The Preparation and Properties of a Shell Structure Ceramsite
Abstract
:1. Introduction
2. Experiment
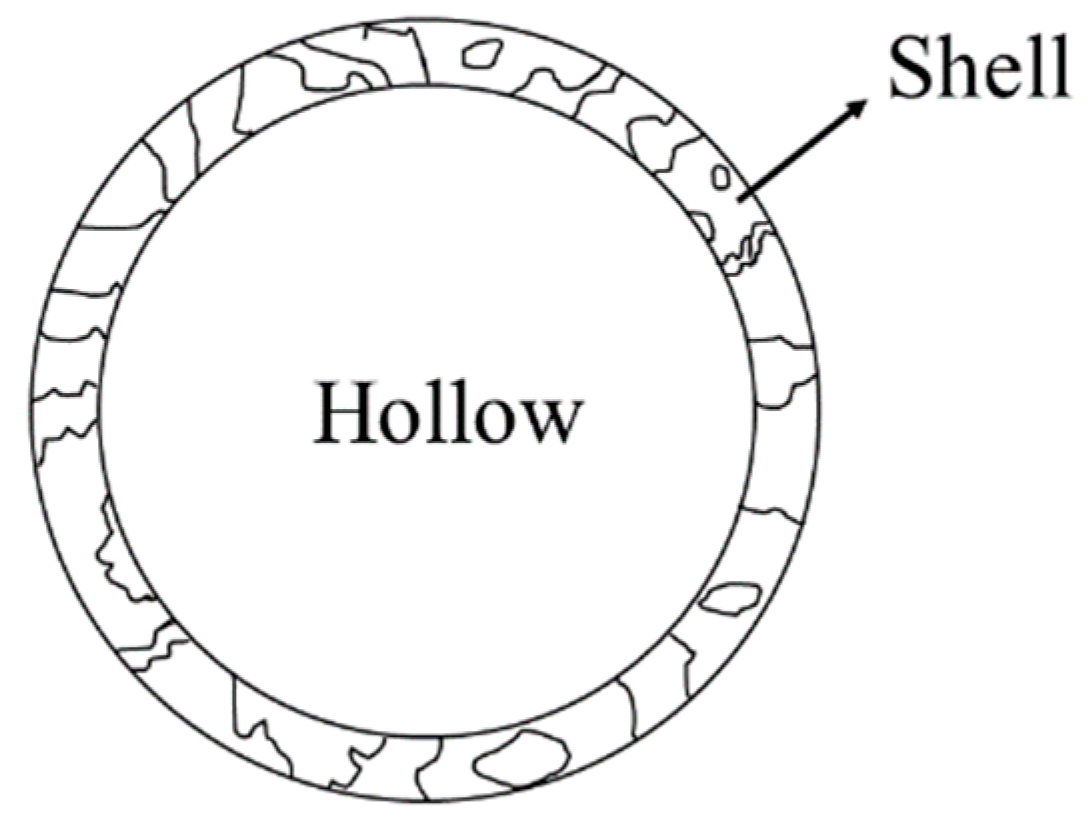
2.1. Shell Structure Ceramsite
2.2. Raw Materials and Equipment
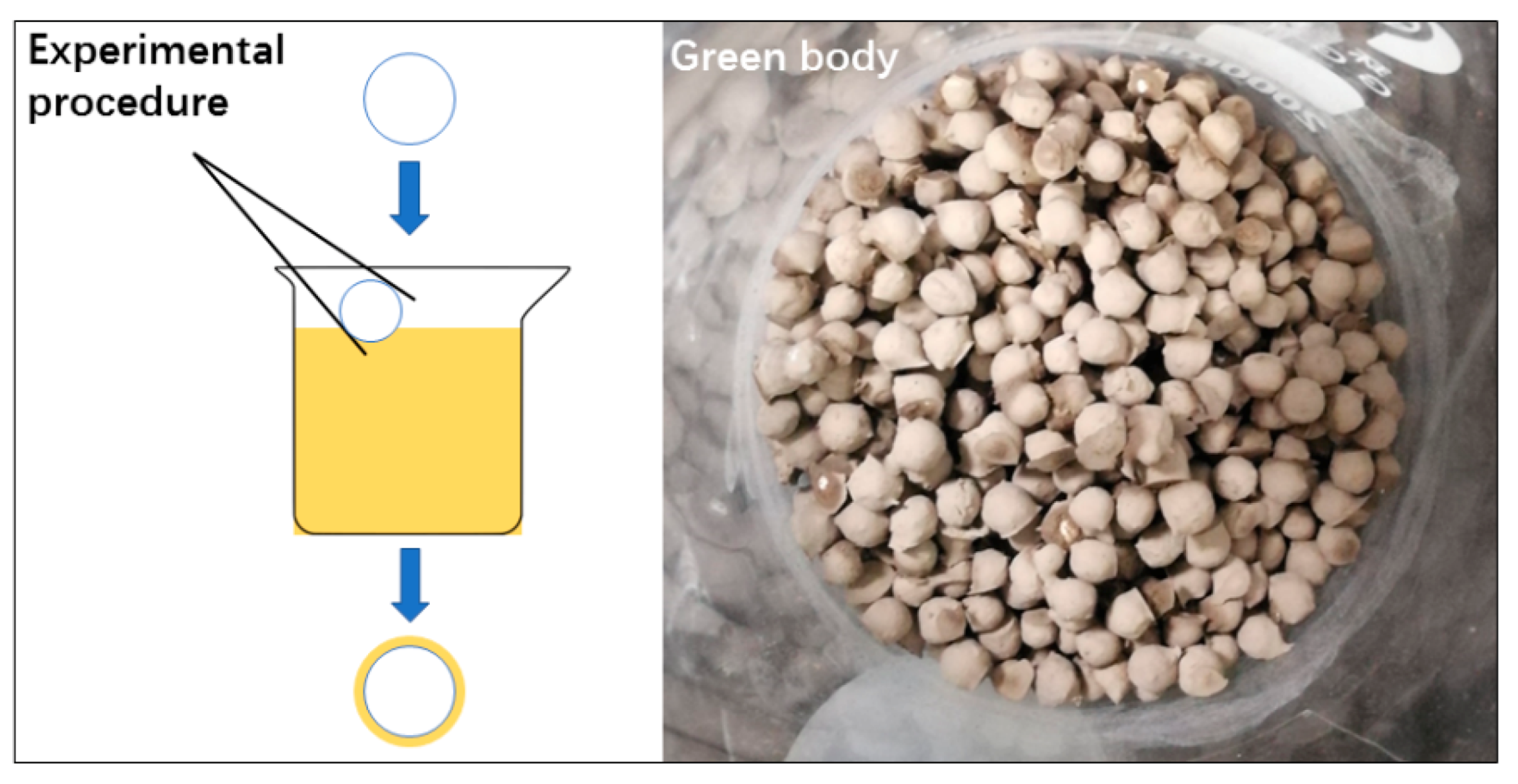
2.3. Experimental Procedures and Method
2.4. Testing Methods
3. Results and Discussion
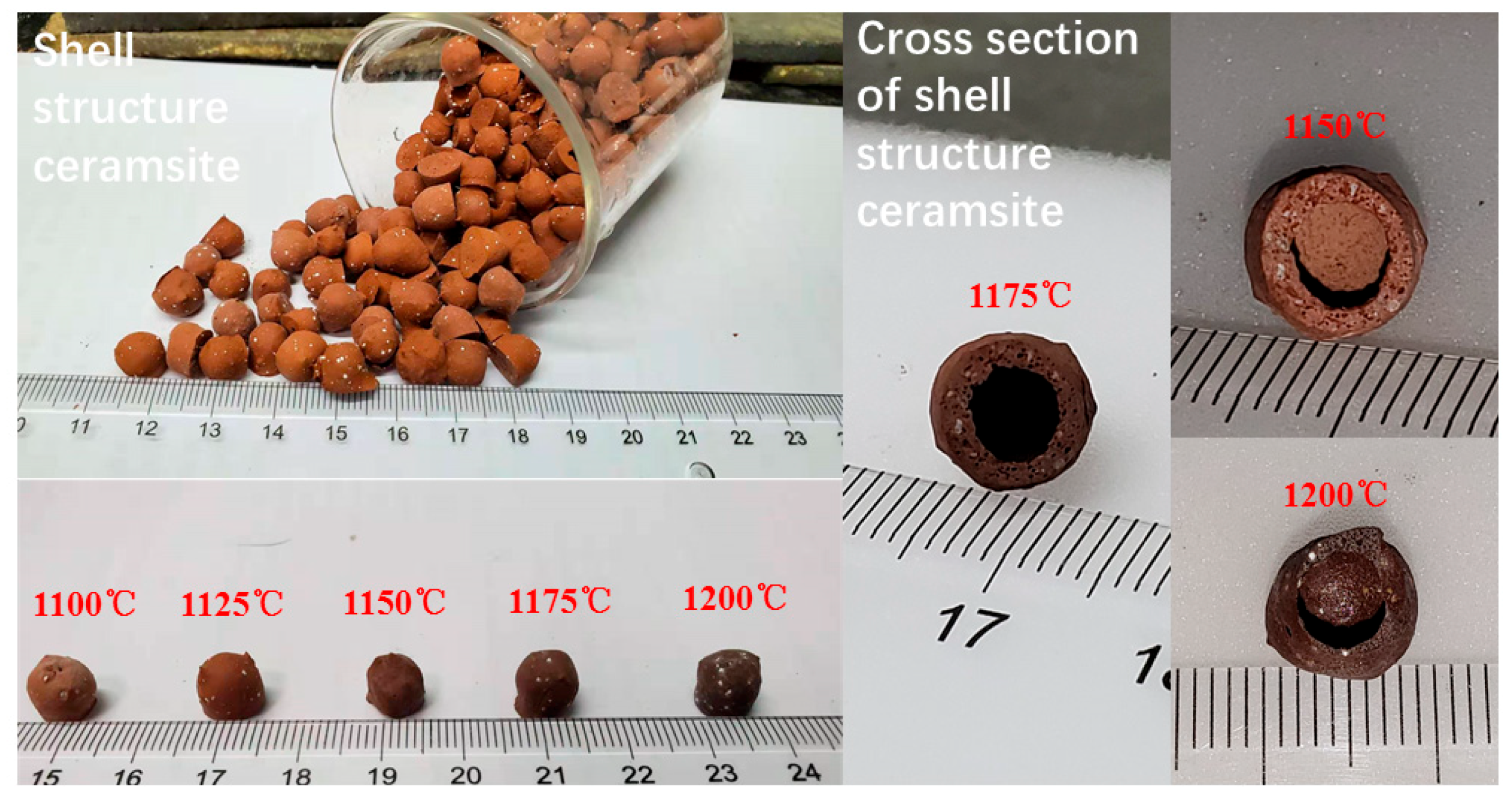
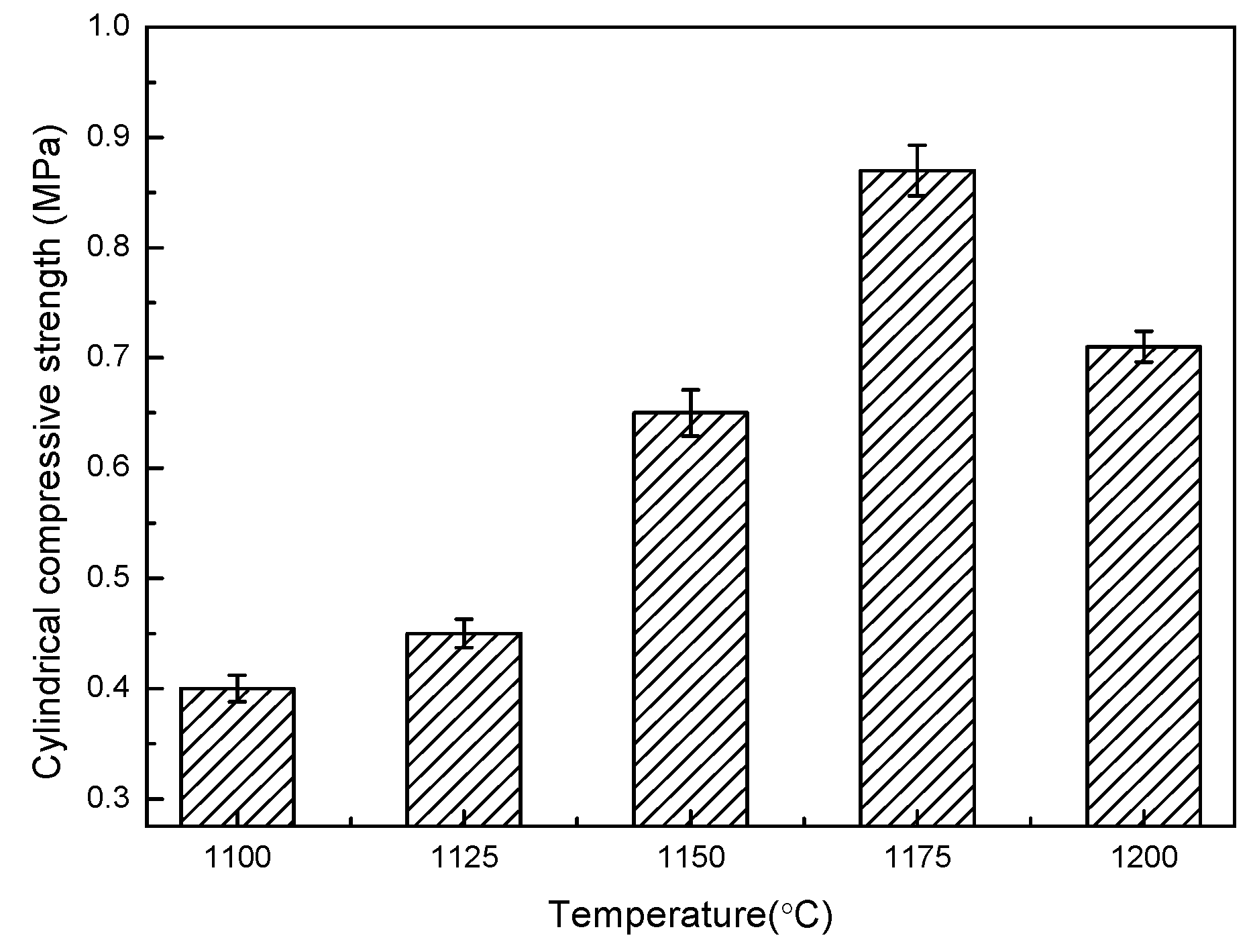
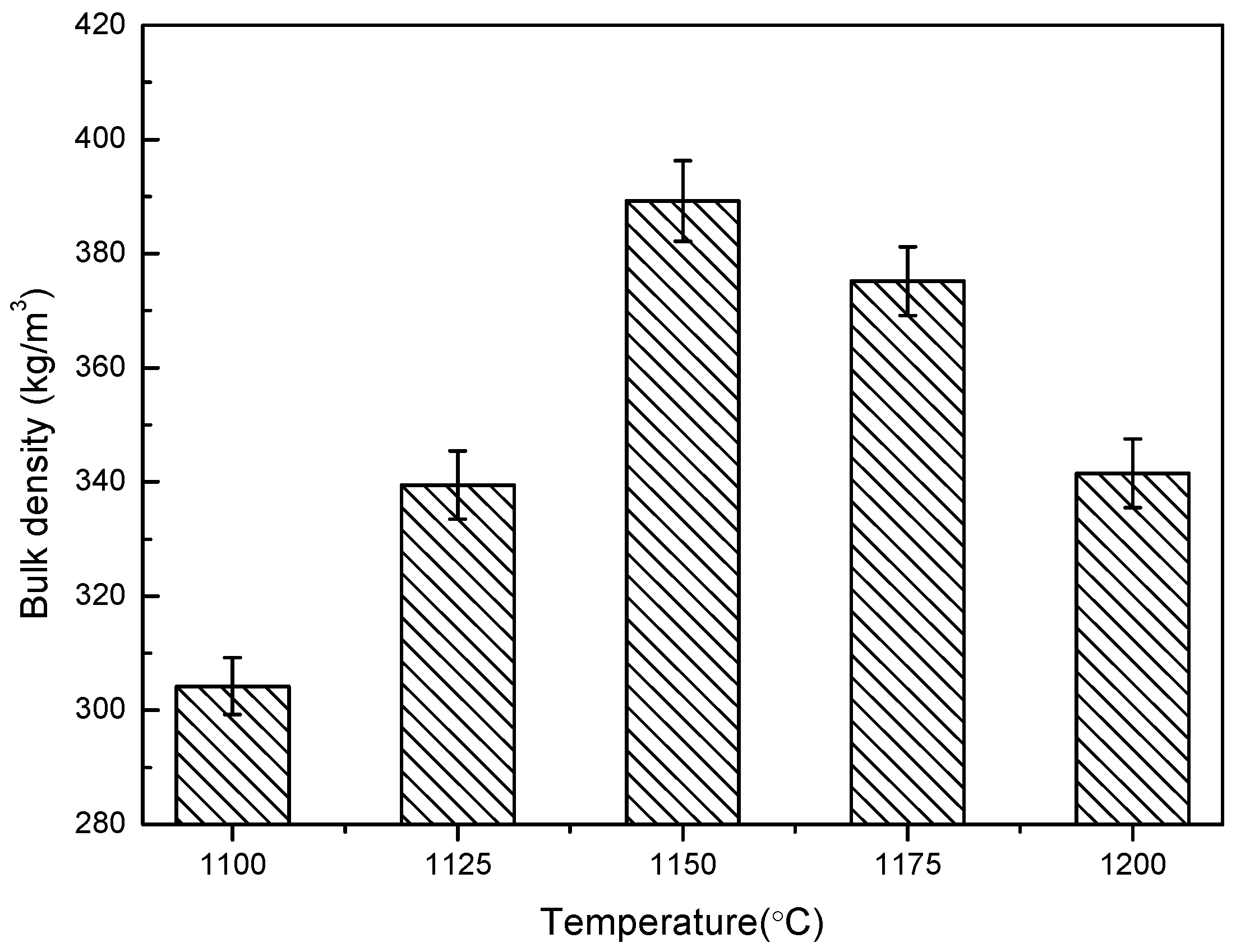
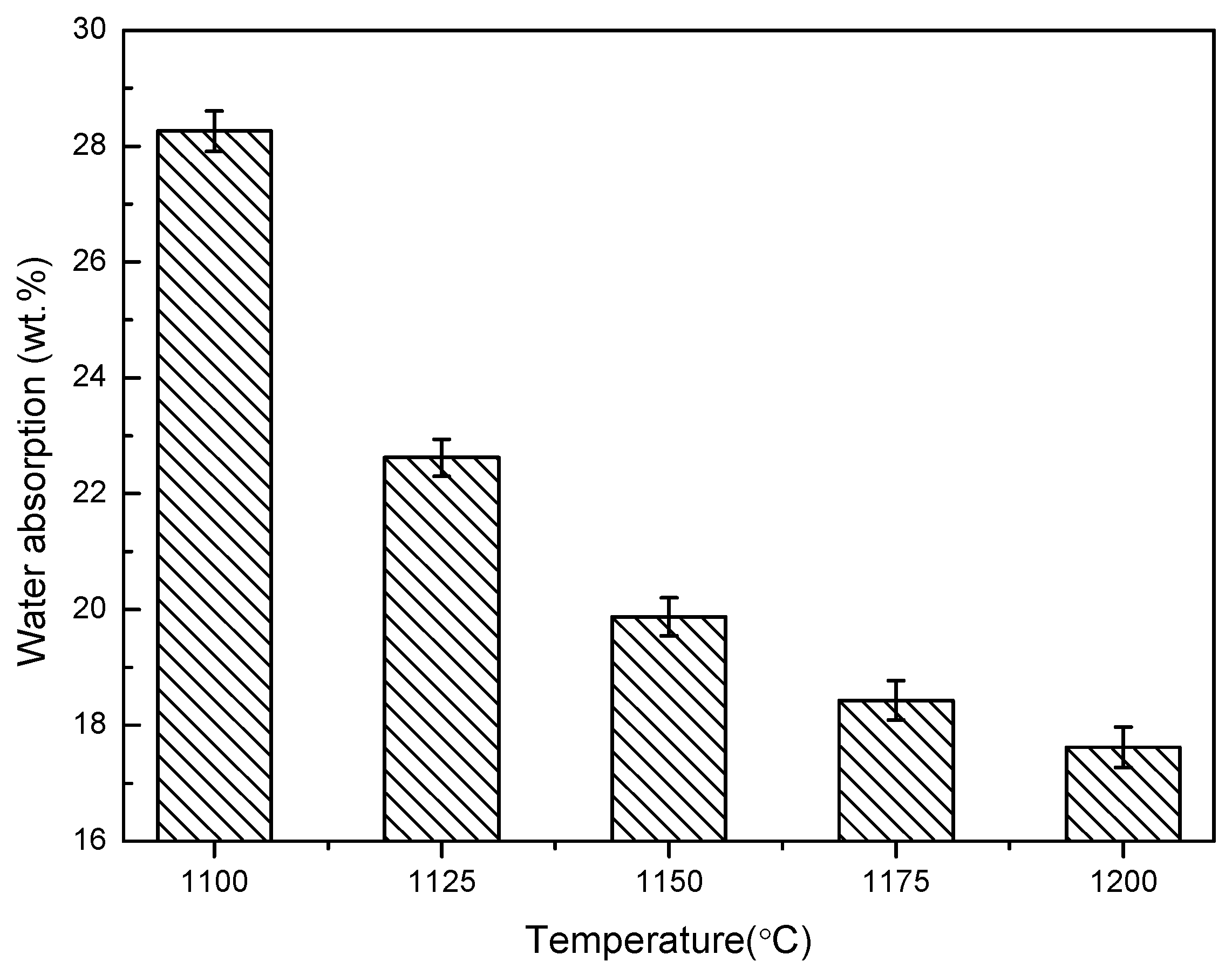
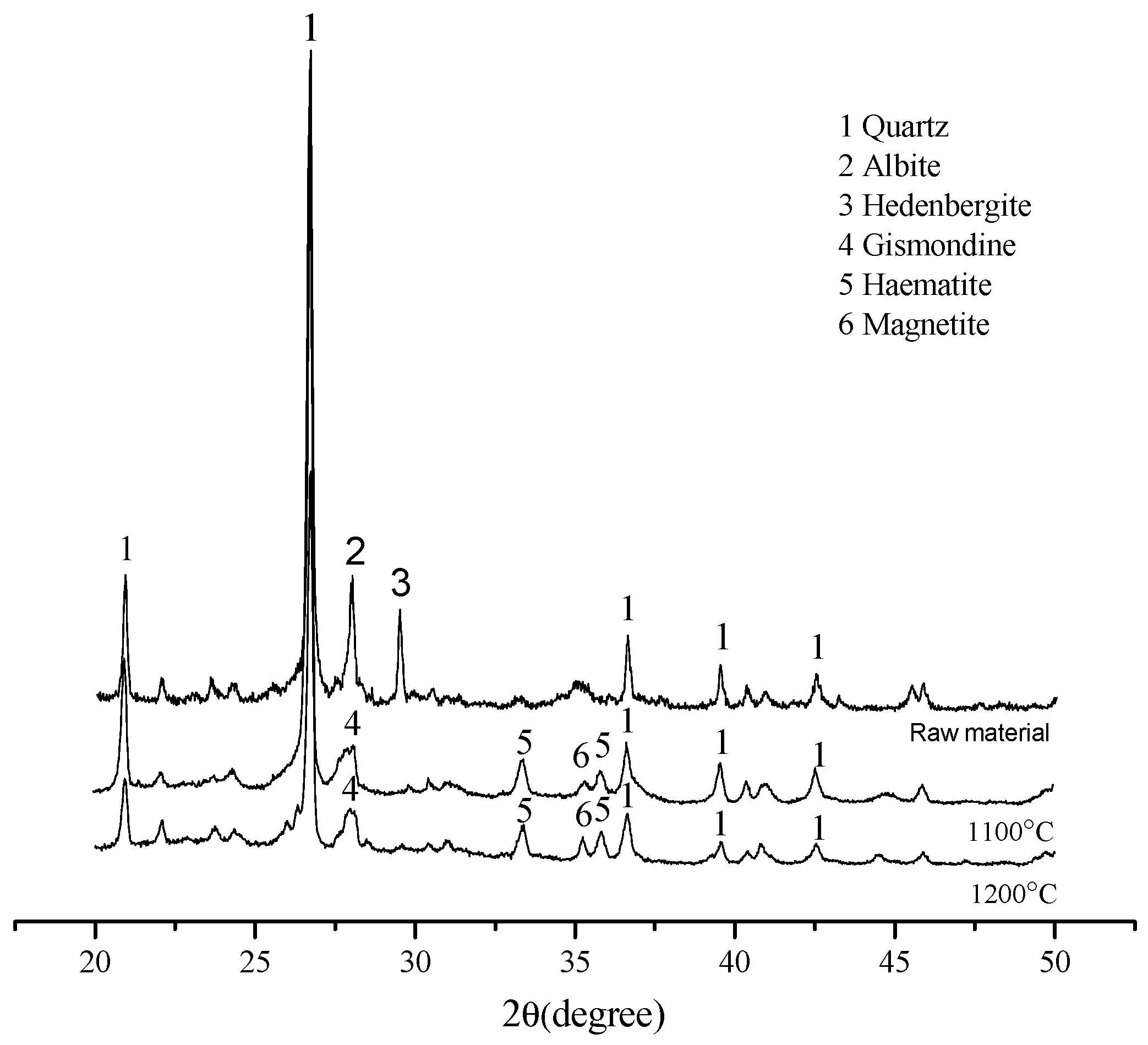
3.1. The Temperature Influence on Physical Properties of Shell Structure Ceramsite
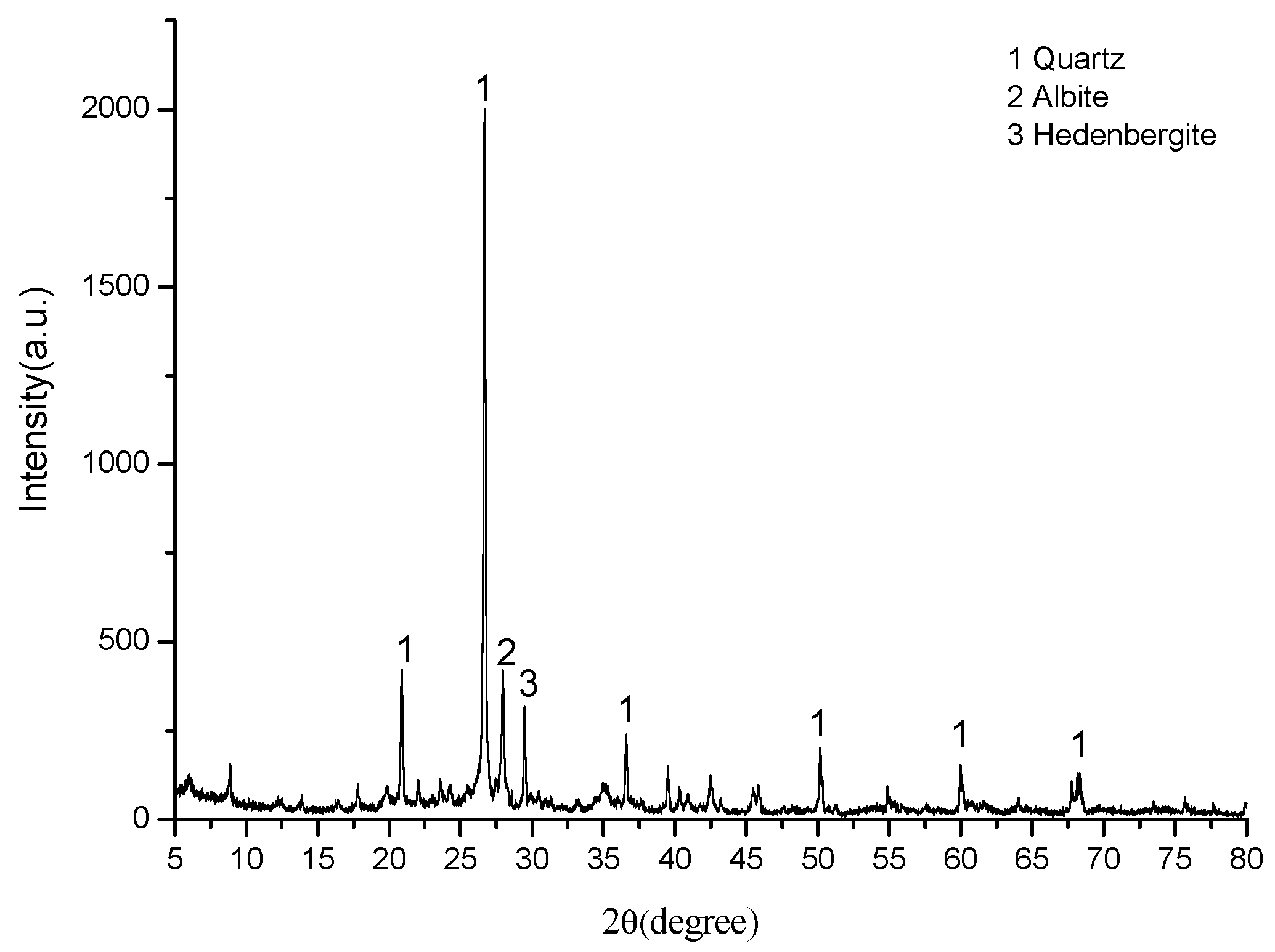
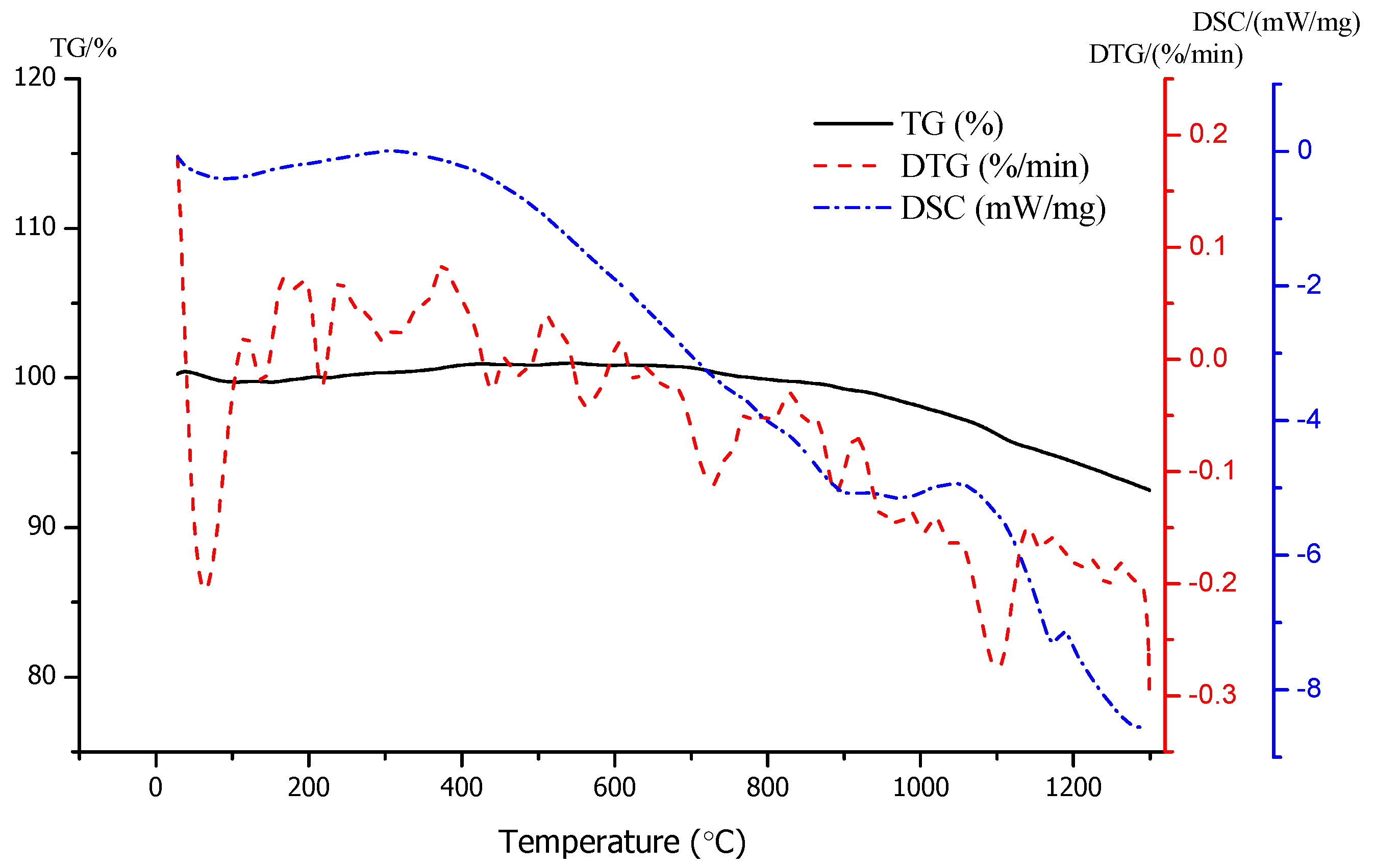
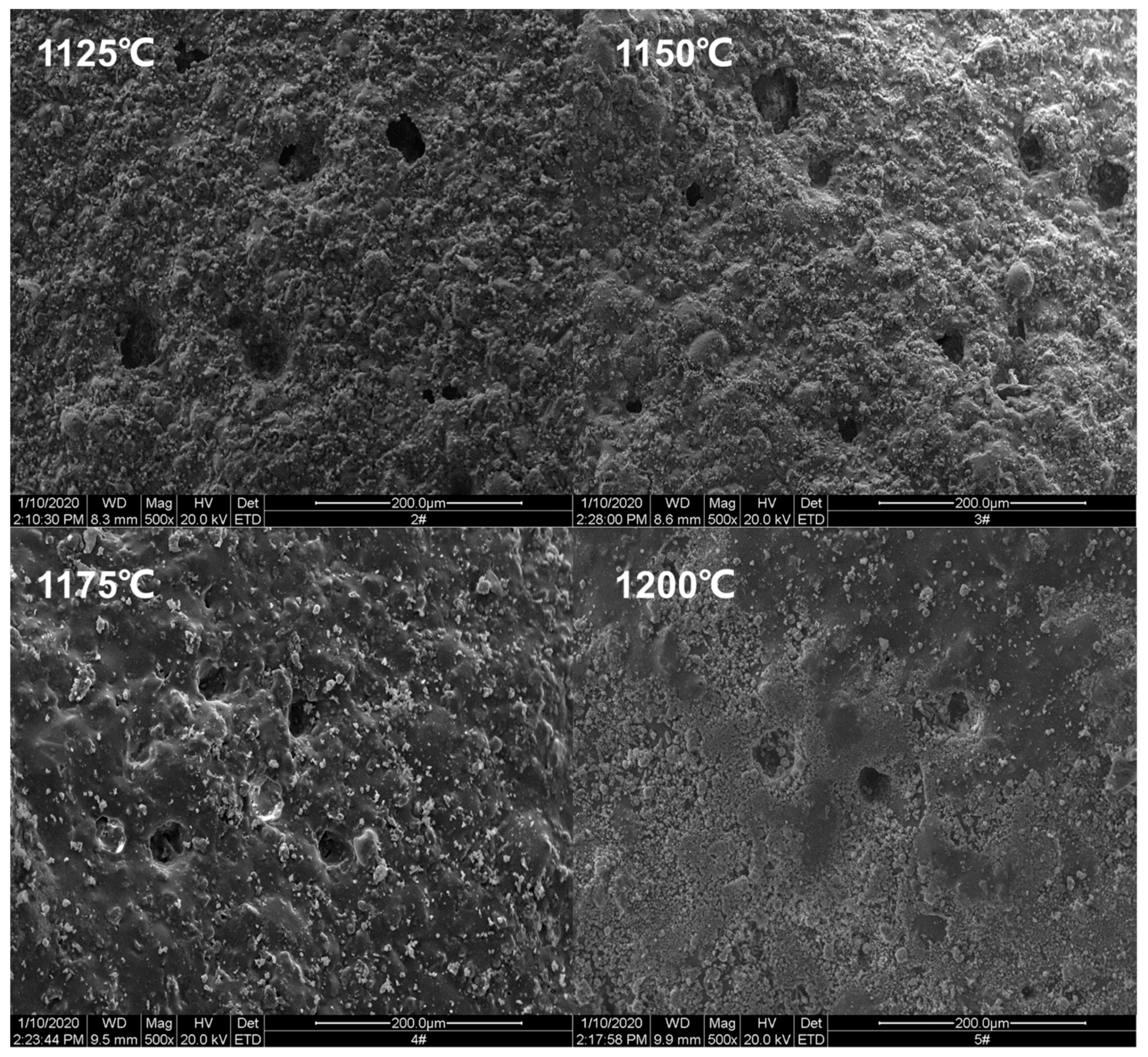
3.2. Micro Analysis of the Sintering Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dai, Z.; Li, Y.; Zhao, T. Development research on sandeich panel of lightweight concrete. Fujian Archit. Constr. 2003, 1, 29–30. [Google Scholar]
