Development of Novel Magnetoliposomes Containing Nickel Ferrite Nanoparticles Covered with Gold for Applications in Thermotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Nickel Ferrite/Gold Nanoparticles
2.1.1. Preparation of Nickel Ferrite Nanoparticles
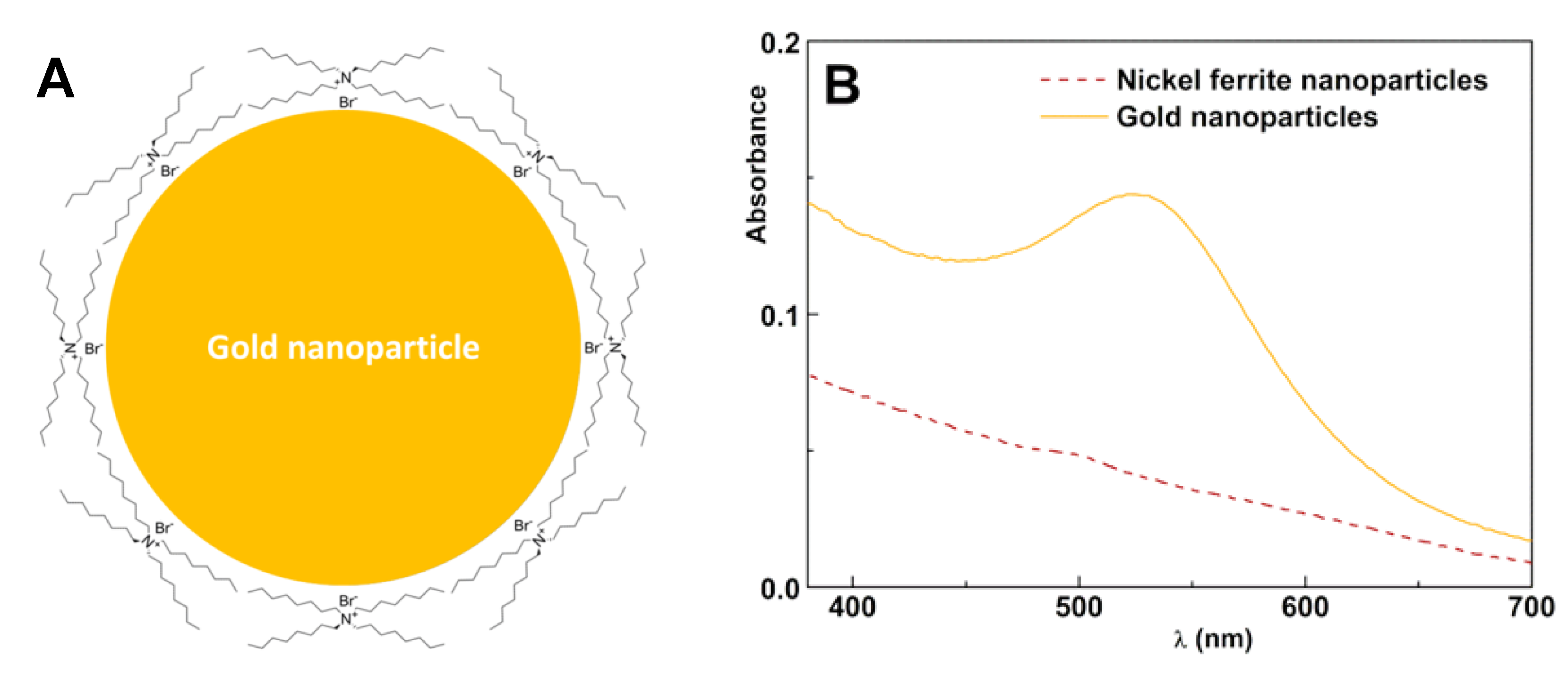
2.1.2. Preparation of Gold Nanoparticles
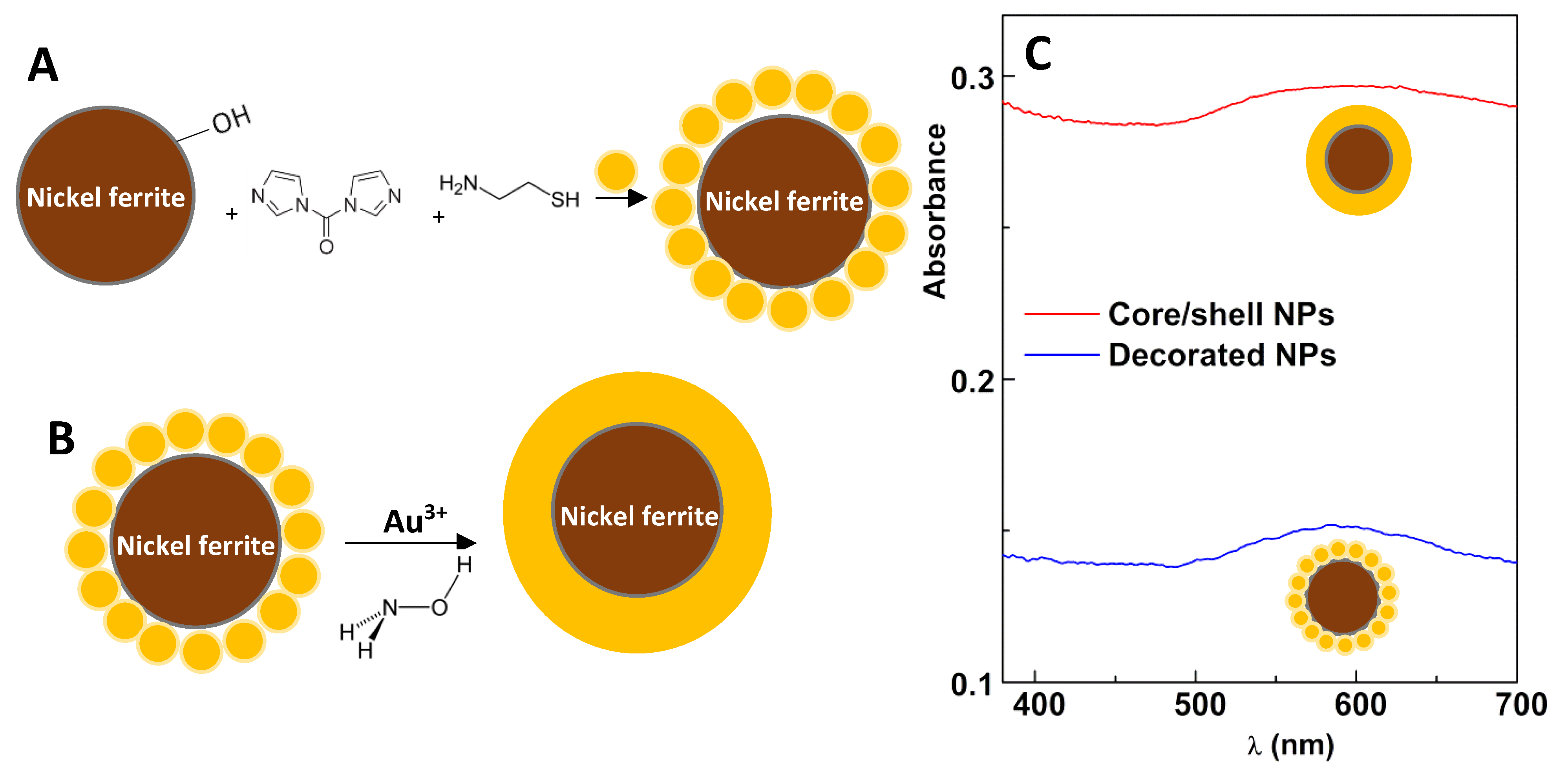
2.1.3. Preparation of Nickel Ferrite Nanoparticles Decorated with Gold
2.1.4. Preparation of Nickel Ferrite/Gold Core/Shell Nanoparticles
2.2. Preparation of Solid Magnetoliposomes
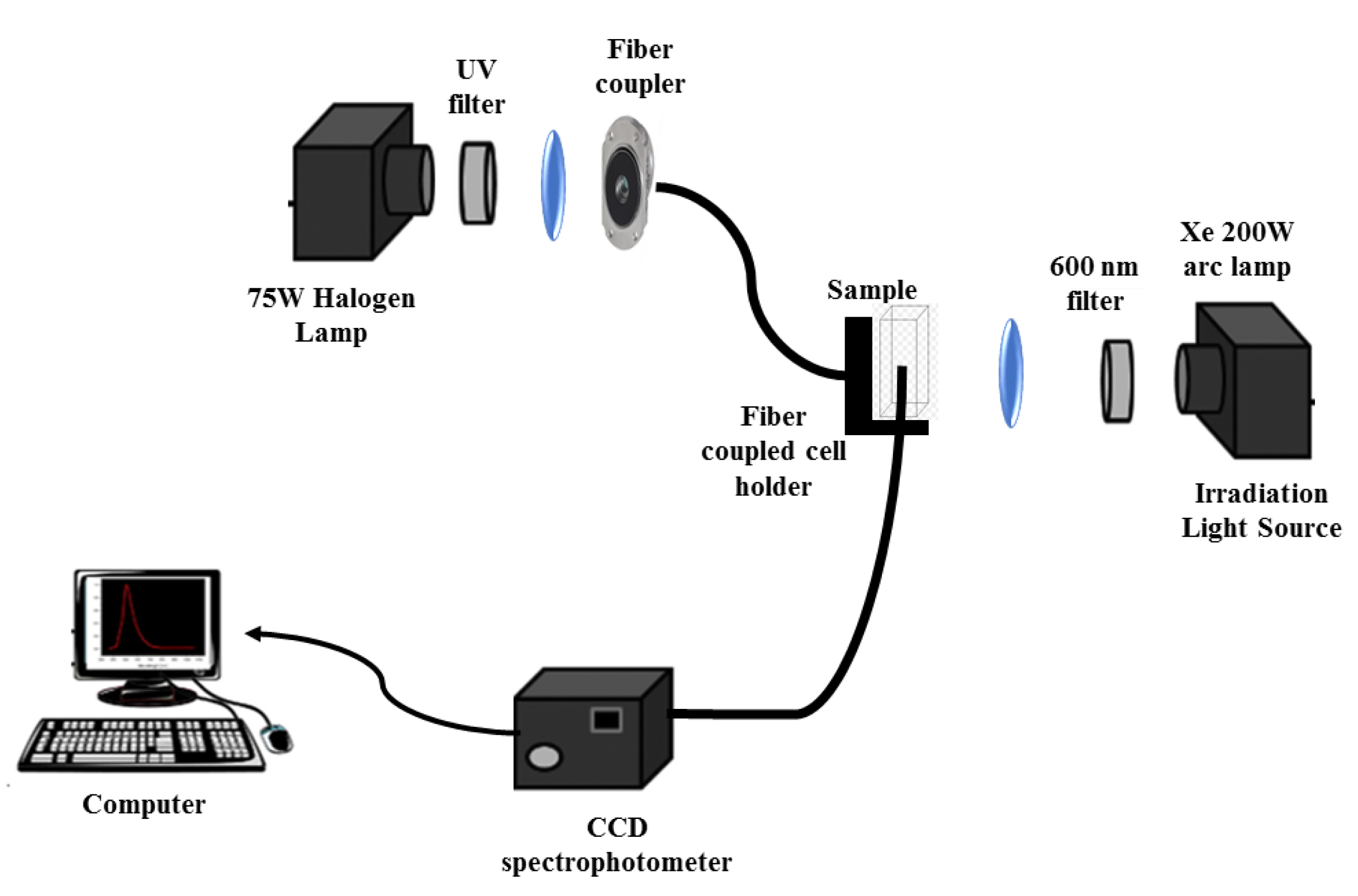
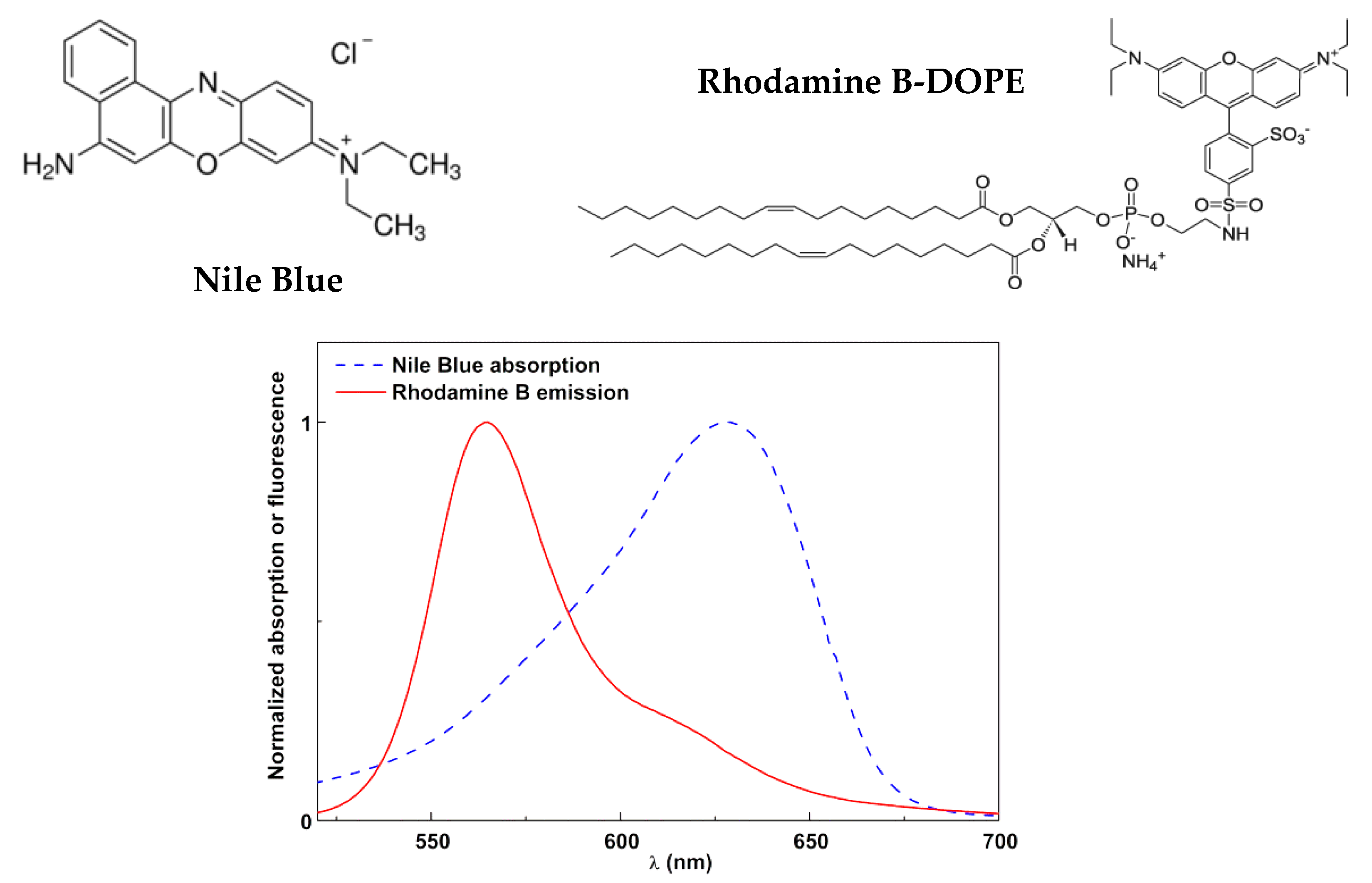
2.3. Spectroscopic Measurements
2.4. Structural and Magnetic Characterization
2.5. Assessment of the Photothermal Effect
3. Results and Discussion
3.1. Nanoparticles Synthesis and Characterization
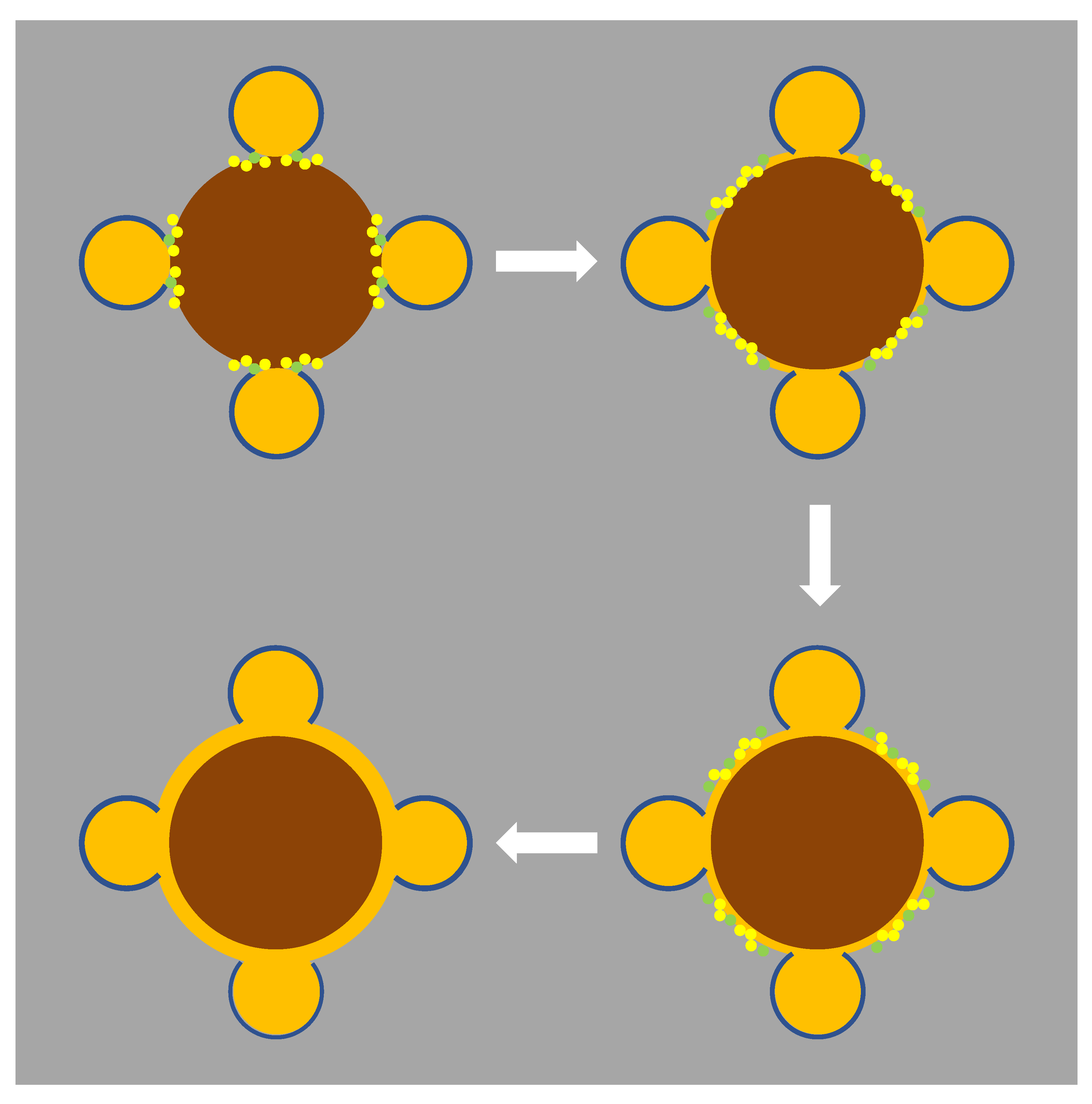
3.1.1. Synthesis of Magnetic/Plasmonic Nanoparticles
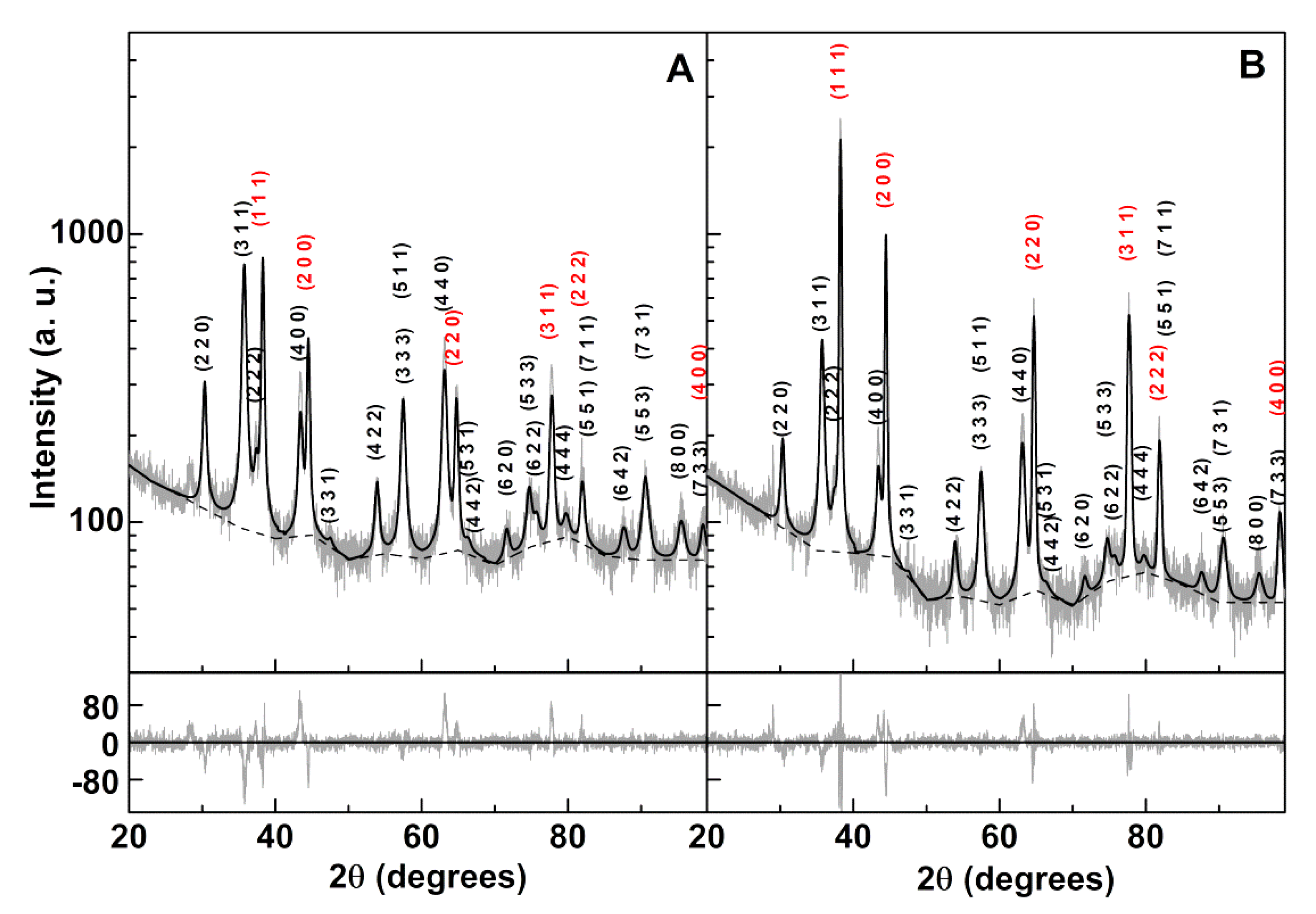
3.1.2. XRD Analysis
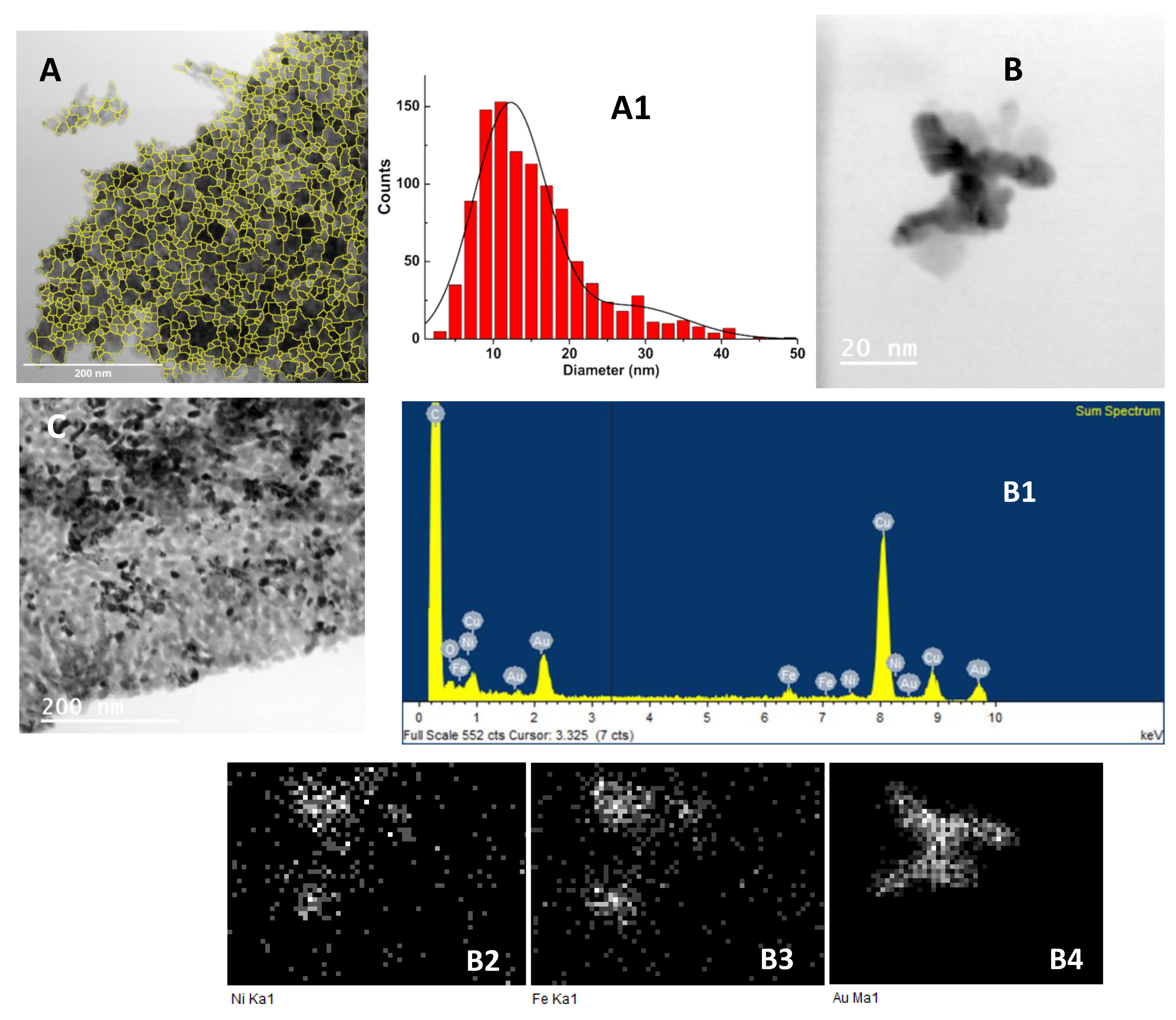
3.1.3. Transmission Electron Microscopy (TEM)
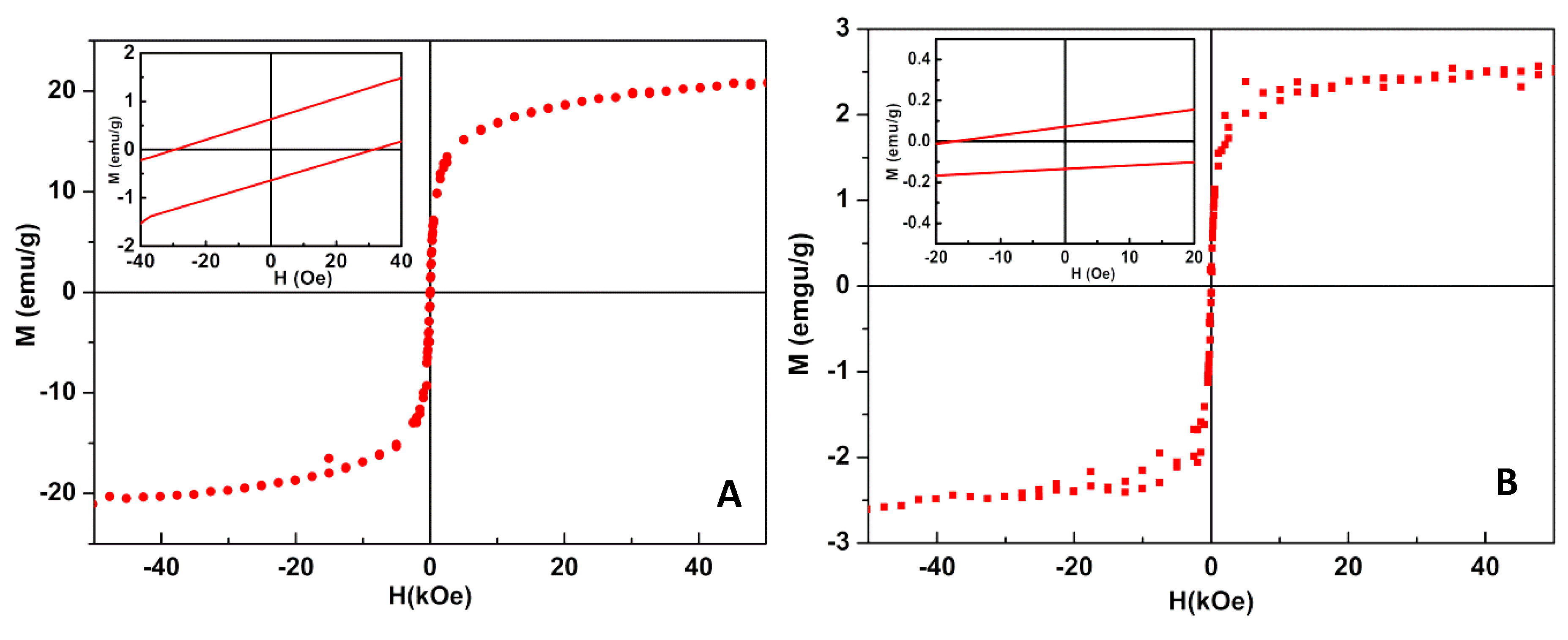
3.1.4. Magnetic Measurements
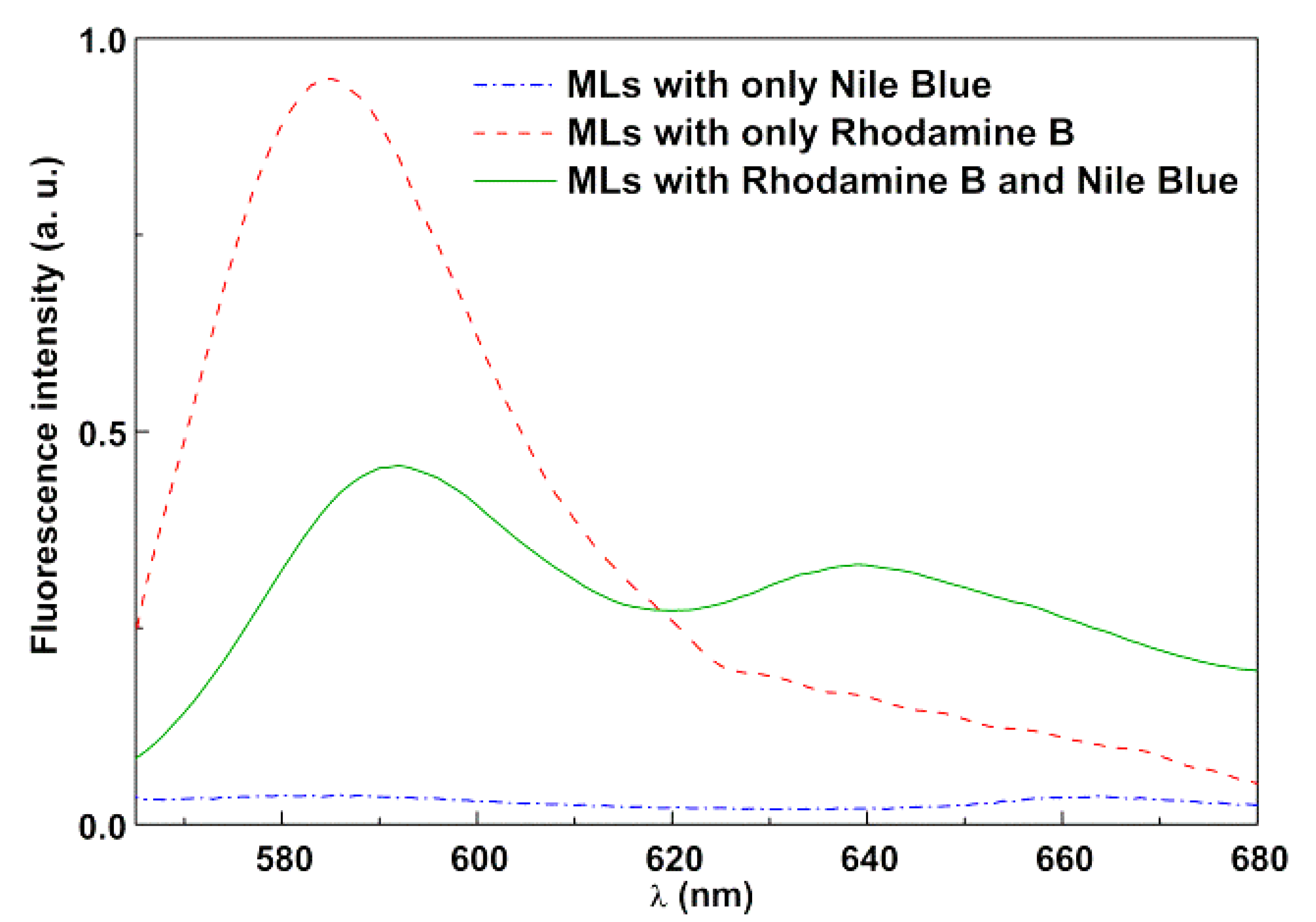
3.2. Magnetoliposome Synthesis and Characterization
Synthesis
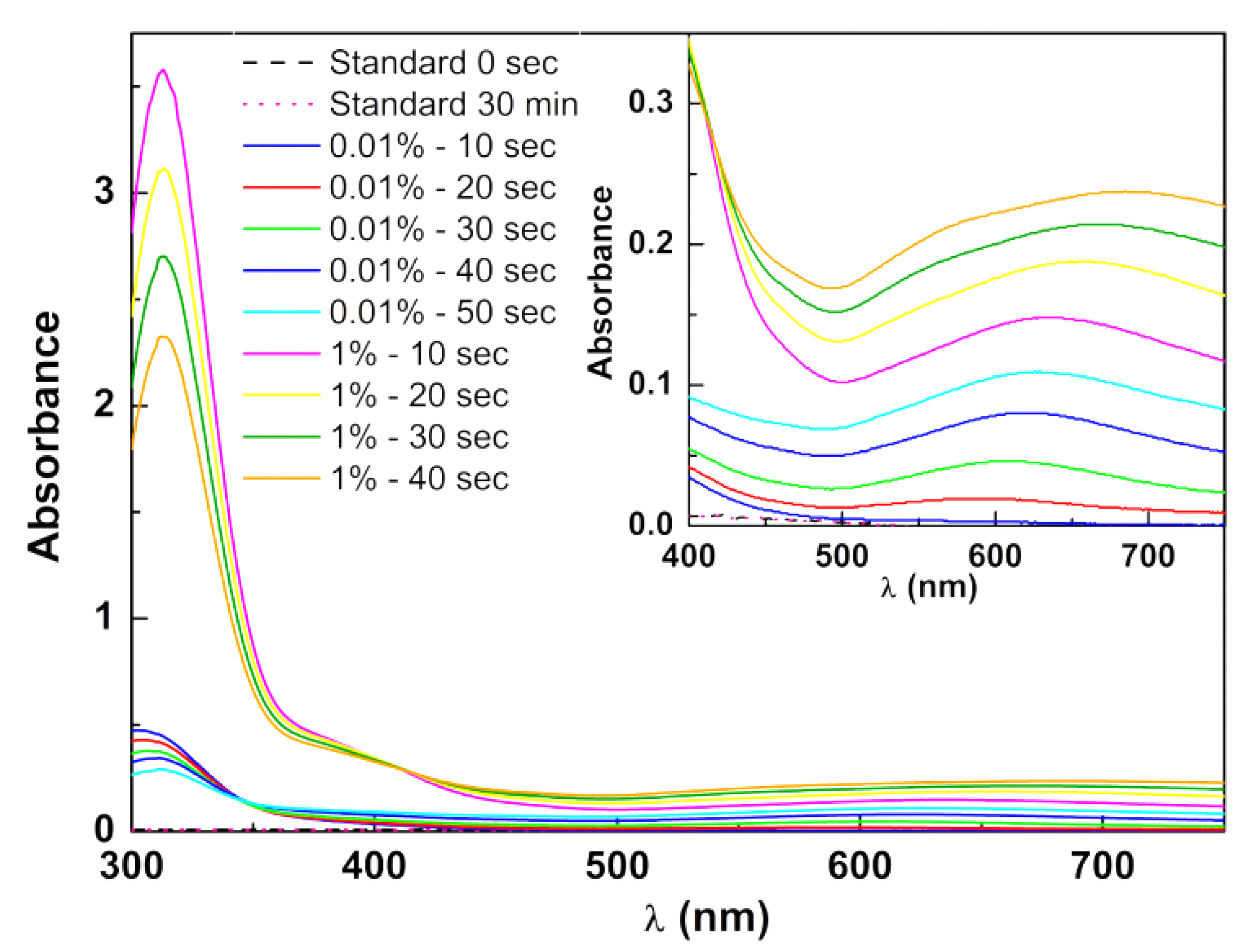
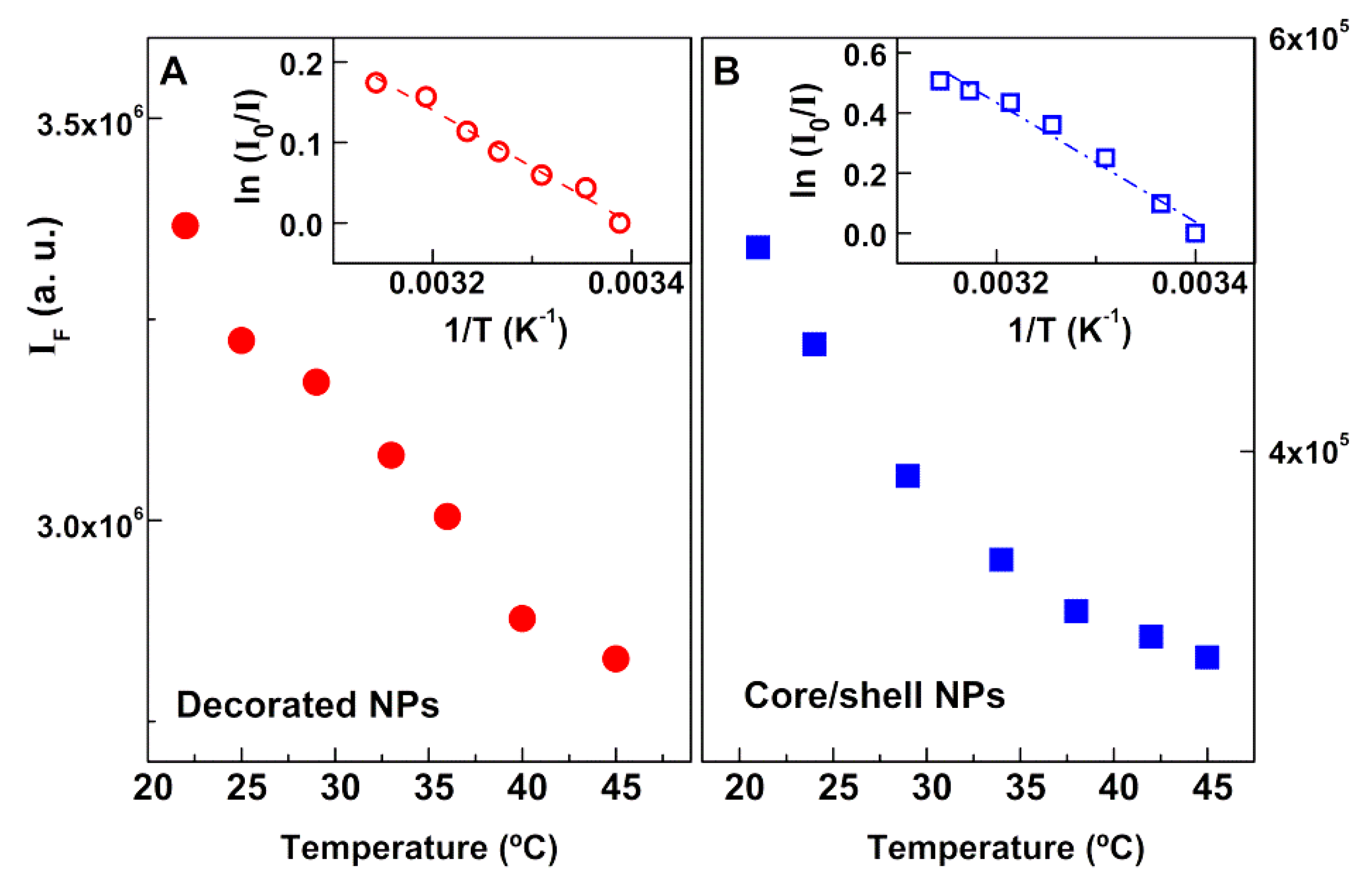
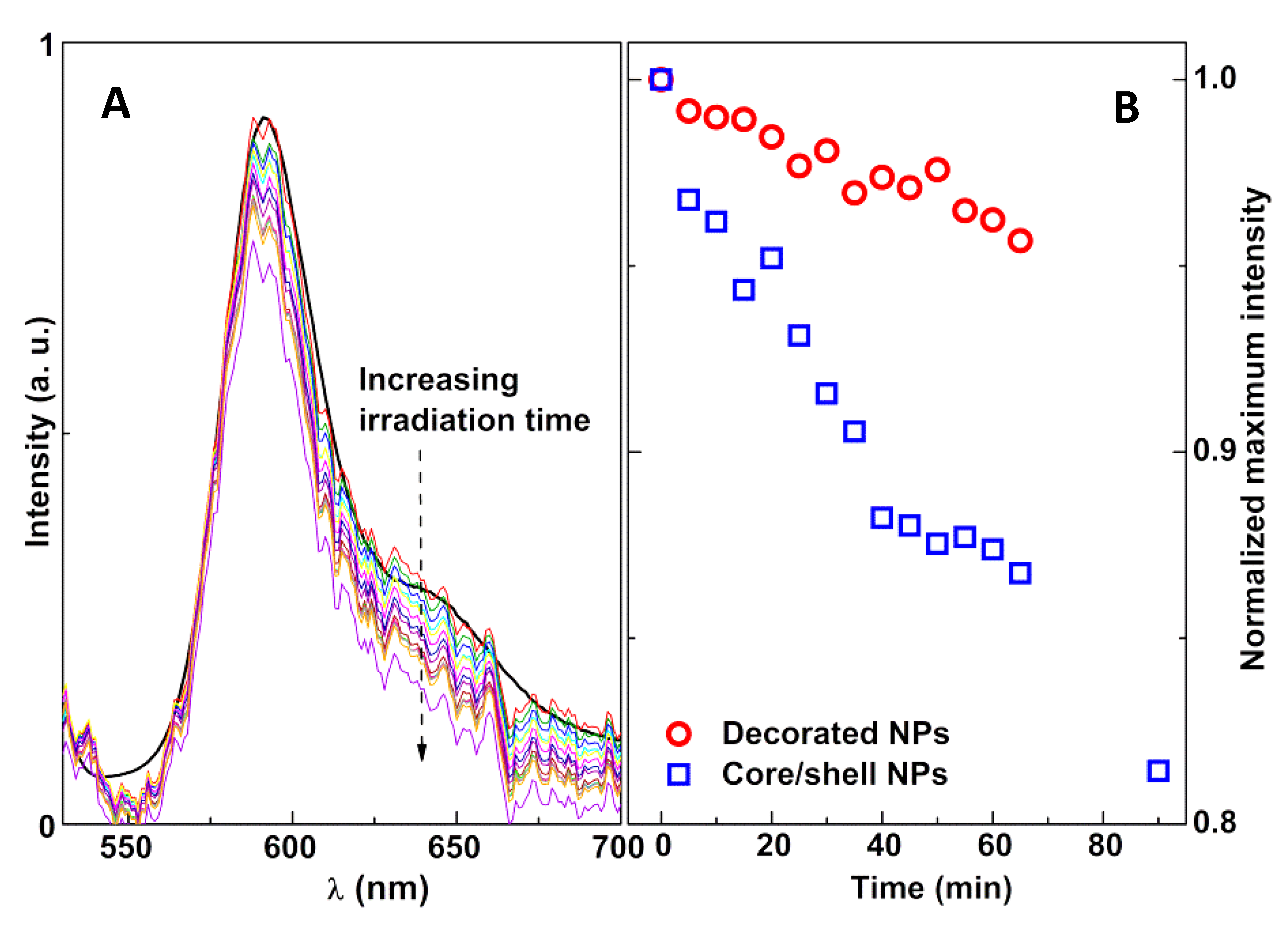
