Mesoporous Acidic Catalysts Synthesis from Dual-Stage and Rising Co-Current Gasification Char: Application for FAME Production from Waste Cooking Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of DSC-SO4 and RCC-SO4 Catalysts
2.3. Produced Catalysts Characterization
2.4. Transesterification Reaction with Produced Catalysts
2.5. Reusability of the Catalyst
2.6. Biodiesel Analysis
3. Results and Discussion
3.1. Characterization of DSC-SO4 and RCC-SO4 Catalysts
3.1.1. Surface Area Analysis
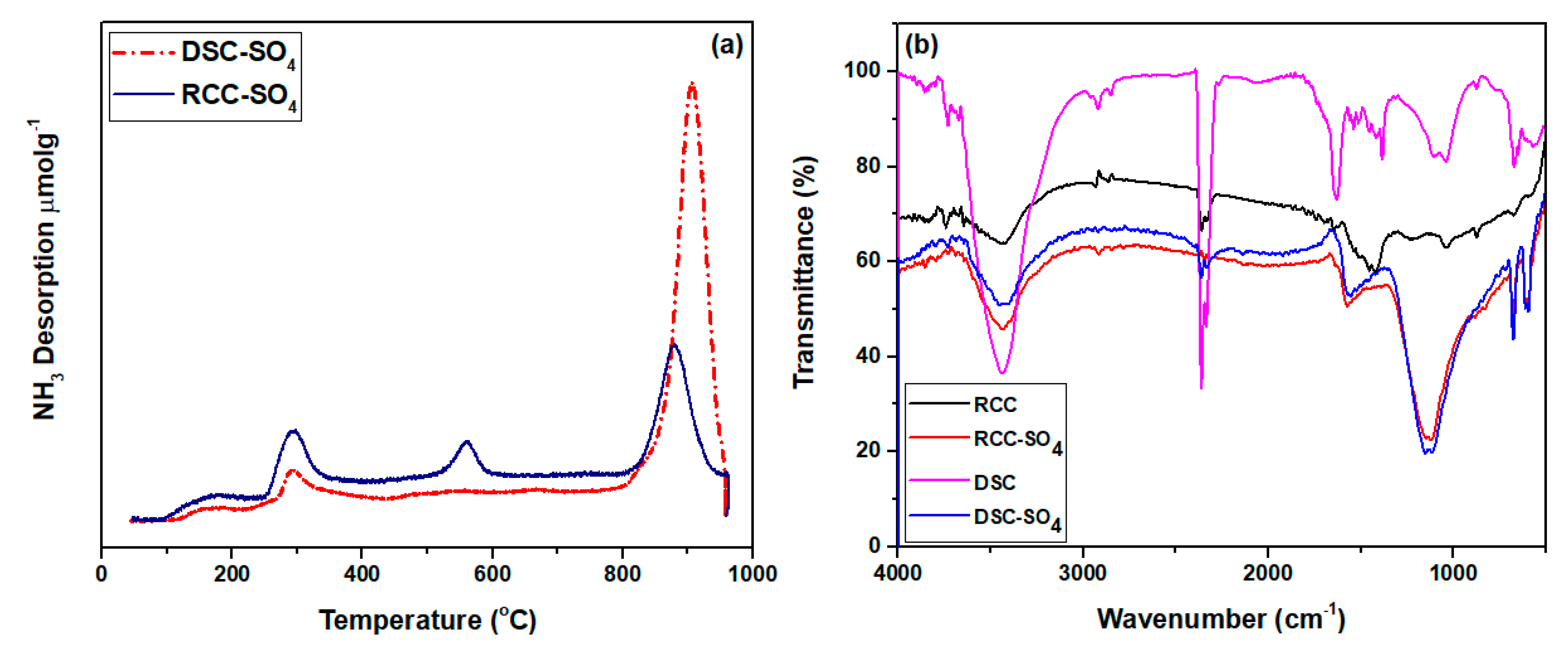
3.1.2. Acid Density Analysis via NH3-TPD
3.1.3. Functional Groups Determination
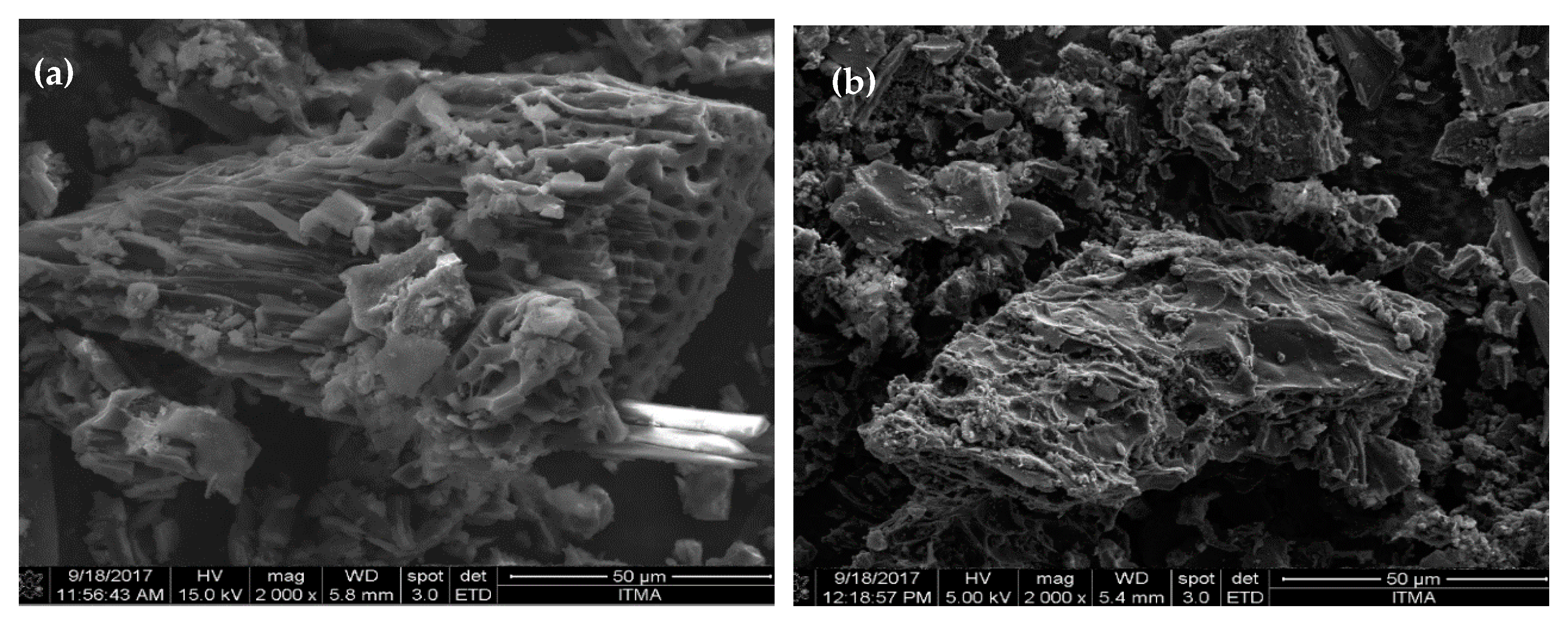
3.1.4. Morphology Evaluation of DSC-SO4 and RCC-SO4 Catalysts
3.2. Optimization Study for FAME Production
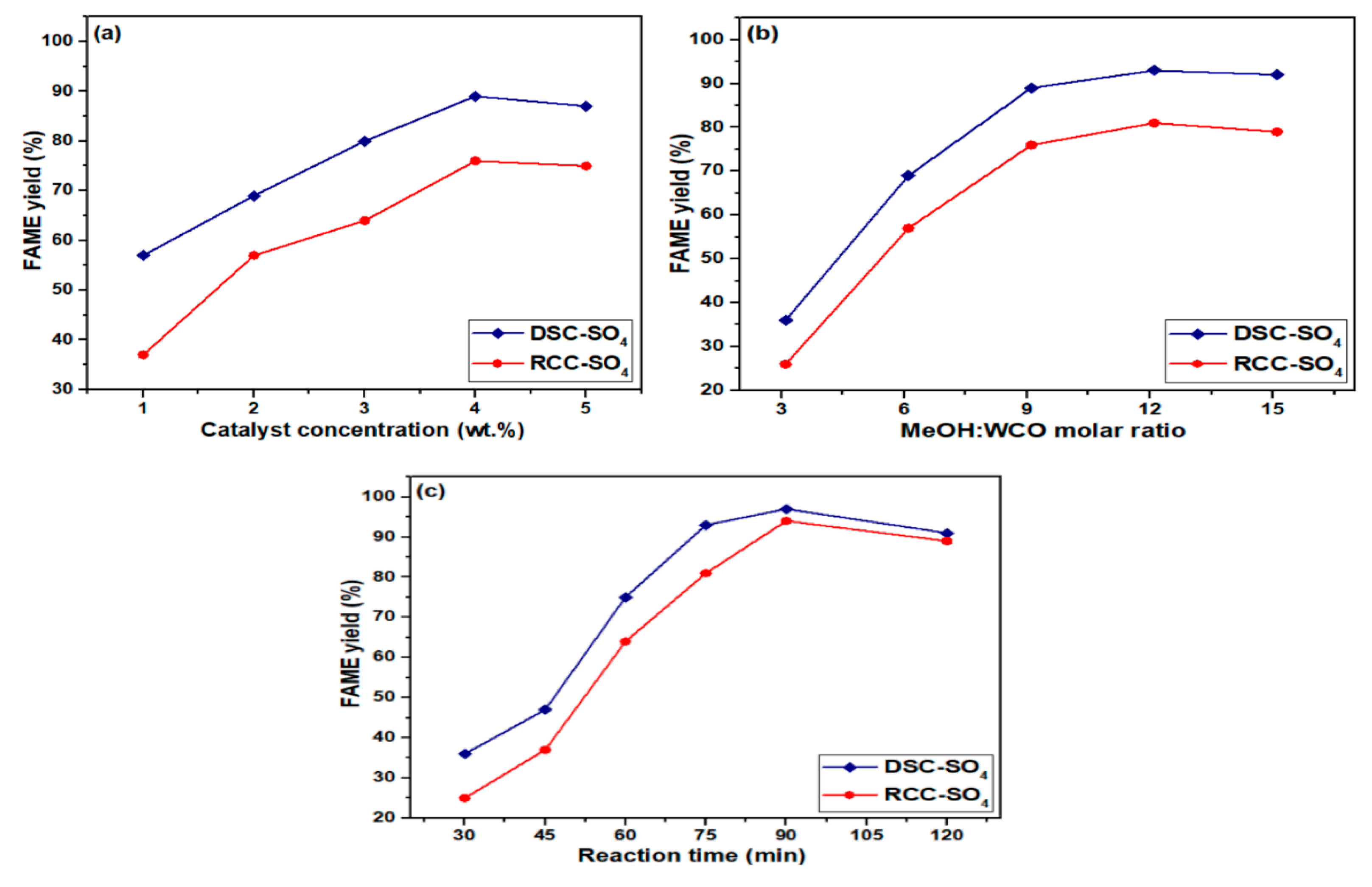
3.2.1. Influence of Catalyst Concentration
3.2.2. Influence of Methanol/Oil Molar Ratio
3.2.3. Influence of Reaction Time
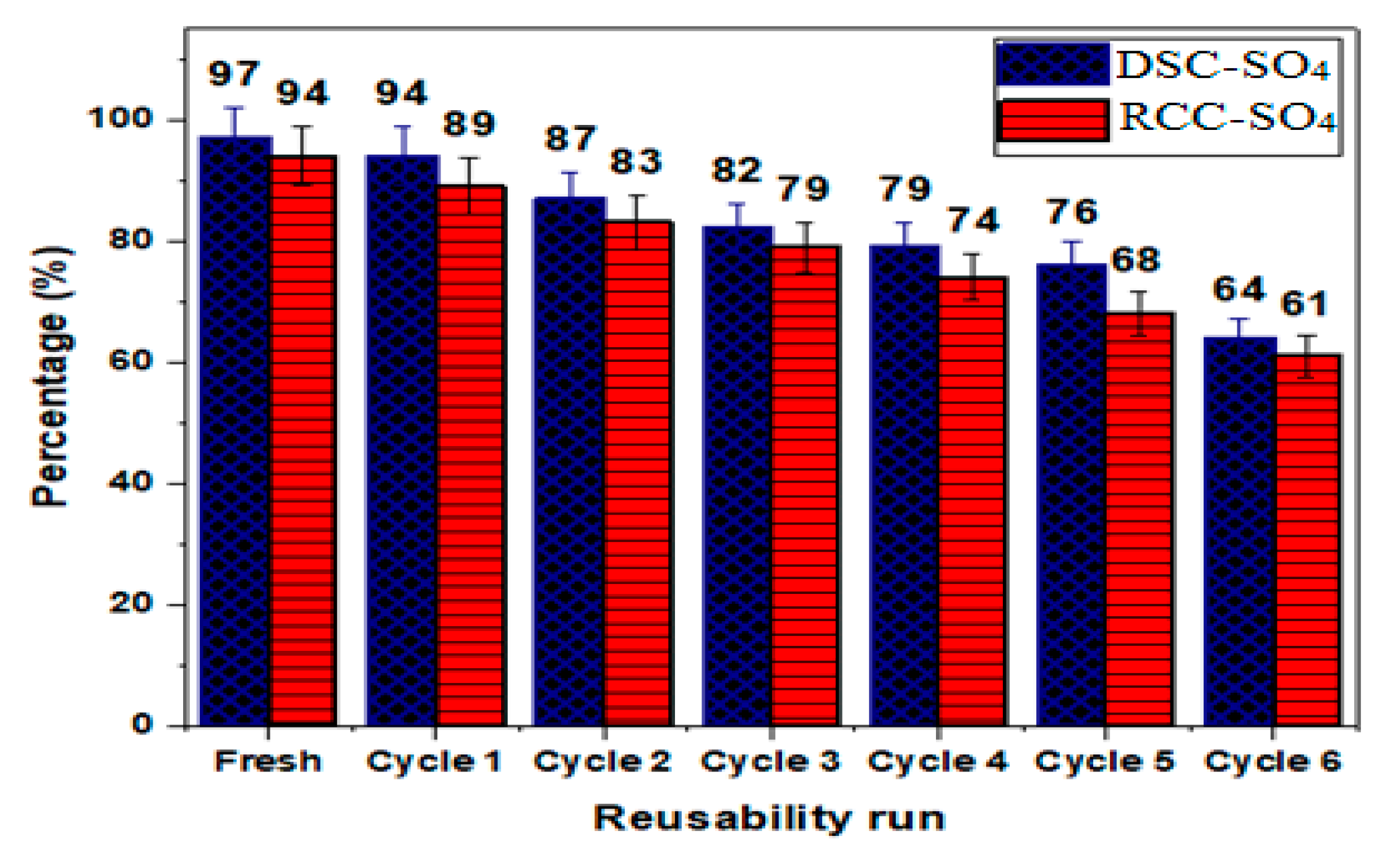
3.2.4. Reusability of DSC-SO4 and RCC-SO4 Catalysts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rashid, U.; Rehman, H.A.; Hussain, I.; Ibrahim, M.; Haider, M.S. Muskmelon (Cucumis melo) seed oil: A potential non-food oil source for biodiesel production. Energy 2011, 36, 5632–5639. [Google Scholar] [CrossRef]
- Soltani, S.; Rashid, U.; Yunus, R.; Taufiq-Yap, Y.H. Biodiesel production in the presence of sulfonated mesoporous ZnAl2O4 catalyst via esterification of palm fatty acid distillate (PFAD). Fuel 2016, 178, 253–262. [Google Scholar] [CrossRef]