- He, H.; Zhao, P.; Yue, Q.; Gao, B.; Li, Q. A novel polynary fatty acid/sludge ceramsite composite phase change materials and its applications in building energy conservation. J. Renew. Energy 2015, 76, 45–52. [Google Scholar] [CrossRef]
- Zhuang, Y.Z.; Chen, C.Y.; Ji, T. Effect of shale ceramsite type on the tensile creep of lightweight aggregate concrete. J. Constr. Build. Mater. 2013, 46, 13–18. [Google Scholar] [CrossRef]
- Fu, J.; Cheng, Y.; Tang, Y.; Liu, M. Application of fly ash porous ceramsite to wastewater treatment. J. Environ. Sci. Technol. 2008, 11. [Google Scholar] [CrossRef]
- Jin, X.; Zeng, L. Sound absroption characteristics and air-flow resistivity determination of metro sound absorbed material. J. Synth. Cryst. 2008, 8, 918–926. [Google Scholar]
- Zhou, D.; Zhang, Z.; Li, F.; Pan, Z.; Zhu, S.; Jin, J. Preparation of cement based on composite porous sound-absorbing material and its properties. Noise Vib. Control 2008, 28, 136–140. [Google Scholar]
- Pan, L.Q.; Yuan, X.L.; He, X.D.; Zhou, L. Identification of medium formula of soilless cultivation for bonsai Buxus silica. J. B. For. Univ. 2011, 33, 104–107. [Google Scholar]