3.3. Photothermal Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Félix, R.; Schlag, P.M. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Rodrigues, A.R.O.; Almeida, B.; Rodrigues, J.M.; Queiroz, M.J.R.P.; Calhelha, R.C.; Ferreira, I.C.F.R.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Coutinho, P.J.G.; et al. Magnetoliposomes as carriers for promising antitumor thieno[3,2-b]pyridin-7-arylamines: photophysical and biological studies. RSC Adv. 2017, 7, 15352–15361. [Google Scholar] [CrossRef]
- Grasset, F.; Duguet, E. Magnetic nanoparticle design for medical diagnosis and therapy. J. Mater. Chem. 2004, 14, 2161–2175. [Google Scholar]
- Carneiro, M.F.H.; Barbosa, F. Gold nanoparticles: A critical review of therapeutic applications and toxicological aspects. J. Toxicol. Environ. Heal. Part B 2016, 19, 129–148. [Google Scholar] [CrossRef]
- Yeh, Y.; Creran, B.; Rotello, V. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in Biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef]
- Govorov, A.O.; Richardson, H.H. Generating heat with metal nanoparticles. Nano Today 2007, 2, 30–38. [Google Scholar] [CrossRef]
- Wu, D.; Xu, X.; Liu, X. Influence of dielectric core, embedding medium and size on the optical properties of gold nanoshells. Solid State Commun. 2008, 146, 7–11. [Google Scholar] [CrossRef]
- Das, M.; Shim, K.H.; An, S.S.A.; Yi, D.K. Review on gold nanoparticles and their applications. Toxicol. Environ. Heal. Sci. 2011, 3, 193–205. [Google Scholar] [CrossRef]
- Krishnan, K.M. Biomedical Nanomagnetics: A Spin Through Possibilities in Imaging, Diagnostics, and Therapy. IEEE Trans. Magn. 2010, 46, 2523–2558. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, S.; Santos, A.; Fessi, H.; Elaissari, A. Stimuli-responsive magnetic particles for biomedical applications. Int. J. Pharm. 2011, 403, 139–161. [Google Scholar] [CrossRef] [PubMed]