- Shi, G.; Yu, F.; Yan, X.; Li, R. Synthesis of tetragonal sulfated zirconia via a novel route for biodiesel production. J. Fuel Chem. Technol. 2017, 45, 311–316. [Google Scholar] [CrossRef]
- Soltani, S.; Rashid, U.; Yunus, R.; Taufiq-Yap, Y.H.; Al-Resayes, S.I. Post-functionalization of polymeric mesoporous C@Zn core–shell spheres used for methyl FAME production. Renew. Energy 2016, 9, 1235–1243. [Google Scholar] [CrossRef]
- Jyoti, M.; Das, A.; Das, V.; Bhuyan, N.; Deka, D. Transesterification of waste cooking oil for biodiesel production catalyzed by Zn substituted waste egg shell derived CaO nanocatalyst. Fuel 2019, 242, 345–354. [Google Scholar] [CrossRef]
- Bazargan, A.; Kostić, M.D.; Stamenković, O.S.; Veljković, V.B.; McKay, G. A calcium oxide-based catalyst derived from palm kernel shell gasification residues for biodiesel production. Fuel 2015, 150, 519–525. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Gu, Y.; Xu, Y.; Zeng, G.; Hu, X. Biochar-based nano-composites for the decontamination of wastewater: A review. Bioresour. Technol. 2016, 212, 318–333. [Google Scholar] [CrossRef]