- Liu, Z.M.; Zheng, W.K.; Li, H.; Yang, Y.X.; Zhang, J.J. Experimental study on the preparation of ceramsite raw ball by disc coating. J. Synth. Cryst. 2018, 6, 1248–1253. [Google Scholar]
- Zhao, W.; Wang, Z.; Huang, H.N. Research on preparation and properties of lightweight high- intensity ceramsites based on gold tailings. J. Synth. Cryst. 2018, 47, 1266–1277. [Google Scholar]
- Shao, Q.; Xu, H.; Tong, M.M. The technical study on the preparation of lightweight ceramsite made from sewage sludge and fly ash. Adv. Mat. Res. 2011, 365, 1871–1875. [Google Scholar] [CrossRef]
- Liu, Y.D.; Yang, D.Y.; Jia, Y.T. Preparation of ultra-lightweight sludge ceramsite and analysis of its inner-structure characteristics. Concrete 2014, 6, 65–68. [Google Scholar]
- Ma, L.; Li, G.Z. The preparation of red mud lightweight ceramsite. Adv. Mat. Res. 2013, 648, 96–99. [Google Scholar] [CrossRef]
- Jia, C.J.; Gan, H.J.; Ma, B.C.; Liu, Y.X. Study on preparation of water retaining aggregate using lake sludge. J. Green Sci. Technol. 2017, 6, 9–11. [Google Scholar]
