Strengthening Behavior of Cemented Paste Backfill Using Alkali-Activated Slag Binders and Bottom Ash Based on the Response Surface Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- (1)
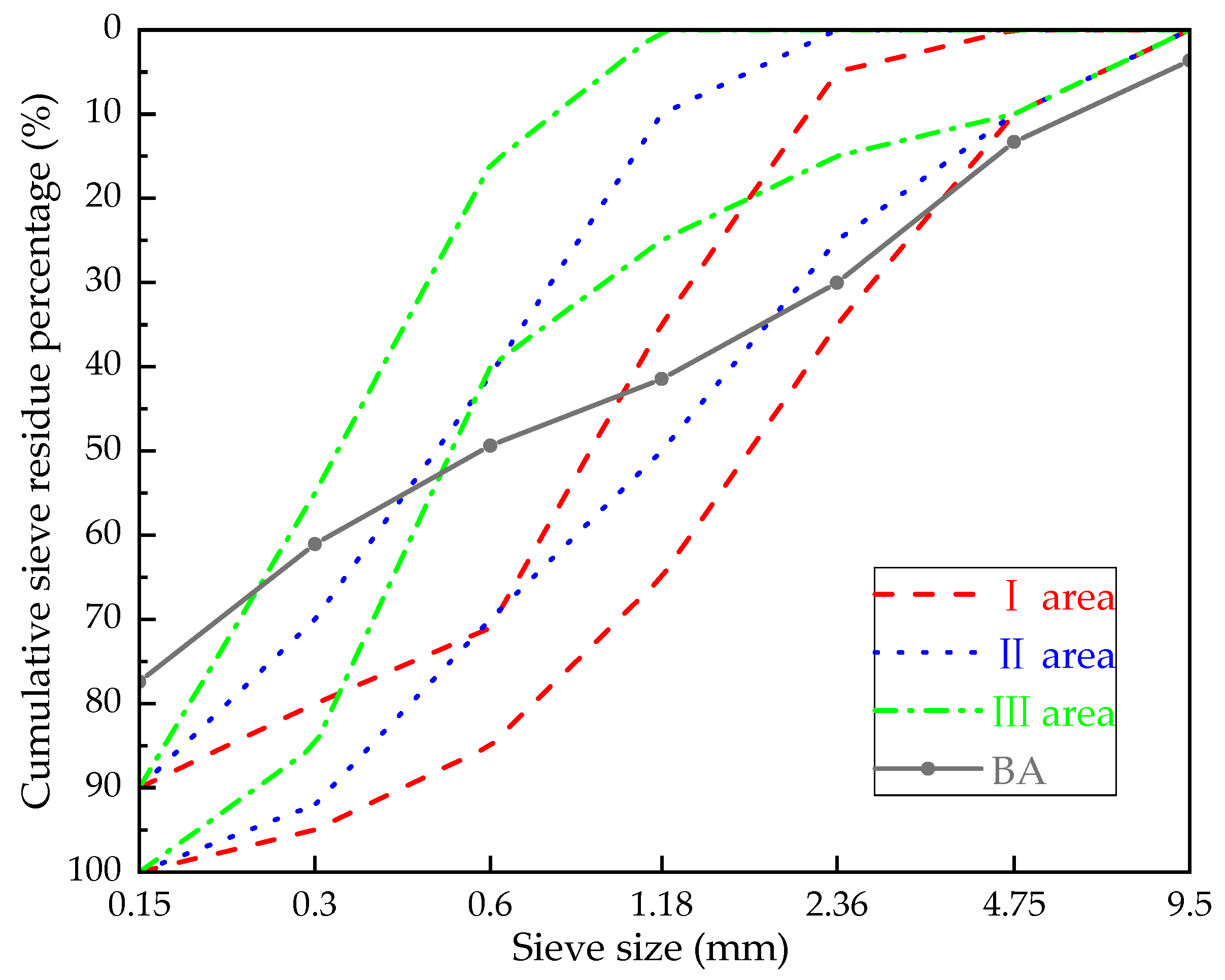
- BA: The BA used in this research was taken from a power plant in Fuxin City, Liaoning Province, China. The main chemical composition obtained by X-ray fluorescence (XRF) analysis is shown in Table 1. According to the Chinese standard “Sand for building” (GB/T14684-2011) [22], the screening curves of BA are shown in Figure 1. The bulk density of BA is 801.7 kg/m3.
- (2)
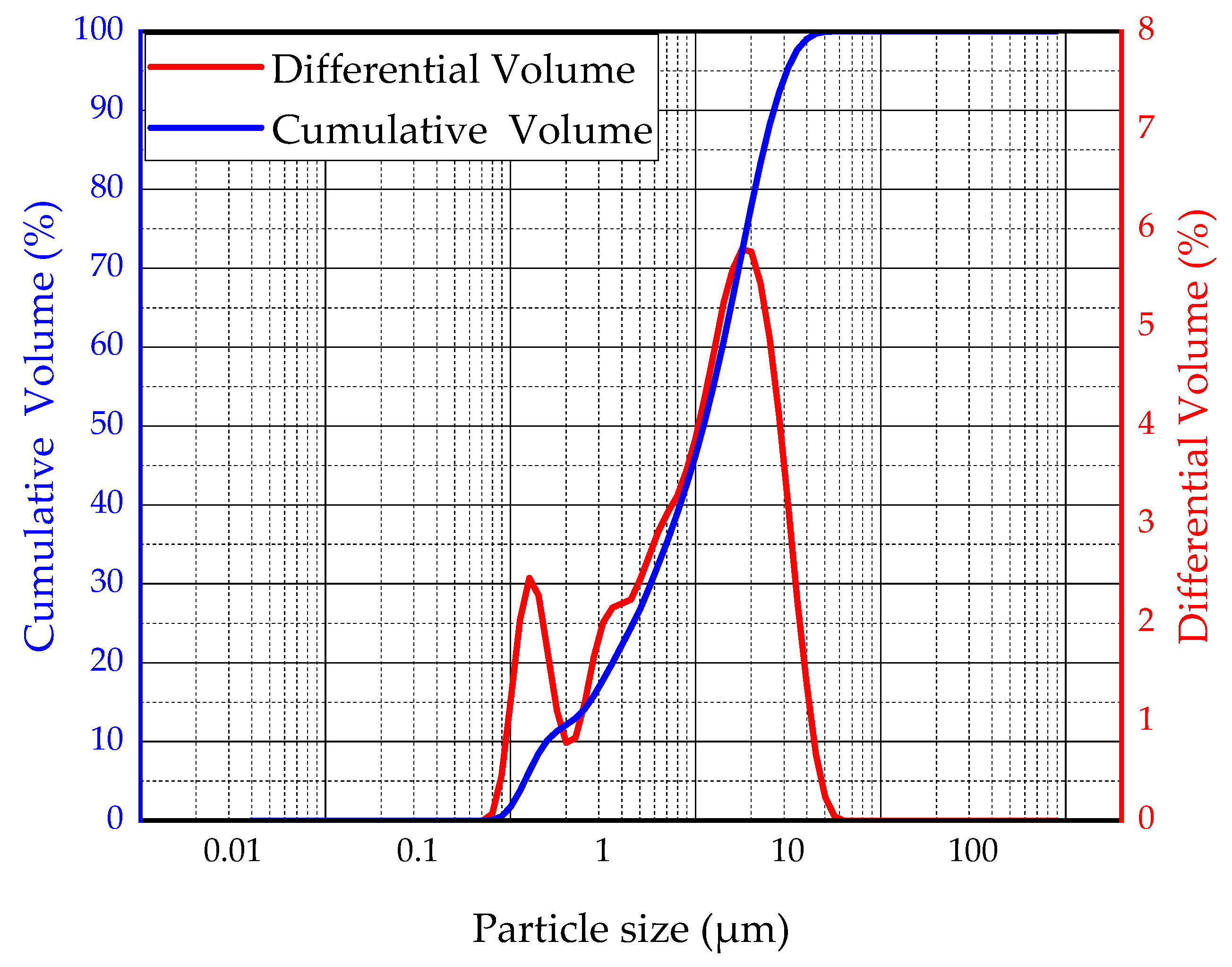
- Granulated blast furnace slag: The slag used in this research is Panlongshan brand S95 slag powder produced by Shandong Kangjing New Material Technology Co., Ltd, Shandong, China. The main chemical composition is shown in Table 1. The particle size distribution of slag was characterized by BT-2003 laser particle size analyzer (Dandong Bettersize Instrument Co., Ltd, Liaoning, China) as shown in Figure 2. The density of slag is 2920 kg/m3, and the specific surface area is 397.6 m2/kg.
