Synthesis and Characterization of the CaTiO3:Eu3+ Red Phosphor by an Optimized Microwave-Assisted Sintering Process
Abstract
1. Introduction
2. Experimental Procedures
2.1. Sample Preparation
2.2. Characterization Techniques
3. Results and Discussion
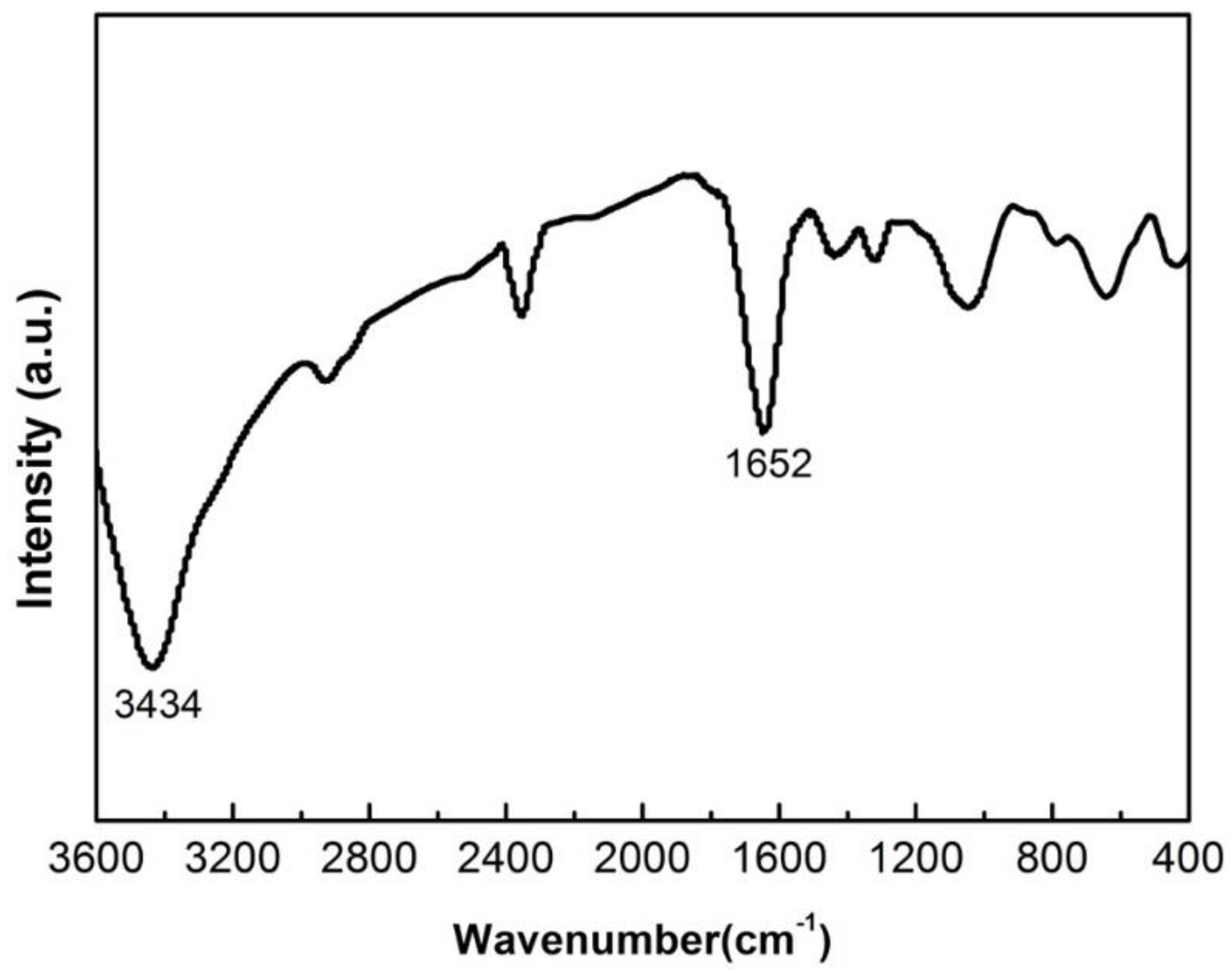
3.1. Precursor Analysis
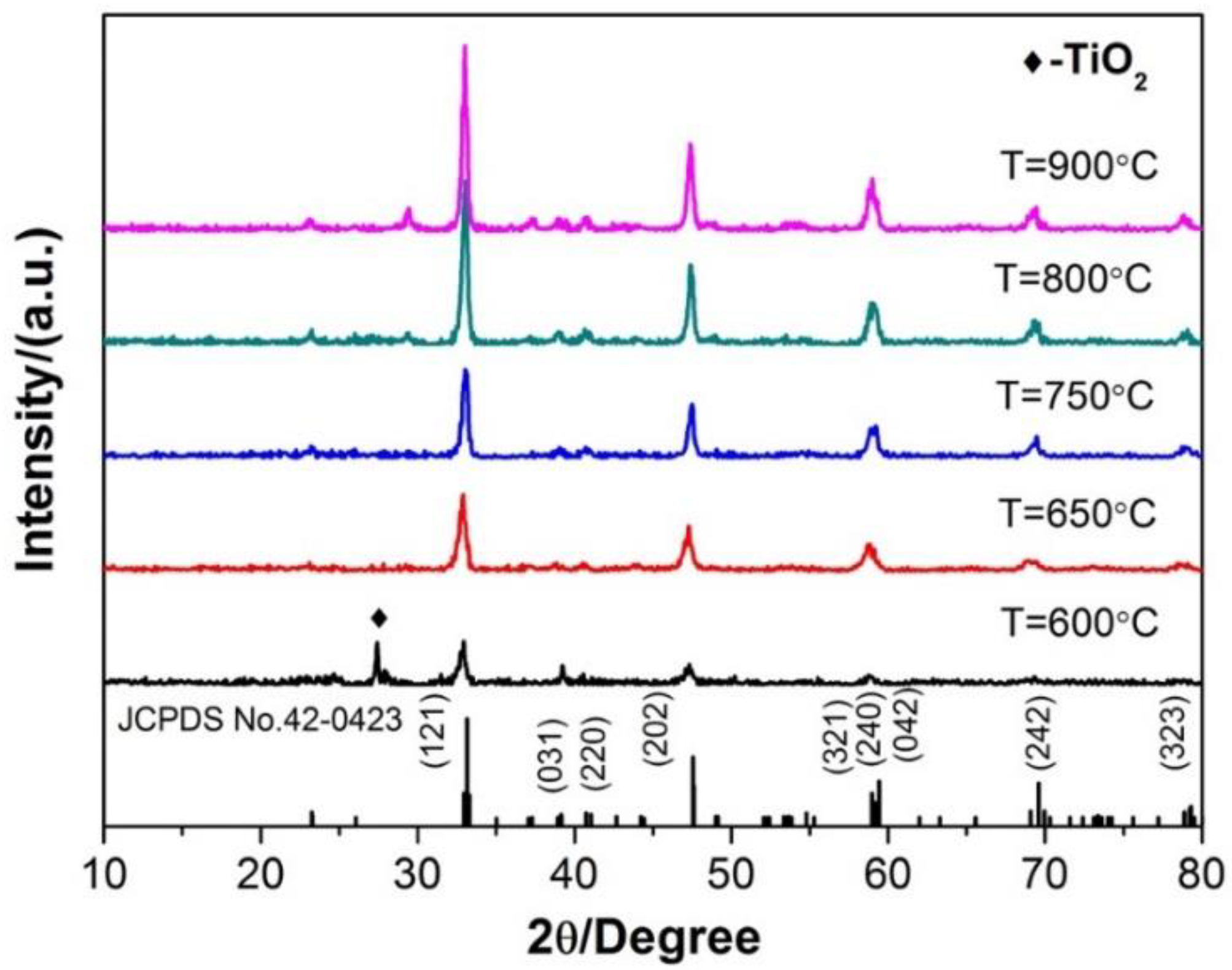
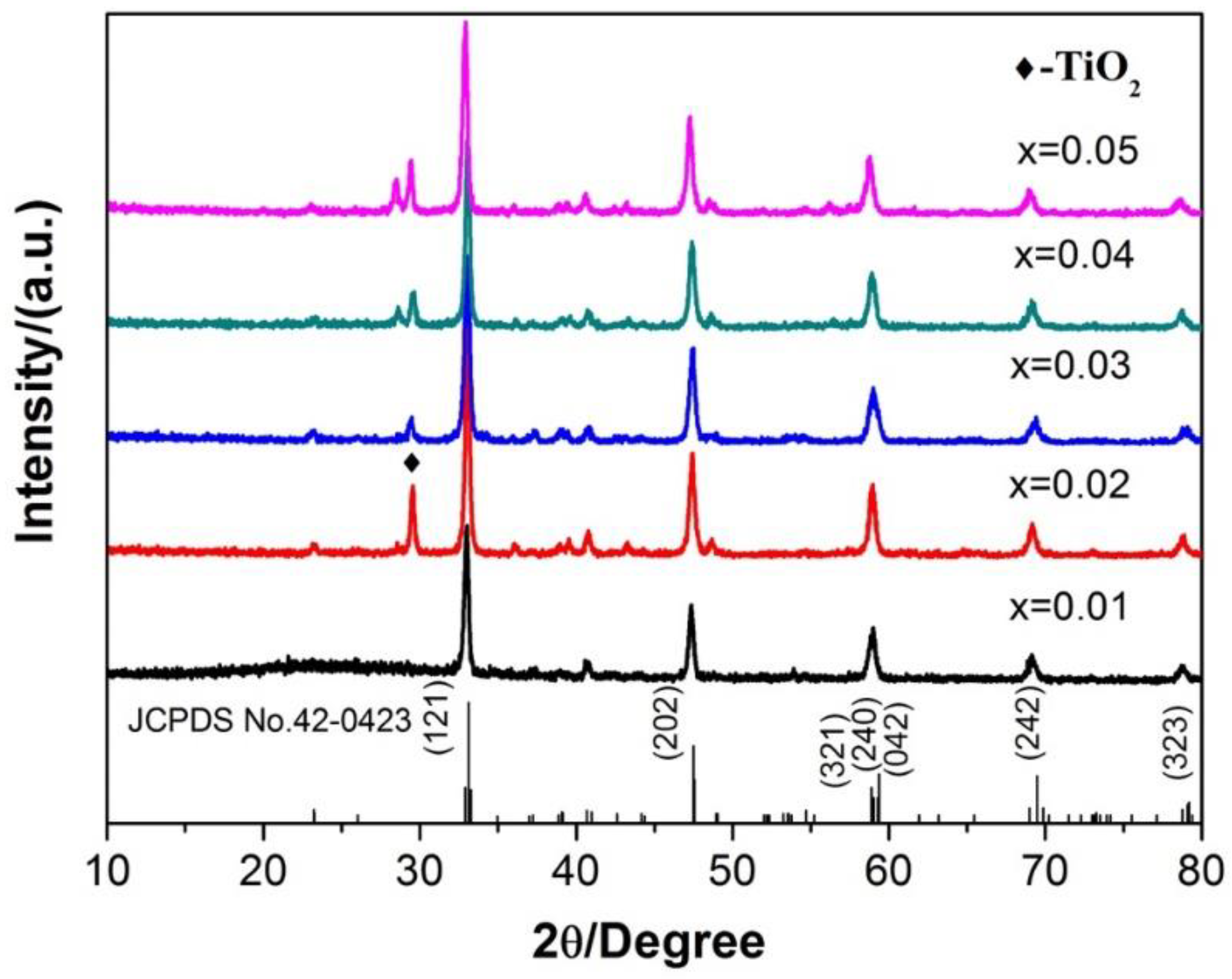
3.2. Ca1-xTiO3:Eu3+x Phosphor Synthesized by the Traditional Sintering Processes
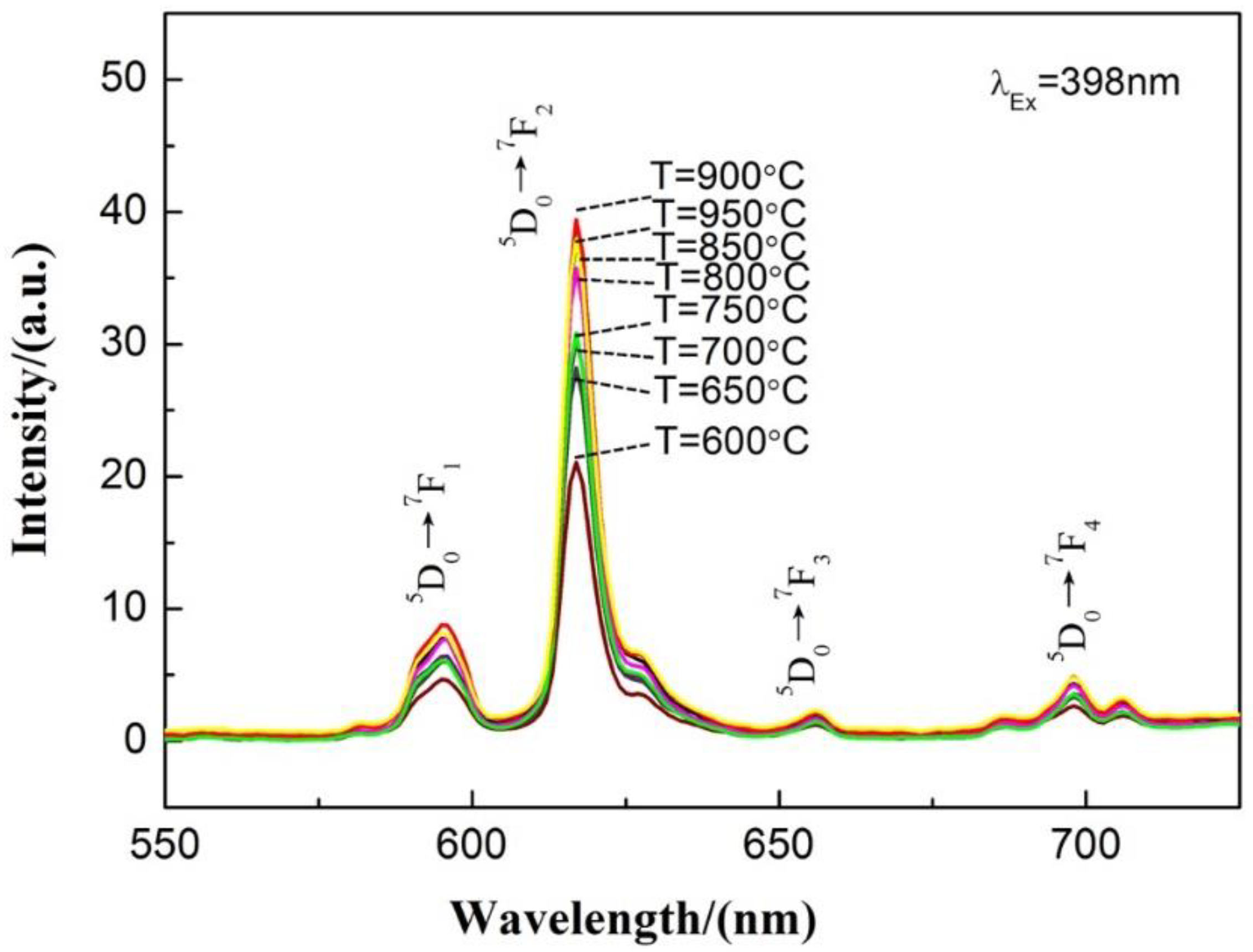
3.3. Ca0.97TiO3: Eu3+0.03 Phosphor Synthesized by the Microwave-Assisted Sintering
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Orihashi, T.; Adachi, S. Synthesis condition and structural/luminescent properties of CaTiO3:Eu3+ red phosphor. J. Alloy. Compd. 2015, 646, 1116. [Google Scholar] [CrossRef]
