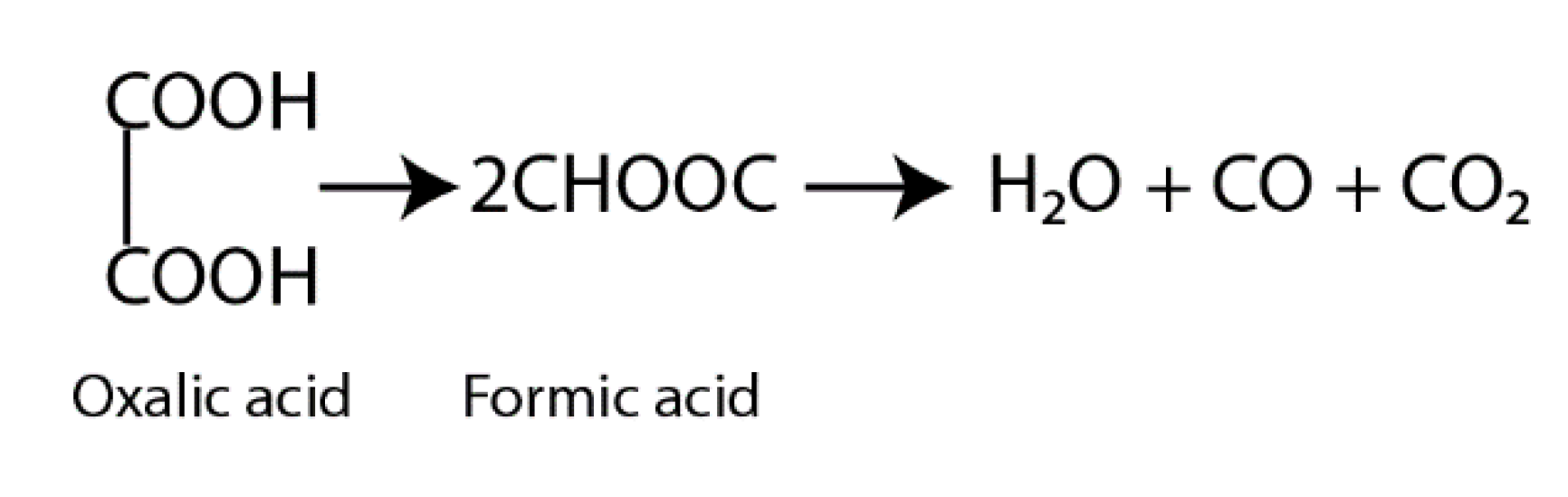
2.3. Photocatalysis Efficiency Evaluation
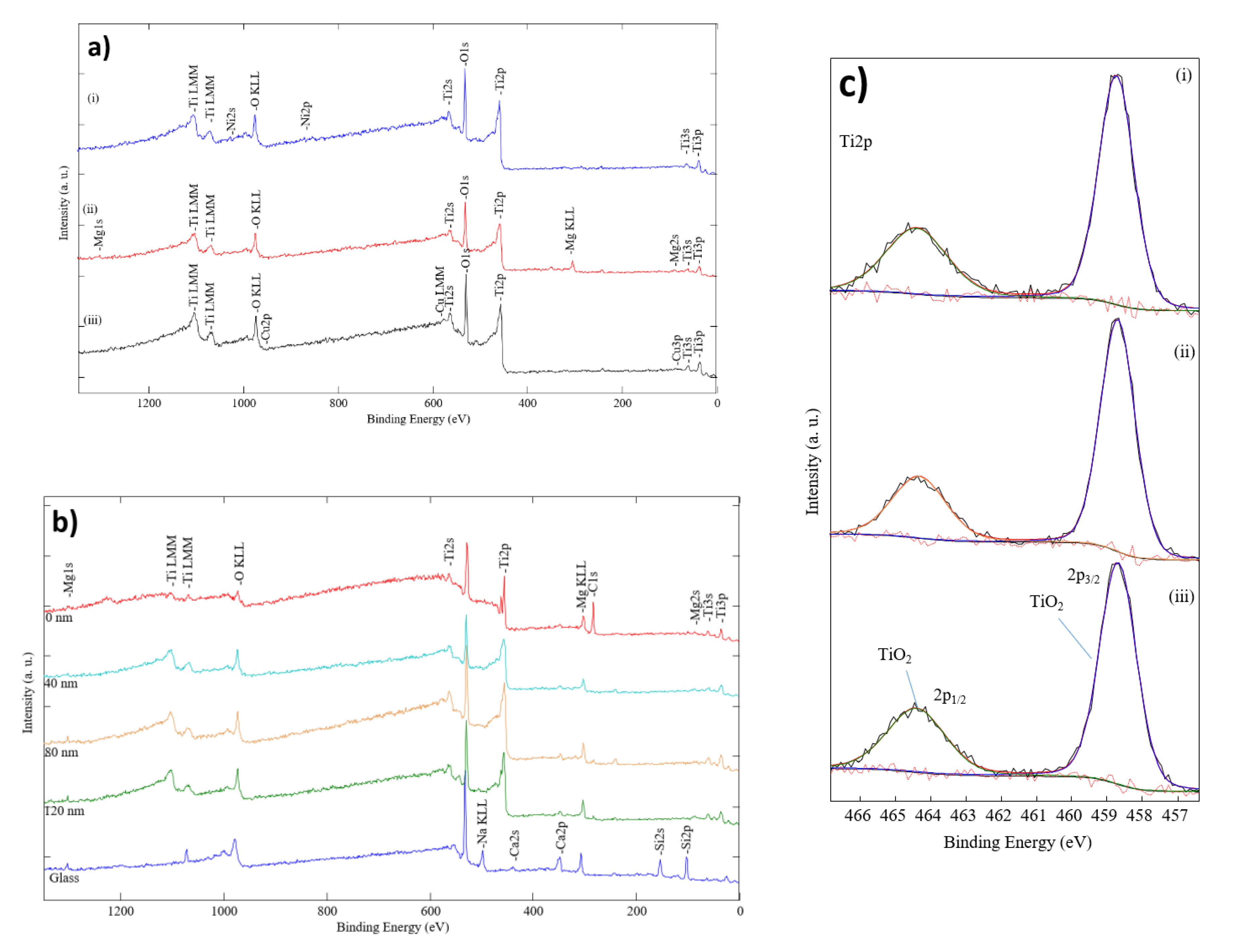
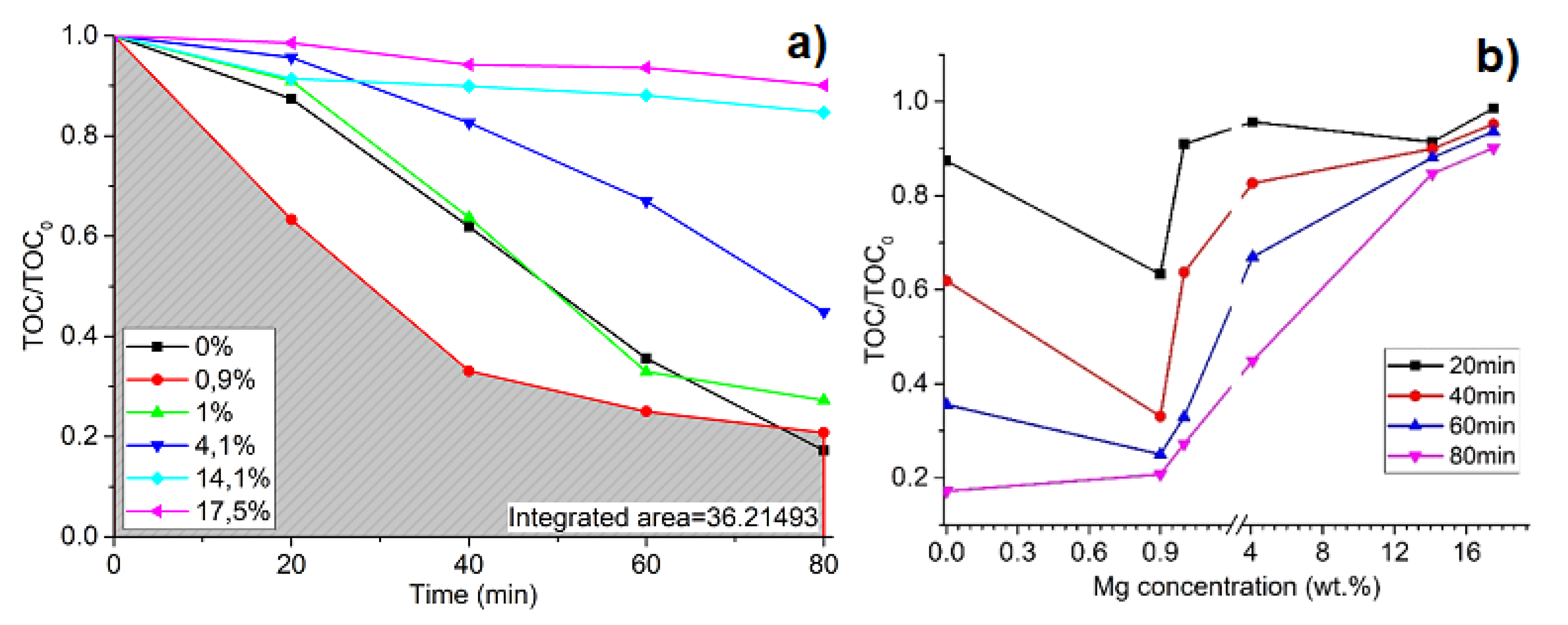
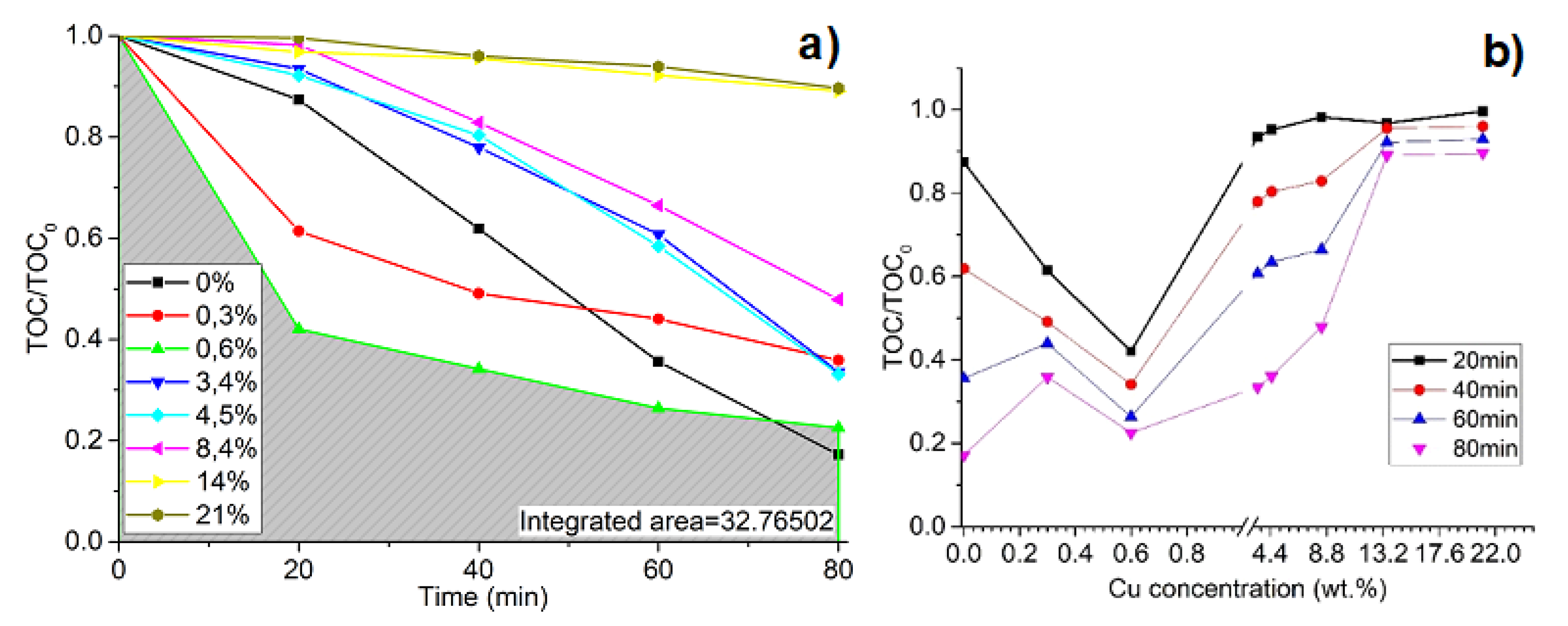
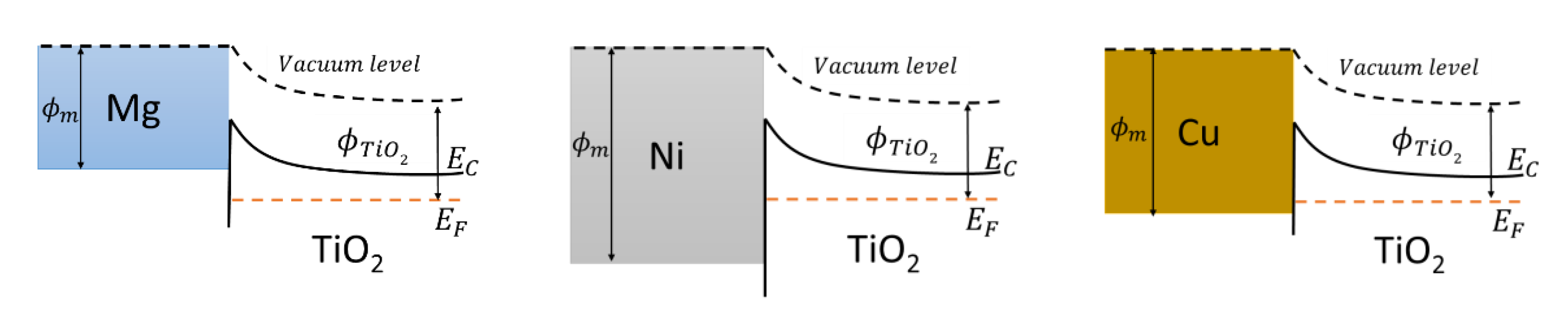
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Food Safety Authority. Food Colours: Titanium Dioxide Marks Re-Evaluation Milestone. Available online: https://www.efsa.europa.eu/en/press/news/160914 (accessed on 14 September 2016).
- Binas, V.; Venieri, D.; Kotzias, D.; Kiriakidis, G. Modified TiO2 based photocatalysts for improved air and health quality. J. Mater. 2017, 3, 3–16. [Google Scholar]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for environmental photocatalytic applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Khairy, M.; Zakaria, W. Effect of metal-doping of TiO2 nanoparticles on their photocatalytic activities toward removal of organic dyes. Egypt. J. Pet. 2014, 23, 419–426. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, X. Photocatalytic oxidation for indoor air purification: A literature review. Build. Environ. 2003, 38, 645–654. [Google Scholar] [CrossRef]
- Li, R. Latest progress in hydrogen production from solar water splitting via photocatalysis, photoelectrochemical, and photovoltaic-photoelectrochemical solutions. Chinese J. Catal. 2017, 38, 5–12. [Google Scholar] [CrossRef]
- Nosaka, Y. Solar cells and photocatalysts. Compr. Nanosci. Technol. 2011, 1, 571–605. [Google Scholar]
- Mansoob Khan, M.; Farooq Adil, S.; Al-Mayouf, A. Metal oxides as photocatalysts. J. Saudi Chem. Soc. 2015, 19, 462–464. [Google Scholar] [CrossRef]
- Yu, S.; Zhou, Y. Photochemical decomposition of hydrogen sulfide. Adv. Catal. Mater. Photocatal. Other Curr. Trends 2016, 3, 269–293. [Google Scholar]
- Wu, C.-H. Comparison of azo dye degradation efficiency using UV/single semiconductor and UV/coupled semiconductor systems. Chemosphere 2004, 57, 601–608. [Google Scholar] [CrossRef]
- Okamoto, K.; Yamamoto, Y.; Tanaka, H.; Itaya, A. Kinetics of Heterogeneous Photocatalytic Decomposition of Phenol over Anatase TiO2 Powder. Bull. Chem. Soc. Jpn. 1985, 58, 2023–2028. [Google Scholar] [CrossRef]
