The Impact of the Vanadium Oxide Addition on the Physicochemical Performance Stability and Intercalation of Lithium Ions of the TiO2-rGO-electrode in Lithium Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of VxOy-TiO2-rGO Oxide Nanocomposites
2.2. Electrode and Electrolyte
2.3. Procedures and Measurements
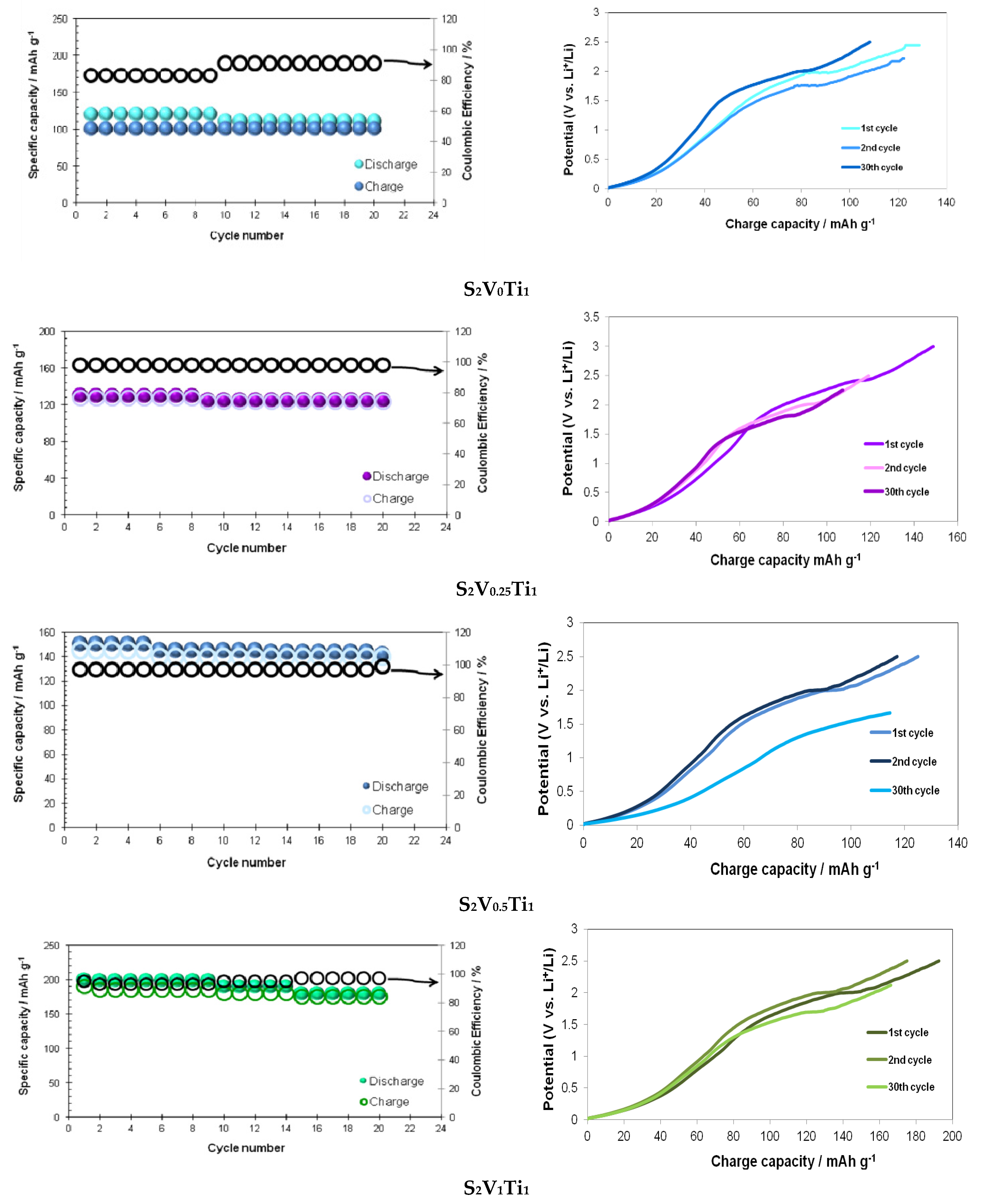
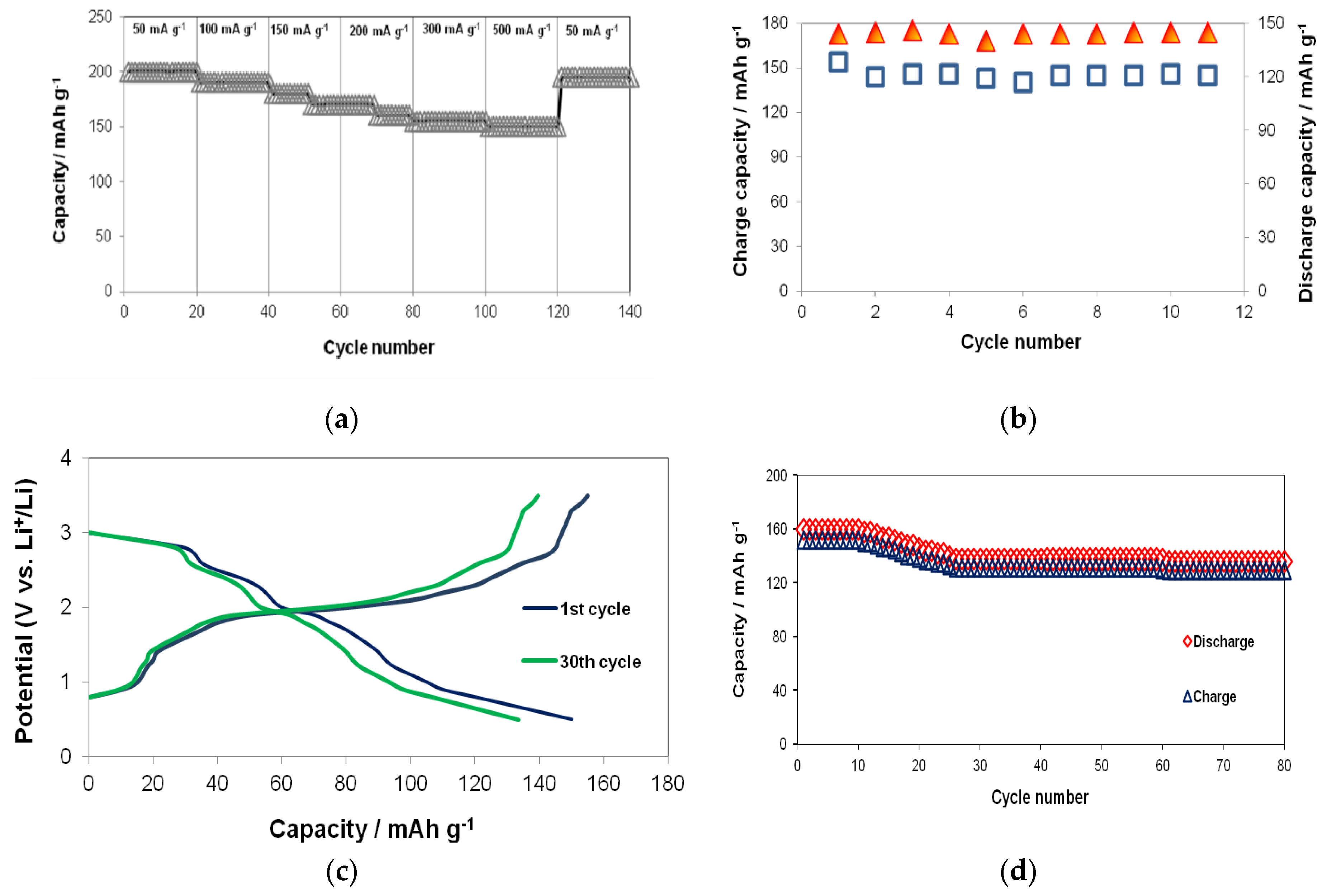
3. Results
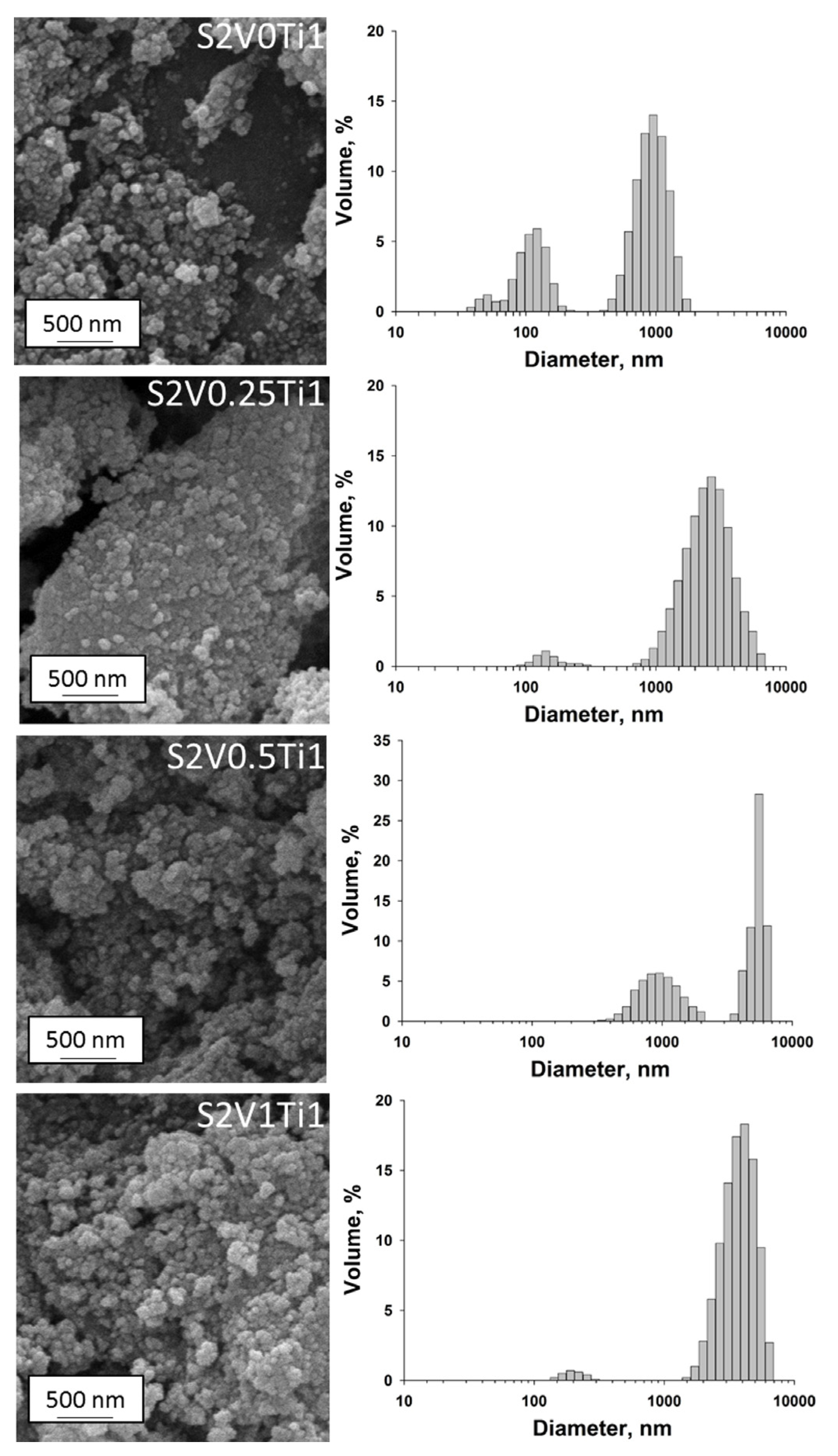
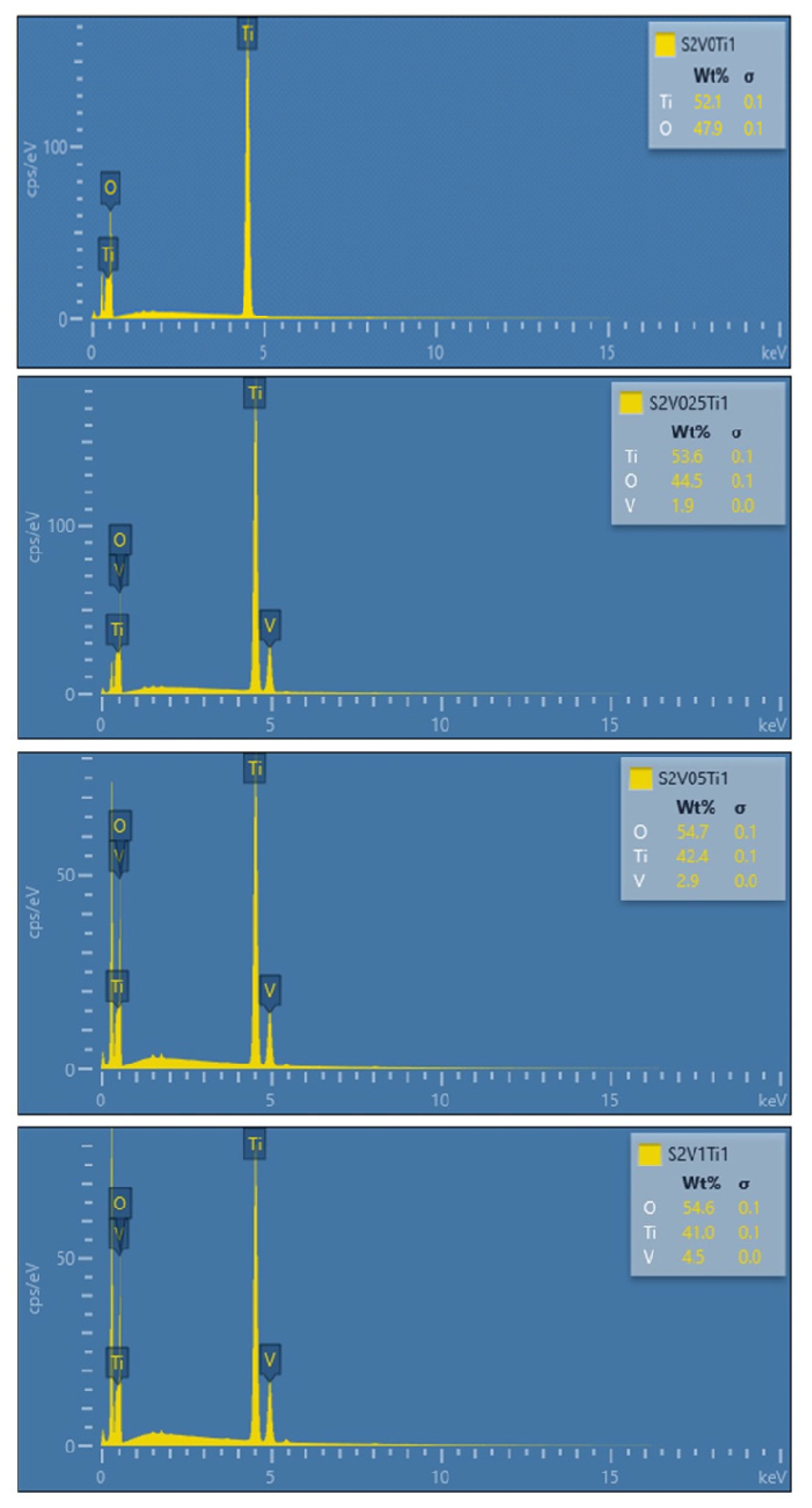
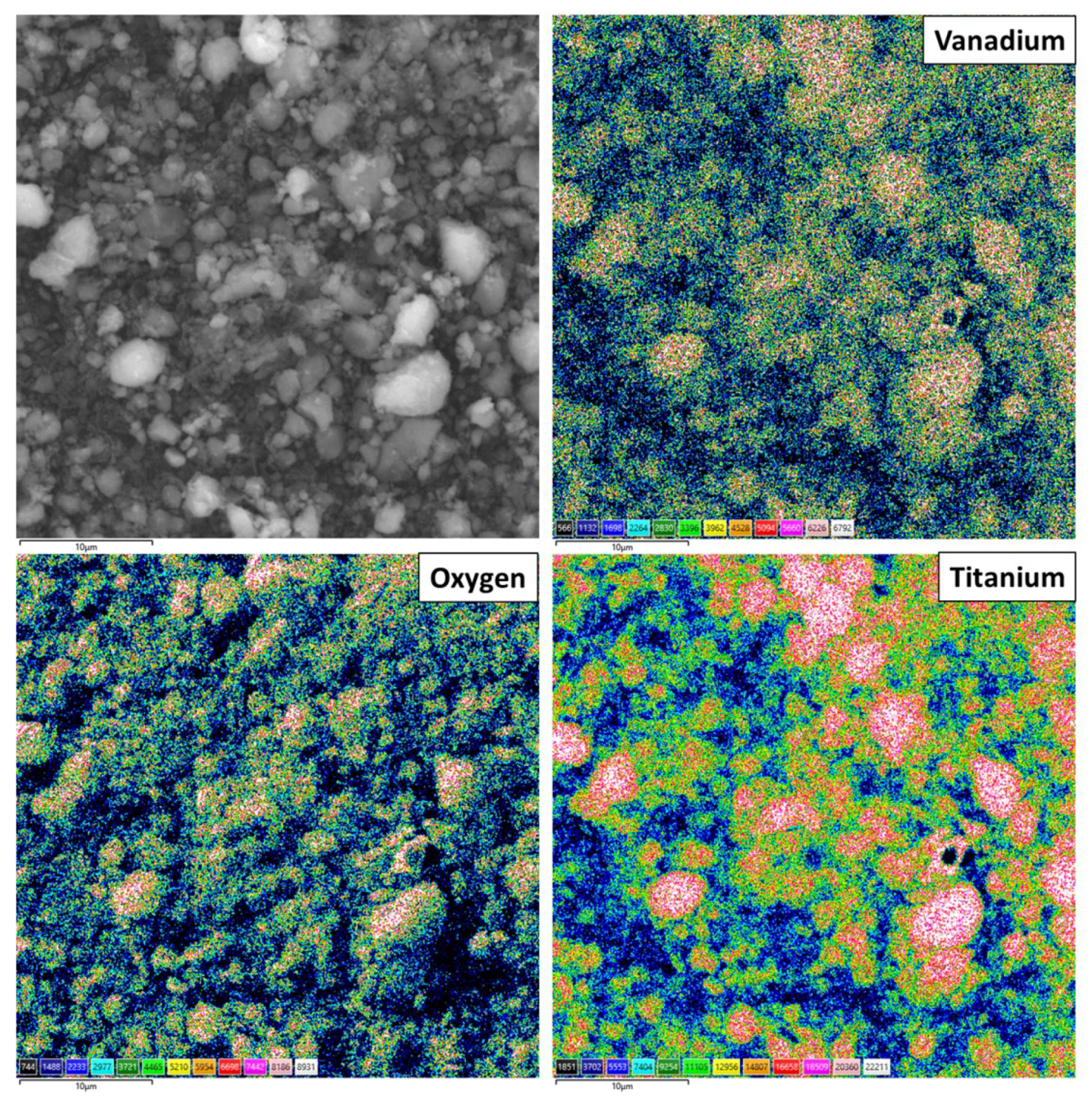
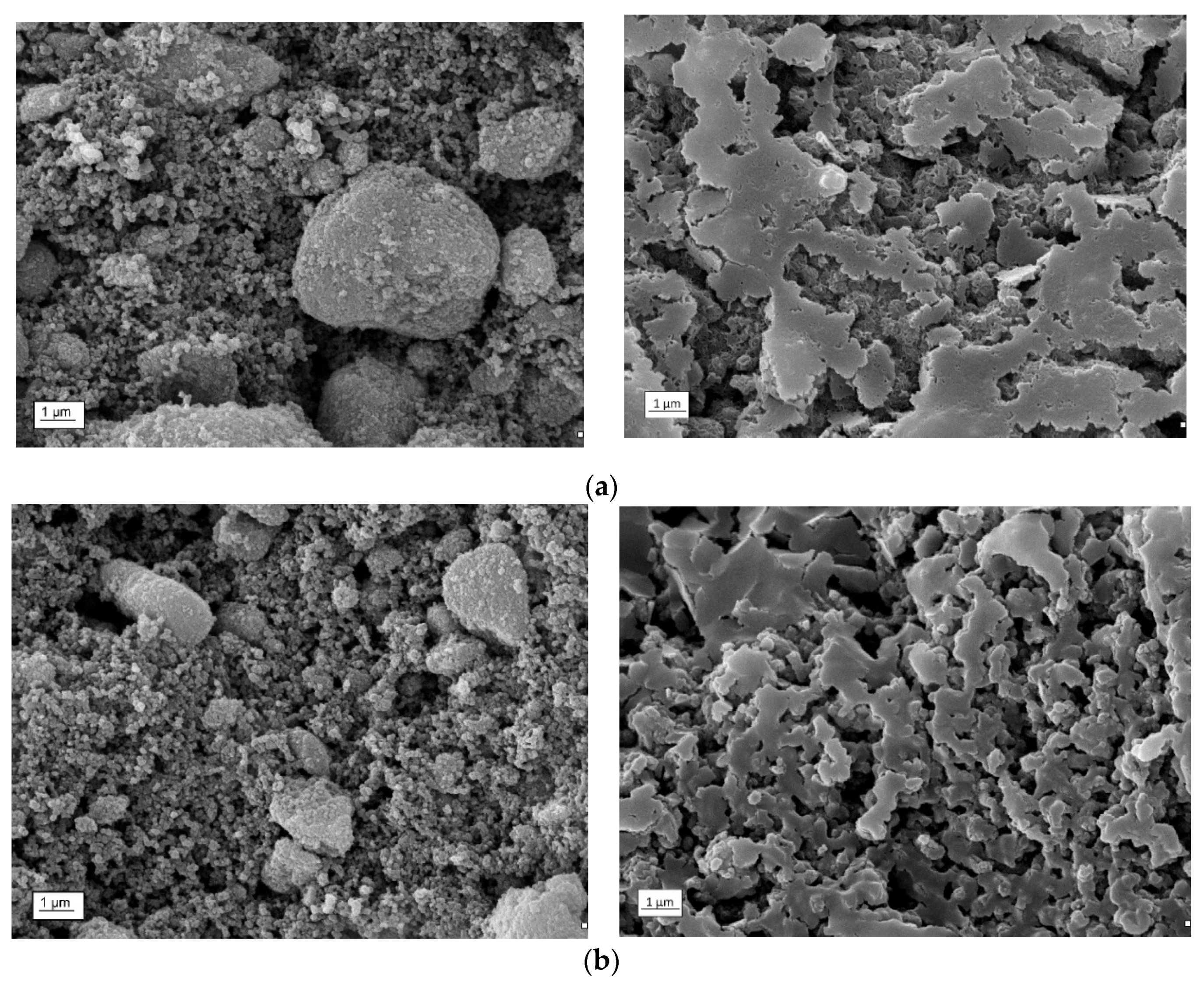
3.1. Dispersive and Structural Properties of the Obtained Materials
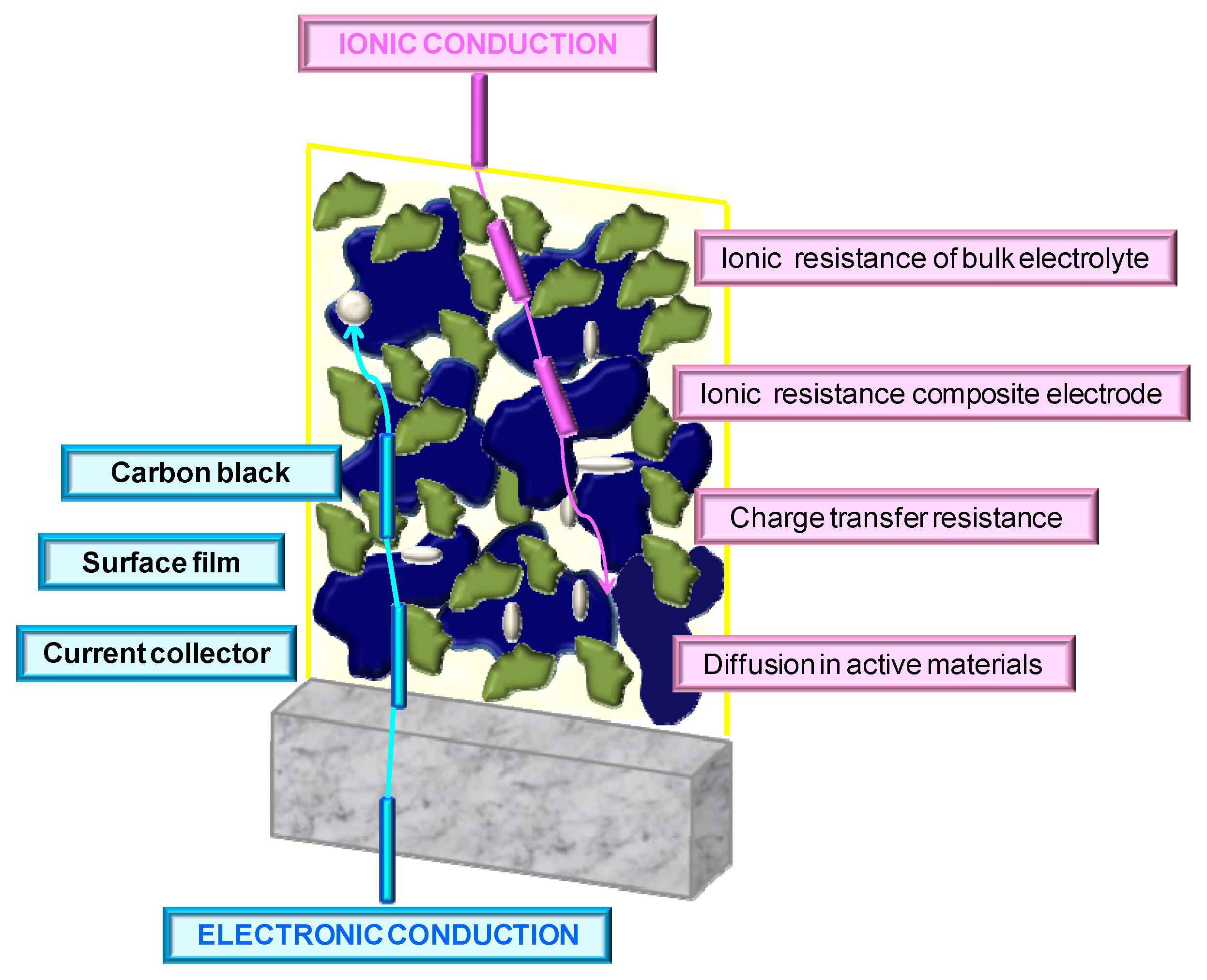
3.2. Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ziolkowski, A. Automotive Thermoelectric Generator impact on the efficiency of a drive system with a combustion engine. MATEC Web Conf. 2017, 118, 00024. [Google Scholar] [CrossRef]
- Bajerlein, M.; Rymaniak, L.; Swiatek, P.; Ziolkowski, A.; Daszkiewicz, P.; Dobrzynski, M. Modification of a Hybrid City Bus Powertrain in the Aspect of Lower Fuel Consumption and Exhaust Emissions. Appl. Mech. Mater. 2014, 518, 108–113. [Google Scholar] [CrossRef]
- Skretowicz, M.; Sroka, Z. Analysis of application of alternative drive systems for international heavy-duty transport on Wroclaw-Dresden-Prague routes. E3S Web Conf. 2017, 22, 00159. [Google Scholar] [CrossRef]
- Gis, W.; Merkisz, W.J. The development status of electric (BEV) and hydrogen (FCEV) passenger cars park in the world and new research possibilities of these cars in real traffic conditions. Combust. Engines 2019, 178, 144–149. [Google Scholar]
- Ubide, D.; Larrodé, E.; de Velasco, J. New Hybrid Bus Prototype for Clean Urban Transportation. SAE Trans. 2003, 6, 704–710. [Google Scholar]