- Mazzo, T.M.; Moreira, M.L.; Pinatti, I.M.; Picon, F.C.; Leite, E.R.; Rosa IL, V.; Varela, J.A.; Perazolli, L.A.; Longo, E. CaTiO3:Eu3+ obtained by microwave assisted hydrothermal method: A photoluminescent approach. Opt. Mater. 2010, 32, 990. [Google Scholar] [CrossRef]
- Liu, P.; Yin, J.; Mi, X.; Zhang, L.; Bie, L. Enhanced photoluminescence of CaTiO3:Eu3+ red phosphors prepared by H3BO3 assisted solid state synthesis. J. Rare Earths 2013, 31, 555. [Google Scholar] [CrossRef]
- Oliveira, L.H.; Savioli, J.; de Moura, A.P.; Nogueira, I.C.; Li, M.S.; Longo, E.; Varela, J.A.; Rosa, I.L.V. Investigation of structural and optical properties of CaTiO3 powders doped with Mg2+ and Eu3+ ions. J. Alloy. Compd. 2015, 647, 265. [Google Scholar] [CrossRef]
- Ha, M.G.; Lee, J.H.; Bae, J.S.; Kim, J.P.; Hong, K.S.; Yang, H.-S. Photophysical properties of highly efficient red-emitting CaTiO3:Eu3+ phosphors under near ultra-violet excitation. Curr. Appl. Phys. 2011, 11, 1379. [Google Scholar] [CrossRef]