- Sakthi, S.; Neppolian, B.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. TiO2 catalysed photodegradation of leather dye, acid green 16. Journ al Sci. Ind. Res. 2000, 59, 556–562. [Google Scholar]
- Yildiz, A.; Lisesivdin, S.B.B.B.; Kasap, M.; Mardare, D. Electrical properties of TiO2 thin films. J. Non. Cryst. Solids 2008, 354, 4944–4947. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/Visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef] [PubMed]
- Dette, C.; Pérez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 anatase with a bandgap in the visible region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef]
- Abdullah, H.; Khan, M.M.R.; Ong, H.R.; Yaakob, Z. Modified TiO2 photocatalyst for CO2 photocatalytic reduction: An overview. J. CO2 Util. 2017, 22, 15–32. [Google Scholar] [CrossRef]
- Evtushenko, Y.M.; Romashkin, S.V.; Trofimov, N.S.; Chekhlova, T.K. Optical properties of TiO2 thin films. Phys. Procedia 2015, 73, 100–107. [Google Scholar] [CrossRef]
- Kaneco, S.; Shimizu, Y.; Ohta, K.; Mizuno, T. Photocatalytic reduction of high pressure carbon dioxide using TiO2 powders with a positive hole scavenger. J. Photochem. Photobiol. A Chem. 1998, 115, 223–226. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Castellote, M.; Bengtsson, N. Principles of TiO2 Photocatalysis. Applications of Titanium Dioxide Photocatalysis to Construction Materials: State-of-the-Art Report of the RILEM Technical Committee 194-TDP; Springer: Dordrecht, the Netherlands, 2011; pp. 5–10. [Google Scholar]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.E.; Cab, C.; de Coss, R.D.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. IOP Publ. Nanotechnol. Nanotechnol. 2008, 19, 145605–145610. [Google Scholar]
- Wan, L.; Li, J.F.F.; Feng, J.Y.Y.; Sun, W.; Mao, Z.Q.Q. Anatase TiO2 films with 2.2 eV band gap prepared by micro-arc oxidation. Mater. Sci. Eng. B 2007, 139, 216–220. [Google Scholar] [CrossRef]