- (3)
- Alkali activator: The alkali activator used in this research is 96% analytical NaOH produced by Liaoning Quanrui Reagent Co., Ltd, Liaoning, China.
- (4)
- Air-entraining agent: The air-entraining agent used in this research is the SY-5 air-entraining agent produced by the Jinan Shunxin Chemical Plant, and its main component is triterpenoid saponin.
- (5)
- Mixing water: The mixing water used in this research was tap water.
2.2. Mix Design
2.3. Experimental Methods
3. Results and Discussion
3.1. The Results of the Response Surface Method
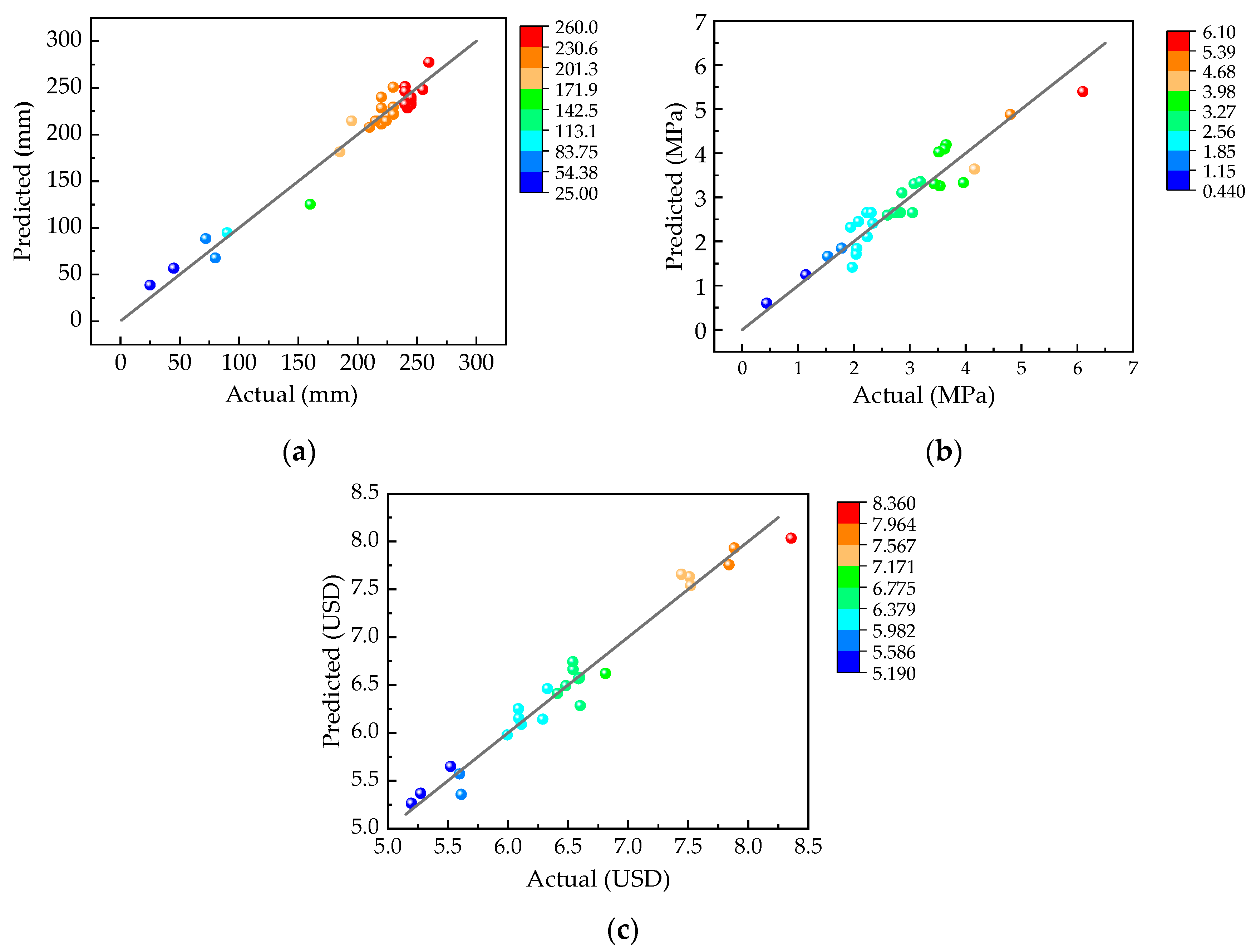