- Hu, Q.; Shao, J.; Yang, H.; Yao, D.; Wang, X.; Chen, H. Effects of binders on the properties of bio-char pellets. Appl. Energy 2015, 157, 508–516. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, P.; Shao, Q.; Ma, D.; Takahashi, F.; Yoshikawa, K. In-situ catalytic conversion of tar using rice husk char-supported nickel-iron catalysts for biomass pyrolysis/gasification. Appl. Catal. B Environ. 2014, 152, 140–151. [Google Scholar] [CrossRef]
- Konwar, L.J. Biochar Supported Cao as heterogeneous catalyst for biodiesel production. Int. J. Innov. Res. Dev. 2012, 1, 186–195. [Google Scholar]
- Dawodu, F.A.; Ayodele, O.; Xin, J.; Zhang, S.; Yan, D. Effective conversion of non-edible oil with high free fatty acid into biodiesel by sulphonated carbon catalyst. Appl. Energy 2014, 114, 819–826. [Google Scholar] [CrossRef]
- Aniq, W.; Zhikeng, Z.; Ruiqi, L.; Di, H.; Yiran, L.; Huixia, L.; Kai, Y. Biomass-derived porous carbon highly efficient for removal of Pb(II) and Cd(II). Green Energy Environ. 2019, 4, 414–423. [Google Scholar]
- Dejean, A.; Ouédraogo, I.W.K.; Mouras, S.; Valette, J.; Blin, J. Sea nut shell based catalysts for the production of ethanolic biodiesel. Energy Sustain. Dev. 2017, 40, 103–111. [Google Scholar] [CrossRef]
- Ahmad, J.; Cordioli, E.; Patuzzi, F.; Prando, D.; Castaldi, M.; Baratieri, M. Possible utilization pathways of char from biomass thermochemical conversion: Char as a catalytic filtering medium for tar cracking. In Proceedings of the 24th European Biomass Conference and Exhibition, Amsterdam, The Netherlands, 6–9 June 2016. [Google Scholar]