- Shang, C.; Zhao, W.-N.; Liu, Z.-P. Searching for new TiO2 crystal phases with better photoactivity. J. Phys. Condens. Matter 2015, 27, 134203. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, F.; Di Valentin, C.; Fantacci, S.; Vittadini, A.; Selloni, A. Theoretical studies on anatase and less common TiO2 phases: Bulk, surfaces, and nanomaterials. Chem. Rev. 2014, 114, 9708–9753. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-T.; Ji, H.-B.; Huang, X.-J. Photocatalytic degradation of methyl orange over metalloporphyrins supported on TiO2 Degussa P25. Molecules 2012, 17, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Rui, Z.; Wu, S.; Peng, C.; Ji, H. Comparison of TiO2 Degussa P25 with anatase and rutile crystalline phases for methane combustion. Chem. Eng. J. 2014, 243, 254–264. [Google Scholar] [CrossRef]
- Bakardjieva, S.; Šubrt, J.; Štengl, V.; Dianez, M.J.; Sayagues, M.J. Photoactivity of anatase–rutile TiO2 nanocrystalline mixtures obtained by heat treatment of homogeneously precipitated anatase. Appl. Catal. B Environ. 2005, 58, 193–202. [Google Scholar] [CrossRef]
- Morgan, B.J.; Watson, G.W. Intrinsic n-type defect formation in TiO2: A comparison of rutile and anatase from GGA+U calculations. J. Phys. Chem. C 2010, 114, 2321–2328. [Google Scholar] [CrossRef]
- Ohno, T.; Tsubota, T.; Toyofuku, M.; Inaba, R. Photocatalytic activity of a TiO2 photocatalyst doped with C4+ and S4+ ions having a rutile phase under visible light. Catal. Letters 2004, 98, 255–258. [Google Scholar] [CrossRef]
- Prasai, B.; Cai, B.; Underwood, M.K.; Lewis, J.P.; Drabold, D.A. Properties of amorphous and crystalline titanium dioxide from first principles. J. Mater. Sci. 2012, 47, 7515–7521. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Lu, L.; Li, L. The photocatalytic properties of amorphous TiO2 composite films deposited by magnetron sputtering. Res. Chem. Intermed. 2012, 38, 487–498. [Google Scholar] [CrossRef]
- Liu, H.Y.; Hsu, Y.L.; Su, H.Y.; Huang, R.C.; Hou, F.Y.; Tu, G.C.; Liu, W.H. A comparative study of amorphous, anatase, rutile, and mixed phase TiO2 films by mist chemical vapor deposition and ultraviolet photodetectors applications. IEEE Sens. J. 2018, 18, 4022–4029. [Google Scholar] [CrossRef]