- Hervault, A.; Thanh, N.T.K. Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nanoscale 2014, 6, 11553–11573. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef]
- Rodrigues, A.R.O.; Gomes, I.; Almeida, B.G.; Araújo, J.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Magnetic liposomes based on nickel ferrite nanoparticles for biomedical applications. Phys. Chem. Chem. Phys. 2015, 17, 18011–18021. [Google Scholar] [CrossRef]
- Markides, H.; Rotherham, M.; El Haj, A.J. Biocompatibility and Toxicity of Magnetic Nanoparticles in Regenerative Medicine. J. Nanomater. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Khairy, M.; Gouda, M.E. Electrical and optical properties of nickel ferrite/polyaniline nanocomposite. J. Adv. Res. 2015, 6, 555–562. [Google Scholar] [CrossRef]
- Pérez-Lorenzo, M.; Vaz, B.; Salgueirino, V.; Correa-Duarte, M.A. Hollow-Shelled Nanoreactors Endowed with High Catalytic Activity. Chem. A Eur. J. 2013, 19, 12196–12211. [Google Scholar] [CrossRef]
- Elsherbini, A.A.; Saber, M.; Aggag, M.; El-Shahawy, A.; Shokier, H.A. Laser and radiofrequency-induced hyperthermia treatment via gold-coated magnetic nanocomposites. Int. J. Nanomed. 2011, 6, 2155–2165. [Google Scholar] [CrossRef]
- Menichetti, L.; Manzoni, L.; Paduano, L.; Flori, A.; Kusmic, C.; De Marchi, D.; Casciaro, S.; Conversano, F.; Lombardi, M.; Positano, V.; et al. Iron Oxide-Gold Core-Shell Nanoparticles as Multimodal Imaging Contrast Agent. IEEE Sensors J. 2013, 13, 2341–2347. [Google Scholar] [CrossRef]