- Marmesat, S.; Rodrigues, E.; Velasco, J.; Dobarganes, C. Quality of used frying fats and oils: Comparison of rapid tests based on chemical and physical oil properties. Int. J. Food Sci. Technol. 2007, 42, 601–608. [Google Scholar] [CrossRef]
- Liang, X.; Xiao, H.; Qi, C. Efficient procedure for biodiesel synthesis from waste oils using novel solid acidic ionic liquid polymer as catalysts. Fuel Process. Technol. 2013, 110, 109–113. [Google Scholar] [CrossRef]
- Fraile, J.M.; García-Bordejé, E.; Pires, E.; Roldán, L. Catalytic performance and deactivation of sulfonated hydrothermal carbon in the Esterification of fatty acids: Comparison with sulfonic solids of different nature. J. Catal. 2015, 324, 107–118. [Google Scholar] [CrossRef]
- Jamil, F.; Ala’a, H.; Naushad, M.; Baawain, M.; Al-Mamun, A.; Saxena, S.K.; Viswanadham, N. Evaluation of synthesized green carbon catalyst from waste date pits for tertiary butylation of phenol. Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; West, A.H.; Ellis, N. Biochar based solid acid catalyst for biodiesel production. Appl. Catal. A Gen. 2010, 382, 197–204. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, J.K.; Hong, S.W.; Yeo, Y.K. Development of a novel process for biodiesel production from palm fatty acid distillate (PFAD). Fuel Process. Technol. 2012, 104, 271–280. [Google Scholar] [CrossRef]