3.2. Response Surface Model Fitting and Verification
− 187.5AD − 4.3BC + 109.4BD + 284.4CD + 118.5A2 + 5.5B2 − 13.2C2 − 2009.4D2,
0.02BC + 0.2BD − 0.3CD + 0.09A2 − 0.08B2 − 0.02C2 + 27.4D2,
− 5.0BC – 0.5BD – 0.5CD + 2.4A2 + 0.04B2 − 0.06C2 – 0.8D2,
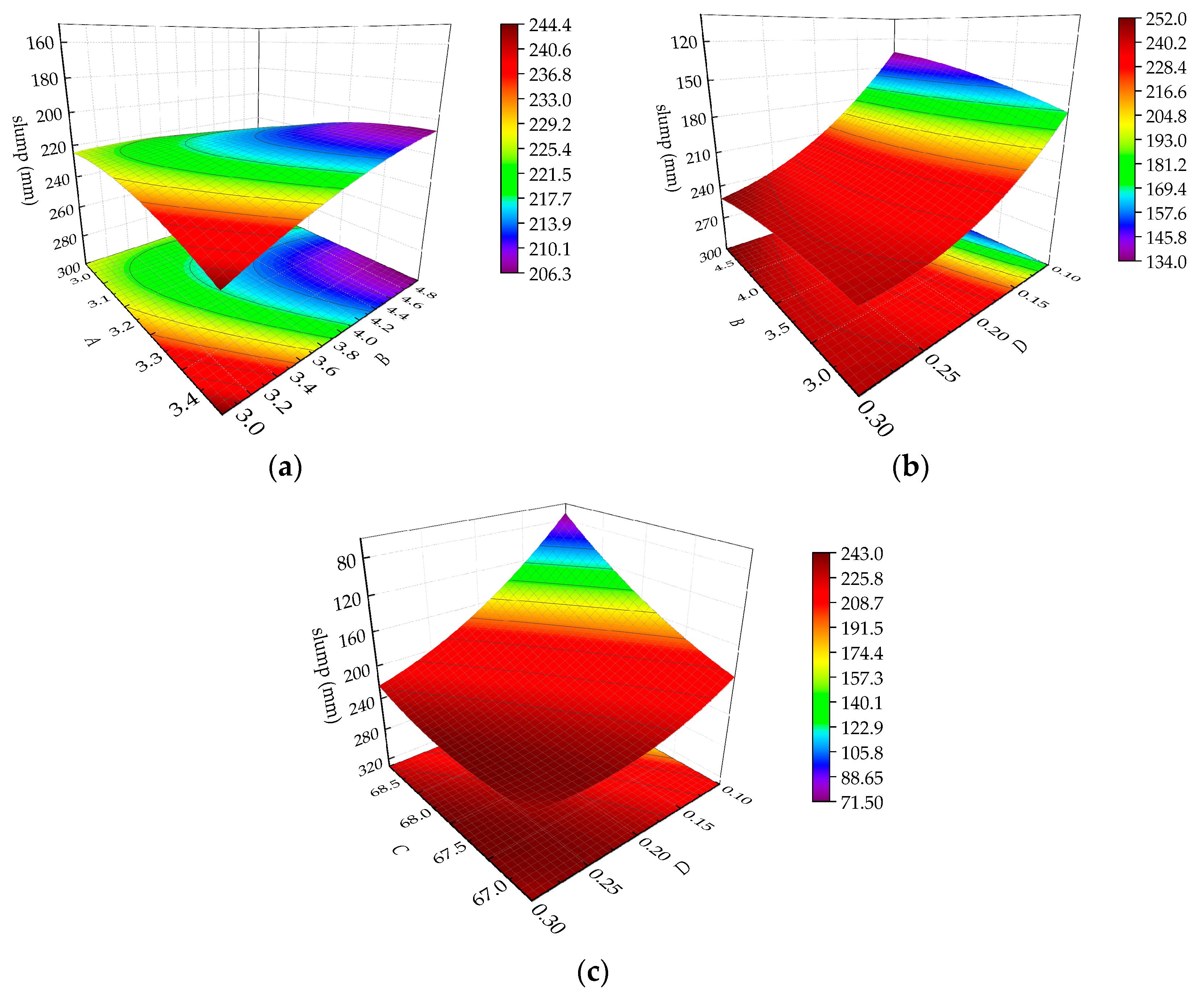
3.3. Effect of Response Surface Parameters on the Slump of the CPB Mix
3.4. Effect of Response Surface Parameters on the 28-d UCS of CPB
3.5. Effect of Response Surface Parameters on the Cost of CPB
3.6. Multi-Objective Optimization
3.7. Microstructural Analysis
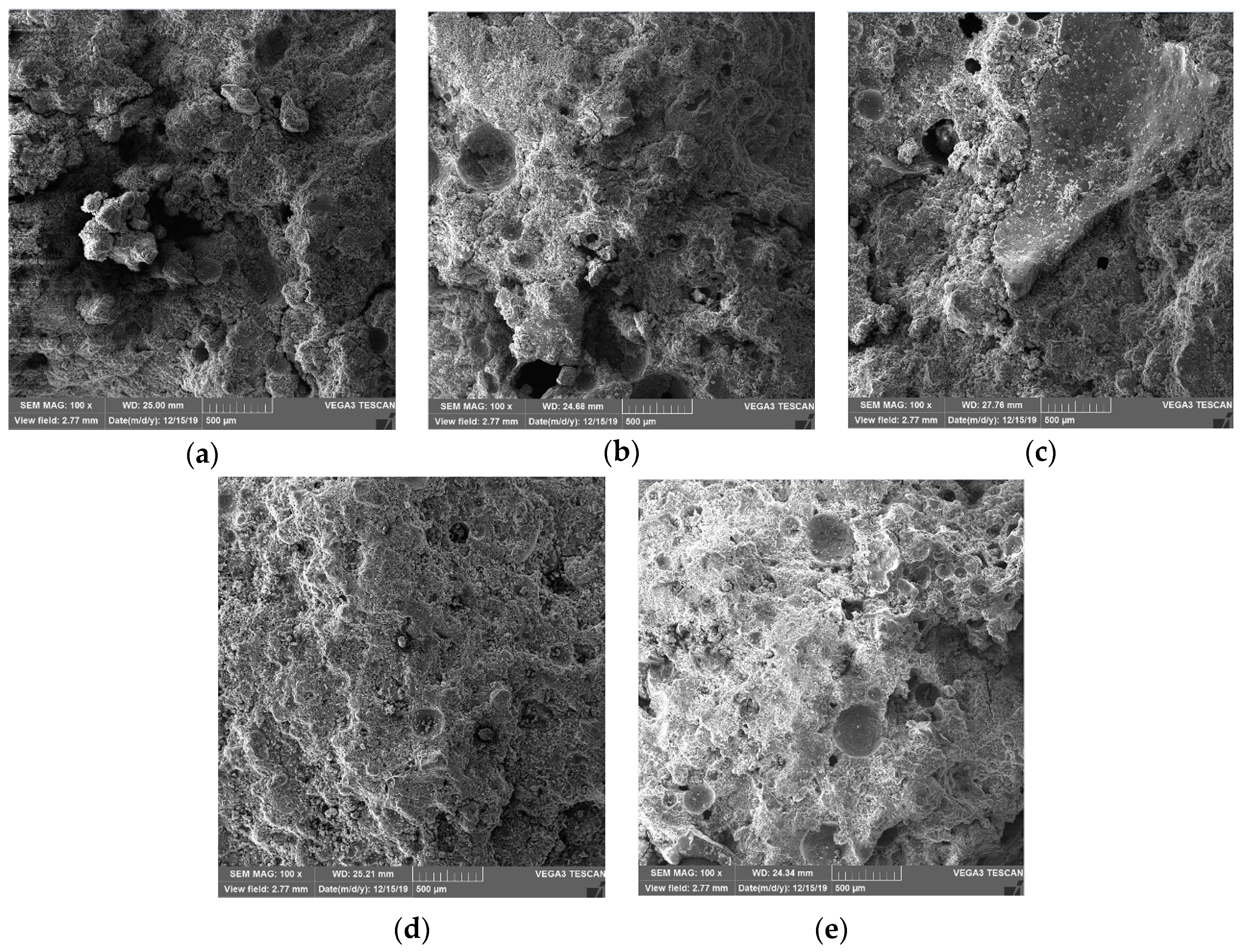