- Sood, A.; Arora, V.; Shah, J.; Kotnala, R.; Jain, T.K. Multifunctional gold coated iron oxide core-shell nanoparticles stabilized using thiolated sodium alginate for biomedical applications. Mater. Sci. Eng. C 2017, 80, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mu, Q.; Revia, R.; Wang, K.; Tian, B.; Lin, G.; Lee, W.; Hong, Y.-K.; Zhang, M. Iron oxide-carbon core-shell nanoparticles for dual-modal imaging-guided photothermal therapy. J. Control. Release 2018, 289, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.R.O.; Matos, J.O.G.; Nova Dias, A.M.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Queiroz, M.J.R.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Development of multifunctional liposomes containing magnetic/plasmonic MnFe₂O₄/Au core/shell nanoparticles. Pharmaceutics 2019, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid? Liquid system. J. Chem. Soc., Chem. Commun. 1994, 7, 801. [Google Scholar] [CrossRef]
- Brown, K.R.; Natan, M.J. Hydroxylamine Seeding of Colloidal Au Nanoparticles in Solution and on Surfaces. Langmuir 1998, 14, 726–728. [Google Scholar] [CrossRef]
- Valeur, B. Molecular Fluorescence—Principles and Applications; Wiley-VCH: Weinheim, Germany, 2001; pp. 247–261. [Google Scholar]
- Fink, J.; Kiely, C.J.; Bethell, D.; Schiffrin, D.J. Self-Organization of Nanosized Gold Particles. Chem. Mater. 1998, 10, 922–926. [Google Scholar] [CrossRef]
- Lee, K.-S.; El-Sayed, M.A. Gold and Silver Nanoparticles in Sensing and Imaging: Sensitivity of Plasmon Response to Size, Shape, and Metal Composition. J. Phys. Chem. B 2006, 110, 19220–19225. [Google Scholar] [CrossRef]