- Huang, M.; Luo, J.; Fang, Z.; Li, H. Biodiesel production catalyzed by highly acidic carbonaceous catalysts synthesized via carbonizing lignin in sub-and super-critical ethanol. Appl. Catal. B Environ. 2016, 190, 103–114. [Google Scholar] [CrossRef]
- Ahmad, J.; Rashid, U.; Patuzzi, F.; Baratieri, M.; Yun Hin, T. Synthesis of char-based acidic catalyst for methanolysis of waste cooking oil: An insight into a possible valorization pathway for the solid by-product of gasification. Energy Convers. Manag. 2018, 158, 186–192. [Google Scholar] [CrossRef]
- Benedetti, V.; Cordioli, E.; Patuzzi, F.; Baratieri, M. CO2 Adsorption study on pure and chemically activated chars derived from commercial biomass gasifiers. J. CO2 Util. 2019, 33, 46–54. [Google Scholar] [CrossRef]
- Istadi, I.; Anggoro, D.D.; Buchori, L.; Rahmawati, D.A.; Intaningrum, D. Active acid catalyst of sulphated zinc oxide for transesterification of soybean oil with methanol to biodiesel. Procedia Environ. Sci. 2015, 23, 385–393. [Google Scholar] [CrossRef]
- Rao, B.V.S.K.; Chandra, M.K.; Rambabu, N.; Dalai, A.K.; Prasad, R.B.N. Carbon-based solid acid catalyst from de-oiled canola meal for biodiesel production. Catal. Commun. 2011, 14, 20–26. [Google Scholar] [CrossRef]
- Soltani, S.; Rashid, U.; Nehdi, I.A.; Al-Resayes, S.I.; Al-Muhtaseb, A.H. Sulfonated mesoporous zinc aluminate catalyst for biodiesel production from high free fatty acid feedstock using microwave heating system. J. Taiwan Inst. Chem. Eng. 2017, 70, 219–228. [Google Scholar] [CrossRef]