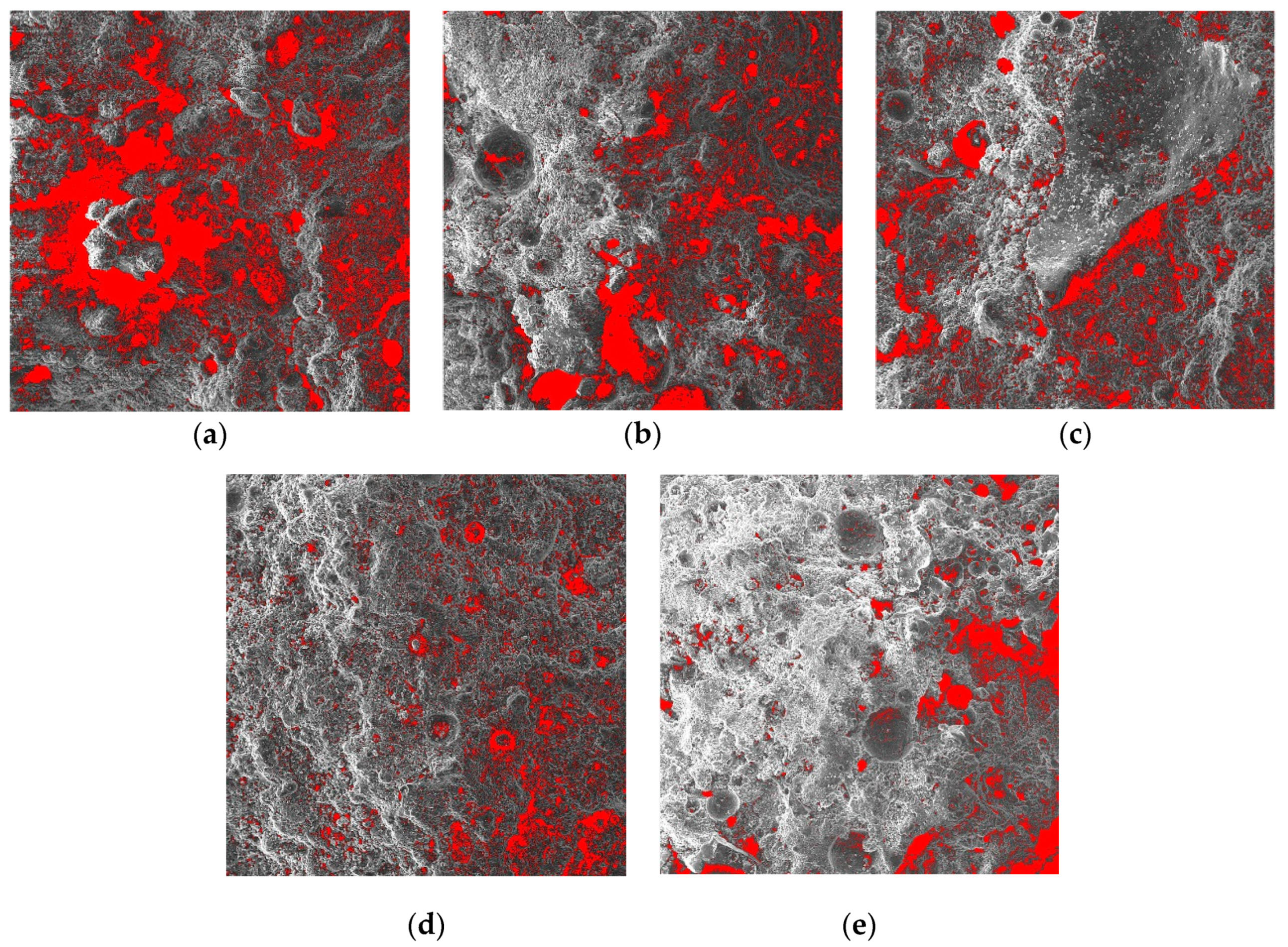
3.7.1. Changes in Porosity with Curing Age
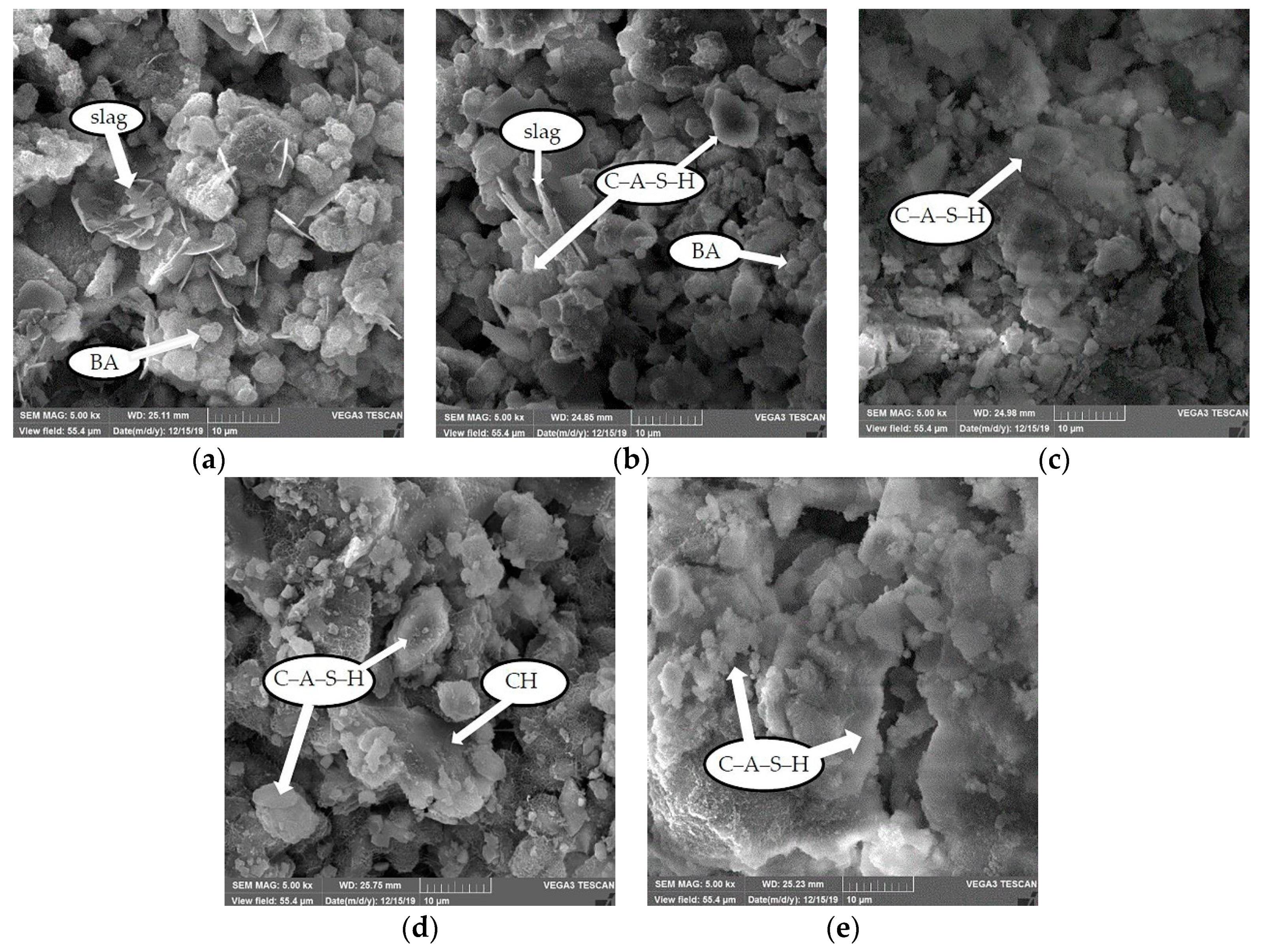
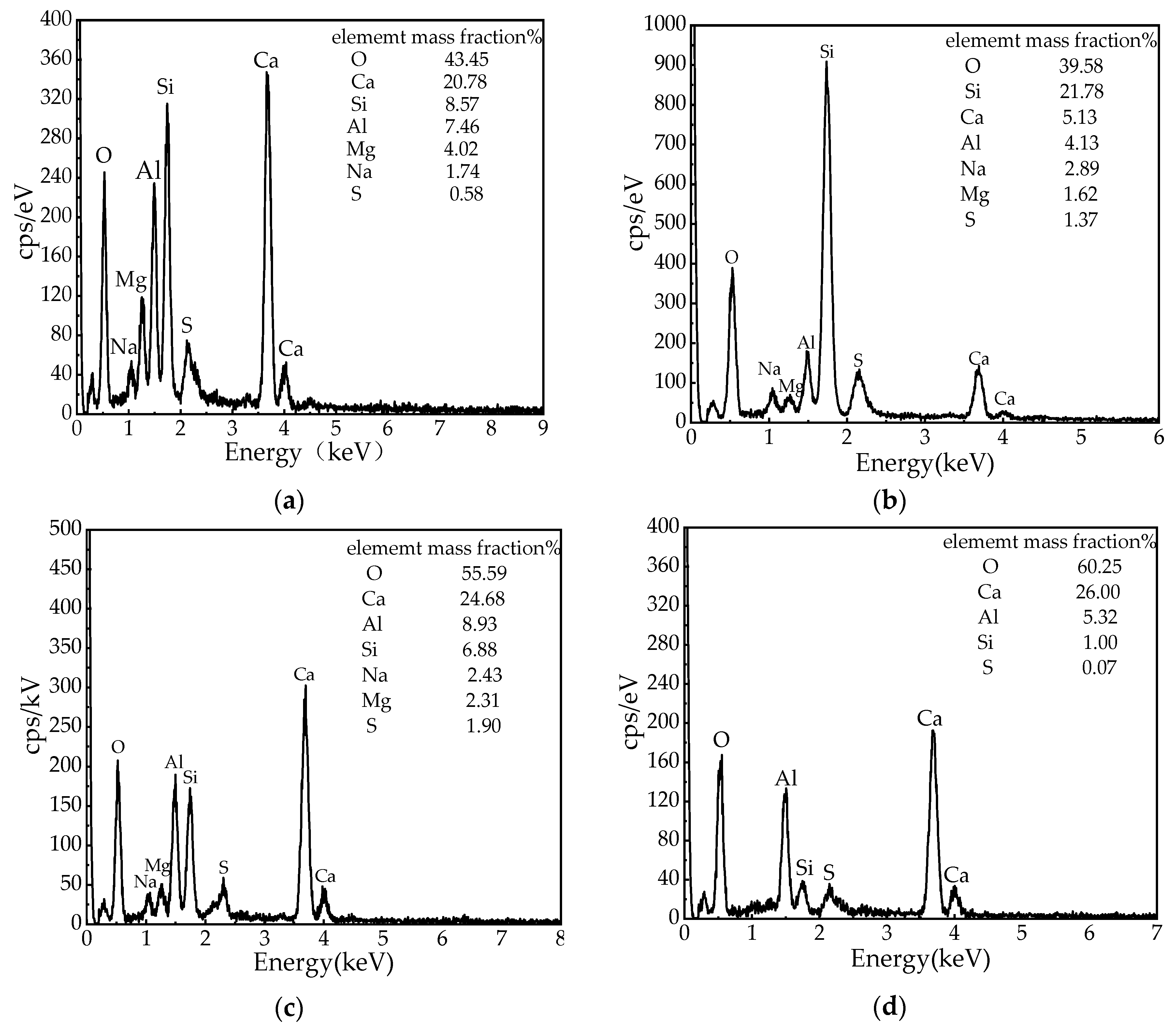
3.7.2. Microstructural Changes and Hydration Product Types Increase with Curing Age
4. Conclusions
- (1)
- Based on the CCD response surface method, mathematical models of the CPB material slump, 28-d UCS, and cost are established. The optimal mix ratio with an aggregate-binder ratio of 3.28, an alkali dosage of 3.00, a solid content of 67.44%, and an air-entraining agent dosage of 0.10% was obtained by the desirability function method. The measured slump is 205 mm, the 28-d UCS is 2.93 MPa, and the cost is 5.70 USD /m3.
- (2)
- The microanalysis of the optimal mix ratio shows that as the curing age increases, the internal porosity of CPB gradually decreases. The hydration products of alkali-activated slag are mainly C-A-S-H gels. At the beginning of the hydration reaction, as the age increases, slag is gradually consumed, and almost all the slag participates in the reaction at 7 d. With the progress of the polycondensation reaction, the C-A-S-H gel continuously wraps the BA surface, thereby increasing the strength.