- Chen, H.; Kou, X.; Yang, Z.; Ni, W.; Wang, J. Shape- and Size-Dependent Refractive Index Sensitivity of Gold Nanoparticles. Langmuir 2008, 24, 5233–5237. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G.; Hanson, J. Determination of Size and Concentration of Gold Nanoparticles from UV−Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Ung, T.; Liz-Marzán, L.M.; Mulvaney, P. Optical Properties of Thin Films of Au@SiO2Particles. J. Phys. Chem. B 2001, 105, 3441–3452. [Google Scholar] [CrossRef]
- Veloso, S.R.S.; Martins, J.A.; Hilliou, L.; Amorim, C.O.; Amaral, V.S.; Almeida, B.G.; Jervis, P.J.; Moreira, R.M.; Pereira, D.M.; Coutinho, P.J.G.; et al. Dehydropeptide-based plasmonic magnetogels: a supramolecular composite nanosystem for multimodal cancer therapy. J. Mater. Chem. B 2020, 8, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, M.H.; Nordhei, C.; Ramstad, A.L.; Nicholson, D.G.; Poliakoff, M.; Cabañas, A. XAS (XANES and EXAFS) Investigations of Nanoparticulate Ferrites Synthesized Continuously in Near Critical and Supercritical Water. J. Phys. Chem. C 2007, 111, 6252–6262. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Xu, Z.; Hou, Y.; Sun, S. Magnetic Core/Shell Fe3O4/Au and Fe3O4/Au/Ag Nanoparticles with Tunable Plasmonic Properties. J. Am. Chem. Soc. 2007, 129, 8698–8699. [Google Scholar] [CrossRef]
- Wang, L.; Luo, J.; Maye, M.M.; Fan, Q.; Rendeng, Q.; Engelhard, M.H.; Wang, C.; Lin, Y.; Zhong, C.-J. Iron oxide–gold core–shell nanoparticles and thin film assembly. J. Mater. Chem. 2005, 15, 1821–1832. [Google Scholar] [CrossRef]