- Lu, M.; Xia, G.H.; Liao, R.H. Preparation and modification of porous lightweight ceramsite and its performance investigation. Desalin. Water Treat. 2013, 51, 22–24. [Google Scholar] [CrossRef]
- Jing, Q.; Wang, Y.; Chai, L.; Chai, L.; Tang, C.; Huang, X.; Gio, H.; Wang, W.; You, W. Adsorption of copper ions on porous ceramsite prepared by diatomite and tungsten residue. T. Nonferr. Metal. Soc. 2018, 28, 1053–1060. [Google Scholar] [CrossRef]
- Zou, J.L.; Xu, G.R.; Li, G.B. Ceramsite obtained from water and wastewater sludge and its characteristics affected by Fe2O3, CaO, and MgO. J. Hazard. Mater. 2009, 165, 995–1001. [Google Scholar] [CrossRef]
- Wang, D.M.; Song, J.P.; Liu, B.H.; Lei, G.Y.; Liu, S.; Luo, W.B.; Cheng, Y.J. Preparation of porous ceramsite with low silica iron tailings and its effects in sewage water treatment. J. Met. Mine. 2012, 12, 148–152. [Google Scholar]
- Sang, D.; Wang, A.G.; Sun, D.S.; Liu, K.W.; Guan, Y.M.; Wang, H. Manufacturing Sintering-Expanded Ceramsite from Industrial Solid Wastes. Mater. Rep. 2016, 30, 110–114. (In Chinese) [Google Scholar]