- Som, S.; Kunti, A.K.; Kumar, V.; Kumar, V.; Dutta, S.; Chowdhury, M.; Sharma, S.K.; Terblans, J.J.; Swart, H.C. Defect correlated fluorescent quenching and electron phonon coupling in the spectral transition of Eu3+ in CaTiO3 for red emission in display application. J. Appl. Phys. 2014, 115. [Google Scholar] [CrossRef]
- Yang, W.; Hu, J. Synthesis and luminescence properties of hexagonal CaTiO3:Eu3+ nanosheets. J. Lumin. 2014, 145, 144. [Google Scholar] [CrossRef]
- Wu, Y.-F.; Nien, Y.-T.; Wang, Y.-J.; Chen, I.-G.; McKittrick, J. Enhancement of Photoluminescence and Color Purity of CaTiO3:Eu Phosphor by Li Doping. J. Am. Ceram. Soc. 2012, 95, 1360. [Google Scholar] [CrossRef]
- Singh, D.K.; Manam, J. Structural and luminescence properties of CaTiO3: Eu3+ phosphor synthesized by chemical co-precipitation method for the application of solid state lighting devices. Aip Conf. Proc. 2016, 1728. [Google Scholar] [CrossRef]
- Đačanin, L.R.; Lukić-Petrović, S.R.; Petrović, D.M.; Nikolić, M.G.; Dramićanin, M.D. Temperature quenching of luminescence emission in Eu3+- and Sm3+- doped YNbO4 powders. J. Lumin. 2014, 151, 82. [Google Scholar]
- Zhang, P.; Xu, M.-X.; Zheng, Z.-T.; Sun, B.; Zhang, Y.-H. Microwave synthesis and characterization of new red long afterglow phosphor Sr3Al2O6: Eu. Trans. Nonferrous Met. Soc. China 2006, 16, S423–S425. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Weng, M.-H.; Chang, S.-J.; Yang, R.-Y. Preparation of Sr2SiO4: Eu3+ phosphors by microwave-assisted sintering and their luminescent properties. Ceram. Int. 2012, 38, 125. [Google Scholar] [CrossRef]