- Mandal, M.; Kundu, S.; Ghosh, S.K.; Panigrahi, S.; Sau, T.K.; Yusuf, S.; Pal, T. Magnetite nanoparticles with tunable gold or silver shell. J. Colloid Interface Sci. 2005, 286, 187–194. [Google Scholar] [CrossRef]
- Legland, D.; Arganda-Carreras, I.; Andrey, P. MorphoLibJ: integrated library and plugins for mathematical morphology with Image. J. Bioinform. 2016, 32, 3532–3534. [Google Scholar] [CrossRef]
- Perron, H.; Mellier, T.; Domain, C.; Roques, J.; Simoni, E.; Drot, R.; Catalette, H. Structural investigation and electronic properties of the nickel ferrite NiFe2O4: A periodic density functional theory approach. J. Phys. Condens. Matter 2007, 19, 346219. [Google Scholar] [CrossRef]
- Chinnasamy, C.N.; Narayanasamy, A.; Ponpandian, N.; Chattopadhyay, K.; Shinoda, K.; Jeyadevan, B.; Tohji, K.; Nakatsuka, K.; Furubayashi, T.; Nakatani, I. Mixed spinel structure in nanocrystalline NiFe2O4. Phys. Rev. B 2001, 63, 184108. [Google Scholar] [CrossRef]
- Nawale, A.B.; Kanhe, N.S.; Patil, K.; Bhoraskar, S.; Mathe, V.; Das, A. Magnetic properties of thermal plasma synthesized nanocrystalline nickel ferrite (NiFe2O4). J. Alloy. Compd. 2011, 509, 4404–4413. [Google Scholar] [CrossRef]
- Jaffari, G.H.; Rumaiz, A.K.; Woicik, J.C.; Shah, S.I. Influence of oxygen vacancies on the electronic structure and magnetic properties of NiFe2O4 thin films. J. Appl. Phys. 2012, 111, 93906. [Google Scholar] [CrossRef]
- Smit, J. Magnetic Properties of Materials; McGraw Hill: New York, NY, USA, 1971; ISBN 978-0070584457. [Google Scholar]
- Demas, J.N.; Crosby, G.A. The measurement of photoluminescence quantum yields—Review. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar] [CrossRef]