- Pandey, G.P.; Liu, T.; Brown, E.; Yang, Y.; Li, Y.; Sun, X.S.; Fang, Y.; Li, J. Mesoporous Hybrids of Reduced Graphene Oxide and Vanadium Pentoxide for Enhanced Performance in Lithium-Ion Batteries and Electrochemical Capacitors. Appl. Mater. Interfaces 2016, 8, 9200–9210. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, D.; Yang, M.; Schmuki, P. Vertically aligned mixed V2O5–TiO2nanotube arrays for supercapacitor applications. Chem. Comm. 2011, 47, 7746–7748. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Nagaraju, D.H.; Beaujuge, P.; Alsharef, H.N. Supercapacitors based on two dimensional VO2 nanosheet electrodes in organic gel electrolyte. Electochim. Acta 2016, 220, 601–608. [Google Scholar] [CrossRef]
- Felix-Quintero, H.; Angulo-Rocha, J.; Murrieta, S.H.; Hernandez, A.J.; Camarillo, G.E.; Flores, J.M.C.; Alejo-Armenta, C.; Garcia-Hipolito, M.; Ramos-Brito, F. Study on grow process and optical properties of ZnO microrods synthesized by hydrothermal method. J. Luminesence 2017, 182, 107–113. [Google Scholar] [CrossRef]
- Li, H.X.; Jiao, L.F.; Yuan, H.T.; Zhao, M.; Zhang, M.; Wang, Y.M. High-performance Cu-doped vanadium oxide (CuxV2O5) prepared by rapid precipitation method for rechargeable batteries. Mater. Lett. 2007, 61, 101–104. [Google Scholar]
- Saravanakumar, B.; Purushothaman, K.K.; Muralidharan, G. Interconnected V2O5 Nanoporous Network for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2012, 4, 4484–4490. [Google Scholar] [CrossRef] [PubMed]
- Gautam, G.S.; Canepa, P.; Abdellahi, A.; Urban, A.; Malik, R.; Ceder, G. The Intercalation Phase Diagram of Mg in V2O5 from First-Principles. Chem. Mater. 2015, 27, 225–230. [Google Scholar] [CrossRef]
- Foo, C.Y.; Sumboja, A.; Tan, D.J.; Wang, J.X.; Lee, P.S. Flexible and Highly Scalable V2O5-rGO Electrodes in an Organic Electrolyte for Supercapacitor Devices. Adv. Energy. Mater. 2014, 4, 1400236–1400241. [Google Scholar] [CrossRef]
- Wang, P.; Han, L.; Zhu, C.; Zhai, Y.; Wang, S.P. Aqueous-phase synthesis of Ag-TiO2-reduced graphene oxide and Pt-TiO2-reduced graphene oxide hybrid nanostructures and their catalytic properties. Nano Res. 2011, 4, 1153–1162. [Google Scholar] [CrossRef]