- Kaur, K.; Singh, C.V. Amorphous TiO2 as a photocatalyst for hydrogen production: A DFT study of structural and electronic properties Selection and/or peer-review under responsibility of Canadian Hydrogen and Fuel Cell Association. Energy Procedia 2012, 29, 291–299. [Google Scholar] [CrossRef]
- Khramov, E.; Kotolevich, Y.; Ramos, J.G.; Pestryakov, A.; Zubavichus, Y.; Bogdanchikova, N. Amorphization of Degussa nanosized TiO2 caused by its modification. Fuel 2018, 234, 312–317. [Google Scholar] [CrossRef]
- Hu, S.; Shaner, M.R.; Beardslee, J.A.; Lichterman, M.; Brunschwig, B.S.; Lewis, N.S. Amorphous TiO2 coatings stabilize Si, GaAs, and GaP photoanodes for efficient water oxidation. Science 2014, 344, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Kato, S.; Aoki, T.; Sirghi, L.; Hatanaka, Y. Role of terminal OH groups on the electrical and hydrophilic properties of hydro-oxygenated amorphous TiOx: OH thin films. J. Appl. Phys. 2001, 90, 3391–3395. [Google Scholar] [CrossRef]
- Ohtani, B.; Ogawa, Y. Nishimoto, Photocatalytic Activity of Amorphous-Anatase Mixture of Titanium(IV) Oxide Particles Suspended in Aqueous Solutions. J. Phys. Chem. B 1997, 5647, 3746–3752. [Google Scholar] [CrossRef]
- Brinker, C.J.; Frye, G.C.; Hurd, A.J.; Ashley, C.S. Fundamentals of sol-gel dip coating. Thin Solid Films 1991, 201, 97–108. [Google Scholar] [CrossRef]
- Kavaliunas, V.; Sestakauskaite, A.; Sriubas, M.; Laukaitis, G. Influence of Deposition Parameters on the Structure of TiO2 thin Films Prepared by Reactive Magnetron Sputtering Technique. Recent Advances in Technology Research and Education; Springer: Cham, Switzerland, 2018; pp. 49–57. [Google Scholar]
- Senthil, T.S.; Muthukumarasamy, N.; Agilan, S.; Thambidurai, M.; Balasundaraprabhu, R. Preparation and characterization of nanocrystalline TiO2 thin films prepared By sol-gel spin coating method. Mater. Sci. Eng. B 2010, 174, 102–104. [Google Scholar] [CrossRef]
- Lin, H.; Huang, C.P.; Li, W.; Ni, C.; IsmatShah, S.; Yao-Hsuan, T. Size dependency of nanocrystalline TiO2 on its optical property and photocatalytic reactivity exemplified by 2-chlorophenol. Appl. Catal. B Environ. 2006, 68, 1–11. [Google Scholar] [CrossRef]
- Tan, R.-J.; Tseng, Y.-H.; Kuo, C.-H. Crystal size control of TiO2 using experimental strategies in sol–gel process. Micro Nano Lett. 2010, 5, 361. [Google Scholar] [CrossRef]