- (3)
- At 14 d, CHwith a high degree of crystallization and a relatively complete morphology is embedded in the hydration products. At 28 d, the C-A-S-H flocs are connected as a whole, forming a dense structure with a high degree of hydration. The formation of microscopic hydration products and the denseness of the pore structure cause the CPB strength to increase with age.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jang, J.G.; Park, S.-M.; Chung, S.; Ahn, J.-W.; Kim, H.-K. Utilization of circulating fluidized bed combustion ash in producing controlled low-strength materials with cement or sodium carbonate as activator. Constr. Build. Mater. 2018, 159, 642–651. [Google Scholar] [CrossRef]
- Yin, K.; Ahamed, A.; Lisak, G. Environmental perspectives of recycling various combustion ashes in cement production–A review. Waste Manag. 2018, 78, 401–416. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.-K. Coal bottom ash in field of civil engineering: A review of advanced applications and environmental considerations. Ksce. J. Civ. Eng. 2015, 19, 1802–1818. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, X.; Querol, X.; Font, O.; Moreno, N. A review on the applications of coal combustion products in China. Int. Geol. Rev. 2018, 60, 671–716. [Google Scholar] [CrossRef]
- Nikbin, I.M.; Rahimi, S.; Allahyari, H.; Damadi, M. A comprehensive analytical study on the mechanical properties of concrete containing waste bottom ash as natural aggregate replacement. Constr. Build. Mater. 2016, 121, 746–759. [Google Scholar] [CrossRef]
- Singh, N.; Mithulraj, M.; Arya, S. Influence of coal bottom ash as fine aggregates replacement on various properties of concretes: A review. Resour. Conserv. Recycl. 2018, 138, 257–271. [Google Scholar] [CrossRef]
- Zhao, Y.; Taheri, A.; Soltani, A.; Karakus, M.; Deng, A. Strength Development and Strain Localization Behavior of Cemented Paste Backfills Using Portland Cement and Fly Ash. Materials 2019, 12, 3282. [Google Scholar] [CrossRef]
- Wu, D.; Hou, Y.; Deng, T.; Chen, Y.; Zhao, X. Thermal, hydraulic and mechanical performances of cemented coal gangue-fly ash backfill. Int. J. Miner. Process. 2017, 162, 12–18. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Q.; Qi, C.; Fourie, A.; Xiao, C. Recycling phosphogypsum and construction demolition waste for cemented paste backfill and its environmental impact. J. Clean. Prod. 2018, 186, 418–429. [Google Scholar] [CrossRef]
- Yılmaz, T.; Ercikdi, B.; Deveci, H. Utilisation of construction and demolition waste as cemented paste backfill material for underground mine openings. J. Environ. Manag. 2018, 222, 250–259. [Google Scholar] [CrossRef]
- Li, X.; Du, J.; Gao, L.; He, S.; Gan, L.; Sun, C.; Shi, Y. Immobilization of phosphogypsum for cemented paste backfill and its environmental effect. J. Clean. Prod. 2017, 156, 137–146. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Q.; Wang, X. Cemented backfilling technology of paste-like based on aeolian sand and tailings. Minerals 2016, 6, 132. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, J.; Klein, B.; Zhou, N.; Dewit, B. Experimental characterization of the influence of solid components on the rheological and mechanical properties of cemented paste backfill. Int. J. Miner. Process. 2017, 168, 116–125. [Google Scholar] [CrossRef]
- Jiao, H.-z.; Wang, S.-f.; Wu, A.-x.; Shen, H.-m.; Wang, J.-d. Cementitious property of NaAlO 2-activated Ge slag as cement supplement. Int. J. Min. Met. Mater. 2019, 26, 1594–1603. [Google Scholar] [CrossRef]
- Jiang, H.; Qi, Z.; Yilmaz, E.; Han, J.; Qiu, J.; Dong, C. Effectiveness of alkali-activated slag as alternative binder on workability and early age compressive strength of cemented paste backfills. Constr. Build. Mater. 2019, 218, 689–700. [Google Scholar] [CrossRef]
- Sun, Q.; Tian, S.; Sun, Q.; Li, B.; Cai, C.; Xia, Y.; Wei, X.; Mu, Q. Preparation and microstructure of fly ash geopolymer paste backfill material. J. Clean. Prod. 2019, 225, 376–390. [Google Scholar] [CrossRef]
- Ercikdi, B.; Cihangir, F.; Kesimal, A.; Deveci, H.; Alp, İ. Utilization of industrial waste products as pozzolanic material in cemented paste backfill of high sulphide mill tailings. J. Hazard. Mater. 2009, 168, 848–856. [Google Scholar] [CrossRef]
- Cihangir, F.; Ercikdi, B.; Kesimal, A.; Turan, A.; Deveci, H. Utilisation of alkali-activated blast furnace slag in paste backfill of high-sulphide mill tailings: effect of binder type and dosage. Miner. Eng. 2012, 30, 33–43. [Google Scholar] [CrossRef]
- Fall, M.; Benzaazoua, M.; Saa, E. Mix proportioning of underground cemented tailings backfill. Tunn. Undergr. Space Technol. 2008, 23, 80–90. [Google Scholar] [CrossRef]
- Bouzalakos, S.; Dudeney, A.; Chan, B. Formulating and optimising the compressive strength of controlled low-strength materials containing mine tailings by mixture design and response surface methods. Miner. Eng. 2013, 53, 48–56. [Google Scholar] [CrossRef]
- Ferdosian, I.; Camões, A. Eco-efficient ultra-high performance concrete development by means of response surface methodology. Cem. Concr. Compos. 2017, 84, 146–156. [Google Scholar] [CrossRef]
- Sand for Building; GB/T 14684 -2001; Standardization Administration of China: Beijing, China, 2001.
- Sun, Q.; Li, T.; Liang, B. Preparation of a New Type of Cemented Paste Backfill with an Alkali-Activated Silica Fume and Slag Composite Binder. Materials 2020, 13, 372. [Google Scholar] [CrossRef] [PubMed]
- Testing of Concrete — Part 2: Properties of Fresh Concrete; ISO1920-2; ISO: Geneva, Switzerland, 2005.
- Standard Method for Compressive Strength of Cylindrical Concrete Specimens; ASTM C39/C39M-15a; ASTM International: West Conshohocken, PA, USA, 2015.
- Test Method for Density (Unit Weight), Yield, and Air Content (Gravimetric) of Concrete; ASTM C138/C138M-14; ASTM International: West Conshohocken, PA, USA, 2014.