- Liang, Z.; Zhao, Y.; Dong, Y.; Kuang, Q.; Lin, X.; Liu, X.; Yan, D. The low and high temperature electrochemical performance of Li3VO4/C anode material for Li-ion batteries. J. Electroanal. Chem. 2015, 745, 1–7. [Google Scholar] [CrossRef]
- Bezrodna, T.; Puchkovska, G.; Shymanovska, V.; Baran, H.; Ratajczak, J. IR-analysis of H-bonded H2O on the pure TiO2 surface. J. Mol. Struct. 2004, 700, 175–181. [Google Scholar] [CrossRef]
- Roşu, M.C.; Socaci, C.; Floare-Avram, V.; Borodi, G.; Pogacean, F.; Coros, M.; Magerusan, L.; Pruneanu, S. Photocatalytic performance of graphene/TiO2-Ag composites on amaranth dye degradation. Mater. Chem. Phys. 2016, 179, 232–241. [Google Scholar] [CrossRef]
- Calvo, F.; Li, Y.; Kiawi, D.M.; Bakker, J.M.; Parneix, P.; Janssens, E. Nonlinear effects in infrared action spectroscopy of silicon and vanadium oxide clusters: Experiment and kinetic modeling. Phys. Chem. Chem. Phys. 2015, 17, 25956–25967. [Google Scholar] [CrossRef]
- Patil, C.E.; Jadhav, P.R.; Tarwal, N.L.; Deshmukh, H.P.; Karanjkar, M.M.; Patil, P.S. Electrochromic performance of mixed V2O5–MoO3 thin films synthesized by pulsed spray pyrolysis technique. Mater. Chem. Phys. 2011, 126, 711–716. [Google Scholar] [CrossRef]
- Mutyala, S.; Mathiyarasu, J. Reagentless non-enzymatic hydrogen peroxide sensor using electrochemically reduced graphene oxide modified glassy carbon electrode. Mater. Sci. Eng. C 2016, 69, 398–406. [Google Scholar] [CrossRef]
- Zhuo, Q.; Ma, Y.; Gao, J.; Zhang, P.; Xia, Y.; Tian, Y.; Sun, X.; Zhong, J.; Sun, X. Facile Synthesis of Graphene/Metal Nanoparticle Composites via Self-Catalysis Reduction at Room Temperature. Inorg. Chem. 2013, 52, 3141–3147. [Google Scholar] [CrossRef]