- Karkare, M.M. Estimation of band gap and particle size of TiO2 nanoparticle synthesized using sol gel technique. In Proceedings of the 2014 International Conference on Advances in Communication and Computing Technologies (ICACACT 2014), Mumbai, India, 10–11 August 2014; pp. 2–6. [Google Scholar]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Damm, C.; Herrmann, R.; Israel, G.; Müller, F.W. Acrylate photopolymerization on heterostructured TiO2 photocatalysts. Dye. Pigment. 2007, 74, 335–342. [Google Scholar] [CrossRef]
- Yuvaraj, H.; Park, E.J.; Gal, Y.-S.; Lim, K.T. Synthesis and characterization of polypyrrole–TiO2 nanocomposites in supercritical CO2. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 313, 300–303. [Google Scholar] [CrossRef]
- Cho, S.; Choi, W. Solid-phase photocatalytic degradation of PVC–TiO2 polymer composites. J. Photochem. Photobiol. A Chem. 2001, 143, 221–228. [Google Scholar] [CrossRef]
- Varnagiris, S.; Medvids, A.; Lelis, M.; Milcius, D.; Antuzevics, A. Black carbon-doped TiO2 films: Synthesis, characterization and photocatalysis. J. Photochem. Photobiol. A Chem. 2019, 382, 111941. [Google Scholar] [CrossRef]
- Dariani, R.S.; Esmaeili, A.; Mortezaali, A.; Dehghanpour, S. Photocatalytic reaction and degradation of methylene blue on TiO2 nano-sized particles. Opt. Int. J. Light Electron Opt. 2016, 127, 7143–7154. [Google Scholar] [CrossRef]
- Al-Shamali, S.S. Photocatalytic degradation of methylene blue in the presence of TiO2 catalyst assisted solar radiation. Aust. J. Basic Appl. Sci. 2013, 7, 172–176. [Google Scholar]
- Huang, H.; Gu, X.; Zhou, J.; Ji, K.; Liu, H.; Feng, Y. Photocatalytic degradation of Rhodamine B on TiO2 nanoparticles modified with porphyrin and iron-porphyrin. Catal. Commun. 2009, 11, 58–61. [Google Scholar] [CrossRef]
- Mazlina, T.; Darus, M.; Muktar, J. Degradation of Rhodamine B dye by TiO2 nanotubes photocatalyst synthesized via alkaline hydrothermal method. MATEC Web Conf. 2015, 27. [Google Scholar] [CrossRef]