- Yang, Y.X.; Li, H.; Zheng, W.K.; Bai, Y.; Liu, Z.M. Experimental study on calcining process of secondary coated ceramsite solidified chromium contaminated soil. Sci. Adv. Mater. 2019, 11, 208–214. [Google Scholar] [CrossRef]
- Yan, C.L. The Preparation of Gold Tailings Roasting Ceramsite and Its Performance Study. Master Thesis, University of Jinan, Jinan, China, 2014. [Google Scholar]
- Wang, L.L.; Yang, D.Y.; Ai, Y.M. Preparation and void structure of sludge ceramsite. Concrete 2016, 1, 103–111. [Google Scholar]
- Li, L. Experimental study on preparation of fly ash ceramsite. B. Chin. Ceram. Soc. 2017, 36, 1577–1589. [Google Scholar]
- Hu, C.G.; Xing, C.E.; Liu, L. Preparation and performance of high-strength ceramisite with core-Shell Structure Based on Iron tailings and alkaline residue. J. Metal. Mine. 2019, 5, 197–203. [Google Scholar]
- Wang, P. Experimental Research on the Fundamental Mechanical Behavior of Ceramisite Concrete. Master Thesis, Changsha University of Science & Technology, Changsha, China, 2008. [Google Scholar]
- Zhang, Y.F. Study on the Reutilization of Municipal Sewage Sludge-Sludge Aggregate Development and Application. Master Thesis, Fuzhou University, Fuzhou, China, 2005. [Google Scholar]
- Lu, S.; Hou, Z.; Wang, F.; Guo, W. Engineering application of pumping ceramsite concrete. Concrete 2015, 4, 148–150. [Google Scholar]
- Wang, S. Analysis on the mechanical properties and the engineering application of fly ash aggregate. Sichuan Build. Mater. 2015, 41, 26–27. [Google Scholar]
- Qian, W.; Fan, C.G.; Ran, S.L.; Guo, W.J. Preparation and Performance of Specified Density Concrete Using Keramzite Made from Municipal Sewage Sludge. Concrete 2012, 4, 122–125. [Google Scholar]
- Li, P.J.; Liu, X.B. Fundamental mechanical proerties of concrete with high strength expanded expanded Shale. J. Build. Mater. 2004, 1, 113–116. [Google Scholar]
- Na, W. Production of sludge ceramsite from sewage sludge, municipal solid waste incineration fly ash and clay. Nat. Environ. Pollut. Technol. 2015, 14, 153–156. [Google Scholar]