- Verma, R.; Samdarshi, S.K.; Sagar, K.; Konwar, B.K. Nanostructured bi-phasic TiO2 nanoparticles grown on reduced graphene oxide with high visible light photocatalytic detoxification. Mater. Chem. Phys. 2017, 186, 202–211. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Rafaja, D.; Koltsov, I.; Stocker, H.; Szalaty, T.J.; Bazhenov, V.V.; Stelling, A.L.; Beyer, J.; Heitmann, J.; et al. Extreme Biomimetic approach for synthesis of nanocrystalline chitin-(Ti,Zr)O2 multiphase composites. Mater. Chem. Phys. 2017, 188, 115–124. [Google Scholar] [CrossRef]
- Jung, H.; Gerasopoulos, K.; Talin, A.A.; Ghodssi, R. In situ characterization of charge rate dependent stress and structure changes in V2O5 cathode prepared by atomic layer deposition. J. Power Sources 2017, 340, 89–97. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, J.Z.; Chou, S.L.; Wexler, D.; Li, H.J.; Ozawa, K.; Liu, H.K.; Wu, Y.P. Hollow Structured Li3VO4 Wrapped with Graphene Nanosheets in Situ Prepared by a One-Pot Template-Free Method as an Anode for Lithium-Ion Batterie. Nano Lett. 2013, 13, 4715–4720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.G.; Liu, H.; Liu, H.; Wei, B. Coaxial MoS2@Carbon Hybrid Fibers: A Low-Cost Anode Material for High-Performance Li-Ion Batteries. Materials 2017, 10, 250–255. [Google Scholar]
- Luo, W.; Zheng, B. Improved electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode material by double-layer coating with graphene oxide and V2O5 for lithium-ion batteries. Appl. Surf. Sci. 2017, 404, 310–317. [Google Scholar]
- Zhang, J.; Ni, S.; Kang, T.; Tang, J.; Yang, X.; Zhang, L. Prominent electrochemical performance of a Li3VO4/C –Ni anode via hierarchically porous architecture design. J. Mater. Chem. A 2016, 4, 14101–14105. [Google Scholar] [CrossRef]
- Przesniak-Welenc, M.; Karczewski, J.; Smalc-Koziorowska, J.; Łapinski, M.; Sadowskia, W.; Koscielska, B. The influence of nanostructure size on V2O5 electrochemical properties as cathode materials for lithium ion batteries. RSC Adv. 2016, 6, 55689–55694. [Google Scholar] [CrossRef]