- Nambiar, E.K.; Ramamurthy, K. Models relating mixture composition to the density and strength of foam concrete using response surface methodology. Cem. Concr. Compos. 2006, 28, 752–760. [Google Scholar] [CrossRef]
- Qiu, J.; Zhao, Y.; Long, H.; Guo, Z.; Xing, J.; Sun, X. Low-Carbon Binder for Cemented Paste Backfill: Flowability, Strength and Leaching Characteristics. Minerals 2019, 9, 707. [Google Scholar] [CrossRef]
- Yin, S.; Wu, A.; Hu, K.; Wang, Y.; Zhang, Y. The effect of solid components on the rheological and mechanical properties of cemented paste backfill. Miner. Eng. 2012, 35, 61–66. [Google Scholar] [CrossRef]
- Kim, H.-K.; Jeon, J.; Lee, H.-K. Workability, and mechanical, acoustic and thermal properties of lightweight aggregate concrete with a high volume of entrained air. Constr. Build. Mater. 2012, 29, 193–200. [Google Scholar] [CrossRef]
- Lee, N.K.; Kim, H.K.; Park, I.; Lee, H.K. Alkali-activated, cementless, controlled low-strength materials (CLSM) utilizing industrial by-products. Constr. Build. Mater. 2013, 49, 738–746. [Google Scholar] [CrossRef]
- Park, S.M.; Lee, N.-K.; Lee, H.-K. Circulating fluidized bed combustion ash as controlled low-strength material (CLSM) by alkaline activation. Constr. Build. Mater. 2017, 156, 728–738. [Google Scholar] [CrossRef]
- Cihangir, F.; Akyol, Y. Mechanical, hydrological and microstructural assessment of the durability of cemented paste backfill containing alkali-activated slag. Int. J. Min. Reclam. Environ. 2018, 32, 123–143. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Zhao, G.; Ma, J.; Peng, K.; Yang, Q.; Zhou, L. Mix ratio optimization of alpine mine backfill based on the response surface method. J. Univ. Sci. Technol. B 2013, 35, 559–565. [Google Scholar]
- Achara, B.E.; Mohammed, B.S.; Liew, M. Bond behaviour of nano-silica-modified self-compacting engineered cementitious composite using response surface methodology. Constr. Build. Mater. 2019, 224, 796–814. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Q.; Zhang, J.; An, S. Microstructure and Composition of Hardened paste of Soda Residue-Slag Complex Binding Materials. J. Build. Mater. 2019. [Google Scholar]
- Xu, W.; Pang, W.; Ding, M. Experiment on evolution of microstructures and long-term strength model of cemented backfill mass. J. Cent. South. Univ. Technol. 2015, 46, 2333–2341. [Google Scholar]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si+Ca) and metakaolin (Si+Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Criado, M.; Aperador, W.; Sobrados, I. Microstructural and mechanical properties of alkali activated Colombian raw materials. Materials 2016, 9, 158. [Google Scholar] [CrossRef]
- Part, W.K.; Ramli, M.; Cheah, C.B. An overview on the influence of various factors on the properties of geopolymer concrete derived from industrial by-products. Constr. Build. Mater. 2015, 77, 370–395. [Google Scholar] [CrossRef]

| Materials | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | SO3 | Na2O |
|---|---|---|---|---|---|---|---|
| BA | 56.7 | 18.9 | 5.3 | 10.3 | 1.9 | 0.5 | 1.1 |
| Slag [23] | 27.5 | 16.2 | 40.8 | 0.5 | 7.8 | 2.9 | 0.3 |
| Factors | Code | Unit | Level | ||||
|---|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | |||
| Aggregate-binder ratio | A | - | 2.75 | 3 | 3.25 | 3.5 | 3.75 |
| Alkali dosage | B | % | 2 | 3 | 4 | 5 | 6 |
| Solid content | C | % | 66 | 67 | 68 | 69 | 70 |
| Air-entraining agent dosage | D | % | 0 | 0.1 | 0.2 | 0.3 | 0.4 |
| Materials | Slag | NaOH | H2O | Air-Entraining Agent |
|---|---|---|---|---|
| Unit Price (USD/kg) | 0.0137 | 0.289 | 0.000665 | 1.44 |
| Test Number | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Response 1 | Response 2 | Response 3 |
|---|---|---|---|---|---|---|---|
| A | B | C | D | Slump | 28-d UCS | Cost | |
| % | % | % | mm | MPa | USD | ||
| 1 | 3 | 5 | 69 | 0.3 | 240 | 3.08 | 7.51 |
| 2 | 3.25 | 4 | 66 | 0.2 | 220 | 2.05 | 6.60 |
| 3 | 3 | 3 | 69 | 0.3 | 242 | 2.08 | 6.33 |
| 4 | 3.5 | 5 | 69 | 0.1 | 45 | 3.65 | 6.60 |
| 5 | 2.75 | 4 | 68 | 0.2 | 255 | 4.16 | 8.36 |
| 6 | 3.25 | 4 | 68 | 0.2 | 220 | 3.05 | 6.41 |
| 7 | 3.25 | 4 | 68 | 0.2 | 218 | 2.83 | 6.41 |
| 8 | 3.25 | 4 | 68 | 0 | 25 | 6.10 | 6.29 |
| 9 | 3 | 5 | 67 | 0.3 | 260 | 2.60 | 7.88 |
| 10 | 3.25 | 2 | 68 | 0.2 | 230 | 1.97 | 5.61 |
| 11 | 3.5 | 3 | 69 | 0.1 | 160 | 2.86 | 5.27 |
| 12 | 3.5 | 5 | 67 | 0.3 | 245 | 1.53 | 6.54 |
| 13 | 3.25 | 4 | 68 | 0.4 | 230 | 2.24 | 6.81 |
| 14 | 3.25 | 6 | 68 | 0.2 | 230 | 3.54 | 7.84 |
| 15 | 3 | 3 | 67 | 0.1 | 220 | 3.19 | 6.09 |
| 16 | 3 | 3 | 67 | 0.3 | 245 | 1.78 | 6.54 |
| 17 | 3.25 | 4 | 68 | 0.2 | 215 | 2.78 | 6.41 |
| 18 | 3.25 | 4 | 70 | 0.2 | 90 | 3.96 | 6.11 |
| 19 | 3.5 | 3 | 67 | 0.3 | 245 | 0.44 | 5.52 |
| 20 | 3.75 | 4 | 68 | 0.2 | 220 | 2.03 | 5.99 |
| 21 | 3.25 | 4 | 68 | 0.2 | 215 | 2.72 | 6.41 |