- Zhou, M. Research on preparation of light and high strength haydite made by kaolin tailings-gangue-fly ash. New Build. Mater. 2013, 2, 59–67. [Google Scholar]
- Zhao, W.; Wang, Y.B.; Zhou, C.S. Research on preparation of light-weight and high-intensity ceramsites with shangluo vanadium tailings. J. Synth. Cryst. 2017, 46, 1858–1863. [Google Scholar]
- Qin, J.; Chong, C.; Xiao, Y. Preparation and characterization of ceramsite from lime mud and coal fly ash. Constr. Build. Mater. 2015, 95, 10–17. [Google Scholar] [CrossRef]
- Li, X.L.; Li, Z.; Wang, L. Preparation of ultralight hollow ceramsite with high carbon fly ash. Sci. Technol. Eng. 2019, 19, 260–264. [Google Scholar]
- Burkhanova, K.M.; Koven-Kina, V.L.; Murtazina, E.M. Production of refractory keramzite. Zaporozhe Ind. Inst. 1981, 1, 47–50. [Google Scholar] [CrossRef]
- Ma, L.; Li, G.Z. Influence of sintering temperature on performance of red mud lightweight ceramsite. Adv. Mat. Res. 2013, 662, 323–326. [Google Scholar] [CrossRef]
- Riley, C.M. Relation of chemical properties to the bloating of clays. J. Am. Ceram. Soc. 1951, 34, 121–128. [Google Scholar] [CrossRef]