- Zhou, X.; He, T.; Chen, X.; Sun, L.; Liu, Z. Influence of TiO2 surface coating on the electrochemical properties of V2O5 micro-particles as a cathode material for lithium ion batteries. RSC Adv. 2016, 6, 53925–53931. [Google Scholar] [CrossRef]
- Niu, C.; Li, J.; Jin, H.; Shi, H.; Zhu, Y.; Wang, W.; Cao, M. Self-template processed hierarchical V2O5 nanobelts as cathode for high performance lithium ion battery. Electrochim. Acta 2015, 182, 621–628. [Google Scholar] [CrossRef]
- Pan, L.; Zhu, X.D.; Xie, X.M.; Liu, Y.T. Delicate ternary heterostructures achieved by hierarchical co-assembly of Ag and Fe3O4 nanoparticles on MoS2 nanosheets: Morphological and compositional synergy in reversible lithium storage. J. Mater. Chem. A 2015, 3, 2726–2730. [Google Scholar] [CrossRef]
- Escamilla-Perez, A.M.; Louvain, N.; Kaschowitz, M.; Freunberger, S.; Fontaine, O.; Boury, B.; Brun, N.; Mutin, P.H. Lithium insertion properties of mesoporous nanocrystalline TiO2 and TiO2–V2O5 microspheres prepared by non-hydrolytic sol–gel. J. Sol-Gel Sci. Technol. 2016; 79, 270–278. [Google Scholar]
- Yan, D.J.; Zhu, X.D.; Wang, K.X.; Gao, X.T.; Feng, Y.J.; Sun, K.N.; Liu, Y.T. Facile and elegant self-organization of Ag nanoparticles and TiO2 nanorods on V2O5 nanosheets as a superior cathode material for lithium-ion batteries. J. Mater. Chem. A 2016, 4, 4900–4909. [Google Scholar] [CrossRef]
- Hu, S.; Song, Y.; Yuan, S.; Liu, H.; Xu, Q.; Wang, Y.; Wang, C.-X.; Xia, Y.-Y. A hierarchical structure of carbon-coated Li3VO4 nanoparticles embedded in expanded graphite for high performance lithium ion battery. J. Power Sour. 2016, 303, 333–339. [Google Scholar] [CrossRef]
- Newman, J.; Tiedemann, W. Potential and Current Distribution in Electrochemical-Cells-Interpretation of the Half-Cell Voltage Measurements as a Function of Reference-Electrode Location. J. Electrochem. Soc. 1993, 140, 1961–1968. [Google Scholar] [CrossRef]