- Jin, H.; Hanif, M.U.; Capareda, S.; Chang, Z.; Huang, H.; Ai, Y. Copper (II) removal potential from aqueous solution by pyrolysis biochar derived from anaerobically digested algae-dairy-manure and effect of KOH activation. J. Environ. Chem. Eng. 2016, 4, 365–372. [Google Scholar] [CrossRef]
- Tran, T.T.V.; Kaiprommarat, S.; Kongparakul, S.; Reubroycharoen, P.; Guan, G.; Nguyen, M.H.; Samart, C. Green biodiesel production from waste cooking oil using an environmentally benign acid catalyst. Waste Manag. 2016, 52, 367–374. [Google Scholar] [CrossRef]
- Lee, H.V.; Taufiq-Yap, Y.H. Optimization study of binary metal oxides catalyzed transesterification system for biodiesel production. Process. Saf. Environ. Prot. 2015, 94, 430–440. [Google Scholar] [CrossRef]
- Azcan, N.; Danisman, A. Microwave assisted transesterification of rapeseed oil. Fuel 2008, 87, 1781–1788. [Google Scholar] [CrossRef]
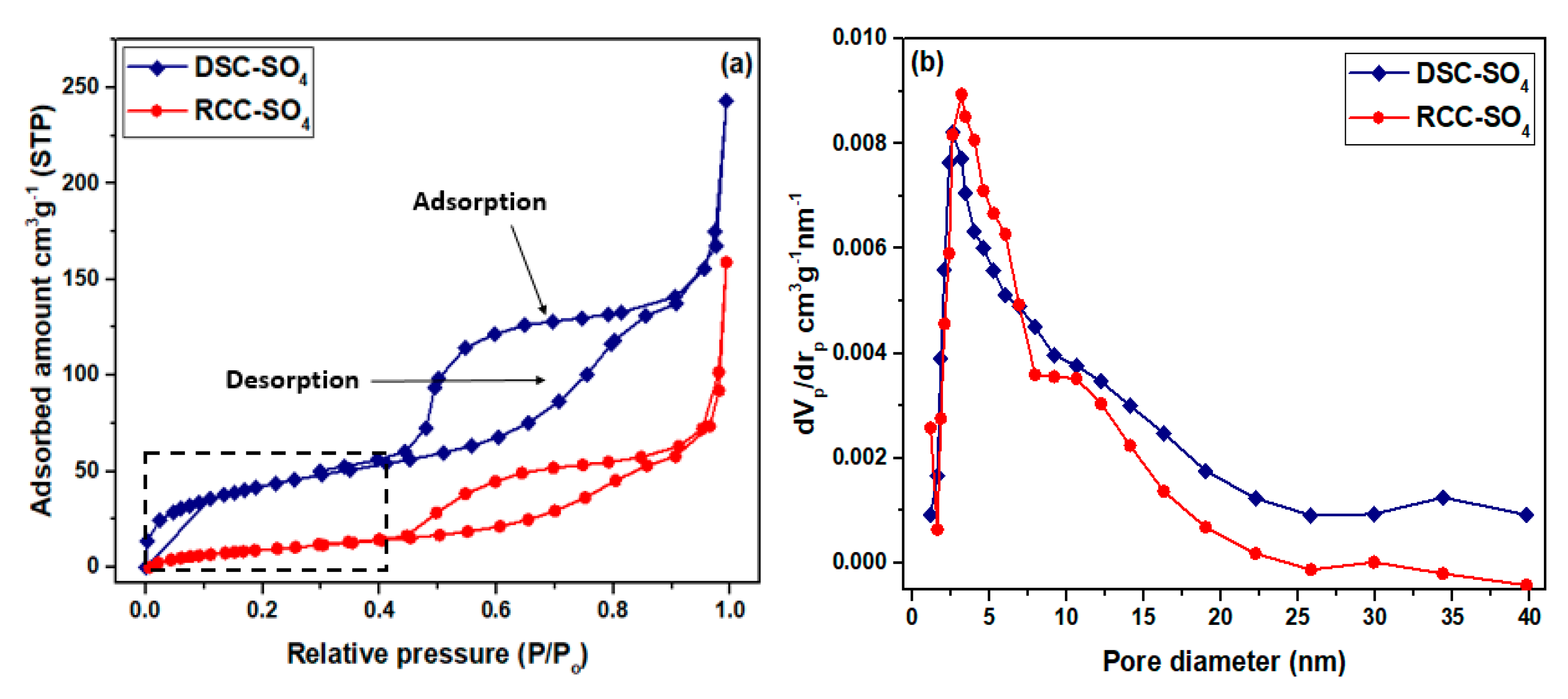
| Catalyst | SBET (m2·g−1) | Dp (nm) | Vp (cm3·g−1) | Acid Density (mmol·g−1) |
|---|---|---|---|---|
| DSC | 587 ± 1.71 | 3.8 ± 0.05 | 0.30 ± 0.03 | 1.62 |
| DSC-SO4 | 527 ± 1.25 | 2.6 ± 0.01 | 0.35 ± 0.05 | 3.38 |
| RCC | 419 ± 1.43 | 3.7 ± 0.07 | 0.37 ± 0.05 | 1.28 |