| Component | SiO2 | Al2O3 | Fe2O3 | CaO | K2O | MgO | Na2O | SO3 | Others |
|---|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 57.14 | 24.03 | 4.979 | 4.77 | 3.39 | 1.53 | 1.02 | 0.036 | 3.105 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, W.; He, D.; Li, H.; Wang, F.; Yang, Y.; Zhang, J. The Preparation and Properties of a Shell Structure Ceramsite. Materials 2020, 13, 1009. https://doi.org/10.3390/ma13041009
Zheng W, He D, Li H, Wang F, Yang Y, Zhang J. The Preparation and Properties of a Shell Structure Ceramsite. Materials. 2020; 13(4):1009. https://doi.org/10.3390/ma13041009
Chicago/Turabian StyleZheng, Wukui, Diyang He, Hui Li, Fei Wang, Yuxuan Yang, and Jingjie Zhang. 2020. "The Preparation and Properties of a Shell Structure Ceramsite" Materials 13, no. 4: 1009. https://doi.org/10.3390/ma13041009
APA StyleZheng, W., He, D., Li, H., Wang, F., Yang, Y., & Zhang, J. (2020). The Preparation and Properties of a Shell Structure Ceramsite. Materials, 13(4), 1009. https://doi.org/10.3390/ma13041009