- Ng, S.H.; La Mantia, F.; Novak, P. A Multiple Working Electrode for Electrochemical Cells: A Tool for Current Density Distribution Studies. Angew. Chem. Int. Ed. 2009, 48, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kunz, M.; Chen, K.; Tamura, N.; Richardson, T.J. Visualization of Charge Distribution in a Lithium Battery Electrode. J. Phys. Chem. Lett. 2010, 1, 2120–2123. [Google Scholar] [CrossRef]
- Nanda, J.; Remillard, J.; O’Neill, A.; Bernardi, D.; Ro, T.; Nietering, K.E.; Go, G.-Y.; Miller, T. Local State-of-Charge Mapping of Lithium-Ion Battery Electrodes. Adv. Funct. Mater. 2011, 21, 3282–3290. [Google Scholar] [CrossRef]
- Yoo, E.; Kim, J.; Hosono, E.; Zhou, H.-S.; Kudo, T.; Honma, I. Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef]
- Wang, X.; Shi, G. Flexible graphene devices related to energy conversion and storage. Energy Environ. Sci. 2015, 8, 790–823. [Google Scholar] [CrossRef]
- Gwon, H.; Kim, H.-S.; Lee, K.U.; Seo, D.-H.; Park, Y.C.; Lee, Y.-S.; Ahn, B.T.; Kang, K. Flexible energy storage devices based on graphene paper. Energy Environ. Sci. 2011, 4, 1277–1283. [Google Scholar] [CrossRef]
- Yang, S.; Gong, Y.; Liu, Z.; Zhan, L.; Hashim, D.P.; Ma, L.; Vajtai, R.; Ajayan, P.M. Bottom-up approach toward single-crystalline VO2-graphene ribbons as cathodes for ultrafast lithium storage. Nano Lett. 2013, 13, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, A.; Liang, S.; Chen, T.; Tang, Y.; Tan, X. Reduced graphene oxide modified V2O3 with enhanced performance for lithium-ion battery. Mater. Lett. 2014, 137, 174–177. [Google Scholar] [CrossRef]
- Xu, J.; Li, Z.; Zhang, X.; Huang, S.; Jiang, S.; Zhu, Q.; Sun, H.; Zakharova, G.S. Self-assembled V3O7/graphene oxide nanocomposites as cathode material for lithium-ion batteries. Int. J. Nanotechnol. 2014, 11, 808–818. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Zhang, Y.; Liang, S.; Pan, A. Controllable Preparation of V2O5/Graphene Nanocomposites as Cathode Materials for Lithium-Ion Batteries. Nanoscale Res. Lett. 2016, 11, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Ren, G.; Hoque, M.N.F.; Bayne, S.; Zhu, K.; Fan, Z. Fast Supercapacitors Based on Graphene-Bridged V2O3/VOx Core–Shell Nanostructure Electrodes with a Power Density of 1 MW kg−1. Adv. Mater. Interfaces 2014, 1, 1400398–1400406. [Google Scholar] [CrossRef]
- Cui, C.J.; Wu, G.M.; Shen, J.; Zhou, B.; Zhang, Z.H.; Yang, H.Y.; She, S.F. Synthesis and electrochemical performance of lithium vanadium oxide nanotubes as cathodes for rechargeable lithium-ion batteries. Electrochim. Acta 2010, 55, 2536–2541. [Google Scholar] [CrossRef]
- Su, D.W.; Dou, S.X.; Wang, G.X. Hierarchical orthorhombic V2O5 hollow nanospheres as high performance cathode materials for sodium-ion batteries. J. Mater. Chem. 2014, 2, 11185–11194. [Google Scholar] [CrossRef]
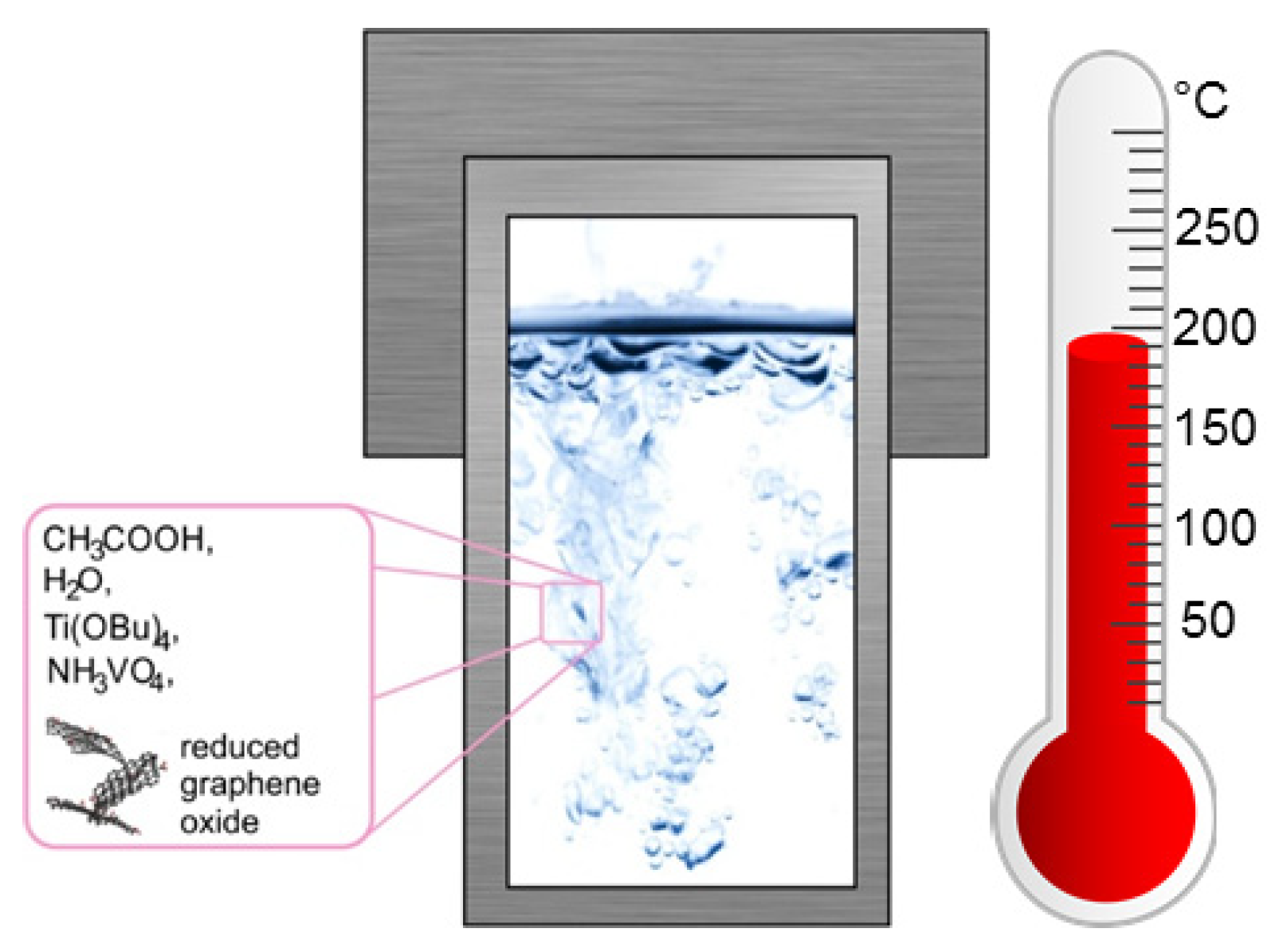


| Sample Name | Volume of H2O/mL | CH3COOH/mL | Amount of NH4VO3/g | Volume of Ti(OC4H7)4 /mL | Temperature/K | Time/h |
|---|---|---|---|---|---|---|
| S2V0Ti1 | 20 | 40 | 0 | 10 | 473 | 24 |
| S2V0.25Ti1 | 0.1 | |||||
| S2V0.5Ti1 | 0.2 | |||||
| S2V1Ti1 | 0.4 |
| Reference | Electrode Materials | Discharge Capacity/mAh g−1 | Current/mA g−1 |
|---|---|---|---|
| [15] | Carbon-coated Li3VO4(Li3VO4/C) | 370 | 15C (C = 394) |
| [24] | LixV2O5 | 116 | C/4 (C = 294) |
| [25] | Li3VO4/graphene nanosheets composite (Li3VO4/graphene) | 223 | 20C (C = 400) |
| [26] | V2O5 | 273 | 0.2C (C = 294) |
| [26] | V2O5-300 | 256 | 0.2C (C = 300) |
| [26] | V2O5-400 | 210 | 0.2C (C = 300) |
| [26] | V2O5-400 | 195 | 0.2C (C = 300) |
| [27] | LiNi0.5Co0.2Mn0.3O2 was surface-modified by double-layer coating: V2O5, graphene oxide (GO-VO-NCM) | 125 | 0.2C (C = 270) |
| [27] | VO-NCM | 130 | 0.2C (C = 270) |
| [28] | Li3VO4/C–Ni composite | 325 | 10C (C = 180) |
| [29] | LiV2O5 | 135 | C/5 (C=294) |
| [6] | rGO-V2O5 | 295 | 10C (C = 394) |