- Bin Mukhlish, M.Z.; Najnin, F.; Rahman, M.M.; Uddin, M.J. Photocatalytic degradation of different dyes using TiO2 with high surface area: A kinetic study. J. Sci. Res. 2013, 5, 301–314. [Google Scholar] [CrossRef]
- Guo, J.; Yuan, S.; Jiang, W.; Yue, H.; Cui, Z.; Liang, B. Adsorption and photocatalytic degradation behaviors of rhodamine dyes on surface-fluorinated TiO2 under visible irradiation. RSC Adv. 2016, 6, 4090–4100. [Google Scholar] [CrossRef]
- Barka, N.; Qourzal, S.; Assabbane, A.; Nounah, A.; Ait-Ichou, Y. Factors influencing the photocatalytic degradation of Rhodamine B by TiO2-coated non-woven paper. J. Photochem. Photobiol. A Chem. 2008, 195, 346–351. [Google Scholar] [CrossRef]
- Ollis, D.F. Kinetics of liquid phase photocatalyzed reactions: An illuminating approach. J. Phys. Chem. B 2005, 109, 2439–2444. [Google Scholar] [CrossRef] [PubMed]
- Emeline, A.V.; Rudakova, A.V.; Ryabchuk, V.K.; Serpone, N. Photostimulated reactions at the surface of wide band-gap metal oxides (ZrO2 and TiO2): Interdependence of rates of reactions on pressure−concentration and on light intensity. J. Phys. Chem. B 1998, 102, 10906–10916. [Google Scholar] [CrossRef]
- Villarreal, T.L.; Gomez, R.; Neumann-Spallart, M.; Alonso-Vante, N.; Salvador, P. Semiconductor photooxidation of pollutants dissolved in water: A kinetic model for distinguishing between direct and indirect interfacial hole transfer. I. Photoelectrochemical experiments with polycrystalline anatase electrodes under current doubling and absence of recombination. J. Phys. Chem. B 2004, 108, 15172–15181. [Google Scholar]
- Manzanares, M.; Fàbrega, C.; Ossó, J.O.; Vega, L.F.; Andreu, T.; Morante, J.R. Engineering the TiO2 outermost layers using magnesium for carbon dioxide photoreduction. Appl. Catal. B Environ. 2014, 150, 57–62. [Google Scholar] [CrossRef]
- Zu, D.; Xu, Z.; Zhang, A.; Wang, H.; Wei, H.; Ou, G.; Huang, K.; Zhang, R.; Li, L.; Hu, S.; et al. Room temperature Mg reduction of TiO2: Formation mechanism and application in photocatalysis. Chem. Commun. 2019, 55, 7675–7678. [Google Scholar] [CrossRef]
- Bensouici, F.; Bououdina, M.; Dakhel, A.A.; Tala-Ighil, R.; Tounane, M.; Iratni, A.; Souier, T.; Liu, S.; Cai, W. Optical, structural and photocatalysis properties of Cu-doped TiO2 thin films. Appl. Surf. Sci. 2017, 395, 110–116. [Google Scholar] [CrossRef]