- Fery-Forgues, S.; Lavabre, D. Are Fluorescence Quantum Yields So Tricky to Measure? A Demonstration Using Familiar Stationery Products. J. Chem. Educ. 1999, 76, 1260. [Google Scholar] [CrossRef]
- Kubin, R.; Fletcher, A. Fluorescence quantum yields of some rhodamine dyes. J. Lumin. 1982, 27, 455–462. [Google Scholar] [CrossRef]
- Curtis, H.; Barnes, N.S. Biology, 5th ed.; Worth Publishers: New York, NY, USA, 1989; part 1; ISBN 978-0879013943. [Google Scholar]
- Sawant, R.R.; Torchilin, V.P. Challenges in Development of Targeted Liposomal Therapeutics. AAPS J. 2012, 14, 303–315. [Google Scholar] [CrossRef]
- Dahl, K.; Biswas, R.; Maroncelli, M. The Photophysics and Dynamics of Diphenylbutadiene in Alkane and Perfluoroalkane Solvents. J. Phys. Chem. B 2003, 107, 7838–7853. [Google Scholar] [CrossRef]
- Hirsch, L.R.; Gobin, A.M.; Lowery, A.R.; Tam, F.; Drezek, R.A.; Halas, N.J.; West, J.L. Metal Nanoshells. Ann. Biomed. Eng. 2006, 34, 15–22. [Google Scholar] [CrossRef]


| Nanoparticles | Intensity Percentages | Phase Size (nm) Lattice Constant (Å) | Quality Parameters NiFe2O4|Au | |||
|---|---|---|---|---|---|---|
| NiFe2O4 | Au | NiFe2O4 | Au | Rf | χ2 | |
| Au@NiFe2O4 | 89.1 | 10.9 | 11.5 8.331 | 22.7 4.071 | 5.16|3.81 | 1.43 |
| NiFe2O4/Au | 70.1 | 29.9 | 11.5 (*) 8.331 (*) | 59.6 4.075 | 5.05|1.36 | 1.42 |
| Nanoparticles | Hc (Oe) | Mr (emu/g) | Ms (emu/g) | Mr/Ms |
|---|---|---|---|---|
| NiFe2O4 NPs decorated with Au NPs | 29.00 | 0.61 | 20.55 | 0.030 |
| NiFe2O4/Au core/shell NPs | 17.19 | 0.06 | 2.65 | 0.023 |