| [31] | TiO2-V2O5 | 319 | 100 |
| [32] | Hierarchical V2O5 nanobelts (V2O5-HNbs) | 248 | 50 |
| [33] | V2O5- TiO2 | 452 | 50 |
| [34] | TiO2-V2O5 | 195 | 17 |
| [35] | Ag-TiO2/V2O5 | 300 | 50 |
| [36] | Li3VO4/C | 360 | 100 |
| Current Rate/mA g−1 | SAMPLE NAME/CAPACITY mAh g−1 | ||
|---|---|---|---|
| S2V0Ti1 | S2V0.25Ti1 | S2V0.5Ti1 | |
| 50 (20th cycle) | 120 | 134 | 155 |
| 100 (40th cycle) | 110 | 122 | 143 |
| 150 (60th cycle) | 100 | 113 | 134 |
| 200 (80th cycle) | 90 | 104 | 121 |
| 300 (100th cycle) | 90 | 100 | 120 |
| 500 (120th cycle) | 85 | 95 | 118 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurc, B.; Wysokowski, M.; Rymaniak, Ł.; Lijewski, P.; Piasecki, A.; Fuć, P. The Impact of the Vanadium Oxide Addition on the Physicochemical Performance Stability and Intercalation of Lithium Ions of the TiO2-rGO-electrode in Lithium Ion Batteries. Materials 2020, 13, 1018. https://doi.org/10.3390/ma13041018
Kurc B, Wysokowski M, Rymaniak Ł, Lijewski P, Piasecki A, Fuć P. The Impact of the Vanadium Oxide Addition on the Physicochemical Performance Stability and Intercalation of Lithium Ions of the TiO2-rGO-electrode in Lithium Ion Batteries. Materials. 2020; 13(4):1018. https://doi.org/10.3390/ma13041018
Chicago/Turabian StyleKurc, Beata, Marcin Wysokowski, Łukasz Rymaniak, Piotr Lijewski, Adam Piasecki, and Paweł Fuć. 2020. "The Impact of the Vanadium Oxide Addition on the Physicochemical Performance Stability and Intercalation of Lithium Ions of the TiO2-rGO-electrode in Lithium Ion Batteries" Materials 13, no. 4: 1018. https://doi.org/10.3390/ma13041018
APA StyleKurc, B., Wysokowski, M., Rymaniak, Ł., Lijewski, P., Piasecki, A., & Fuć, P. (2020). The Impact of the Vanadium Oxide Addition on the Physicochemical Performance Stability and Intercalation of Lithium Ions of the TiO2-rGO-electrode in Lithium Ion Batteries. Materials, 13(4), 1018. https://doi.org/10.3390/ma13041018