- Cai, W.; Fu, C.; Hu, W.; Chen, G.; Deng, X. Effects of microwave sintering power on microstructure, dielectric, ferroelectric and magnetic properties of bismuth ferrite ceramics. J. Alloy. Compd. 2013, 554, 64. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Liu, L.; Xie, R.-J.; Huang, Q. Microwave assisted sintering of thermally stable BaMgAl10O17: Eu2+ phosphors. Ecs J. Solid State Sci. Technol. 2013, 2, 196. [Google Scholar] [CrossRef]
- Rybakov, K.I.; Olevsky, E.A.; Krikun, E.V. Microwave sintering: Fundamentals and modeling. J. Am. Ceram. Soc. 2013, 96, 1003. [Google Scholar] [CrossRef]
- Potdar, H.; Deshpande, S.; Deshpande, A.; Khollam, Y.; Patil, A.; Pradhan, S.; Date, S. Simplified chemical route for the synthesis of barium titanyl oxalate (BTO). Int. J. Inorg. Mater. 2001, 3, 613. [Google Scholar] [CrossRef]
- Malghe, Y.; Gurjar, A.; Dharwadkar, S. Synthesis of BaTiO3 powder from barium titanyl oxalate (BTO) precursor employing microwave heating technique. Bull. Mater. Sci. 2004, 27, 217. [Google Scholar] [CrossRef]
- Feng, H.; Li, X.; Ji, H.; Zhou, Z. Preparation of Ba0.55Sr0.4Ca0.05TiO3 Powders by Chemical Co-Precipitation for Low Temperature Sintering Ferroelectric Ceramics. Integr. Ferroelectr. 2011, 129, 95. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, X.; Guo, J.; Liu, F.; Li, Z.; Xu, G.; Cui, P. Fabrication of uniform core-shell structural calcium and titanium precipitation particles and enhanced electrorheological activities. Nanotechnology 2009, 20. [Google Scholar] [CrossRef]
- Lee, H.-G.; Gopalan, A.-I.; Sai-Anand, G.; Lee, B.-C.; Kang, S.-W.; Lee, K.-P. Facile synthesis of functionalized graphene-palladium nanoparticle incorporated multicomponent TiO2 composite nanofibers. Mater. Chem. Phys. 2015, 154, 125–136. [Google Scholar] [CrossRef]