| 22 | 3.5 | 3 | 69 | 0.3 | 240 | 1.14 | 5.60 |
| 23 | 3.5 | 5 | 67 | 0.1 | 185 | 3.44 | 6.48 |
| 24 | 3 | 5 | 69 | 0.1 | 80 | 4.80 | 7.52 |
| 25 | 3 | 3 | 69 | 0.1 | 72 | 3.62 | 6.09 |
| 26 | 3.25 | 4 | 68 | 0.2 | 224 | 2.23 | 6.41 |
| 27 | 3 | 5 | 67 | 0.1 | 210 | 3.52 | 7.44 |
| 28 | 3.5 | 3 | 67 | 0.1 | 240 | 1.94 | 5.20 |
| 29 | 3.5 | 5 | 69 | 0.3 | 230 | 2.34 | 6.59 |
| 30 | 3.25 | 4 | 68 | 0.2 | 195 | 2.31 | 6.41 |
| Response | Slump | 28-d UCS | Cost |
|---|---|---|---|
| Degree of freedom | |||
| Regression | 14 | 14 | 14 |
| Residual error | 15 | 15 | 15 |
| Standard deviation | 17.55 | 0.48 | 0.19 |
| R-Squared | 0.9631 | 0.9039 | 0.9683 |
| Adj R-Squared | 0.9287 | 0.8142 | 0.9386 |
| F value | 27.97 | 10.08 | 32.69 |
| p value | <0.001 | <0.0001 | <0.0001 |
| Significance | Yes | Yes | Yes |
| Variation Source | Sum of Squares | df | Mean Square | F Value | P Value |
|---|---|---|---|---|---|
| Model | 120,600 | 14 | 8613.00 | 27.97 | <0.0001 |
| A | 100.04 | 1 | 100.04 | 0.32 | 0.5771 |
| B | 1190.04 | 1 | 1190.04 | 3.86 | 0.0681 |
| C | 26,733.37 | 1 | 26,733.37 | 86.82 | <0.0001 |
| D | 54,626.04 | 1 | 54,626.04 | 177.41 | <0.0001 |
| AB | 2280.06 | 1 | 2280.06 | 7.40 | 0.0158 |
| AC | 232.56 | 1 | 232.56 | 0.76 | 0.3985 |
| AD | 351.56 | 1 | 351.56 | 1.14 | 0.3022 |
| BC | 297.56 | 1 | 297.56 | 0.97 | 0.3412 |
| BD | 1914.06 | 1 | 1914.06 | 6.22 | 0.0248 |
| CD | 12,939.06 | 1 | 12,939.06 | 42.02 | <0.0001 |
| A2 | 1504.53 | 1 | 1504.53 | 4.89 | 0.043 |
| B2 | 839.17 | 1 | 839.17 | 2.73 | 0.1195 |
| C2 | 4792.74 | 1 | 4792.74 | 15.57 | 0.0013 |
| D2 | 11,074.53 | 1 | 11,074.53 | 35.97 | <0.0001 |
| Residual | 4618.75 | 15 | 307.92 | - | - |
| Lack of Fit | 4105.25 | 10 | 419.52 | 4.00 | 0.0697 |
| Pure Error | 513.50 | 5 | 102.70 | - | - |
| Cor Total | 125,200 | 29 | - | - | - |
| Variation Source | Sum of Squares | df | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| Model | 32.97 | 14 | 2.35 | 10.08 | <0.0001 |
| A | 5.60 | 1 | 5.60 | 23.95 | 0.0002 |
| B | 5.09 | 1 | 5.09 | 21.77 | 0.0003 |
| C | 3.34 | 1 | 3.34 | 14.28 | 0.0018 |
| D | 16.25 | 1 | 16.25 | 69.56 | <0.0001 |
| AB | 0.098 | 1 | 0.098 | 0.42 | 0.5277 |
| AC | 0.00141 | 1 | 0.00141 | 0.0062 | 0.9392 |
| AD | 0.045 | 1 | 0.045 | 0.19 | 0.6665 |
| BC | 0.012 | 1 | 0.012 | 0.049 | 0.827 |
| BD | 0.00601 | 1 | 0.00601 | 0.026 | 0.8748 |
| CD | 0.019 | 1 | 0.019 | 0.081 | 0.78 |
| A2 | 0.000774 | 1 | 0.000774 | 0.000774 | 0.9549 |
| B2 | 0.17 | 1 | 0.17 | 0.75 | 0.4015 |
| C2 | 0.0081 | 1 | 0.0081 | 0.035 | 0.8548 |
| D2 | 2.06 | 1 | 2.06 | 8.82 | 0.0096 |
| Residual | 350 | 15 | 0.23 | - | - |
| Lack of Fit | 3.00 | 10 | 0.30 | 2.96 | 0.1211 |
| Pure Error | 0.51 | 5 | 0.10 | - | - |
| Cor Total | 36.47 | 29 | - | - | - |
| Variation Source | Sum of Squares | df | Mean Square | F Value | P Value |
|---|---|---|---|---|---|
| Model | 16.34 | 14 | 1.17 | 32.69 | <0.0001 |
| A | 6.34 | 1 | 6.34 | 177.57 | <0.0001 |
| B | 8.63 | 1 | 8.63 | 241.74 | <0.0001 |
| C | 0.058 | 1 | 0.058 | 1.61 | 0.2238 |
| D | 0.34 | 1 | 0.34 | 9.60 | 0.0073 |
| AB | 0.03 | 1 | 0.03 | 0.85 | 0.3704 |
| AC | 0.042 | 1 | 0.042 | 1.17 | 0.2972 |
| AD | 0.011 | 1 | 0.011 | 0.31 | 0.5862 |
| BC | 0.0004 | 1 | 0.0004 | 0.011 | 0.9177 |
| BD | 0.047 | 1 | 0.047 | 1.31 | 0.2701 |
| CD | 0.034 | 1 | 0.034 | 0.94 | 0.3478 |
| A2 | 0.61 | 1 | 0.61 | 16.99 | 0.0009 |
| B2 | 0.036 | 1 | 0.036 | 1.01 | 0.3316 |
| C2 | 0.086 | 1 | 0.086 | 2.42 | 0.1408 |
| D2 | 0.0015 | 1 | 0.0015 | 0.043 | 0.8379 |
| Residual | 0.54 | 15 | 0.54 | ||
| Lack of Fit | 0.54 | 10 | 0.54 | ||
| Pure Error | 0.00 | 5 | 0.00 | ||
| Cor Total | 16.88 | 29 |
| Term | Slump(mm) | 28d-UCS(MPa) | Cost(USD) |
|---|---|---|---|
| Experimental | 205 | 2.93 | 5.70 |
| Predicted | 200 | 2.94 | 5.59 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Wei, X.; Li, T.; Zhang, L. Strengthening Behavior of Cemented Paste Backfill Using Alkali-Activated Slag Binders and Bottom Ash Based on the Response Surface Method. Materials 2020, 13, 855. https://doi.org/10.3390/ma13040855
Sun Q, Wei X, Li T, Zhang L. Strengthening Behavior of Cemented Paste Backfill Using Alkali-Activated Slag Binders and Bottom Ash Based on the Response Surface Method. Materials. 2020; 13(4):855. https://doi.org/10.3390/ma13040855
Chicago/Turabian StyleSun, Qi, Xueda Wei, Tianlong Li, and Lu Zhang. 2020. "Strengthening Behavior of Cemented Paste Backfill Using Alkali-Activated Slag Binders and Bottom Ash Based on the Response Surface Method" Materials 13, no. 4: 855. https://doi.org/10.3390/ma13040855
APA StyleSun, Q., Wei, X., Li, T., & Zhang, L. (2020). Strengthening Behavior of Cemented Paste Backfill Using Alkali-Activated Slag Binders and Bottom Ash Based on the Response Surface Method. Materials, 13(4), 855. https://doi.org/10.3390/ma13040855