- Jung, M.; Scott, J.; Ng, Y.H.; Jiang, Y.; Amal, R. CuOx dispersion and reducibility on TiO2 and its impact on photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2014, 39, 12499–12506. [Google Scholar] [CrossRef]
- Tasbihi, M.; Kočí, K.; Troppová, I.; Edelmannová, M.; Reli, M.; Čapek, L.; Schomäcker, R. Photocatalytic reduction of carbon dioxide over Cu/TiO2 photocatalysts. Environ. Sci. Pollut. Res. 2018, 25, 34903–34911. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.T.; Chan, A.; Sun-Waterhouse, D.; Moriga, T.; Idriss, H.; Waterhouse, G.I. Ni/TiO2: A promising low-cost photocatalytic system for solar H2 production from ethanol-water mixtures. J. Catal. 2015, 326, 43–53. [Google Scholar] [CrossRef]
- Yu, J.; Hai, Y.; Cheng, B. Enhanced photocatalytic H2-production activity of TiO2 by Ni(OH)2 cluster modification. J. Phys. Chem. C 2011, 115, 4953–4958. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Xiong, X.; Liu, S.; Xu, Y. Role of Ni2+ ions in TiO2 and Pt/TiO2 photocatalysis for phenol degradation in aqueous suspensions. Appl. Catal. B Environ. 2019, 258, 117903. [Google Scholar] [CrossRef]
- Miranda-García, N.; Suárez, S.; Maldonado, M.I.; Malato, S.; Sánchez, B. Regeneration approaches for TiO2 immobilized photocatalyst used in the elimination of emerging contaminants in water. Catal. Today 2014, 230, 27–34. [Google Scholar] [CrossRef]
- Cunha, D.L.; Kuznetsov, A.; Achete, C.A.; da Hora Machado, A.E.; Marques, M. Immobilized TiO2 on glass spheres applied to heterogeneous photocatalysis: Photoactivity, leaching and regeneration process. PeerJ 2018, 6, e4464. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Srikanth, B.; Goutham, R.; Narayan, R.B.; Ramprasath, A.; Gopinath, K.P.; Sankaranarayanan, A.R. Recent advancements in supporting materials for immobilised photocatalytic applications in waste water treatment. J. Environ. Manage. 2017, 200, 60–78. [Google Scholar] [CrossRef]