- Hezam, M.; Qaid SM, H.; Bedja, I.M.; Alharbi, F.; Nazeeruddin, M.K.; Aldwayyan, A. Synthesis of Pure Brookite Nanorods in a Nonaqueous Growth Environment. Crystals 2019, 9, 562. [Google Scholar] [CrossRef]


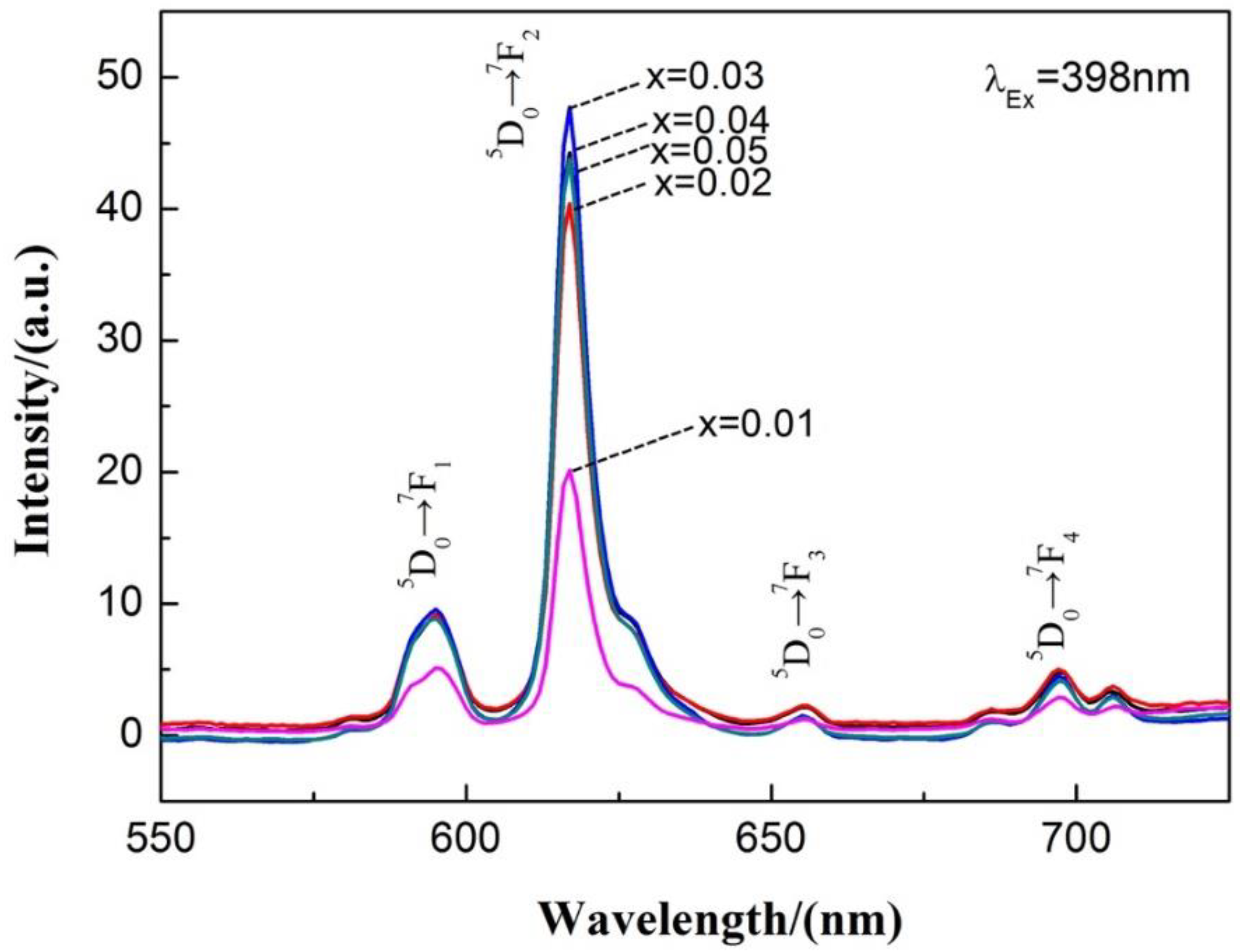
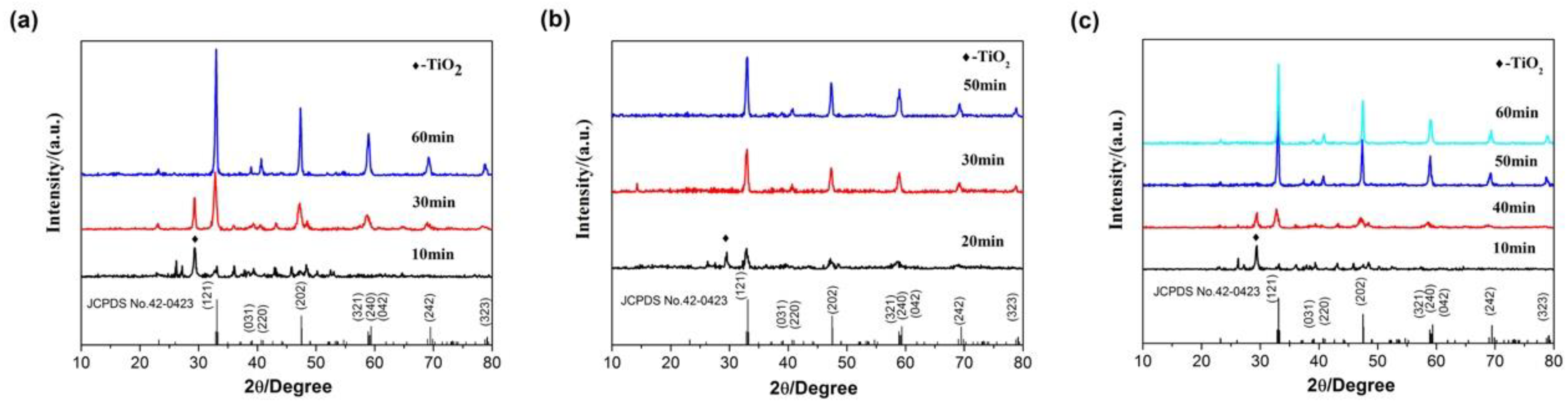

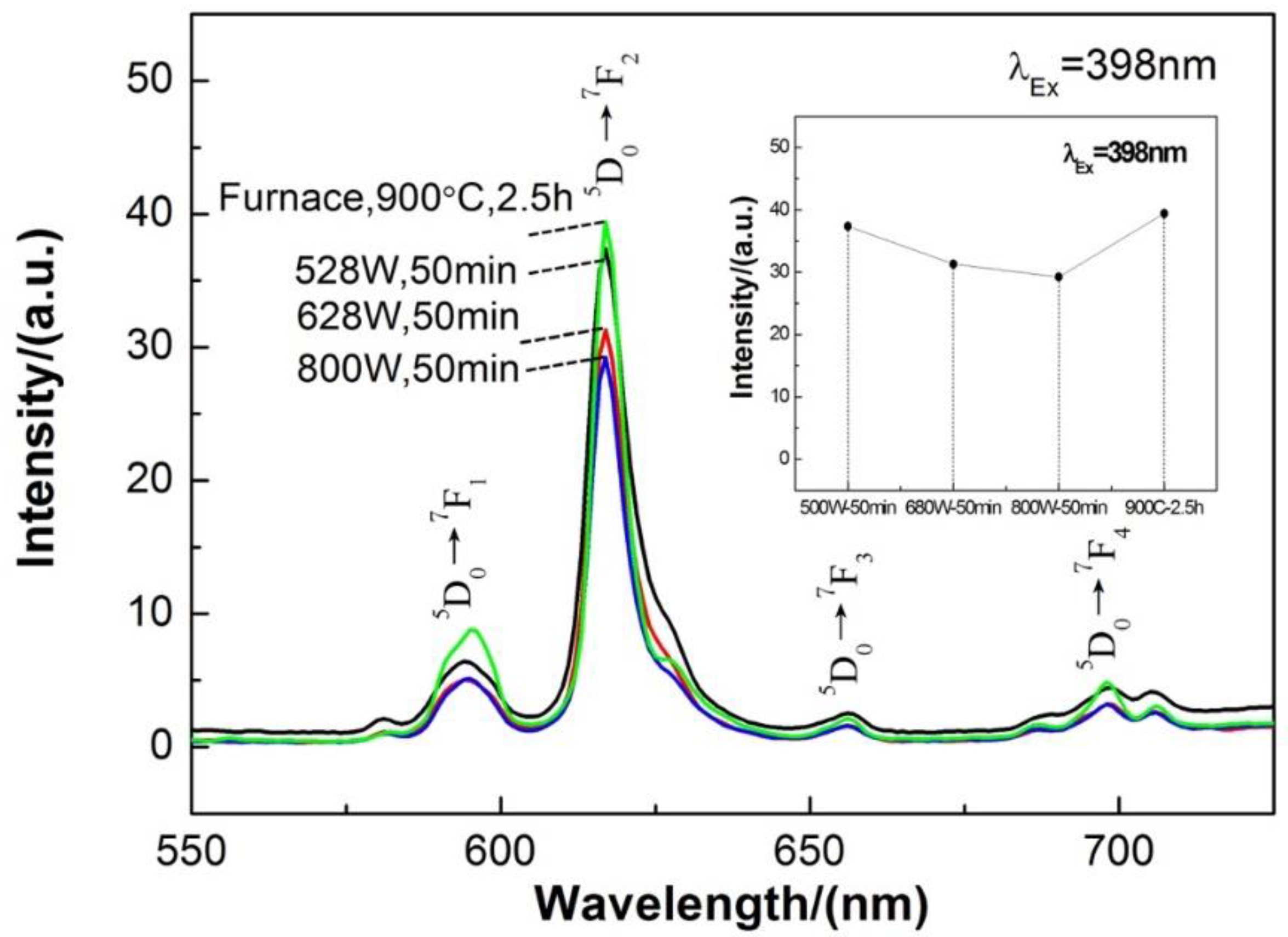

| Phosphor | Lattice Parameters | ||||
|---|---|---|---|---|---|
| X | a(Å) | b(Å) | c(Å) | v(Å3) | |
| Ca1-xTiO3: Eu3+x | 0.01 | 5.442 | 7.649 | 5.421 | 225.654 |
| 0.02 | 5.440 | 7.665 | 5.404 | 225.334 | |
| 0.03 | 5.439 | 7.645 | 5.403 | 224.927 | |
| 0.04 | 5.419 | 7.662 | 5.406 | 224.460 | |
| 0.05 | 5.433 | 7.662 | 5.377 | 223.832 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Lu, J.; Wang, R.; Dong, Y.; Ding, L. Synthesis and Characterization of the CaTiO3:Eu3+ Red Phosphor by an Optimized Microwave-Assisted Sintering Process. Materials 2020, 13, 874. https://doi.org/10.3390/ma13040874
Wang H, Lu J, Wang R, Dong Y, Ding L. Synthesis and Characterization of the CaTiO3:Eu3+ Red Phosphor by an Optimized Microwave-Assisted Sintering Process. Materials. 2020; 13(4):874. https://doi.org/10.3390/ma13040874
Chicago/Turabian StyleWang, Haifeng, Jianwei Lu, Ruoxuan Wang, Yungu Dong, and Linfeng Ding. 2020. "Synthesis and Characterization of the CaTiO3:Eu3+ Red Phosphor by an Optimized Microwave-Assisted Sintering Process" Materials 13, no. 4: 874. https://doi.org/10.3390/ma13040874
APA StyleWang, H., Lu, J., Wang, R., Dong, Y., & Ding, L. (2020). Synthesis and Characterization of the CaTiO3:Eu3+ Red Phosphor by an Optimized Microwave-Assisted Sintering Process. Materials, 13(4), 874. https://doi.org/10.3390/ma13040874