| RCC-SO4 | 348 ± 1.76 | 3.2 ± 0.09 | 0.32 ± 0.02 | 2.68 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, J.; Rashid, U.; Patuzzi, F.; Alamoodi, N.; Choong, T.S.Y.; Soltani, S.; Ngamcharussrivichai, C.; Nehdi, I.A.; Baratieri, M. Mesoporous Acidic Catalysts Synthesis from Dual-Stage and Rising Co-Current Gasification Char: Application for FAME Production from Waste Cooking Oil. Materials 2020, 13, 871. https://doi.org/10.3390/ma13040871
Ahmad J, Rashid U, Patuzzi F, Alamoodi N, Choong TSY, Soltani S, Ngamcharussrivichai C, Nehdi IA, Baratieri M. Mesoporous Acidic Catalysts Synthesis from Dual-Stage and Rising Co-Current Gasification Char: Application for FAME Production from Waste Cooking Oil. Materials. 2020; 13(4):871. https://doi.org/10.3390/ma13040871
Chicago/Turabian StyleAhmad, Junaid, Umer Rashid, Francesco Patuzzi, Nahla Alamoodi, Thomas Shean Yaw Choong, Soroush Soltani, Chawalit Ngamcharussrivichai, Imededdine Arbi Nehdi, and Marco Baratieri. 2020. "Mesoporous Acidic Catalysts Synthesis from Dual-Stage and Rising Co-Current Gasification Char: Application for FAME Production from Waste Cooking Oil" Materials 13, no. 4: 871. https://doi.org/10.3390/ma13040871
APA StyleAhmad, J., Rashid, U., Patuzzi, F., Alamoodi, N., Choong, T. S. Y., Soltani, S., Ngamcharussrivichai, C., Nehdi, I. A., & Baratieri, M. (2020). Mesoporous Acidic Catalysts Synthesis from Dual-Stage and Rising Co-Current Gasification Char: Application for FAME Production from Waste Cooking Oil. Materials, 13(4), 871. https://doi.org/10.3390/ma13040871