- Navabpour, P.; Ostovarpour, S.; Tattershall, C.; Cooke, K.; Kelly, P.; Verran, J.; Whitehead, K.; Hill, C.; Raulio, M.; Priha, O. Photocatalytic TiO2 and doped TiO2 coatings to improve the hygiene of surfaces used in food and beverage processing—a study of the physical and chemical resistance of the coatings. Coatings 2014, 4, 433–449. [Google Scholar] [CrossRef]
- Bloh, J.Z.; Dillert, R.; Bahnemann, D.W. Designing optimal metal-doped photocatalysts: Correlation between photocatalytic activity, doping ratio, and particle size. J. Phys. Chem. C 2012, 116, 25558–25562. [Google Scholar] [CrossRef]
- Michaelson, H.B. The work function of the elements and its periodicity. J. Appl. Phys. 1977, 48, 4729–4733. [Google Scholar] [CrossRef]

| Mg/TiO2 | Cu/TiO2 | Ni/TiO2 | TiO2 | OA | |||
|---|---|---|---|---|---|---|---|
| Conc., % | kapp, min−1 | Conc., % | kapp, min−1 | Conc., % | kapp, min−1 | kapp, min−1 | kapp, min−1 |
| 0.9 | 0.01866 | 0.3 | 0.01373 | 0.5 | 0.01317 | 0.01160 | 0.00080 |
| 1 | 0.01015 | 0.6 | 0.02221 | 1.4 | 0.00519 | ||
| 4.1 | 0.00474 | 3.4 | 0.00631 | 3.6 | 0.00214 | ||
| 14.1 | 0.00227 | 4.5 | 0.00646 | 11.6 | 0.00170 | ||
| 17.5 | 0.00092 | 8.4 | 0.00432 | 17.9 | 0.00152 | ||
| 13.5 | 0.00111 | 20.2 | 0.00114 | ||||
| 21 | 0.00073 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavaliunas, V.; Krugly, E.; Sriubas, M.; Mimura, H.; Laukaitis, G.; Hatanaka, Y. Influence of Mg, Cu, and Ni Dopants on Amorphous TiO2 Thin Films Photocatalytic Activity. Materials 2020, 13, 886. https://doi.org/10.3390/ma13040886
Kavaliunas V, Krugly E, Sriubas M, Mimura H, Laukaitis G, Hatanaka Y. Influence of Mg, Cu, and Ni Dopants on Amorphous TiO2 Thin Films Photocatalytic Activity. Materials. 2020; 13(4):886. https://doi.org/10.3390/ma13040886
Chicago/Turabian StyleKavaliunas, Vytautas, Edvinas Krugly, Mantas Sriubas, Hidenori Mimura, Giedrius Laukaitis, and Yoshinori Hatanaka. 2020. "Influence of Mg, Cu, and Ni Dopants on Amorphous TiO2 Thin Films Photocatalytic Activity" Materials 13, no. 4: 886. https://doi.org/10.3390/ma13040886
APA StyleKavaliunas, V., Krugly, E., Sriubas, M., Mimura, H., Laukaitis, G., & Hatanaka, Y. (2020). Influence of Mg, Cu, and Ni Dopants on Amorphous TiO2 Thin Films Photocatalytic Activity. Materials, 13(4), 886. https://doi.org/10.3390/ma13040886