| Nanoparticles | Size ± SD (nm) | PDI ± SD |
|---|---|---|
| Nickel ferrite decorated with gold NPs | 118.5 ± 8 | 0.171 ± 0.03 |
| Nickel ferrite/gold core/shell NPs | 158 ± 12 | 0.184 ± 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rio, I.S.R.; Rodrigues, A.R.O.; Rodrigues, C.P.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Development of Novel Magnetoliposomes Containing Nickel Ferrite Nanoparticles Covered with Gold for Applications in Thermotherapy. Materials 2020, 13, 815. https://doi.org/10.3390/ma13040815
Rio ISR, Rodrigues ARO, Rodrigues CP, Almeida BG, Pires A, Pereira AM, Araújo JP, Castanheira EMS, Coutinho PJG. Development of Novel Magnetoliposomes Containing Nickel Ferrite Nanoparticles Covered with Gold for Applications in Thermotherapy. Materials. 2020; 13(4):815. https://doi.org/10.3390/ma13040815
Chicago/Turabian StyleRio, Irina S. R., Ana Rita O. Rodrigues, Carolina P. Rodrigues, Bernardo G. Almeida, A. Pires, A. M. Pereira, J. P. Araújo, Elisabete M. S. Castanheira, and Paulo J. G. Coutinho. 2020. "Development of Novel Magnetoliposomes Containing Nickel Ferrite Nanoparticles Covered with Gold for Applications in Thermotherapy" Materials 13, no. 4: 815. https://doi.org/10.3390/ma13040815
APA StyleRio, I. S. R., Rodrigues, A. R. O., Rodrigues, C. P., Almeida, B. G., Pires, A., Pereira, A. M., Araújo, J. P., Castanheira, E. M. S., & Coutinho, P. J. G. (2020). Development of Novel Magnetoliposomes Containing Nickel Ferrite Nanoparticles Covered with Gold for Applications in Thermotherapy. Materials, 13(4), 815. https://doi.org/10.3390/ma13040815






